Development of Fermented Kombucha Tea Beverage Enriched with Inulin and B Vitamins
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
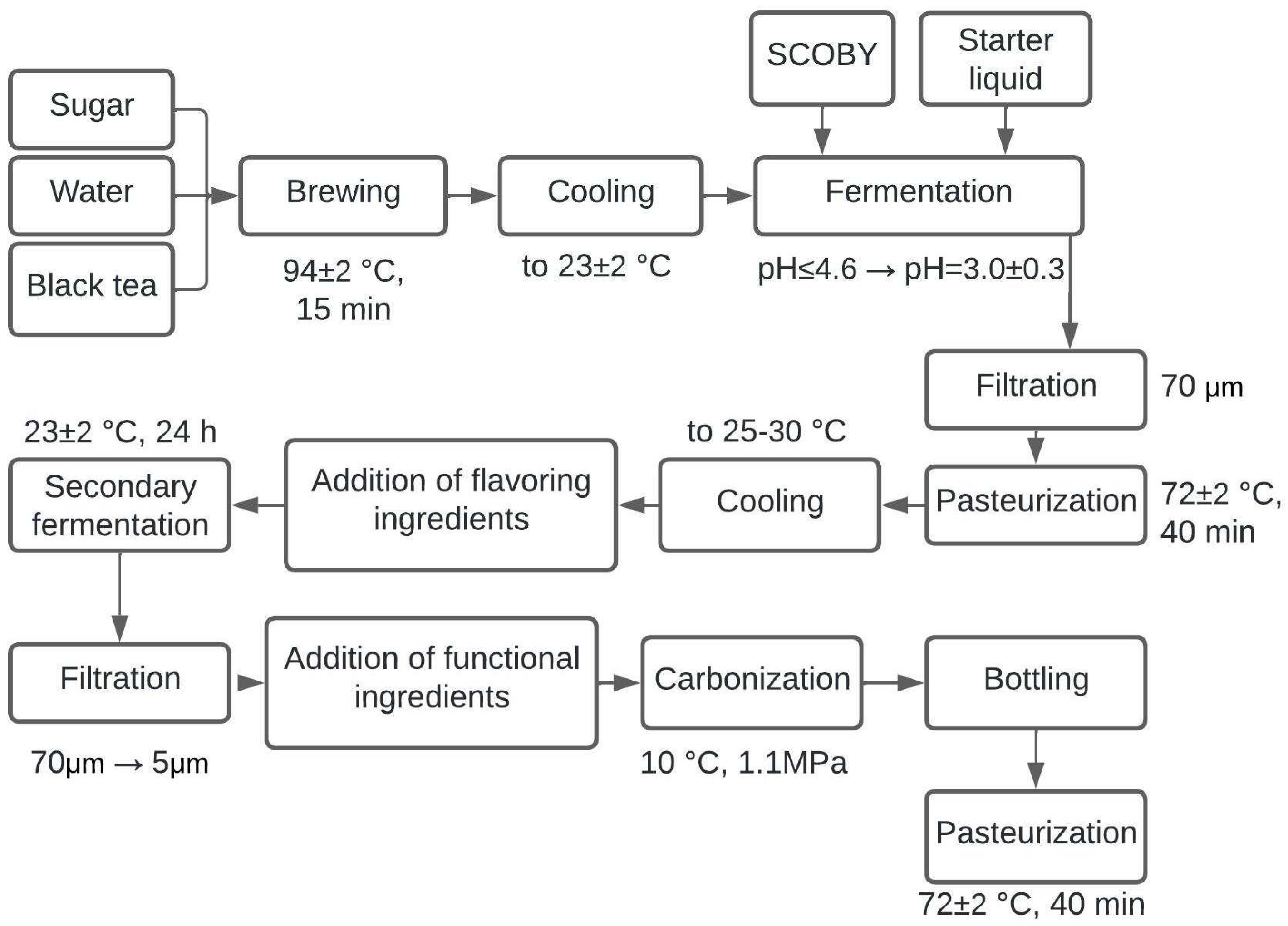
2.2. Fermented Beverage Base Production Technology
2.3. Methods
2.3.1. Titratable Acidity Determination
2.3.2. pH Determination
2.3.3. Determination of Carbohydrates Profile
2.3.4. Determination of the Dry Matter Content
2.3.5. Determination of Ethanol Content
2.3.6. Determination of Organic Acids
2.3.7. Determination of Total Polyphenol Content
2.3.8. Determination of Volatile Substances
2.3.9. Modeling of Reaction Kinetics
2.4. The Technology of Fermented Beverage Production
2.5. Methods for Investigation of Fermented Beverages
2.5.1. Determination of Inulin Content
2.5.2. Determination of Vitamin Content
2.5.3. Assessment of Antioxidant Activity
2.5.4. Organoleptic Evaluation
2.6. Statistical Processing
3. Results and Discussion
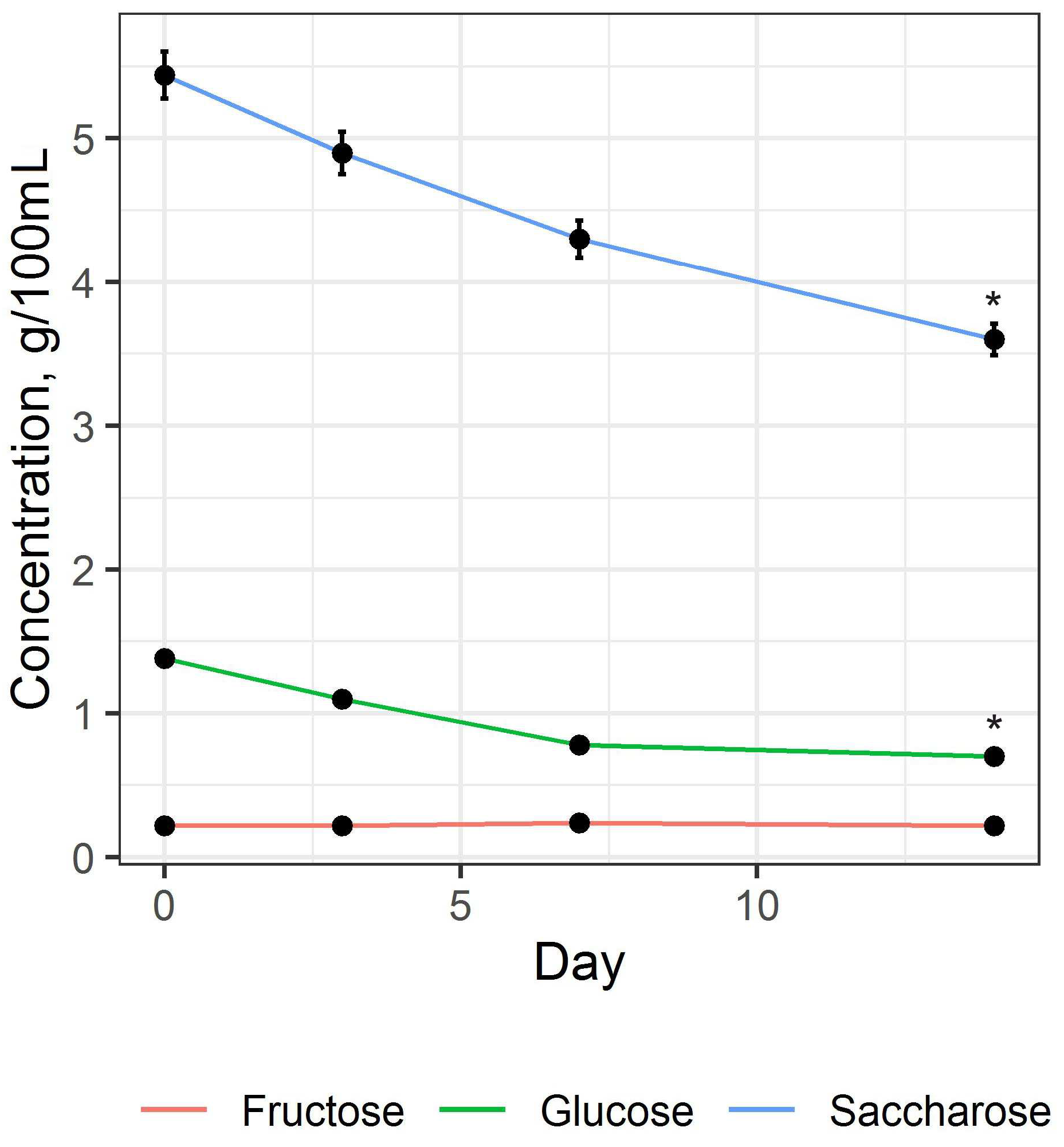
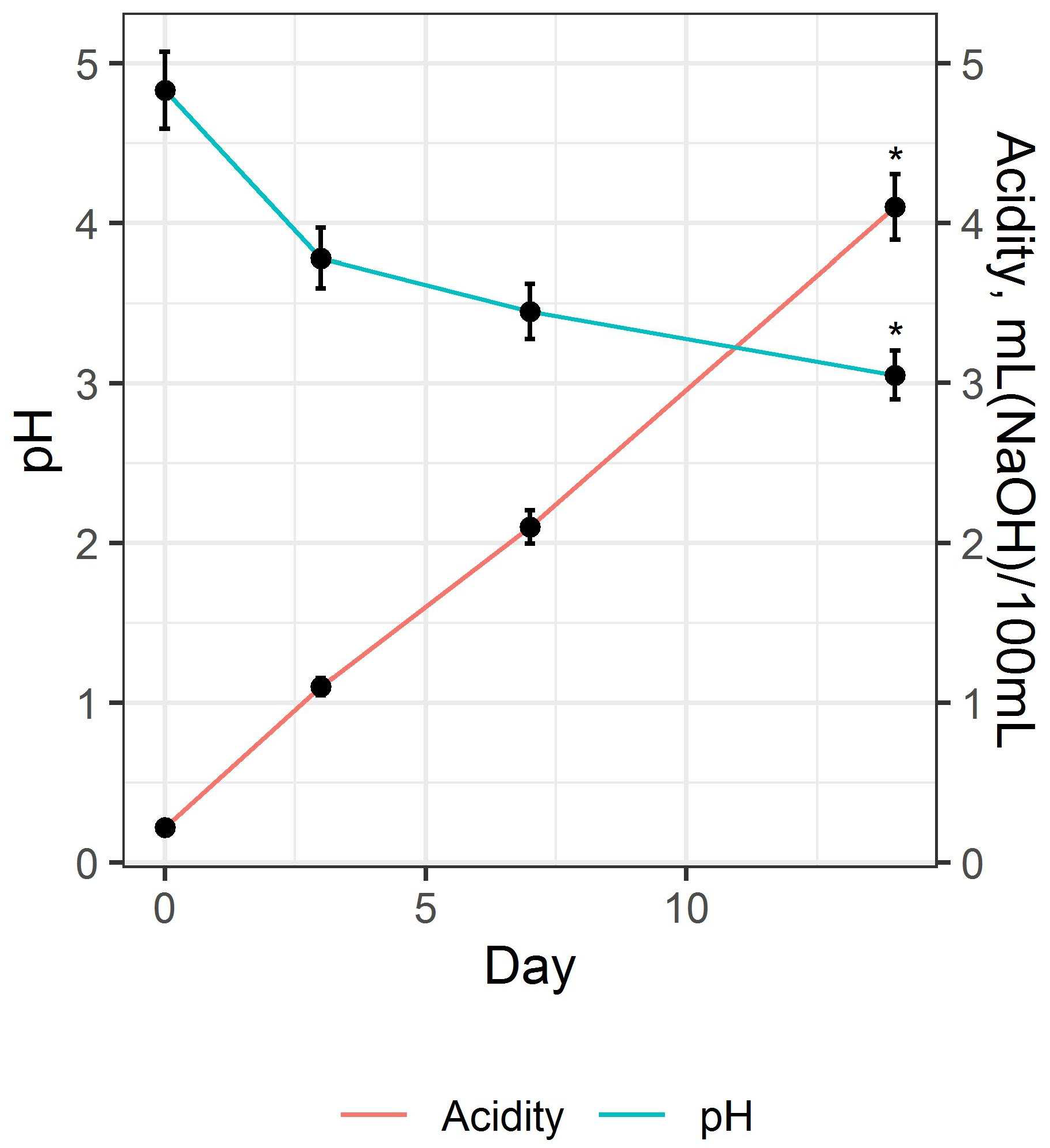
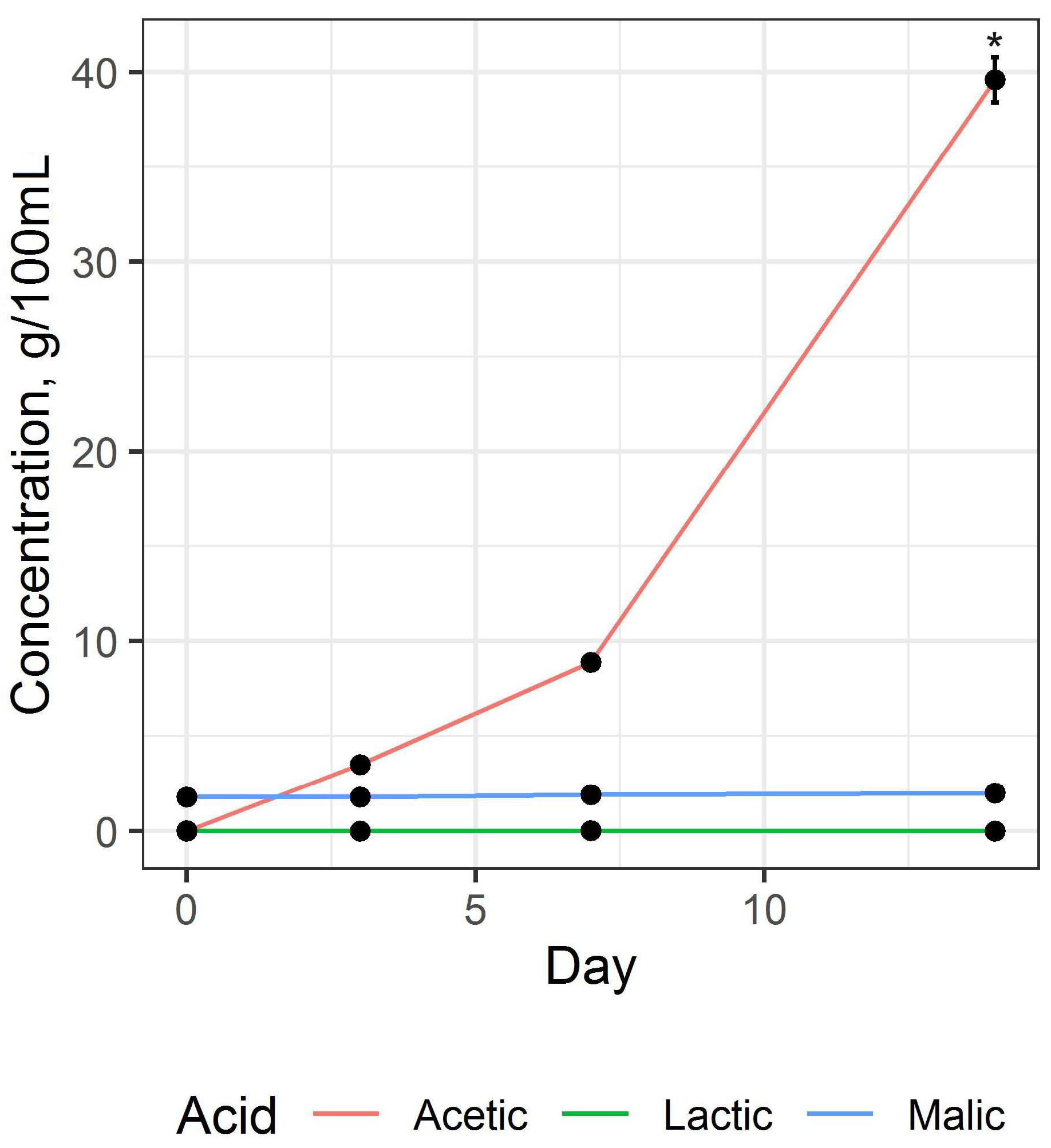
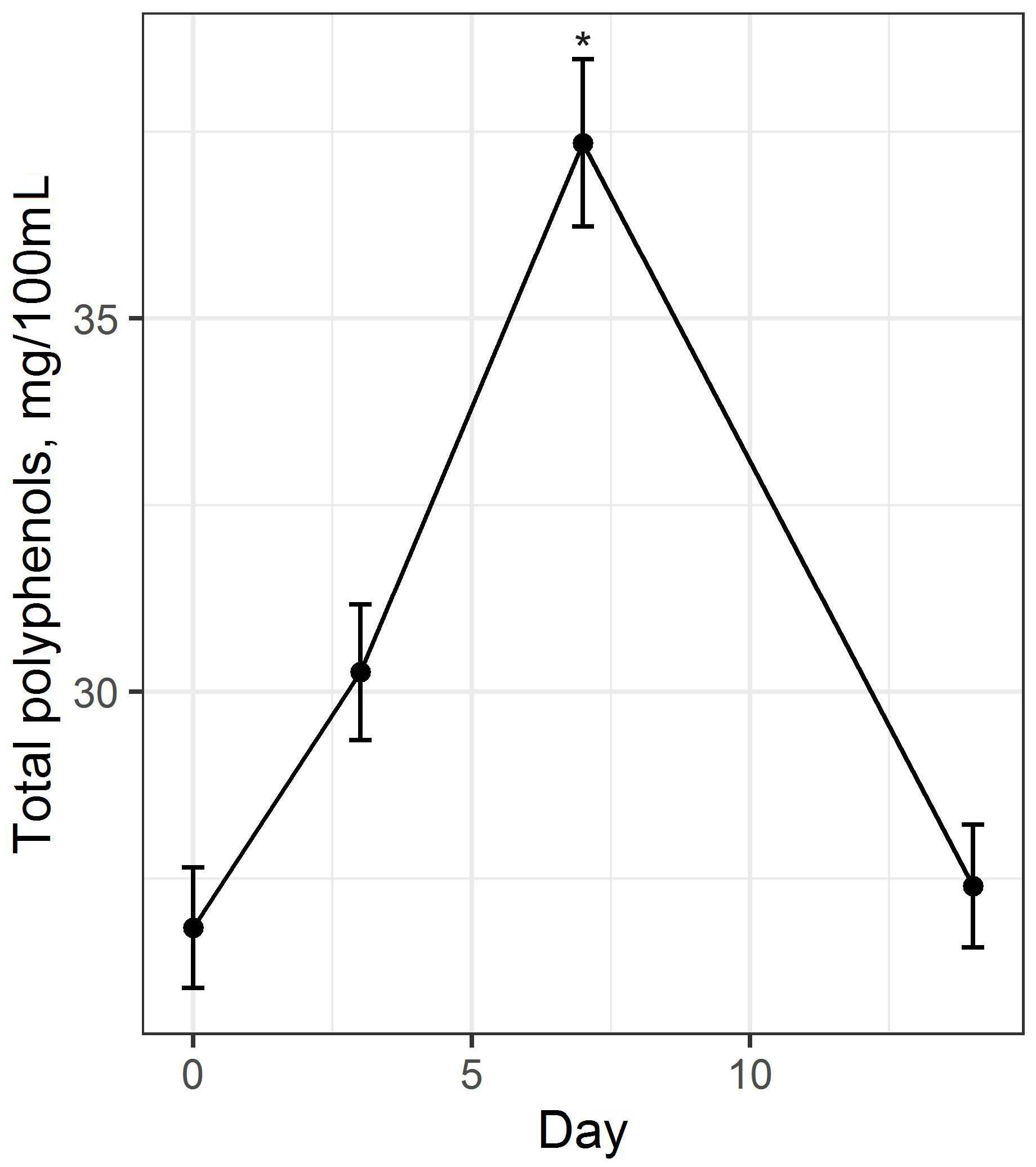
3.1. Preparation and Study of Fermented Beverage Bases
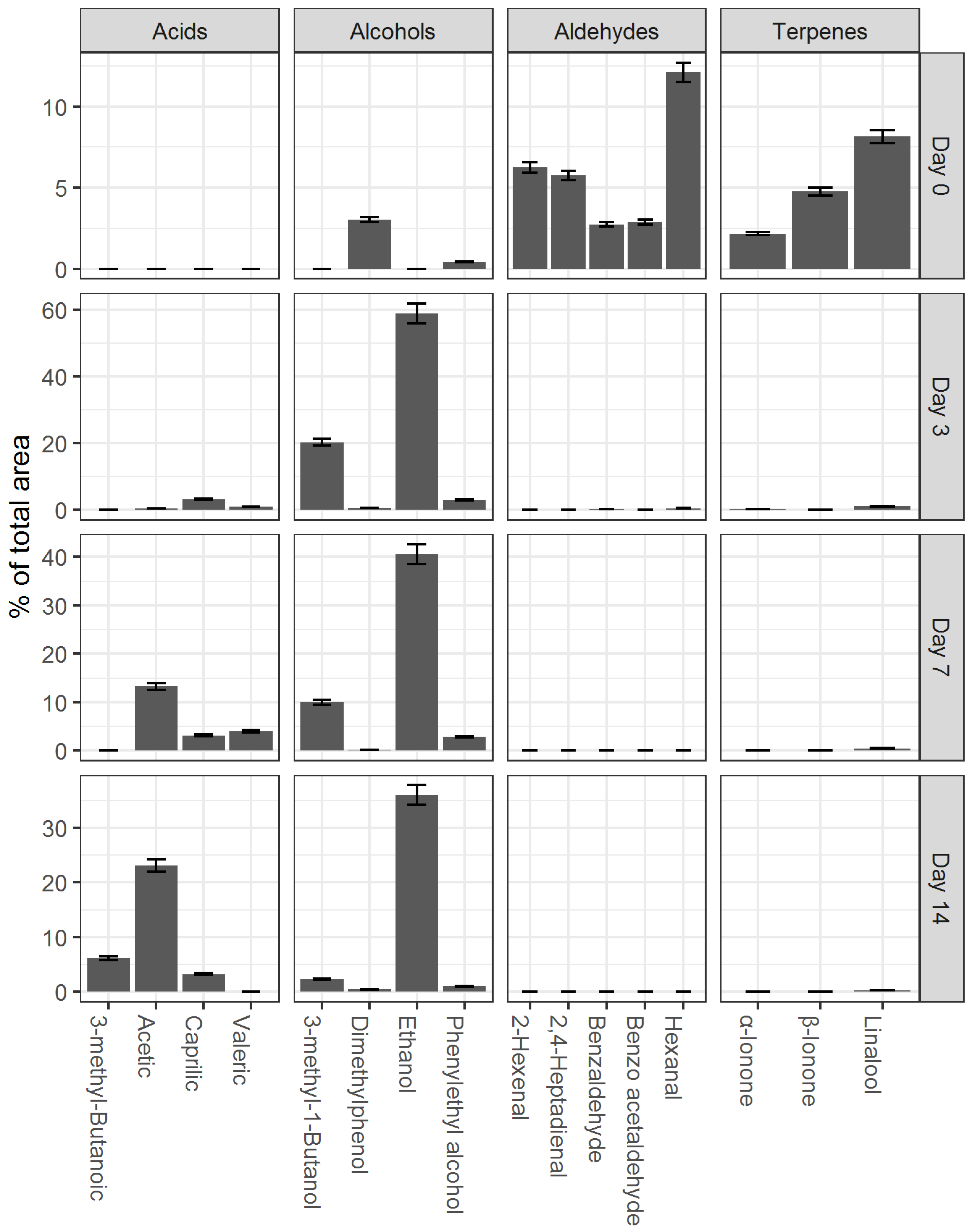
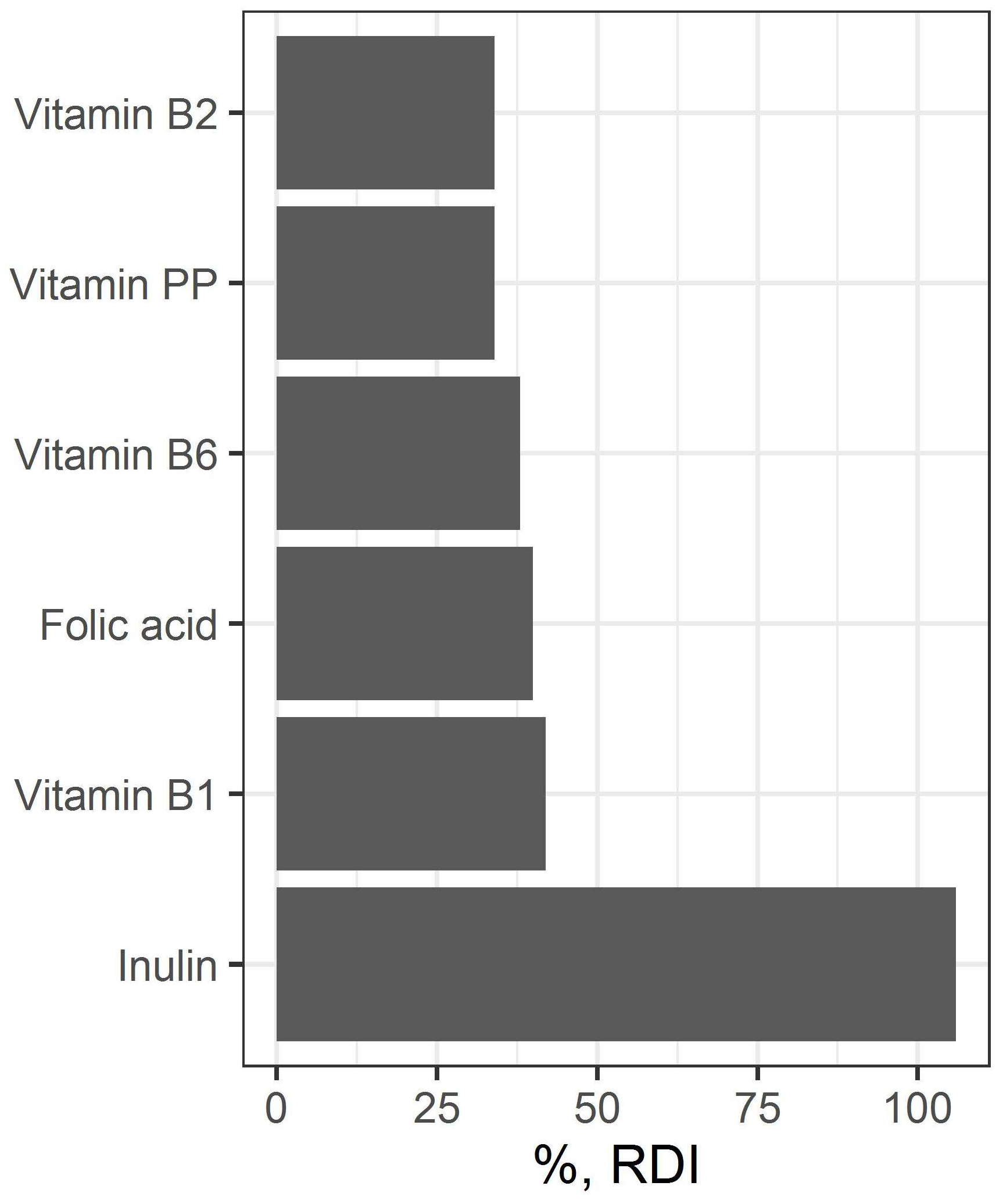
3.2. Preparation and Study of the Fermented Beverage
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nyhan, L.M.; Lynch, K.M.; Sahin, A.W.; Arendt, E.K. Advances in kombucha tea fermentation: A review. Appl. Microbiol. 2022, 2, 73–103. [Google Scholar] [CrossRef]
- Bortolomedi, B.M.; Paglarin, C.S.; Brod, F.C.A. Bioactive compounds in kombucha: A review of substrate effect and fermentation conditions. Food Chem. 2022, 385, 132719. [Google Scholar] [CrossRef] [PubMed]
- de Miranda, J.F.; Ruiz, L.F.; Silva, C.B.; Uekane, T.M.; Silva, K.A.; Gonzalez, A.G.M.; Fernandes, F.F.; Lima, A.R. Kombucha: A review of substrates, regulations, composition, and biological properties. J. Food Sci. 2022, 87, 503–527. [Google Scholar] [CrossRef] [PubMed]
- Soares, M.G.; de Lima, M.; Schmidt, V.C.R. Technological aspects of kombucha, its applications and the symbiotic culture (SCOBY), and extraction of compounds of interest: A literature review. Trends Food Sci. Technol. 2021, 110, 539–550. [Google Scholar] [CrossRef]
- Antolak, H.; Piechota, D.; Kucharska, A. Kombucha tea—A double power of bioactive compounds from tea and symbiotic culture of bacteria and yeasts (SCOBY). Antioxidants 2021, 10, 1541. [Google Scholar] [CrossRef] [PubMed]
- Vargas, B.K.; Fabricio, M.F.; Ayub, M.A.Z. Health effects and probiotic and prebiotic potential of Kombucha: A bibliometric and systematic review. Food Biosci. 2021, 44, 101332. [Google Scholar] [CrossRef]
- Kim, J.; Adhikari, K. Current trends in kombucha: Marketing perspectives and the need for improved sensory research. Beverages 2020, 6, 15. [Google Scholar] [CrossRef]
- Frolova, Y.V. Russian market of fermented kombucha beverages. Vopr. Pitan. 2022, 91, 115–118. [Google Scholar] [CrossRef]
- Higuchi, K. A Two-Step Approach to Quantitative Content Analysis: KH Coder Tutorial using Anne of Green Gables (Part II). Ritsumeikan Soc. Sci. Rev. 2017, 53, 137–147. [Google Scholar]
- Li, S.; Zhang, Y.; Gao, J.; Li, T.; Li, H.; Mastroyannis, A.; He, S.; Rahaman, A.; Chang, K. Effect of Fermentation Time on Physiochemical Properties of Kombucha Produced from Different Teas and Fruits: Comparative Study. J. Food Qual. 2022, 2022, 2342954. [Google Scholar] [CrossRef]
- Villarreal-Soto, S.A.; Beaufort, S.; Bouajila, J.; Souchard, J.P.; Taillandier, P. Understanding kombucha tea fermentation: A review. J. Food Sci. 2018, 83, 580–588. [Google Scholar] [CrossRef] [PubMed]
- La Torre, C.; Fazio, A.; Caputo, P.; Plastina, P.; Caroleo, M.C.; Cannataro, R.; Cione, E. Effects of long-term storage on radical scavenging properties and phenolic content of Kombucha from black tea. Molecules 2021, 26, 5474. [Google Scholar] [CrossRef] [PubMed]
- Kluz, M.I.; Pietrzyk, K.; Pastuszczak, M.; Kacaniova, M.; Kita, A.; Kapusta, I.; Zaguła, G.; Zagrobelna, E.; Struś, K.; Marciniak-Lukasiak, K.; et al. Microbiological and physicochemical composition of various types of homemade kombucha beverages using alternative kinds of sugars. Foods 2022, 11, 1523. [Google Scholar] [CrossRef] [PubMed]
- Vitas, J.S.; Cvetanović, A.D.; Mašković, P.Z.; Švarc-Gajić, J.V.; Malbaša, R.V. Chemical composition and biological activity of novel types of kombucha beverages with yarrow. J. Funct. Foods 2018, 44, 95–102. [Google Scholar] [CrossRef]
- Abuduaibifu, A.; Tamer, C.E. Evaluation of physicochemical and bioaccessibility properties of goji berry kombucha. J. Food Process. Preserv. 2019, 43, e14077. [Google Scholar] [CrossRef]
- Francisco, Á.R.; Igor, H. Development of a no added sugar kombucha beverage based on germinated corn. Int. J. Gastron. Food Sci. 2021, 24, 100355. [Google Scholar] [CrossRef]
- Li, R.; Xu, Y.; Chen, J.; Wang, F.; Zou, C.; Yin, J. Enhancing the proportion of gluconic acid with a microbial community reconstruction method to improve the taste quality of Kombucha. LWT 2022, 155, 112937. [Google Scholar] [CrossRef]
- da Silva Júnior, J.C.; Magnani, M.; da Costa, W.K.A.; Madruga, M.S.; Olegário, L.S.; Borges, G.D.S.C.; Dantas, A.M.; Lima, M.S.; de Lima, L.C.; de Limo Brinto, I.; et al. Traditional and flavored kombuchas with pitanga and umbu-caja pulps: Chemical properties, antioxidants, and bioactive compounds. Food Biosci. 2021, 44, 101380. [Google Scholar] [CrossRef]
- Zubaidah, E.; Yurista, S.; Rahmadani, N.R. Characteristic of physical, chemical, and microbiological kombucha from various varieties of apples. IOP Conf. Ser. Earth Environ. Sci. 2018, 131, 012040. [Google Scholar] [CrossRef]
- Liamkaew, R.; Chattrawanit, J.; Danvirutai, P. Kombucha production by combinations of black tea and apple juice. Prog. Appl. Sci. Technol. 2016, 6, 139–146. [Google Scholar]
- Zazueta, A.C.L.; Zavala, P.G.; Arguilez, C.G.Z. una Estandarización química de una bebida fermentada de Kombucha a base de té verde, té de limón ye infusión hojas de guayaba. Rev. De Investig. Académica Sin Front. Div. De Cienc. Económicas Y Soc. 2022, 38. [Google Scholar] [CrossRef]
- Zubaidah, E.; Dewantari, F.J.; Novitasari, F.R.; Srianta, I.; Blanc, P.J. Potential of snake fruit (Salacca zalacca (Gaerth.) Voss) for the development of a beverage through fermentation with the Kombucha consortium. Biocatal. Agric. Biotechnol. 2018, 13, 198–203. [Google Scholar] [CrossRef]
- Yavari, N.; Assadi, M.M.; Larijani, K.; Moghadam, M.B. Response surface methodology for optimization of glucuronic acid production using kombucha layer on sour cherry juice. Aust. J. Basic Appl. Sci. 2010, 4, 3250–3256. [Google Scholar]
- Tang, Z.; Zhao, Z.; Chen, S.; Lin, W.; Wang, Q.; Shen, N.; Qin, Y.; Xiao, Y.; Chen, H.; Bu, T.; et al. Dragon fruit-kiwi fermented beverage: In vitro digestion, untargeted metabolome analysis and anti-aging activity in Caenorhabditis elegans. Front. Nutr. 2022, 9, 1052818. [Google Scholar] [CrossRef] [PubMed]
- Ulusoy, A.; Tamer, C.E. Determination of suitability of black carrot (Daucus carota L. spp. sativus var. atrorubens Alef.) juice concentrate, cherry laurel (Prunus laurocerasus), blackthorn (Prunus spinosa) and red raspberry (Rubus ideaus) for kombucha beverage production. J. Food Meas. Charact. 2019, 13, 1524–1536. [Google Scholar]
- Tejedor-Calvo, E.; Morales, D. Chemical and Aromatic Changes during Fermentation of Kombucha Beverages Produced Using Strawberry Tree (Arbutus unedo) Fruits. Fermentation 2023, 9, 326. [Google Scholar] [CrossRef]
- Budiari, S.; Mulyani, H.; Maryati, Y.; Filailla, E.; Devi, A.F.; Melanie, H. Chemical properties and antioxidant activity of sweetened red ginger extract fermented with kombucha culture. Agrointek: J. Teknol. Ind. Pertan. 2023, 17, 60–69. [Google Scholar] [CrossRef]
- Nintiasari, J.; Ramadhani, M.A. Uji Kuantitatif flavonoid dan Aktivitas Antioksidan Teh Kombucha Daun Kersen (Muntingia calabura). Indones. J. Pharm. Nat. Prod. 2022, 5, 174–183. [Google Scholar]
- Kayisoglu, S.; Coskun, F. Determination of physical and chemical properties of kombucha teas prepared with different herbal teas. Food Sci. Technol. 2020, 41, 393–397. [Google Scholar] [CrossRef]
- Velićanski, A.; Cvetković, D.; Markov, S. Characteristics of Kombucha fermentation on medicinal herbs from Lamiaceae family. Rom. Biotechnol. Lett. 2013, 18, 8034–8042. [Google Scholar]
- Tanticharakunsiri, W.; Mangmool, S.; Wongsariya, K.; Ochaikul, D. Characteristics and upregulation of antioxidant enzymes of kitchen mint and oolong tea kombucha beverages. J. Food Biochem. 2021, 45, e13574. [Google Scholar] [CrossRef] [PubMed]
- Shahbazi, H.; Hashemi Gahruie, H.; Golmakani, M.T.; Eskandari, M.H.; Movahedi, M. Effect of medicinal plant type and concentration on physicochemical, antioxidant, antimicrobial, and sensorial properties of kombucha. Food Sci. Nutr. 2018, 6, 2568–2577. [Google Scholar] [CrossRef]
- Özyurt, H. Changes in the content of total polyphenols and the antioxidant activity of different beverages obtained by Kombucha ‘tea fungus’. Int. J. Agric. Environ. Food Sci. 2020, 4, 255–261. [Google Scholar] [CrossRef]
- Tu, C.; Tang, S.; Azi, F.; Hu, W.; Dong, M. Use of kombucha consortium to transform soy whey into a novel functional beverage. J. Funct. Foods 2019, 52, 81–89. [Google Scholar] [CrossRef]
- Martínez Leal, J.; Valenzuela Suárez, L.; Jayabalan, R.; Huerta Oros, J.; Escalante-Aburto, A. A review on health benefits of kombucha nutritional compounds and metabolites. CyTA-J. Food 2018, 16, 390–399. [Google Scholar] [CrossRef]
- Kapp, J.M.; Sumner, W. Kombucha: A systematic review of the empirical evidence of human health benefit. Ann. Epidemiol. 2019, 30, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Bishop, P.; Pitts, E.R.; Budner, D.; Thompson-Witrick, K.A. Chemical composition of kombucha. Beverages 2022, 8, 45. [Google Scholar] [CrossRef]
- Godswill, A.G.; Somtochukwu, I.V.; Ikechukwu, A.O.; Kate, E.C. Health benefits of micronutrients (vitamins and minerals) and their associated deficiency diseases: A systematic review. Int. J. Food Sci. 2020, 3, 1–32. [Google Scholar] [CrossRef]
- Kodentsova, V.M.; Vrzhesinskaya, O.A.; Nikityuk, D.V.; Tutelyan, V.A. Vitamin status of adult population of the Russian Federation: 1987-2017. Vopr. Pitan. 2018, 87, 62–68. [Google Scholar]
- Pietrangelo, L.; Magnifico, I.; Petronio Petronio, G.; Cutuli, M.A.; Venditti, N.; Nicolosi, D.; Perna, A.; Guerra, G.; Di Marco, R. A Potential “Vitaminic Strategy” against Caries and Halitosis. Appl. Sci. 2022, 12, 2457. [Google Scholar] [CrossRef]
- Gondivkar, S.M.; Gadbail, A.R.; Gondivkar, R.S.; Sarode, S.C.; Sarode, G.S.; Patil, S.; Awan, K.H. Nutrition and oral health. Disease-a-Month 2019, 65, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Sewón, L.A.; Karjalainen, S.M.; Söderling, E.; Lapinleimu, H.; Simell, O. Associations between salivary calcium and oral health. J. Clin. Periodontol. 1998, 25, 915–919. [Google Scholar] [CrossRef] [PubMed]
- Lattimer, J.M.; Haub, M.D. Effects of dietary fiber and its components on metabolic health. Nutrients 2010, 2, 1266–1289. [Google Scholar] [CrossRef] [PubMed]
- Myhrstad, M.C.; Tunsjø, H.; Charnock, C.; Telle-Hansen, V.H. Dietary fiber, gut microbiota, and metabolic regulation—Current status in human randomized trials. Nutrients 2020, 12, 859. [Google Scholar] [CrossRef]
- Murga-Garrido, S.M.; Hong, Q.; Cross, T.W.L.; Hutchison, E.R.; Han, J.; Thomas, S.P.; Vivas, E.I.; Denu, J.; Ceschin, D.G.; Tang, Z.Z.; et al. Gut microbiome variation modulates the effects of dietary fiber on host metabolism. Microbiome 2021, 9, 117. [Google Scholar] [CrossRef]
- Pyryeva, E.A.; Safronova, A.I. The role of dietary fibers in the nutrition of the population. Vopr. Pitan. 2019, 88, 5–11. [Google Scholar]
- Wan, X.; Guo, H.; Liang, Y.; Zhou, C.; Liu, Z.; Li, K.; Niu, F.; Zhai, X.; Wang, L. The physiological functions and pharmaceutical applications of inulin: A review. Carbohydr. Polym. 2020, 246, 116589. [Google Scholar] [CrossRef]
- Redondo-Cuenca, A.; Herrera-Vázquez, S.E.; Condezo-Hoyos, L.; Gómez-Ordóñez, E.; Rupérez, P. Inulin extraction from common inulin-containing plant sources. Ind. Crops Prod. 2021, 170, 113726. [Google Scholar] [CrossRef]
- Illippangama, A.U.; Jayasena, D.D.; Jo, C.; Mudannayake, D.C. Inulin as a functional ingredient and their applications in meat products. Carbohydr. Polym. 2022, 275, 118706. [Google Scholar] [CrossRef]
- Gupta, N.; Jangid, A.K.; Pooja, D.; Kulhari, H. Inulin: A novel and stretchy polysaccharide tool for biomedical and nutritional applications. Int. J. Biol. Macromol. 2019, 132, 852–863. [Google Scholar] [CrossRef]
- Ni, D.; Xu, W.; Zhu, Y.; Zhang, W.; Zhang, T.; Guang, C.; Mu, W. Inulin and its enzymatic production by inulosucrase: Characteristics, structural features, molecular modifications and applications. Biotechnol. Adv. 2019, 37, 306–318. [Google Scholar] [CrossRef] [PubMed]
- Lončar, E.S.; Kanurić, K.G.; Malbaša, R.V.; Đurić, M.S.; Milanović, S.D. Kinetics of saccharose fermentation by Kombucha. Chem. Ind. Chem. Eng. Q. 2014, 20, 345–352. [Google Scholar] [CrossRef]
- Bokov, D.O.; Sergunova, E.V.; Marakhova, A.I.; Morokhina, S.L.; Plakhotnaia, O.N.; Krasnyuk, I.I.; Bessonov, V.V. Determination of Sugar Profile in Viburnum Fruits and its Dosage Forms by HPLC-RID. Pharmacogn. J. 2020, 12, 103–108. [Google Scholar] [CrossRef]
- Gamboa-Gómez, C.I.; Simental-Mendía, L.E.; González-Laredo, R.F.; Alcantar-Orozco, E.J.; Monserrat-Juarez, V.H.; Ramírez-España, J.C.; Gallegos-Infante, J.A.; Moreno-Jiménez, M.R.; Rocha-Guzmán, N.E. In vitro and in vivo assessment of anti-hyperglycemic and antioxidant effects of Oak leaves (Quercus convallata and Quercus arizonica) infusions and fermented beverages. Food Res. Int. 2017, 102, 690–699. [Google Scholar] [CrossRef] [PubMed]
- Borai, A.; Sebaih, S.; Alshargi, A.; Albarzan, F.; Al-Ghamdi, A.; Al-Ghamdi, S.; Boraie, S.; Bahijri, S.; Al-Shareef, A.S.; Al-Armi, A.; et al. Ethanol content of a traditional Saudi beverage Sobia. Int. J. Food Prop. 2021, 24, 1790–1798. [Google Scholar] [CrossRef]
- Tytelyan, V.A. Guidance on Methods of Quality Control and Safety of Biologically Active Food Supplements; Federal Center for State Sanitary and Epidemiological Surveillance of the Ministry of Health of Russia: Moscow, Russia, 2004; 240p.
- Chu, S.C.; Chen, C. Effects of origins and fermentation time on the antioxidant activities of kombucha. Food Chem. 2006, 98, 502–507. [Google Scholar] [CrossRef]
- Zou, C.; Li, R.Y.; Chen, J.X.; Wang, F.; Gao, Y.; Fu, Y.Q.; Xu, Y.Q.; Yin, J.F. Zijuan tea-based kombucha: Physicochemical, sensorial, and antioxidant profile. Food Chem. 2021, 363, 130322. [Google Scholar] [CrossRef]
- Espenson, J.H. Chemical Kinetics and Reaction Mechanisms; McGraw-Hill: New York, NY, USA, 1995; p. 296. [Google Scholar]
- Bokov, D.O.; Khromchenkova, E.P.; Sokurenko, M.S.; Vasilev, A.V.; Bessonov, V.V. Development of a technique for the determination of inulin in natural instant chicory after enzymatic hydrolysis by high-performance liquid chromatography. Vopr. Pitan. 2017, 86, 50–55. [Google Scholar]
- Bendryshev, A.A.; Pashkova, E.B.; Pirogov, A.V.; Shpigun, O.A. Determination of water-soluble vitamins in vitamin premixes, bioacitve dietary supplements, and pharmaceutical preparations using high-efficiency liquid chromatography with gradient elution. Mosc. Univ. Chem. Bull. 2010, 65, 260–268. [Google Scholar] [CrossRef]
- Polak, J.; Bartoszek, M.; Chorązewski, M. Antioxidant capacity: Experimental determination by EPR spectroscopy and mathematical modeling. J. Agric. Food Chem. 2015, 63, 6319–6324. [Google Scholar] [CrossRef]
- ISO 8586-1:1996; Sensory Analysis-General Guidance for the Selection, Training and Monitoring of Assessors-Part 1: Selected Assessors. ISO: Geneva, Switzerland, 1996.
- ISO 8586-2:1996; Sensory Analysis-General Guidance for the Selection, Training and Monitoring of Assessors-Part 2: Expert Sensory Assessors. ISO: Geneva, Switzerland, 1996.
- ISO 8589:2007; General Guidance for the Design of Test Rooms. International Organization for Standardization. ISO: Geneva, Switzerland, 2007.
- ISO 13299:2016; Sensory Analysis. Methodology. General Guidance for Establishing a Sensory Profile. International Organization for Standardization (ISO): Geneva, Switzerland, 2016.
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 5 June 2023).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Granato, D.; de Araújo Calado, V.M.; Jarvis, B. Observations on the use of statistical methods in food science and technology. Food Res. Int. 2014, 55, 137–149. [Google Scholar] [CrossRef]
- Cvetković, D.; Markov, S.; Djurić, M.; Savić, D.; Velićanski, A. Specific interfacial area as a key variable in scaling-up Kombucha fermentation. J. Food Eng. 2008, 85, 387–392. [Google Scholar] [CrossRef]
- Malbaša, R.; Lončar, E.; Djurić, M.; Klašnja, M.; Kolarov, L.J.; Markov, S. Scale-up of black tea batch fermentation by kombucha. Food Bioprod. Process. 2006, 84, 193–199. [Google Scholar] [CrossRef]
- Diez-Ozaeta, I.; Astiazaran, O.J. Recent advances in Kombucha tea: Microbial consortium, chemical parameters, health implications and biocellulose production. Int. J. Food Microbiol. 2022, 377, 109783. [Google Scholar] [CrossRef]
- Vorobyeva, V.M.; Vorobyeva, I.S.; Sarkisyan, V.A.; Frolova, Y.V.; Kochetkova, A.A. Technological features of fermented beverages production using kombucha. Vopr. Pitan. 2022, 91, 115–120. [Google Scholar] [CrossRef]
- Tran, T.; Grandvalet, C.; Verdier, F.; Martin, A.; Alexandre, H.; Tourdot-Maréchal, R. Microbiological and technological parameters impacting the chemical composition and sensory quality of kombucha. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2050–2070. [Google Scholar] [CrossRef]
- Chen, C.; Liu, B.Y. Changes in major components of tea fungus metabolites during prolonged fermentation. J. Appl. Microbiol. 2000, 89, 834–839. [Google Scholar] [CrossRef]
- Jayabalan, R.; Subathradevi, P.; Marimuthu, S.; Sathishkumar, M.; Swaminathan, K. Changes in free-radical scavenging ability of kombucha tea during fermentation. Food Chem. 2008, 109, 227–234. [Google Scholar] [CrossRef]
- Jakubczyk, K.; Kałduńska, J.; Kochman, J.; Janda, K. Chemical profile and antioxidant activity of the kombucha beverage derived from white, green, black and red tea. Antioxidants 2020, 9, 447. [Google Scholar] [CrossRef]
- Gaggìa, F.; Baffoni, L.; Galiano, M.; Nielsen, D.S.; Jakobsen, R.R.; Castro-Mejía, J.L.; Bosi, S.; Truzzi, F.; Musumeci, F.; Dinelli, G.; et al. Kombucha beverage from green, black and rooibos teas: A comparative study looking at microbiology, chemistry and antioxidant activity. Nutrients 2018, 11, 1. [Google Scholar] [CrossRef]
- Laureys, D.; Britton, S.J.; De Clippeleer, J. Kombucha tea fermentation: A review. J. Am. Soc. Brew. Chem. 2020, 78, 165–174. [Google Scholar] [CrossRef]
- Jayabalan, R.; Malbaša, R.V.; Sathishkumar, M. Kombucha tea: Metabolites. In Fungal Metabolites; Springer: Cham, Switzerland, 2017; pp. 965–978. [Google Scholar]
- Reiss, J. Influence of different sugars on the metabolism of the tea fungus. Z. Für Lebensm.-Unters. Und-Forsch. 1994, 198, 258–261. [Google Scholar] [CrossRef]
- Kaewkod, T.; Bovonsombut, S.; Tragoolpua, Y. Efficacy of kombucha obtained from green, oolong, and black teas on inhibition of pathogenic bacteria, antioxidation, and toxicity on colorectal cancer cell line. Microorganisms 2019, 7, 700. [Google Scholar] [CrossRef]
- Liu, Z.; Bruins, M.E.; de Bruijn, W.J.; Vincken, J.P. A comparison of the phenolic composition of old and young tea leaves reveals a decrease in flavanols and phenolic acids and an increase in flavonols upon tea leaf maturation. J. Food Compos. Anal. 2020, 86, 103385. [Google Scholar] [CrossRef]
- Meng, X.H.; Li, N.; Zhu, H.T.; Wang, D.; Yang, C.R.; Zhang, Y.J. Plant resources, chemical constituents, and bioactivities of tea plants from the genus Camellia section Thea. J. Agric. Food Chem. 2018, 67, 5318–5349. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, L.; Qi, L.; Liang, H.; Lin, X.; Li, S.; Yu, C.; Ji, C. Effect of synthetic microbial community on nutraceutical and sensory qualities of kombucha. Int. J. Food Sci. Technol. 2020, 55, 3327–3333. [Google Scholar] [CrossRef]
- Chakravorty, S.; Bhattacharya, S.; Chatzinotas, A.; Chakraborty, W.; Bhattacharya, D.; Gachhui, R. Kombucha tea fermentation: Microbial and biochemical dynamics. Int. J. Food Microbiol. 2016, 220, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Li, Y.; Li, M.; Wang, Y.; Zhang, L.; Wan, X.; Yang, X. Tea aroma formation from six model manufacturing processes. Food Chem. 2019, 285, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Bishop, P.; Pitts, E.R.; Budner, D.; Thompson-Witrick, K.A. Kombucha: Biochemical and microbiological impacts on the chemical and flavor profile. Food Chem. Adv. 2022, 1, 100025. [Google Scholar] [CrossRef]
- Jayabalan, R.; Marimuthu, S.; Swaminathan, K. Changes in content of organic acids and tea polyphenols during kombucha tea fermentation. Food Chem. 2007, 102, 392–398. [Google Scholar] [CrossRef]
- Matsushita, K.; Adachi, O. Quinoprotein aldehyde dehydrogenases in microorganisms. In Principles and Applications of Quinoproteins; CRC Press: Boca Raton, FL, USA, 2020; pp. 65–72. [Google Scholar]
- Akimov, M.Y.; Bessonov, V.V.; Kodentsova, V.M.; Eller, K.I.; Vrzhesinskaya, O.A.; Beketova, N.A.; Kosheleva, O.V.; Bogachuk, M.N.; Malinkin, A.D.; Makarenko, M.A.; et al. Biological value of fruits and berries of Russian production. Vopr. Pitan. 2020, 89, 220–232. [Google Scholar] [PubMed]
- Tutel'ian, V.A.; Lashneva, N.V. Biologically active substances of plant origin. Flavonols and flavones: Prevalence, dietary sourses and consumption. Vopr. Pitan. 2013, 82, 4–22. [Google Scholar] [PubMed]
- Ikegaya, A.; Toyoizumi, T.; Ohba, S.; Nakajima, T.; Kawata, T.; Ito, S.; Arai, E. Effects of distribution of sugars and organic acids on the taste of strawberries. Food Sci. Nutr. 2019, 7, 2419–2426. [Google Scholar] [CrossRef]
- Koyuncu, M.A.; Dilmaçünal, T. Determination of vitamin C and organic acid changes in strawberry by HPLC during cold storage. Not. Bot. Horti Agrobot. Cluj-Napoca 2010, 38, 95–98. [Google Scholar]
- Mukherjee, A.; Gómez-Sala, B.; O’connor, E.M.; Kenny, J.G.; Cotter, P.D. Global regulatory frameworks for fermented foods: A review. Front. Nutr. 2022, 9, 902642. [Google Scholar] [CrossRef] [PubMed]
- Pilipenko, V.I.; Isakov, V.A.; Morozov, S.V.; Vlasova, A.V.; Kochetkova, A.A. Efficacy of newly developed kombucha-based specialized food product for treatment of constipation-predominant irritable bowel syndrome. Vopr. Pitan. 2022, 91, 95–104. [Google Scholar]

| Compound | Concentration, mg/kg |
|---|---|
| Thiamine (Vitamin B1) | 48,750 |
| Riboflavin | 44,000 |
| Pyridoxine (Vitamin B6) | 57,000 |
| Niacin | 479,998 |
| Folic acid | 7500 |
| Compound | Reaction Order | r2 | K | t1/2, Days |
|---|---|---|---|---|
| Acetic acid | Zero | 0.960 ± 0.012 | 1.22 ± 0.03 (M∙d−1) | - |
| Ethanol | Second | 0.854 ± 0.010 | 9.39 ± 0.11 × 10−4 (M−1·d−1) | 18.05 ± 1.28 |
| 3-methyl-1-Butanol | First | 0.999 ± 0.014 | 5.99 ± 0.02 × 10−1 (d−1) | 1.15 ± 0.16 |
| Linalool | Second | 0.996 ± 0.013 | 2.95 ± 0.07 × 10−1 (M−1·d−1) | 0.42 ± 0.09 |
| Hexanal | Second | 0.984 ± 0.021 | 1.4 ± 0.06 (M−1·d−1) | 0.06 ± 0.01 |
| Ingredient | Amount, g |
|---|---|
| Kombucha base | 1038.0 |
| Strawberry | 173.0 |
| Kaffir lime leaf | 3.7 |
| Total | 1214.7 |
| Yield after filtration | 1000 |
| Parameter | Value | Value |
|---|---|---|
| Kombucha Base | Fermented Beverages | |
| Acidity, mL 1 mole/L NaOH/100 mL | 4.10 ± 0.26 | 4.72 ± 0.38 |
| pH | 3.31 ± 0.08 | 3.57 ± 0.07 |
| Solids content, °Brix | 4.60 ± 0.25 | 4.95 ± 0.25 |
| Ethanol content, % | 0.22 ± 0.01 | 0.43 ± 0.01 |
| Content of organic acids, mg/100 mL | ||
| 0.025 ± 0.001 | 43.80 ± 4.82 |
| 39.80 ± 1.82 | 205.00 ± 16.40 |
| - | 2.00 ± 0.14 |
| - | 65.10 ± 5.86 |
| 2.04 ± 0.11 | 45.50 ± 6.37 |
| Carbohydrates contents (mono-, disaccharides), g/100 mL | ||
| 0.70 ± 0.11 | 1.45 ± 0.13 |
| 0.22 ± 0.12 | 1.47 ± 0.12 |
| 3.60 ± 0.04 | 0.18 ± 0.02 |
| Ingredient | Amount, g |
|---|---|
| Filtered fermented beverage | 903.34 |
| Water | 84.0 |
| Inulin | 12.6 |
| Vitamin premix | 0.06 |
| Total | 1000 |
| Parameter | Description | Value | |
|---|---|---|---|
| Kombucha Base | Fermented Beverages | ||
| Appearance | Non-transparent liquid. Sludge due to the characteristics of the raw material used is allowed, without foreign inclusions. | 5.00 ± 0.00 | 5.00 ± 0.00 |
| Color | Red with a brownish hue due to the color of the raw material used. | 5.00 ± 0.00 | 5.00 ± 0.00 |
| Odor | Inherent in the ingredients used with the aroma of a fermented beverage. | 4.25 ± 0.71 | 4.71 ± 0.48 |
| Taste | Sweet and sour with strawberry flavor and pronounced kaffir lime leaf flavor. | 4.25 ± 0.71 | 4.71 ± 0.48 |
| Consistency | Liquid with low viscosity and rich body. | 4.38 ± 0.50 | 5.00 ± 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frolova, Y.; Vorobyeva, V.; Vorobyeva, I.; Sarkisyan, V.; Malinkin, A.; Isakov, V.; Kochetkova, A. Development of Fermented Kombucha Tea Beverage Enriched with Inulin and B Vitamins. Fermentation 2023, 9, 552. https://doi.org/10.3390/fermentation9060552
Frolova Y, Vorobyeva V, Vorobyeva I, Sarkisyan V, Malinkin A, Isakov V, Kochetkova A. Development of Fermented Kombucha Tea Beverage Enriched with Inulin and B Vitamins. Fermentation. 2023; 9(6):552. https://doi.org/10.3390/fermentation9060552
Chicago/Turabian StyleFrolova, Yuliya, Valentina Vorobyeva, Irina Vorobyeva, Varuzhan Sarkisyan, Alexey Malinkin, Vasily Isakov, and Alla Kochetkova. 2023. "Development of Fermented Kombucha Tea Beverage Enriched with Inulin and B Vitamins" Fermentation 9, no. 6: 552. https://doi.org/10.3390/fermentation9060552
APA StyleFrolova, Y., Vorobyeva, V., Vorobyeva, I., Sarkisyan, V., Malinkin, A., Isakov, V., & Kochetkova, A. (2023). Development of Fermented Kombucha Tea Beverage Enriched with Inulin and B Vitamins. Fermentation, 9(6), 552. https://doi.org/10.3390/fermentation9060552







