Enhancement of Bioactive Properties in Momordica charantia by Leuconostoc Fermentation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials and Chemicals
2.2. Preparation of Fermentation
2.3. Measurement of Free Sugar, Organic Acid, and Alcohol
2.4. Measurement of Dextran Contents
- W1: weight of dextran produced of fermented sample (g);
- W2: weight of dextran produced of non-fermented sample (g);
- A: amount of sucrose added to the medium (g).
2.5. Measurement of Carbohydrate Pattern
2.6. Measurement of Carbohydrate-Hydrolyzing Enzyme Inhibitory Activity
2.6.1. α-Glucosidase Inhibitory Activity
- ABSsample: absorbance of the experimental sample;
- ABSsample blank: absorbance of the sample blank;
- ABScontrol: absorbance of the control;
- ABSblank: absorbance of the blank.
2.6.2. α-Amylase Inhibitory Activity
2.7. Measurement of Acetylcholinesterase (AChE) Inhibitory Activity and Butyrylcholinesterase (BuChE) Inhibitory Activity
- Ssample: Slope of the sample;
- Scontrol: Slope of the control.
2.8. Measurement of SOD-Like Activity
2.9. Correlation Analysis, Principal Component Analysis, and Statistical Analysis
2.10. Statistical Analysis
3. Results
3.1. Contents of Free Sugar and Metabolites in Fermented MC
3.2. Carbohydrate-Hydrolyzing Enzyme Inhibitory Activity of Fermented MC
3.3. Inhibitory Activity of Cholinesterase in MC Fermentation
3.4. Antioxidant Activity of MC Fermented by Leuconostoc Strains
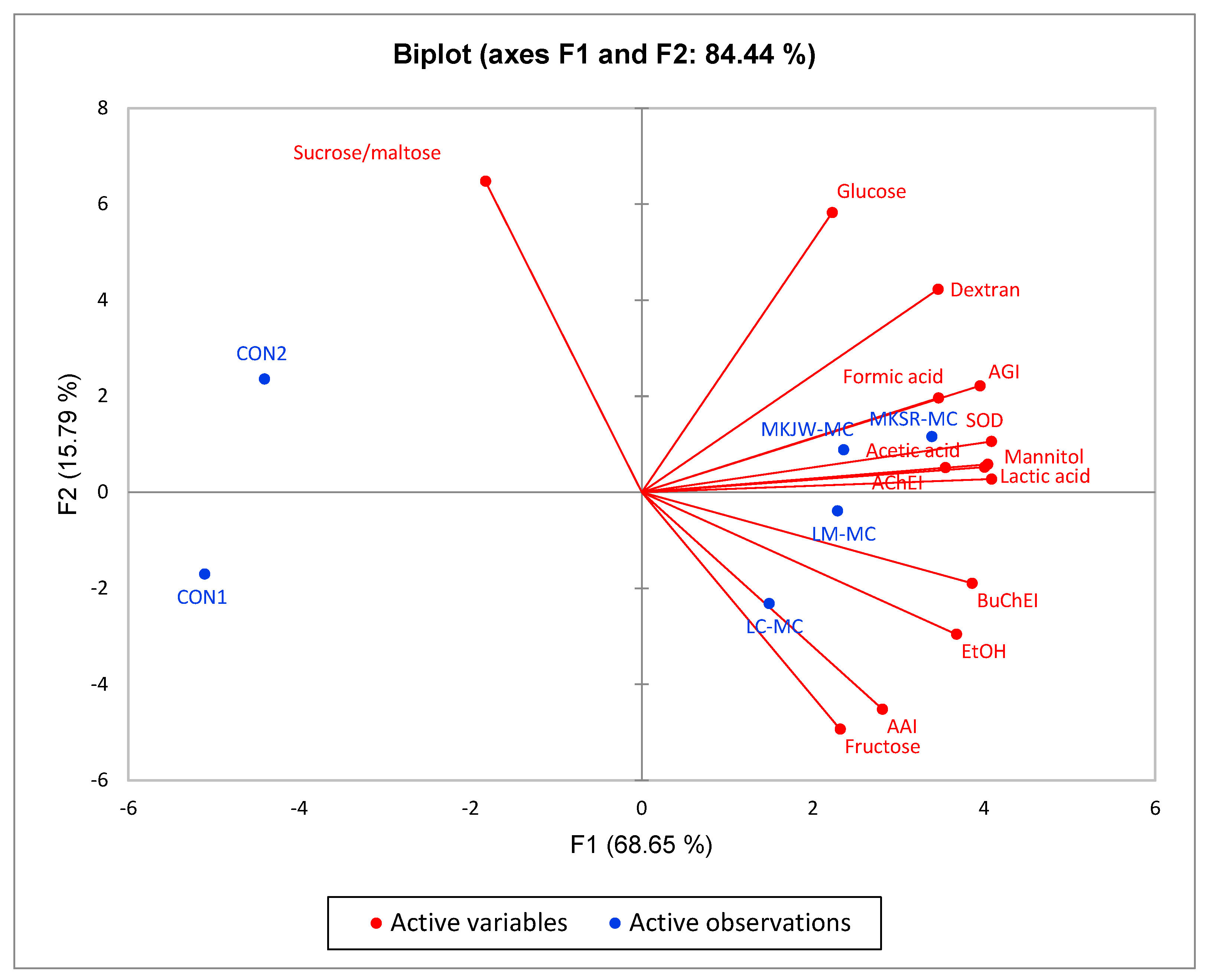
3.5. Correlation Analysis of Enzyme Inhibitory Activities, Dextran Yield, Free Sugar, Organic Acid, and Antioxidant Activity of Fermented MC by Leuconostoc Strains
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Dementia. Available online: https://www.who.int/news-room/fact-sheets/detail/dementia (accessed on 15 March 2023).
- Goyal, R.; Jialal, I. Diabetes mellitus type 2. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2018. [Google Scholar]
- Ahtiluoto, S.; Polvikoski, T.; Peltonen, M.; Solomon, A.; Tuomilehto, J.; Winblad, B.; Sulkava, R.; Kivipelto, M. Diabetes, Alzheimer disease, and vascular dementia: A population-based neuropathologic study. Neurology 2010, 75, 1195–1202. [Google Scholar] [CrossRef] [PubMed]
- Feil, D.G.; Zhu, C.W.; Sultzer, D.L. The relationship between cognitive impairment and diabetes self-management in a population-based community sample of older adults with Type 2 diabetes. J. Behav. Med. 2012, 35, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Whitmer, R.A.; Gunderson, E.P.; Quesenberry, C.P.; Zhou, J.; Yaffe, K. Body mass index in midlife and risk of Alzheimer disease and vascular dementia. Curr. Alzheimer Res. 2007, 4, 103–109. [Google Scholar] [CrossRef]
- Reddy, V.P.; Zhu, X.; Perry, G.; Smith, M.A. Oxidative stress in diabetes and Alzheimer’s disease. J. Alzheimer’s Dis. 2009, 16, 763–774. [Google Scholar] [CrossRef] [PubMed]
- Anwar, S.; Rivest, S. Alzheimer’s disease: Microglia targets and their modulation to promote amyloid phagocytosis and mitigate neuroinflammation. Expert Opin. Ther. Targets 2020, 24, 331–344. [Google Scholar] [CrossRef]
- Leblhuber, F.; Steiner, K.; Schuetz, B.; Fuchs, D.; Gostner, J.M. Probiotic supplementation in patients with Alzheimer’s dementia-an explorative intervention study. Curr. Alzheimer Res. 2018, 15, 1106–1113. [Google Scholar] [CrossRef]
- Firouzi, S.; Majid, H.A.; Ismail, A.; Kamaruddin, N.A.; Barakatun-Nisak, M. Effect of multi-strain probiotics (multi-strain microbial cell preparation) on glycemic control and other diabetes-related outcomes in people with type 2 diabetes: A randomized controlled trial. Eur. J. Nutr. 2017, 56, 1535–1550. [Google Scholar] [CrossRef]
- Kim, M.; Yoon, J.; Seong, H.; Jeong, Y. Novel Leconostoc mesemteroides MKJW, and uses thereof. 10-2021-0014624, 2 February 2021. [Google Scholar]
- Lee, S.; Kim, M. Leuconostoc mesenteroides MKSR isolated from kimchi possesses α-glucosidase inhibitory activity, antioxidant activity, and cholesterol-lowering effects. LWT 2019, 116, 108570. [Google Scholar] [CrossRef]
- Campanella, D.; Rizzello, C.G.; Fasciano, C.; Gambacorta, G.; Pinto, D.; Marzani, B.; Scarano, N.; De Angelis, M.; Gobbetti, M. Exploitation of grape marc as functional substrate for lactic acid bacteria and bifidobacteria growth and enhanced antioxidant activity. Food Microbiol. 2017, 65, 25–35. [Google Scholar] [CrossRef]
- Marco, M.L.; Heeney, D.; Binda, S.; Cifelli, C.J.; Cotter, P.D.; Foligné, B.; Gänzle, M.; Kort, R.; Pasin, G.; Pihlanto, A.; et al. Health benefits of fermented foods: Microbiota and beyond. Curr. Opin. Biotechnol. 2017, 44, 94–102. [Google Scholar] [CrossRef]
- Yoon, J.; Kim, M. In vitro evaluation of antidiabetic, antidementia, and antioxidant activity of Artemisia capillaris fermented by Leuconostoc spp. LWT 2022, 172, 114163. [Google Scholar] [CrossRef]
- Oh, Y.J.; Kim, T.S.; Moon, H.W.; Lee, S.Y.; Lee, S.Y.; Ji, G.E.; Hwang, K.T. Lactobacillus plantarum PMO 08 as a probiotic starter culture for plant-based fermented beverages. Molecules 2020, 25, 5056. [Google Scholar] [CrossRef] [PubMed]
- Chavan, M.; Gat, Y.; Harmalkar, M.; Waghmare, R. Development of non-dairy fermented probiotic drink based on germinated and ungerminated cereals and legume. LWT 2018, 91, 339–344. [Google Scholar] [CrossRef]
- Demarinis, C.; Verni, M.; Pinto, L.; Rizzello, C.G.; Baruzzi, F. Use of Selected Lactic Acid Bacteria for the Fermentation of Legume-Based Water Extracts. Foods 2022, 11, 3346. [Google Scholar] [CrossRef]
- Kubola, J.; Siriamornpun, S. Phenolic contents and antioxidant activities of bitter gourd (Momordica charantia L.) leaf, stem and fruit fraction extracts in vitro. Food Chem. 2008, 110, 881–890. [Google Scholar] [CrossRef]
- Yeo, Y.L.; Chia, Y.Y.; Lee, C.H.; Sow, H.S.; Yap, W.S. Effectiveness of Maceration Periods with Different Extraction Solvents on in-vitro Antimicrobial Activity from Fruit of Momordica charantia L. J. Appl. Pharm. Sci. 2014, 4, 016–023. [Google Scholar] [CrossRef]
- Bortolotti, M.; Mercatelli, D.; Polito, L. Momordica charantia, a nutraceutical approach for inflammatory related diseases. Front. Pharmacol. 2019, 10, 486. [Google Scholar] [CrossRef]
- Cho, I.S.; Yoon, H.Y.; Hong, K.W. Quality Characteristics of Teriyaki Sauce added with Bitter Melon (Momoridaica charantia L.) Powder. Culin. Sci. Hosp. Res. 2017, 23, 176–182. [Google Scholar]
- Desai, S.; Tatke, P. Charantin: An important lead compound from Momordica charantia for the treatment of diabetes. J. Pharmacogn. Phytochem. 2015, 3, 163–166. [Google Scholar]
- Shu, C.H.; Jaiswal, R.; Peng, Y.Y.; Liu, T.H. Improving bioactivities of Momordica charantia broth through fermentation using mixed cultures of Lactobacillus plantarum, Gluconacetobacter sp. and Saccharomyces cerevisiae. Process Biochem. 2022, 117, 142–152. [Google Scholar] [CrossRef]
- Gao, H.; Wen, J.J.; Hu, J.L.; Nie, Q.X.; Chen, H.H.; Nie, S.P.; Xiong, T.; Xie, M.Y. Momordica charantia juice with Lactobacillus plantarum fermentation: Chemical composition, antioxidant properties and aroma profile. Food Biosci. 2019, 29, 62–72. [Google Scholar] [CrossRef]
- Gao, H.; Wen, J.J.; Hu, J.L.; Nie, Q.X.; Chen, H.H.; Nie, S.P.; Xiong, T.; Xie, M.Y. Fermented Momordica charantia L. juice modulates hyperglycemia, lipid profile, and gut microbiota in type 2 diabetic rats. Food Res. Int. 2019, 121, 367–378. [Google Scholar] [CrossRef]
- Wen, J.J.; Li, M.Z.; Gao, H.; Hu, J.L.; Nie, Q.X.; Chen, H.H.; Zhang, Y.L.; Xie, M.Y.; Nie, S.P. Polysaccharides from fermented Momordica charantia L. with Lactobacillus plantarum NCU116 ameliorate metabolic disorders and gut microbiota change in obese rats. Food Funct. 2021, 12, 2617–2630. [Google Scholar] [CrossRef]
- Ilaslan, K.; Boyaci, I.H.; Topcu, A. Rapid analysis of glucose, fructose and sucrose contents of commercial soft drinks using Raman spectroscopy. Food Control 2015, 48, 56–61. [Google Scholar] [CrossRef]
- da Costa, M.P.; da Silva Frasao, B.; da Costa Lima, B.R.C.; Rodrigues, B.L.; Junior, C.A.C. Simultaneous analysis of carbohydrates and organic acids by HPLC-DAD-RI for monitoring goat’s milk yogurts fermentation. Talanta 2016, 152, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Diana, C.R.; Humberto, H.S.; Jorge, Y.F. Structural characterization and rheological properties of dextran produced by native strains isolated of Agave salmiana. Food Hydrocoll. 2019, 90, 1–8. [Google Scholar] [CrossRef]
- Oshima, N.; Saito, M.; Niino, M.; Hiraishi, Y.; Ueki, K.; Okoshi, K.; Hakamatsuka, T.; Hada, N. Elucidation of Chemical Interactions between Crude Drugs Using Quantitative Thin-Layer Chromatography Analysis. Molecules 2022, 27, 593. [Google Scholar] [CrossRef]
- Chen, P.; Zhang, Q.; Dang, H.; Liu, X.; Tian, F.; Zhao, J.; Chen, Y.; Zhang, H.; Chen, W. Screening for potential new probiotic based on probiotic properties and α-glucosidase inhibitory activity. Food Control 2014, 35, 65–72. [Google Scholar] [CrossRef]
- Sarmadi, B.; Aminuddin, F.; Hamid, M.; Saari, N.; Abdul-Hamid, A.; Ismail, A. Hypoglycemic effects of cocoa (Theobroma cacao L.) autolysates. Food Chem. 2012, 134, 905–911. [Google Scholar] [CrossRef]
- Rana, Z.H.; Alam, M.K.; Akhtaruzzaman, M. Nutritional composition, total phenolic content, antioxidant and α-amylase inhibitory activities of different fractions of selected wild edible plants. Antioxidants 2019, 8, 203. [Google Scholar] [CrossRef]
- Sharififar, F.; Moshafi, M.H.; Shafazand, E.; Koohpayeh, A. Acetyl cholinesterase inhibitory, antioxidant and cytotoxic activity of three dietary medicinal plants. Food Chem. 2012, 130, 20–23. [Google Scholar] [CrossRef]
- Kandylis, P.; Pissaridi, K.; Bekatorou, A.; Kanellaki, M.; Koutinas, A.A. Dairy and non-dairy probiotic beverages. Curr. Opin. Food Sci. 2016, 7, 58–63. [Google Scholar] [CrossRef]
- Kumar, B.; Tharumasivam, S.V.; Boominathan, V.; Perumal, E.; Dhandapani, P.; Kaliyaperumal, K.; Arumugam, S.; Subramanian, K.; Renuga, P.S.; Shakthivel, V.; et al. A pilot study on nanotherapy of Momordica charantia against trimethyltin chloride-induced neurotoxicity in Danio rerio (zebrafish). J. Nanomater. 2021, 2021, 2180638. [Google Scholar] [CrossRef]
- Rice, T.; Sahin, A.W.; Heitmann, M.; Lynch, K.M.; Jacob, F.; Arendt, E.K.; Coffey, A. Application of mannitol producing Leuconostoc citreum TR116 to reduce sugar content of barley, oat and wheat malt-based worts. Food Microbiol. 2020, 90, 103464. [Google Scholar] [CrossRef]
- Paschou, S.A.; Papadopoulou-Marketou, N.; Chrousos, G.P.; Kanaka-Gantenbein, C. On type 1 diabetes mellitus pathogenesis. Endocr. Connect. 2018, 7, R38–R46. [Google Scholar] [CrossRef]
- Apostolidis, E.; Lee, C. In vitro potential of Ascophyllum nodosum phenolic antioxidant-mediated α-glucosidase and α-amylase inhibition. J. Food Sci. 2010, 75, H97–H102. [Google Scholar] [CrossRef]
- Zhou, S.; Huang, G. The biological activities of butyrylcholinesterase inhibitors. Biomed. Pharmacother. 2022, 146, 112556. [Google Scholar] [CrossRef]
- Ahmed, S.; Khan, S.T.; Zargaham, M.K.; Khan, A.U.; Khan, S.; Hussain, A.; Uddin, J.; Khan, A.; Al-Harrasi, A. Potential therapeutic natural products against Alzheimer’s disease with Reference of Acetylcholinesterase. Biomed. Pharmacother. 2021, 139, 111609. [Google Scholar] [CrossRef]
- Adewusi, E.A.; Moodley, N.; Steenkamp, V. Antioxidant and acetylcholinesterase inhibitory activity of selected southern African medicinal plants. S. Afr. J. Bot. 2011, 77, 638–644. [Google Scholar] [CrossRef]
- Tamfu, A.N.; Kucukaydin, S.; Yeskaliyeva, B.; Ozturk, M.; Dinica, R.M. Non-alkaloid cholinesterase inhibitory compounds from natural sources. Molecules 2021, 26, 5582. [Google Scholar] [CrossRef]
- Nagarani, G.; Abirami, A.; Siddhuraju, P. A comparative study on antioxidant potentials, inhibitory activities against key enzymes related to metabolic syndrome, and anti-inflammatory activity of leaf extract from different Momordica species. Food Sci. Hum. Wellness 2014, 3, 36–46. [Google Scholar] [CrossRef]
- Perumal, V.; Khatib, A.; Ahmed, Q.U.; Uzir, B.F.; Abas, F.; Murugesu, S.; Saiman, M.Z.; Primaharinastiti, R.; El-Seedi, H. Correlation of the GC-MS-based metabolite profile of Momordica charantia fruit and its antioxidant activity. Int. Food Res. J. 2022, 29, 58–66. [Google Scholar] [CrossRef]

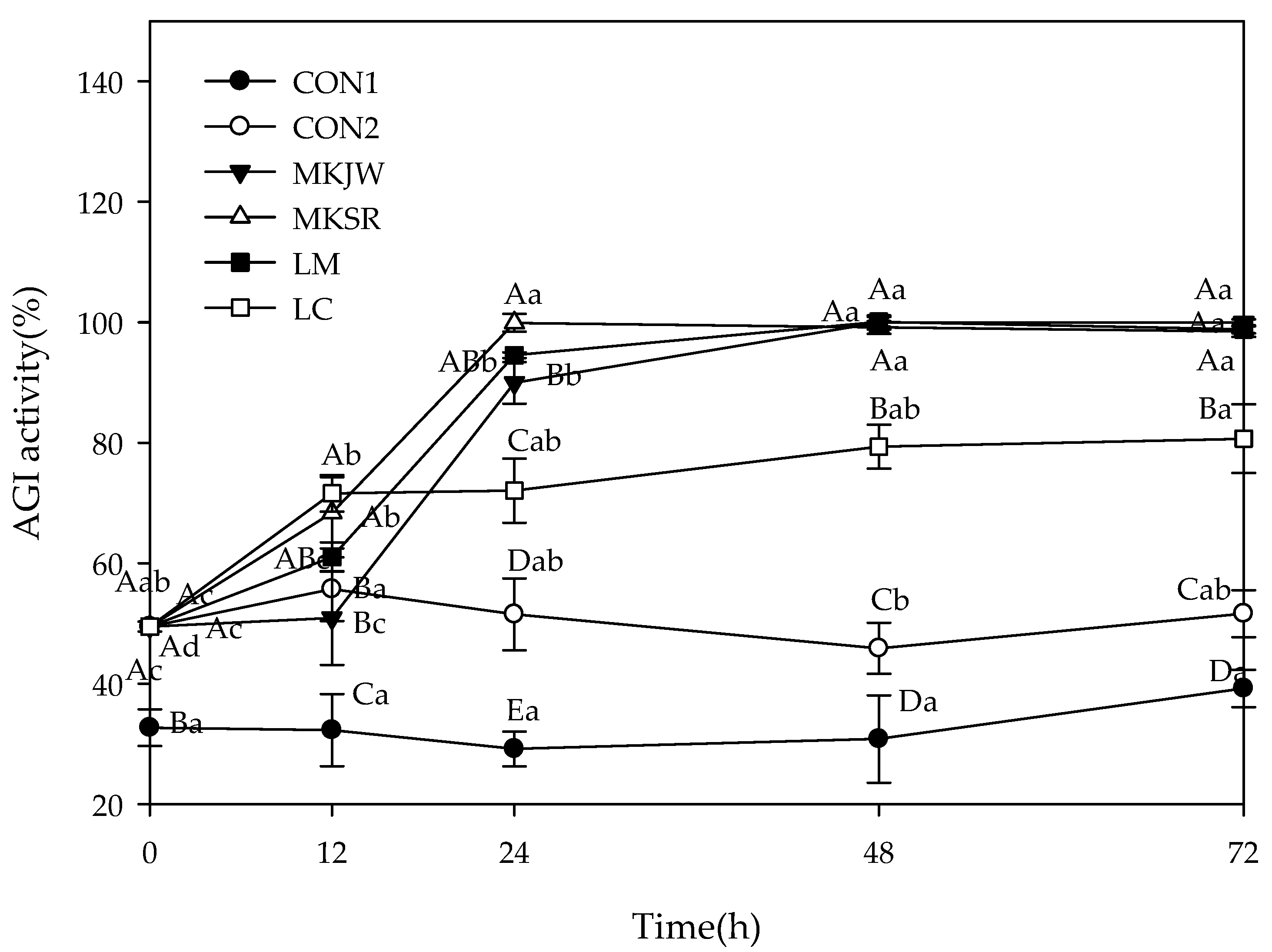
| Free Sugar (mg/mL) | Organic Acid (mg/mL) | Alcohol (mg/mL) | Dextran (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sucrose or Maltose | Glucose | Fructose | Lactic Acid | Formic Acid | Acetic Acid | Mannitol | Ethanol | 0 h | 24 h | |
| CON1 (1) | 0 | 3.54 ± 0.68 BC | 2.28 ± 0.39 C | 0 | 0.11 ± 0.04 A | 0.11 ± 0.00 D | 0 | 0 | 0 | 1.54 ± 1.84 C |
| CON2 | 189.80 ± 22.08 A(2)(3) | 9.33 ± 0.99 B | 3.43 ± 0.48 C | 0 | 0.15 ± 0.08 A | 0.18 ± 0.02 D | 0 | 0 | 0 | 0.47 ± 0.50 C |
| MKJW-MC | 22.29 ± 3.02 BCD | 21.75 ± 3.11 A | 19.77 ± 1.72 B | 8.27 ± 0.06 B | 0.18 ± 0.08 A | 5.03 ± 0.19 B | 23.63 ± 3.05 B | 0.67 ± 0.04 B | 0 | 22.37 ± 0.77 A |
| MKSR-MC | 30.73 ± 7.09 BC | 21.81 ± 4.97 A | 8.86 ± 0.65 C | 10.42 ± 0.20 A | 0.18 ± 0.08 A | 6.94 ± 0.17 A | 34.76 ± 5.99 A | 0.68 ± 0.06 B | 0 | 18.28 ± 3.54 A |
| LM-MC | 32.81 ± 9.16 B | 8.74 ± 3.35 B | 26.85 ± 3.26 B | 8.55 ± 0.14 B | 0.17 ± 0.08 A | 5.38 ± 0.22 B | 27.77 ± 4.69 AB | 0.72 ± 0.08 B | 0 | 10.66 ± 1.56 B |
| LC-MC | 4.25 ± 0.71 CD | 0.51 ± 0.23 C | 48.43 ± 6.38 A | 6.43 ± 0.03 C | 0.16 ± 0.13 A | 3.85 ± 0.10 C | 18.74 ± 3.02 B | 1.00 ± 0.11 A | 0 | 1.99 ± 1.46 C |
| 0 h | 24 h | |
|---|---|---|
| CON1 (1) | 10.20 ± 1.22 Bb | 14.72 ± 1.66 Ac |
| CON2 | 20.14 ± 3.07 Aa | 15.26 ± 1.17 Ac |
| MKJW-MC | 20.14 ± 3.07 Aa | 22.82 ± 0.86 Abc |
| MKSR-MC | 20.14 ± 3.07 Aa | 24.43 ± 3.25 Abc |
| LM-MC | 20.14 ± 3.07 Ba | 29.93 ± 5.18 Aab |
| LC-MC | 20.14 ± 3.07 Ba | 36.61 ± 6.93 Aa |
| AChE Inhibition (%) | BuChE Inhibition (%) | |||
|---|---|---|---|---|
| 0 h | 24 h | 0 h | 24 h | |
| CON1 (1) | 28.50 ± 3.47 Aa | 26.68 ± 2.29 Ac | 73.74 ± 0.95 Ba | 79.37 ± 4.23 Ab |
| CON2 | 19.40 ± 3.23 Ab | 17.79 ± 2.00 Ac | 71.16 ± 3.76 Aa | 74.10 ± 0.95 Ab |
| MKJW-MC | 19.40 ± 3.23 Bb | 40.59 ± 6.82 Ab | 71.16 ± 3.76 Ba | 87.90 ± 0.66 Aa |
| MKSR-MC | 19.40 ± 3.23 Bb | 55.24 ± 7.31 Aa | 71.16 ± 3.76 Ba | 88.27 ± 3.19 Aa |
| LM-MC | 19.40 ± 3.23 Bb | 44.46 ± 7.24 Aab | 71.16 ± 3.76 Ba | 87.86 ± 1.65 Aa |
| LC-MC | 19.40 ± 3.23 Bb | 31.21 ± 7.94 Abc | 71.16 ± 3.76 Ba | 85.28 ± 1.33 Aa |
| Galantamine (2) | 96.80 ± 0.59 | 96.84 ± 0.71 | ||
| 0 h | 24 h | |
|---|---|---|
| CON1 (1) | −18.40 ± 2.20 Bb | 3.78 ± 2.73 Ad |
| CON2 | 7.66 ± 3.64 Aa | 14.46 ± 3.28 Ac |
| MKJW-MC | 7.66 ± 3.64 Ba | 41.68 ± 3.01 Aab |
| MKSR-MC | 7.66 ± 3.64 Ba | 47.51 ± 4.61 Aa |
| LM-MC | 7.66 ± 3.64 Ba | 39.79 ± 0.70 Aab |
| LC-MC | 7.66 ± 3.64 Ba | 36.81 ± 6.14 Ab |
| Dextran | Sucrose and Maltose | Glucose | Fructose | Mannitol | EtOH | Lactic Acid | Formic Acid | Acetic Acid | AGI | AAI | AChEI | BuChEI | SOD | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dextran | 1 | |||||||||||||
| Sucrose and maltose | −0.065 | 1 | ||||||||||||
| Glucose | 0.849 * | 0.026 | 1 | |||||||||||
| Fructose | 0.176 | −0.425 | −0.341 | 1 | ||||||||||
| Mannitol | 0.819 * | −0.432 | 0.609 | 0.396 | 1 | |||||||||
| EtOH (1) | 0.565 | −0.533 | 0.132 | 0.863 * | 0.787 | 1 | ||||||||
| Lactic acid | 0.841 * | −0.461 | 0.601 | 0.458 | 0.993 *** | 0.832 * | 1 | |||||||
| Formic acid | 0.795 | 0.01 | 0.4 | 0.627 | 0.736 | 0.815 * | 0.774 | 1 | ||||||
| Acetic acid | 0.84 * | −0.438 | 0.628 | 0.407 | 0.998 *** | 0.8 | 0.997 *** | 0.756 | 1 | |||||
| AGI | 0.917 * | −0.207 | 0.661 | 0.395 | 0.956 ** | 0.756 | 0.959 ** | 0.858 * | 0.959 ** | 1 | ||||
| AAI | 0.257 | −0.455 | −0.23 | 0.962 ** | 0.560 | 0.929 ** | 0.6 | 0.692 | 0.564 | 0.524 | 1 | |||
| AChEI | 0.734 | −0.514 | 0.68 | 0.155 | 0.948 ** | 0.604 | 0.923 ** | 0.498 | 0.941 ** | 0.847 * | 0.342 | 1 | ||
| BuChEI | 0.711 | −0.703 | 0.476 | 0.512 | 0.927 ** | 0.83 * | 0.947 ** | 0.609 | 0.932 ** | 0.84 * | 0.613 | 0.895 * | 1 | |
| SOD | 0.881 * | −0.314 | 0.587 | 0.521 | 0.957 ** | 0.861 * | 0.975 ** | 0.893 * | 0.968 ** | 0.976 ** | 0.646 | 0.828 | 0.876 * | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Yu, S.; Jeong, Y.; Kim, M. Enhancement of Bioactive Properties in Momordica charantia by Leuconostoc Fermentation. Fermentation 2023, 9, 523. https://doi.org/10.3390/fermentation9060523
Kim J, Yu S, Jeong Y, Kim M. Enhancement of Bioactive Properties in Momordica charantia by Leuconostoc Fermentation. Fermentation. 2023; 9(6):523. https://doi.org/10.3390/fermentation9060523
Chicago/Turabian StyleKim, Jiwoo, Sungryul Yu, Yoonhwa Jeong, and Misook Kim. 2023. "Enhancement of Bioactive Properties in Momordica charantia by Leuconostoc Fermentation" Fermentation 9, no. 6: 523. https://doi.org/10.3390/fermentation9060523
APA StyleKim, J., Yu, S., Jeong, Y., & Kim, M. (2023). Enhancement of Bioactive Properties in Momordica charantia by Leuconostoc Fermentation. Fermentation, 9(6), 523. https://doi.org/10.3390/fermentation9060523







