Calcium Propionate Supplementation Mitigated Adverse Effects of Incubation Temperature Shift on In Vitro Fermentation by Modulating Microbial Composition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design and Diets
2.2. Experiment Procedure and Sampling
2.3. Chemical Analysis
2.3.1. Gas Production
2.3.2. Fermentation Parameters
2.3.3. DNA Extraction and Illumina Sequencing of Archaeal and Bacterial 16S rRNA Genes
2.4. Statistical Analysis
3. Results
3.1. Fermentation Parameters
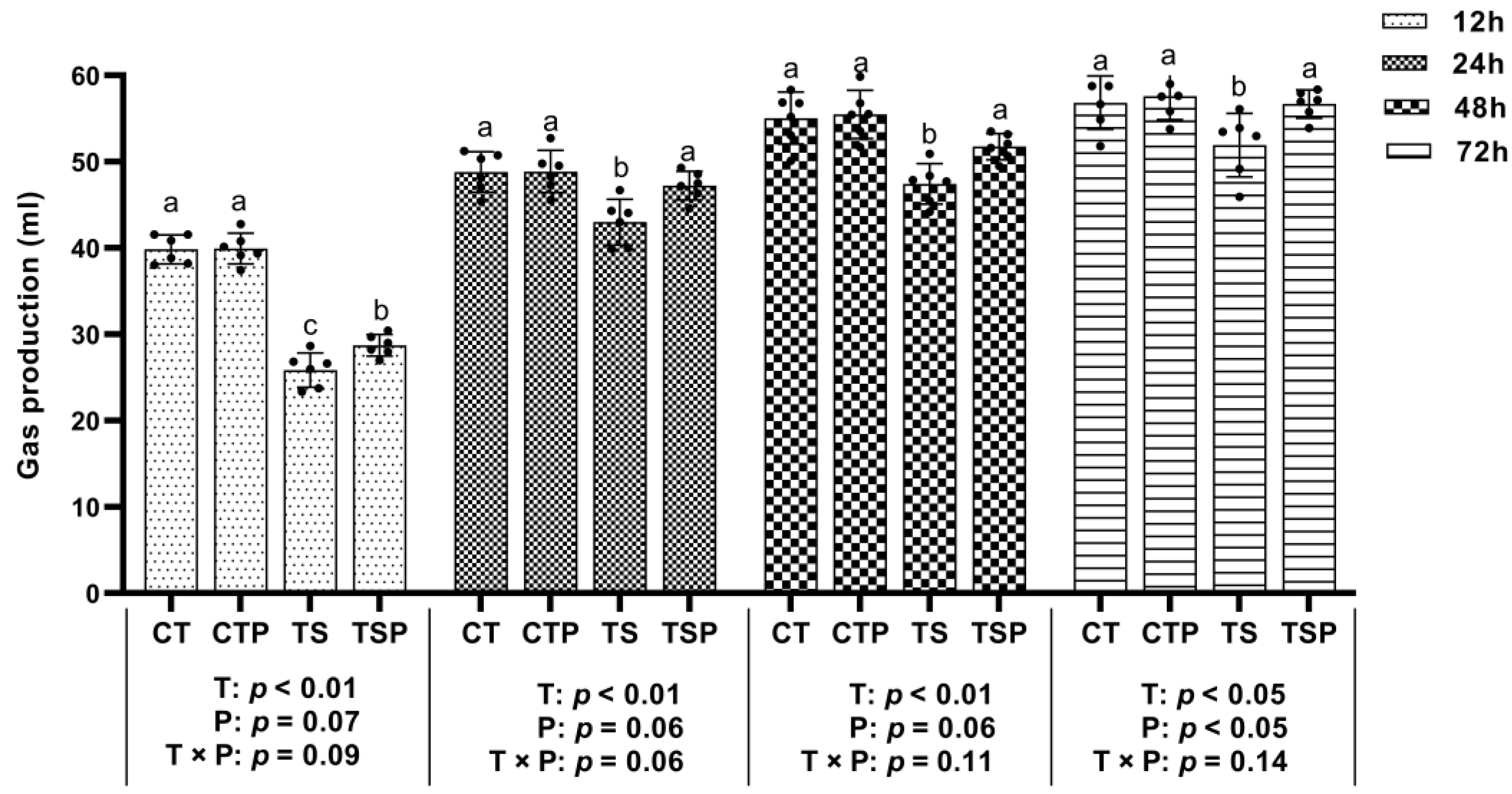
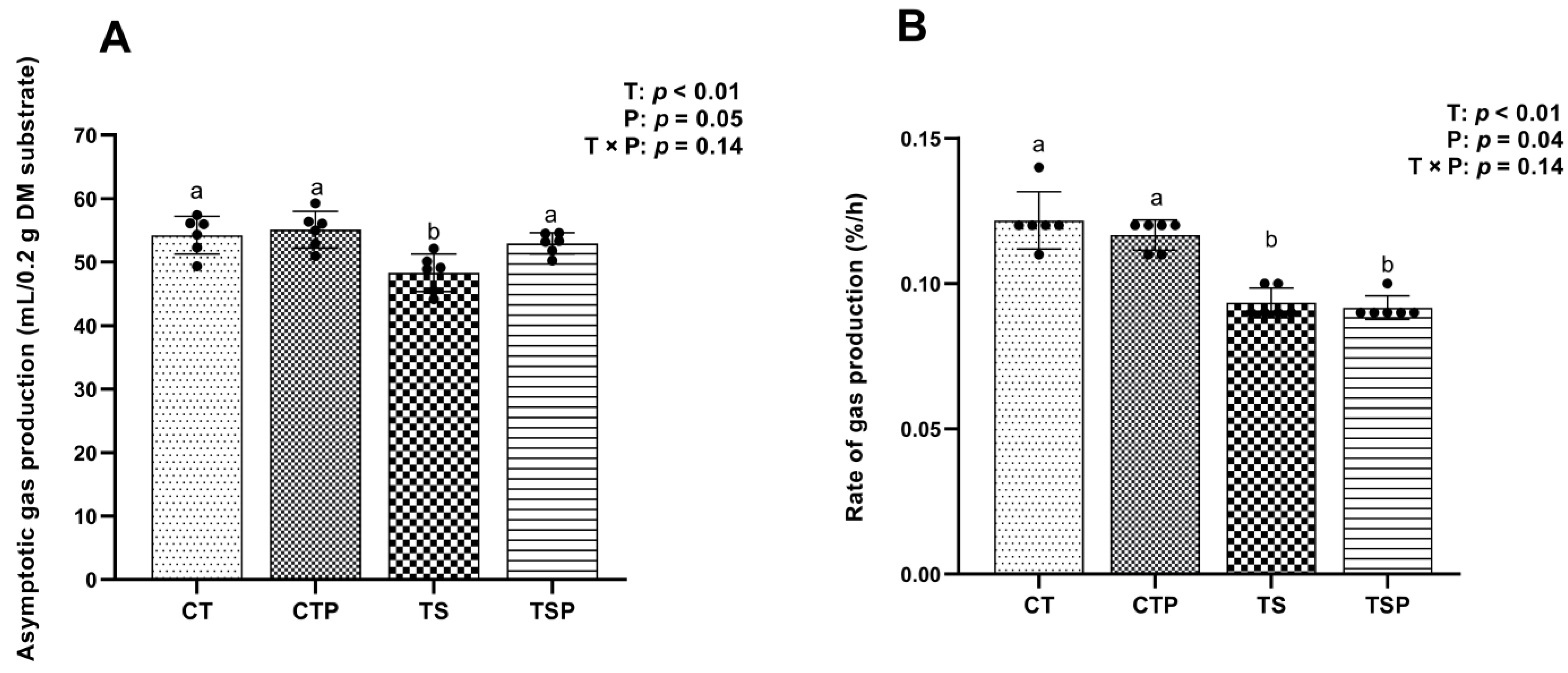
3.2. Gas Production
3.2.1. Cumulative Gas Production
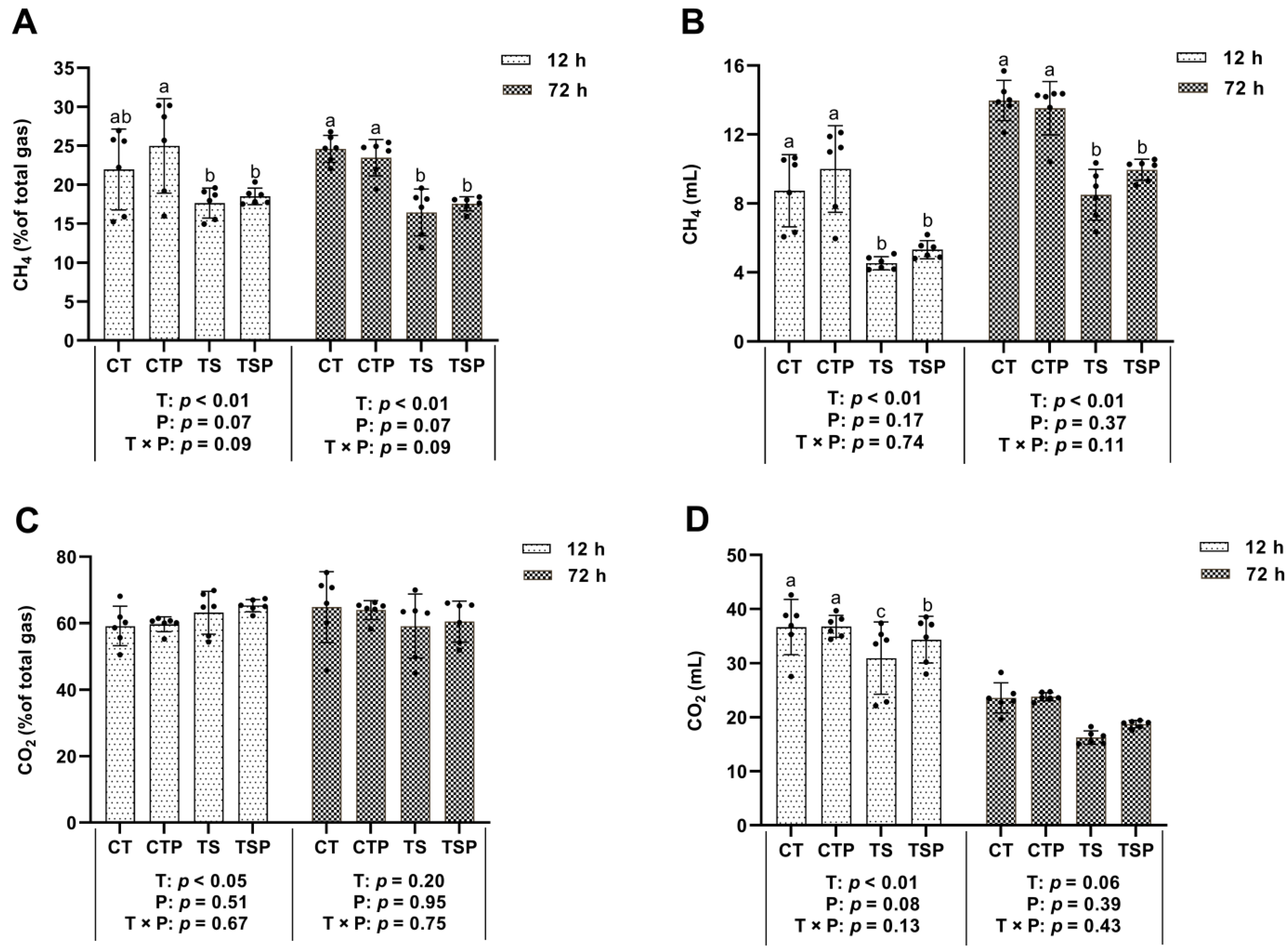
3.2.2. CH4 and CO2 Production
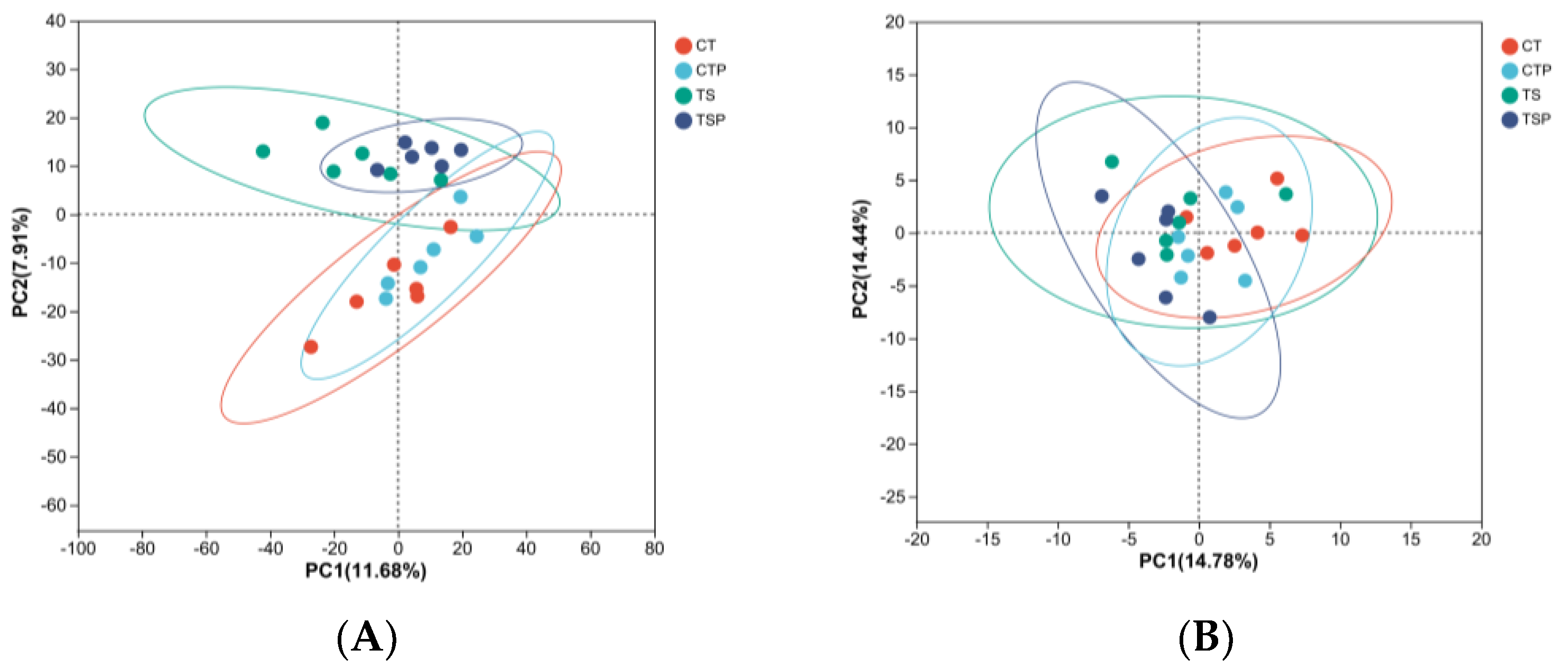
3.3. Bacterial and Archaeal Diversity Analysis
3.4. Microflora Composition of Bacteria and Archaea
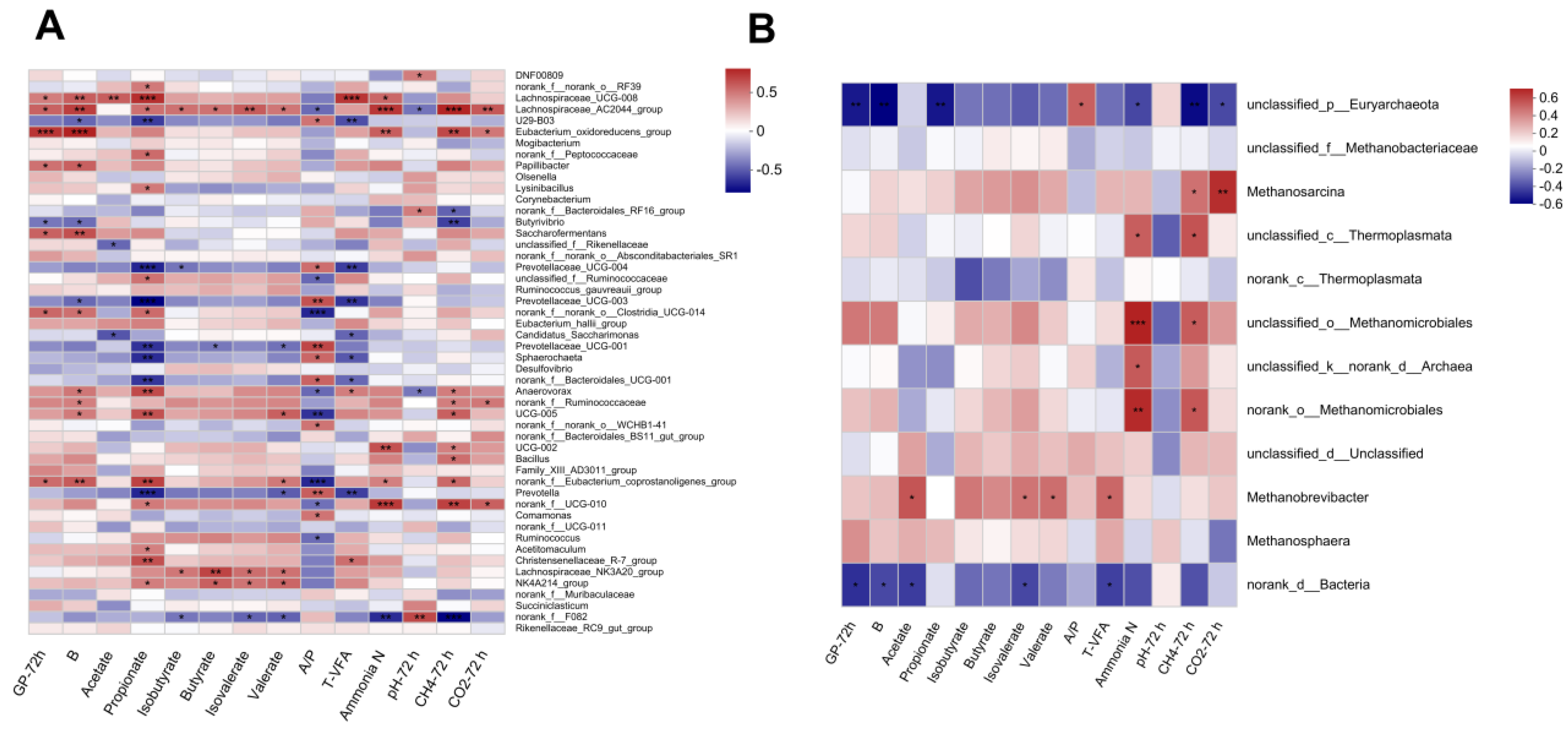
3.5. Spearman Correlation Analysis of the Top 50 Bacteria or Top 12 Archaea Genus with Gas Production, Fermentation Parameters, and CH4 Production
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Intergovernmental Panel on Climate Change. Summary for Policymakers. In Climate Change 2013—The Physical Science Basis: Working Group I Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2014; pp. 1–30. [Google Scholar] [CrossRef]
- Bridgham, S.D.; Cadillo-Quiroz, H.; Keller, J.K.; Zhuang, Q. Methane emissions from wetlands: Biogeochemical, microbial, and modeling perspectives from local to global scales. Glob. Chang. Biol. 2013, 19, 1325–1346. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Xu, X.; Fang, C.; Li, B.; Nie, M. Differences in the temperature dependence of wetland CO2 and CH4 emissions vary with water table depth. Nat. Clim. Chang. 2021, 11, 766–771. [Google Scholar] [CrossRef]
- Beauchemin, K.A.; Ungerfeld, E.M.; Abdalla, A.L.; Alvarez, C.; Arndt, C.; Becquet, P.; Benchaar, C.; Berndt, A.; Mauricio, R.M.; McAllister, T.A.; et al. Invited review: Current enteric methane mitigation options. J. Dairy Sci. 2022, 105, 9297–9326. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.A.; Johnson, D.E. Methane emissions from cattle. J. Anim. Sci. 1995, 73, 2483–2492. [Google Scholar] [CrossRef]
- Linville, K.; Casper, D.P.; Osorio, J.S. In vitro analysis of rumen microbial fermentation at different temperatures. J. Anim. Sci. 2017, 95, 182–183. [Google Scholar] [CrossRef]
- Ramos, S.C.; Jeong, C.D.; Mamuad, L.L.; Kim, S.H.; Kang, S.H.; Kim, E.T.; Cho, Y.I.; Lee, S.S.; Lee, S.S. Diet transition from high-forage to high-concentrate alters rumen bacterial community composition, epithelial transcriptomes and ruminal fermentation parameters in dairy cows. Animals 2021, 11, 838. [Google Scholar] [CrossRef]
- Barnett, M.C.; McFarlane, J.R.; Hegarty, R.S. Low ambient temperature elevates plasma triiodothyronine concentrations while reducing digesta mean retention time and methane yield in sheep. J. Anim. Physiol. Anim. Nutr. 2015, 99, 483–491. [Google Scholar] [CrossRef]
- Duarte, A.C.; Holman, D.B.; Alexander, T.W.; Kiri, K.; Breves, G.; Chaves, A.V. Incubation temperature, but not pequi oil supplementation, affects methane production, and the ruminal microbiota in a rumen simulation technique (Rusitec) System. Front. Microbiol. 2017, 8, 1076. [Google Scholar] [CrossRef] [Green Version]
- Grossi, S.; Rossi, L.; Dell’Anno, M.; Biffani, S.; Sgoifo Rossi, C.A. Effects of heated drinking water on the growth performance and rumen functionality of fattening charolaise beef cattle in winter. Animals 2021, 11, 2218. [Google Scholar] [CrossRef]
- Cantor, M.C.; Costa, J.H.; Bewley, J.M. Impact of observed and controlled water intake on reticulorumen temperature in lactating dairy cattle. Animals 2018, 8, 194. [Google Scholar] [CrossRef] [Green Version]
- Petersen, M.K.; Muscha, J.M.; Mulliniks, J.T.; Roberts, A.J. Water temperature impacts water consumption by range cattle in winter. J. Anim. Sci. 2016, 94, 4297–4306. [Google Scholar] [CrossRef] [Green Version]
- Kang, H.J.; Piao, M.Y.; Park, S.J.; Na, S.W.; Kim, H.J.; Baik, M. Effects of ambient temperature and rumen-protected fat supplementation on growth performance, rumen fermentation and blood parameters during cold season in Korean cattle steers. Asian-Australas. J. Anim. Sci. 2019, 32, 657–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Igoshin, A.; Yudin, N.; Aitnazarov, R.; Yurchenko, A.A.; Larkin, D.M. Whole-genome resequencing points to candidate DNA loci affecting body temperature under cold dtress in Siberian cattle populations. Life 2021, 11, 959. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Zhou, G.; Tian, G.; Liu, Y.; Dong, N.; Li, L.; Zhang, S.; Chai, H.; Chen, Y.; Yang, Y. Changes in rumen microbiota affect metabolites, immune responses and antioxidant enzyme activities of sheep under cold stimulation. Animals 2021, 11, 712. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Haga, S.S.; Kimura, K.J.; Matsuyama, S.C. Propionate and butyrate induce gene expression of monocarboxylate transporter 4 and cluster of differentiation 147 in cultured rumen epithelial cells derived from preweaning dairy calves. J. Anim. Sci. 2018, 96, 4902–4911. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, C.; Guo, G.; Yang, W.Z.; Dong, K.H.; Huang, Y.X.; Yang, X.M.; He, D.C. Effects of calcium propionate on rumen fermentation, urinary excretion of purine derivatives and feed digestibility in steers. J. Agric. Sci. 2009, 147, 201–209. [Google Scholar] [CrossRef]
- Menke, K.H.; Raab, L.; Salewski, A.; Steingass, H.; Fritz, D.; Schneider, W. The estimation of the digestibility and metabolizable energy content of ruminant feedingstuffs from the gas production when they are incubated with rumen liquor in vitro. J. Agric. Sci. 1979, 93, 217–222. [Google Scholar] [CrossRef] [Green Version]
- Ørskov, E.R.; McDonald, I. The estimation of protein degradability in the rumen from incubation measurements weighted according to rate of passage. J. Agric. Sci. 1979, 92, 499–503. [Google Scholar] [CrossRef] [Green Version]
- Broderick, G.A.; Kang, J.H. Automated simultaneous determination of ammonia and total amino acids in ruminal fluid and in vitro media. J. Dairy Sci. 1980, 63, 64–75. [Google Scholar] [CrossRef]
- Cole, J.R.; Wang, Q.; Fish, J.A.; Chai, B.; McGarrell, D.M.; Sun, Y.; Brown, C.T.; Porras-Alfaro, A.; Kuske, C.R.; Tiedje, J.M. Ribosomal Database Project: Data and tools for high throughput rRNA analysis. Nucleic Acids Res. 2014, 42, D633–D642. [Google Scholar] [CrossRef] [Green Version]
- Mahnert, A.; Moissl-Eichinger, C.; Berg, G. Microbiome interplay: Plants alter microbial abundance and diversity within the built environment. Front. Microbiol. 2015, 6, 887. [Google Scholar] [CrossRef] [PubMed]
- Jin, D.; Zhao, S.; Wang, P.; Zheng, N.; Bu, D.; Beckers, Y.; Wang, J. Insights into abundant rumen ureolytic bacterial community using rumen simulation system. Front. Microbiol. 2016, 7, 1006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tagliapietra, F.; Cattani, M.; Bailoni, L.; Schiavon, S. In vitro rumen fermentation: Effect of headspace pressure on the gas production kinetics of corn meal and meadow hay. Anim. Feed Sci. Technol. 2010, 158, 197–201. [Google Scholar] [CrossRef]
- Cattani, M.; Tagliapietra, F.; Maccarana, L.; Hansen, H.H.; Bailoni, L.; Schiavon, S. Technical note: In vitro total gas and methane production measurements from closed or vented rumen batch culture systems. J. Dairy Sci. 2014, 97, 1736–1741. [Google Scholar] [CrossRef] [Green Version]
- Wahrmund, J.L.; Ronchesel, J.R.; Krehbiel, C.R.; Goad, C.L.; Trost, S.M.; Richards, C.J. Ruminal acidosis challenge impact on ruminal temperature in feedlot cattle. J. Anim. Sci. 2012, 90, 2794–2801. [Google Scholar] [CrossRef] [PubMed]
- Roger, V.; Fonty, G.; Komisarczuk-Bony, S.; Gouet, P. Effects of physicochemical factors on the adhesion to cellulose avicel of the ruminal bacteria Ruminococcus flavefaciens and Fibrobacter succinogenes subsp. succinogenes. Appl. Environ. Microbiol. 1990, 56, 3081–3087. [Google Scholar] [CrossRef] [Green Version]
- Cunningham, M.D.; Martz, F.A.; Merilan, C.P. Effect of drinking-water temperature upon ruminant digestion, intraruminal temperature, and water consumption of nonlactating dairy cows. J. Dairy Sci. 1964, 47, 382–385. [Google Scholar] [CrossRef]
- Brod, D.L.; Bolsen, K.K.; Brent, B.E. Effect of water temperature in rumen temperature, digestion and rumen fermentation in sheep. J. Anim. Sci. 1982, 54, 179–182. [Google Scholar] [CrossRef] [Green Version]
- Sheperd, A.C.; Combs, D.K. Long-term effects of acetate and propionate on voluntary feed intake by midlactation Cows. J. Dairy Sci. 1998, 81, 2240–2250. [Google Scholar] [CrossRef]
- Zhang, F.; Zhao, Y.; Wang, Y.; Wang, H.; Nan, X.; Guo, Y.; Xiong, B. Dietary supplementation with calcium propionate could beneficially alter rectal microbial composition of early lactation dairy cows. Front. Vet. Sci. 2022, 9, 1–13. [Google Scholar] [CrossRef]
- Patra, A.K.; Yu, Z. Essential oils affect populations of some rumen bacteria in vitro as revealed by microarray (RumenBactArray) analysis. Front. Microbiol. 2015, 6, 297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antanaitis, R.; Anskienė, L.; Rapaliutė, E.; Bilskis, R.; Džermeikaitė, K.; Bačėninaitė, D.; Juškienė, V.; Juška, R.; Meškinytė, E. Relationship between reticulorumen parameters measured in real time and methane emission and heat stress risk in dairy cows. Animals 2022, 12, 3257. [Google Scholar] [CrossRef] [PubMed]
- Bhatta, R.; Tajima, K.; Kurihara, M. Influence of temperature and pH on germentation pattern and methane production in the rumen simulating fermenter (RUSITEC). Asian-Australas. J. Anim. Sci. 2006, 19, 376–380. [Google Scholar] [CrossRef]
- Wang, M.; Wang, R.; Xie, T.Y.; Janssen, P.H.; Sun, X.Z.; Beauchemin, K.A.; Tan, Z.L.; Gao, M. Shifts in rumen fermentation and microbiota are associated with dissolved ruminal hydrogen concentrations in lactating dairy cows fed different types of carbohydrates. J. Nutr. 2016, 146, 1714–1721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hook, S.E.; Wright, A.-D.G.; McBride, B.W. Methanogens: Methane producers of the rumen and mitigation strategies. Archaea 2010, 2010, 945785. [Google Scholar] [CrossRef] [Green Version]
- Sun, B.; Wang, X.; Bernstein, S.; Huffman, M.A.; Xia, D.-P.; Gu, Z.; Chen, R.; Sheeran, L.K.; Wagner, R.S.; Li, J. Marked variation between winter and spring gut microbiota in free-ranging Tibetan Macaques (Macaca thibetana). Sci. Rep. 2016, 6, 26035. [Google Scholar] [CrossRef] [Green Version]
- Li, A.; Wang, Y.; Li, Z.; Qamar, H.; Mehmood, K.; Zhang, L.; Liu, J.; Zhang, H.; Li, J. Probiotics isolated from yaks improves the growth performance, antioxidant activity, and cytokines related to immunity and inflammation in mice. Microb. Cell Fact. 2019, 18, 112. [Google Scholar] [CrossRef] [Green Version]
- Garneau, J.E.; Tremblay, D.M.; Moineau, S. Characterization of 1706, a virulent phage from Lactococcus lactis with similarities to prophages from other Firmicutes. Virology 2008, 373, 298–309. [Google Scholar] [CrossRef] [Green Version]
- Mao, S.Y.; Zhang, R.Y.; Wang, D.S.; Zhu, W.Y. Impact of subacute ruminal acidosis (SARA) adaptation on rumen microbiota in dairy cattle using pyrosequencing. Anaerobe 2013, 24, 12–19. [Google Scholar] [CrossRef]
- Wetzels, S.U.; Mann, E.; Metzler-Zebeli, B.U.; Wagner, M.; Klevenhusen, F.; Zebeli, Q.; Schmitz-Esser, S. Pyrosequencing reveals shifts in the bacterial epimural community relative to dietary concentrate amount in goats. J. Dairy Sci. 2015, 98, 5572–5587. [Google Scholar] [CrossRef] [Green Version]
- Yao, Q.; Li, Y.; Meng, Q.; Zhou, Z. The effect of calcium propionate on the ruminal bacterial community composition in finishing bulls. Asian-Australas. J. Anim. Sci. 2016, 30, 495–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, F.; Wang, Y.; Wang, H.; Nan, X.; Guo, Y.; Xiong, B. Calcium propionate supplementation has minor effects on major ruminal bacterial community composition of early lactation dairy cows. Front. Microbiol. 2022, 13, 847488. [Google Scholar] [CrossRef] [PubMed]
- McCann, J.C.; Wiley, L.M.; Forbes, T.D.; Rouquette, F.M.; Tedeschi, L.O. Relationship between the rumen microbiome and residual feed intake-efficiency of brahman bulls stocked on bermudagrass pastures. PLoS ONE 2014, 9, e91864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyu, J.Y.; Yang, Z.T.; Wang, E.D.; Liu, G.K.; Wang, Y.J.; Wang, W.; Li, S.L. Possibility of using by-products with high NDF content to alter the fecal short chain fatty acid profiles, bacterial community, and digestibility of lactating dairy cows. Microorganisms 2022, 10, 1731. [Google Scholar] [CrossRef]
- Rathert-Williams, A.R.; McConnell, H.L.; Salisbury, C.M.; Lindholm-Perry, A.K.; Lalman, D.L.; Pezeshki, A.; Foote, A.P. Effects of adding ruminal propionate on dry matter intake and glucose metabolism in steers fed a finishing ration. J. Anim. Sci. 2023, 101, skad072. [Google Scholar] [CrossRef]
- Dieho, K.; van Baal, J.; Kruijt, L.; Bannink, A.; Schonewille, J.T.; Carreño, D.; Hendriks, W.H.; Dijkstra, J. Effect of supplemental concentrate during the dry period or early lactation on rumen epithelium gene and protein expression in dairy cattle during the transition period. J. Dairy Sci. 2017, 100, 7227–7245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Ingredient Composition | Content |
|---|---|
| Corn | 28.15 |
| Soybean meal | 8.64 |
| Jujube powder | 7.64 |
| Corn silage | 32.96 |
| Corn stalk | 17.04 |
| Salt | 1.13 |
| Premix 1 | 2.22 |
| Calcium hydrogen phosphate | 1.10 |
| Sodium bicarbonate | 1.12 |
| Nutrient composition | |
| DM | 48.62 |
| EE | 2.95 |
| Lignin | 4.16 |
| CP | 12.05 |
| NDF | 42.98 |
| ADF | 23.46 |
| Ca | 0.53 |
| P | 0.33 |
| Items | CT | CTP | TS | TSP | SEM | p-Value | ||
|---|---|---|---|---|---|---|---|---|
| T | P | T × P | ||||||
| pH | 6.62 b | 6.68 b | 6.69 b | 6.81 a | 0.03 | 0.06 | 0.08 | 0.13 |
| NH3-N (mmol/L) | 62.78 a | 59.16 b | 51.23 c | 53.33 c | 1.08 | <0.01 | 0.49 | 0.02 |
| Acetate (mmol/L) | 74.83 | 76.73 | 70.85 | 74.28 | 3.43 | 0.36 | 0.45 | 0.83 |
| Propionate (mmol/L) | 23.71 a | 26.55 a | 17.91 b | 24.24 a | 0.98 | <0.01 | <0.01 | 0.10 |
| Acetate:propionate | 3.15 ab | 2.90 b | 4.04 a | 3.11 b | 0.22 | 0.02 | 0.01 | 0.15 |
| Isobutyrate (mmol/L) | 1.84 a | 1.68 bc | 1.56 c | 1.73 ab | 0.04 | 0.01 | 0.90 | <0.01 |
| Butyrate (mmol/L) | 15.43 a | 14.09 a | 11.43 b | 14.35 a | 0.63 | 0.01 | 0.23 | <0.01 |
| Iso-valerate (mmol/L) | 4.69 a | 4.29 a | 3.66 b | 4.27 a | 0.16 | <0.01 | 0.52 | <0.01 |
| Valerate (mmol/L) | 1.97 a | 1.91 a | 1.48 b | 1.90 a | 0.08 | <0.01 | 0.04 | 0.01 |
| Total volatile fatty acid (mmol/L) | 122 a | 125 a | 106 b | 120 a | 3.97 | 0.02 | 0.05 | 0.18 |
| Items | CT | CTP | TS | TSP | SEM | p-Value | ||
|---|---|---|---|---|---|---|---|---|
| T | P | T × P | ||||||
| Bacterial | ||||||||
| Sobs | 1474 | 1486 | 1509 | 1444 | 32.81 | 0.44 | 0.91 | 0.26 |
| Shannon | 5.74 | 5.71 | 5.86 | 5.74 | 0.05 | 0.15 | 0.15 | 0.36 |
| Simpson | 0.013 | 0.011 | 0.010 | 0.010 | 0.00 | 0.74 | 0.32 | 0.72 |
| Ace | 1869 | 1849 | 1887 | 1821 | 34.40 | 0.24 | 0.89 | 0.52 |
| Chao | 1862 | 1834 | 1892 | 1820 | 35.92 | 0.18 | 0.94 | 0.55 |
| Archaeal | ||||||||
| Sobs | 75.17 a | 71.17 bc | 72.00 b | 68.83 c | 0.82 | <0.01 | <0.01 | 0.62 |
| Shannon | 2.225 | 2.199 | 2.272 | 2.182 | 0.05 | 0.29 | 0.79 | 0.56 |
| Simpson | 0.241 | 0.243 | 0.212 | 0.237 | 0.02 | 0.43 | 0.30 | 0.49 |
| Ace | 79.21 | 76.20 | 78.30 | 80.85 | 2.07 | 0.91 | 0.38 | 0.20 |
| Chao | 79.77 | 75.09 | 77.15 | 79.58 | 2.35 | 0.64 | 0.70 | 0.15 |
| Items | CT | CTP | TS | TSP | SEM | p-Value | ||
|---|---|---|---|---|---|---|---|---|
| T | P | T × P | ||||||
| Phylum level of Bacteria | ||||||||
| Firmicutes | 54.58 a | 59.72 a | 45.91 b | 54.13 a | 1.789 | <0.01 | <0.01 | 0.40 |
| Bacteroidota | 34.82 b | 33.12 b | 42.26 a | 37.94 ab | 1.548 | <0.01 | 0.07 | 0.41 |
| Proteobacteria | 4.19 | 1.30 | 5.33 | 1.36 | 1.648 | 0.72 | 0.06 | 0.75 |
| Actinobacteriota | 1.22 b | 1.58 b | 1.39 b | 2.21 a | 0.211 | 0.08 | 0.01 | 0.29 |
| Genus level of Bacteria | ||||||||
| Rikenellaceae_RC9 | 15.21 | 14.42 | 14.87 | 16.82 | 1.106 | 0.37 | 0.61 | 0.24 |
| F082 | 7.77 b | 7.54 b | 11.34 a | 10.77 a | 0.554 | <0.01 | 0.49 | 0.77 |
| Succiniclasticum | 5.02 | 7.04 | 4.95 | 7.74 | 1.529 | 0.84 | 0.14 | 0.80 |
| Muribaculaceae | 4.16 | 5.77 | 4.99 | 4.90 | 1.174 | 0.99 | 0.53 | 0.48 |
| NK4A214 | 4.90 | 4.94 | 4.38 | 5.24 | 0.301 | 0.73 | 0.16 | 0.20 |
| Lachnospiraceae_NK3A20 | 5.20 | 4.63 | 4.14 | 4.94 | 0.393 | 0.35 | 0.77 | 0.10 |
| Christensenellaceae_R-7 | 4.52 | 5.15 | 3.88 | 4.43 | 0.482 | 0.18 | 0.24 | 0.93 |
| Prevotella | 1.74 b | 1.08 b | 3.33 a | 1.18 b | 0.458 | 0.09 | <0.01 | 0.13 |
| Bacillus | 2.29 | 3.04 | 0.41 | 0.15 | 0.969 | 0.03 | 0.80 | 0.61 |
| Bacteroidales_BS11 | 1.08 | 0.81 | 1.03 | 0.87 | 0.085 | 0.97 | 0.03 | 0.51 |
| Ruminococcaceae | 0.80 a | 0.95 a | 0.65 b | 0.66 b | 0.076 | 0.01 | 0.31 | 0.36 |
| Prevotellaceae_UCG-001 | 0.59 ab | 0.52 ab | 1.03 a | 0.34 b | 0.144 | 0.37 | 0.02 | 0.05 |
| Ruminococcus | 0.47 b | 0.52 ab | 0.48 b | 0.74 a | 0.065 | 0.11 | 0.04 | 0.13 |
| Prevotellaceae_UCG-003 | 0.53 ab | 0.30 b | 1.04 a | 0.37 b | 0.176 | 0.12 | 0.02 | 0.23 |
| Prevotellaceae_UCG-004 | 0.54 | 0.37 | 0.78 | 0.39 | 0.111 | 0.26 | 0.02 | 0.33 |
| Butyrivibrio | 0.34 | 0.49 | 0.41 | 0.50 | 0.091 | 0.69 | 0.20 | 0.77 |
| Lachnospiraceae_UCG-008 | 0.32 | 0.49 | 0.23 | 0.29 | 0.07 | 0.06 | 0.13 | 0.47 |
| Phylum level of Archaea | ||||||||
| norank_d_Bacteria | 91.61 | 92.33 | 92.86 | 92.37 | 0.445 | 0.17 | 0.80 | 0.91 |
| Euryarchaeota | 6.61 | 6.21 | 5.68 | 6.58 | 0.416 | 0.51 | 0.57 | 0.14 |
| unclassified_d_Unclassified | 1.11 | 1.01 | 1.01 | 0.84 | 0.101 | 0.20 | 0.23 | 0.73 |
| Genus level of Archaea | ||||||||
| Bacteria | 91.61 | 92.33 | 92.86 | 92.37 | 0.445 | 0.17 | 0.80 | 0.20 |
| Methanosphaera | 3.88 | 3.92 | 3.76 | 5.01 | 0.33 | 0.16 | 0.07 | 0.09 |
| Methanobrevibacter | 1.63 | 1.56 | 1.59 | 1.40 | 0.206 | 0.64 | 0.53 | 0.77 |
| Methanomicrobiales | 0.81 a | 0.53 ab | 0.31 b | 0.16 b | 0.108 | <0.01 | 0.06 | 0.58 |
| Archaea | 0.63 a | 0.41 ab | 0.41 ab | 0.19 b | 0.095 | 0.04 | 0.04 | 0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, T.; Wang, X.; Long, S.; Li, J.; Wu, Z.; Guo, Y.; Sun, F.; Chen, Z. Calcium Propionate Supplementation Mitigated Adverse Effects of Incubation Temperature Shift on In Vitro Fermentation by Modulating Microbial Composition. Fermentation 2023, 9, 544. https://doi.org/10.3390/fermentation9060544
He T, Wang X, Long S, Li J, Wu Z, Guo Y, Sun F, Chen Z. Calcium Propionate Supplementation Mitigated Adverse Effects of Incubation Temperature Shift on In Vitro Fermentation by Modulating Microbial Composition. Fermentation. 2023; 9(6):544. https://doi.org/10.3390/fermentation9060544
Chicago/Turabian StyleHe, Tengfei, Xilin Wang, Shenfei Long, Jiangong Li, Zhenlong Wu, Yao Guo, Fang Sun, and Zhaohui Chen. 2023. "Calcium Propionate Supplementation Mitigated Adverse Effects of Incubation Temperature Shift on In Vitro Fermentation by Modulating Microbial Composition" Fermentation 9, no. 6: 544. https://doi.org/10.3390/fermentation9060544
APA StyleHe, T., Wang, X., Long, S., Li, J., Wu, Z., Guo, Y., Sun, F., & Chen, Z. (2023). Calcium Propionate Supplementation Mitigated Adverse Effects of Incubation Temperature Shift on In Vitro Fermentation by Modulating Microbial Composition. Fermentation, 9(6), 544. https://doi.org/10.3390/fermentation9060544






