Development of Blood Sugar Regulatory Products from Momordica cochininensis via Probiotic Fermentation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation and Identification of Lactic Acid Bacteria (LAB)
2.1.1. Isolation of LAB
2.1.2. Gram Staining, Catalase, and Oxidase Tests
2.1.3. α-Glucosidase Inhibitory Activity Assay
- Asample: Absorbance of sample
- Ablank: Absorbance of mixture with sample and α-glucosidase
- Acontrol: Absorbance of control
2.2. 16S rRNA Gene Sequencing Analysis
2.3. Scanning Electron Microscopy of L. plantarum GBI 001
2.4. Optimal Culture Conditions of L. plantarum GBI 001 for Producing α-Glucosidase Fermentation Activity
2.5. LAB Cell Count
2.6. pH
2.7. Statistical Analysis
3. Results and Discussion
3.1. Isolation and Identification of LAB with α-Glucosidase-Inhibitory Activity
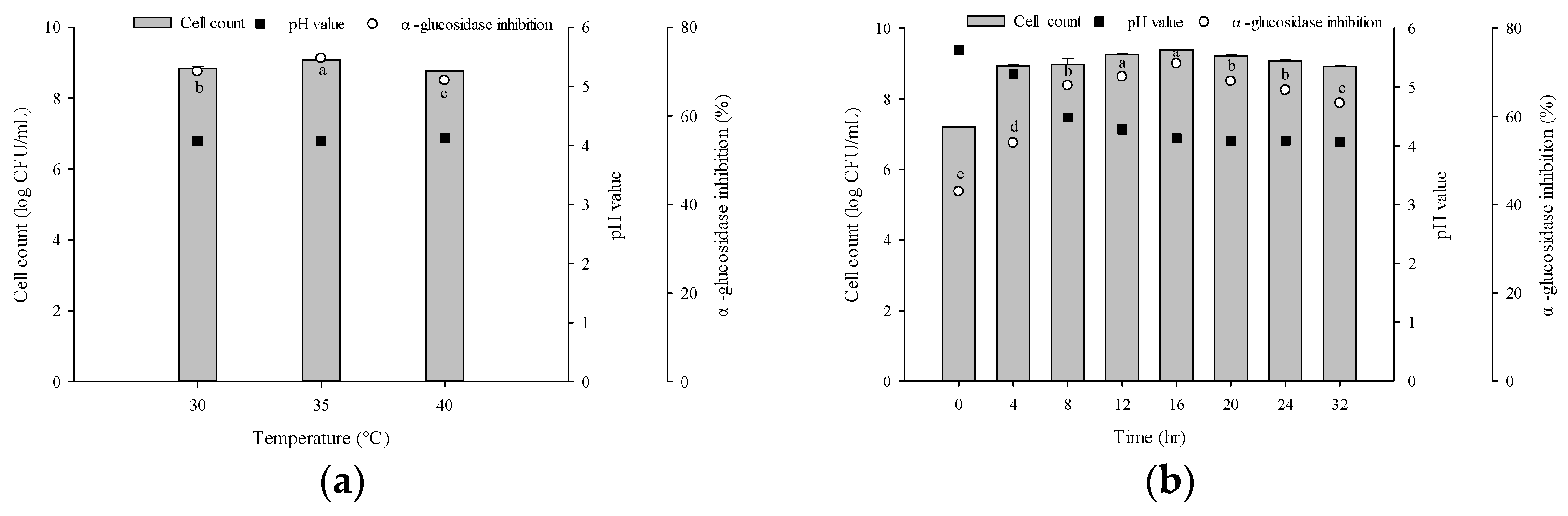
3.2. Optimal Culture Conditions for Producing α-Glucosidase-Inhibitory Activity
3.3. Optimal Fermentation Conditions of Gac Pulp
3.3.1. Matrix Content
3.3.2. Fermentation Temperature, Time, and Initial pH
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, C.-J.; Chiu, W.-C.; Tseng, Y.-H.; Lin, C.-M.; Yang, H.-Y.; Yang, Y.-H.; Chen, P.-C. Aristolochic acid and the risk of cancers in patients with type 2 diabetes: Nationwide population-based cohort study. Phytomedicine 2022, 99, 154023. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-R.; Jia, R.-B.; Luo, D.; Lin, L.; Zheng, Q.; Zhao, M. The positive effects and underlying mechanisms of Undaria pinnatifida polysaccharides on type 2 diabetes mellitus in rats. Food Funct. 2021, 12, 11898–11912. [Google Scholar] [CrossRef] [PubMed]
- Papoutsis, K.; Zhang, J.; Bowyer, M.C.; Brunton, N.; Gibney, E.R.; Lyng, J. Fruit, vegetables, and mushrooms for the preparation of extracts with α-amylase and α-glucosidase inhibition properties: A review. Food Chem. 2021, 338, 128119. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Xu, T.; Mao, G.; Chen, Y.; Qiu, X.; Yang, L.; Zhao, T.; Xu, X.; Feng, W.; Wu, X. Di-(2-ethylhexyl) phthalate-induced hepatotoxicity exacerbated type 2 diabetes mellitus (T2DM) in female pubertal T2DM mice. Food Chem. Toxicol. 2021, 149, 112003. [Google Scholar] [CrossRef]
- Wu, R.; Zhou, L.; Chen, Y.; Ding, X.; Liu, Y.; Tong, B.; Lv, H.; Meng, X.; Li, J.; Jian, T. Sesquiterpene glycoside isolated from loquat leaf targets gut microbiota to prevent type 2 diabetes mellitus in db/db mice. Food Funct. 2022, 13, 1519–1534. [Google Scholar] [CrossRef]
- Jiang, H.; Yao, Q.; An, Y.; Fan, L.; Wang, J.; Li, H. Baicalin suppresses the progression of Type 2 diabetes-induced liver tumor through regulating METTL3/m6A/HKDC1 axis and downstream p-JAK2/STAT1/clevaged Capase3 pathway. Phytomedicine 2022, 94, 153823. [Google Scholar] [CrossRef]
- Kurniawan, A.H.; Suwandi, B.H.; Kholili, U. Diabetic gastroenteropathy: A complication of diabetes mellitus. Acta Med. Indones 2019, 51, 263–271. [Google Scholar]
- Luo, D.; Mu, T.; Sun, H. Sweet potato (Ipomoea batatas L.) leaf polyphenols ameliorate hyperglycemia in type 2 diabetes mellitus mice. Food Funct. 2021, 12, 4117–4131. [Google Scholar] [CrossRef]
- Ghani, U. Re-exploring promising α-glucosidase inhibitors for potential development into oral anti-diabetic drugs: Finding needle in the haystack. Eur. J. Med. Chem. 2015, 103, 133–162. [Google Scholar] [CrossRef]
- Dirir, A.M.; Daou, M.; Yousef, A.F.; Yousef, L.F. A review of alpha-glucosidase inhibitors from plants as potential candidates for the treatment of type-2 diabetes. Phytochem. Rev. 2022, 21, 1049–1079. [Google Scholar] [CrossRef]
- Alongi, M.; Verardo, G.; Gorassini, A.; Anese, M. Effect of pasteurization on in vitro α-glucosidase inhibitory activity of apple juice. LWT 2018, 98, 366–371. [Google Scholar] [CrossRef]
- Yusuf, D.; Nuraida, L.; Dewanti-Hariyadi, R.; Hunaefi, D. In vitro Antioxidant and α-glucosidase inhibitory activities of Lactobacillus spp. isolated from indonesian kefir grains. Appl. Food Biotechnol. 2021, 8, 39–46. [Google Scholar]
- Baba, W.N.; Mudgil, P.; Kamal, H.; Kilari, B.P.; Gan, C.-Y.; Maqsood, S. Identification and characterization of novel α-amylase and α-glucosidase inhibitory peptides from camel whey proteins. J. Dairy Sci. 2021, 104, 1364–1377. [Google Scholar] [CrossRef] [PubMed]
- Si, L.; Lin, R.; Jia, Y.; Jian, W.; Yu, Q.; Wang, M.; Yang, S. Lactobacillus bulgaricus improves antioxidant capacity of black garlic in the prevention of gestational diabetes mellitus: A randomized control trial. Biosci. Rep. 2019, 39, BSR20182254. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Wang, L.; Cheng, S.; Zhang, Y.; Yang, M.; Fang, R.; Li, H.; Man, C.; Jiang, Y. A potential synbiotic strategy for the prevention of type 2 diabetes: Lactobacillus paracasei JY062 and exopolysaccharide isolated from Lactobacillus plantarum JY039. Nutrients 2022, 14, 377. [Google Scholar] [CrossRef]
- Olvera-Sandoval, C.; Fabela-Illescas, H.E.; Fernández-Martínez, E.; Ortiz-Rodríguez, M.A.; Cariño-Cortés, R.; Ariza-Ortega, J.A.; Hernández-González, J.C.; Olivo, D.; Valadez-Vega, C.; Belefant-Miller, H. Potential mechanisms of the improvement of glucose homeostasis in type 2 diabetes by pomegranate juice. Antioxidants 2022, 11, 553. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-H.; Guo, H.-R.; Patel, A.K.; Singhania, R.R.; Chen, Y.-A.; Kuo, J.-M.; Dong, C.-D. Production and characterization of lucrative hypoglycemic collagen-peptide-chromium from tilapia scale. Process Biochem. 2022, 115, 10–18. [Google Scholar] [CrossRef]
- Mokgalaboni, K.; Ntamo, Y.; Ziqubu, K.; Nyambuya, T.M.; Nkambule, B.B.; Mazibuko-Mbeje, S.E.; Gabuza, K.B.; Chellan, N.; Tiano, L.; Dludla, P.V. Curcumin supplementation improves biomarkers of oxidative stress and inflammation in conditions of obesity, type 2 diabetes and NAFLD: Updating the status of clinical evidence. Food Funct. 2021, 12, 12235–12249. [Google Scholar] [CrossRef]
- Yang, J.; He, Q.; Wang, Y.; Pan, Z.; Zhang, G.; Liang, J.; Su, L.; Wang, A.; Zeng, C.; Luo, H. Gegen Qinlian Decoction ameliorates type 2 diabetes osteoporosis via IGFBP3/MAPK/NFATc1 signaling pathway based on cytokine antibody array. Phytomedicine 2022, 94, 153810. [Google Scholar] [CrossRef]
- Yong, Z.; Yanan, Y.; Zhi, Z.; Yinfeng, T.; Lin, D.; Yiying, L.; Weiying, L.; Chongming, W.; Xiaopo, Z. Laurolitsine ameliorates type 2 diabetes by regulating the hepatic LKB1-AMPK pathway and gut microbiota. Phytomedicine 2022, 106, 154423. [Google Scholar] [CrossRef]
- Baralić, K.; Živančević, K.; Jorgovanović, D.; Javorac, D.; Radovanović, J.; Gojković, T.; Djordjevic, A.B.; Ćurčić, M.; Mandinić, Z.; Bulat, Z. Probiotic reduced the impact of phthalates and bisphenol A mixture on type 2 diabetes mellitus development: Merging bioinformatics with in vivo analysis. Food Chem. Toxicol. 2021, 154, 112325. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Park, S.; Mariadoss, A.V.A.; Sathiyaseelan, A.; Veeraraghavan, V.P.; Kim, S.; Wang, M.-H. Chemical composition, antioxidant, and anti-diabetic activities of ethyl acetate fraction of Stachys riederi var. japonica (Miq.) in streptozotocin-induced type 2 diabetic mice. Food Bioprocess Technol. 2021, 155, 112374. [Google Scholar] [CrossRef]
- Marcia, J.; Aleman, R.S.; Montero-Fernández, I.; Martín-Vertedor, D.; Manrique-Fernández, V.; Moncada, M.; Kayanush, A.J.F. Attributes of Lactobacillus acidophilus as Effected by Carao (Cassia grandis) Pulp Powder. Fermentation 2023, 9, 408. [Google Scholar] [CrossRef]
- Abdulqader, A.; Ali, F.; Ismail, A.; Esa, N.M. Antioxidant compounds and capacities of Gac (Momordica cochinchinensis Spreng) fruits. Asian Pac. J. Trop. Biomed. 2019, 9, 158. [Google Scholar]
- Yu, J.S.; Sahar, N.E.; Bi, Y.-R.; Jung, K.; Pang, C.; Huh, J.Y.; Kim, K.H. The effects of triterpenoid saponins from the seeds of Momordica cochinchinensis on adipocyte differentiation and mature adipocyte inflammation. Plants 2020, 9, 984. [Google Scholar] [CrossRef] [PubMed]
- Wimalasiri, D.; Dekiwadia, C.; Fong, S.Y.; Piva, T.J.; Huynh, T. Anticancer activity of Momordica cochinchinensis (red gac) aril and the impact of varietal diversity. BMC Complement. Med. Ther. 2020, 20, 365. [Google Scholar] [CrossRef] [PubMed]
- Kubola, J.; Siriamornpun, S. Phytochemicals and antioxidant activity of different fruit fractions (peel, pulp, aril and seed) of Thai gac (Momordica cochinchinensis Spreng). Food Chem. 2011, 127, 1138–1145. [Google Scholar] [CrossRef]
- Fu, M.; Shen, W.; Gao, W.; Namujia, L.; Yang, X.; Cao, J.; Sun, L. Essential moieties of myricetins, quercetins and catechins for binding and inhibitory activity against α-Glucosidase. Bioorganic Chem. 2021, 115, 105235. [Google Scholar] [CrossRef] [PubMed]
- Aleixandre, A.; Gil, J.V.; Sineiro, J.; Rosell, C.M. Understanding phenolic acids inhibition of α-amylase and α-glucosidase and influence of reaction conditions. Food Chem. 2022, 372, 131231. [Google Scholar] [CrossRef] [PubMed]
- Moradabadi, L.; Kouhsari, S.M.; Sani, M.F. Hypoglycemic effects of three medicinal plants in experimental diabetes: Inhibition of rat intestinal α-glucosidase and enhanced pancreatic Insulin and cardiac Glut-4 mRNAs expression. Iran. J. Pharm. Res. IJPR 2013, 12, 387. [Google Scholar]
- Abdulqader, A.; Ali, F.; Ismail, A.; Esa, N.M. Gac fruit extracts ameliorate proliferation and modulate angiogenic markers of human retinal pigment epithelial cells under high glucose conditions. Asian Pac. J. Trop. Biomed. 2018, 8, 571. [Google Scholar]
- Li, H.; Zhu, J.; Xiao, Y.; Zhang, S.; Sun, Y.; Liu, Z.; Chu, C.; Hu, X.; Yi, J.J.F. Biodiversity of Lactic Acid Bacteria in Traditional Fermented Foods in Yunnan Province, China, and Comparative Genomics of Lactobacillus plantarum. Fermentation 2023, 9, 402. [Google Scholar] [CrossRef]
- Khan, A.N.; Yasmin, H.; Ghazanfar, S.; Hassan, M.N.; Keyani, R.; Khan, I.; Gohar, M.; Shahzad, A.; Hashim, M.J.; Ahmad, A. Antagonistic, anti-oxidant, anti-inflammatory and anti-diabetic probiotic potential of lactobacillus agilis isolated from the rhizosphere of the medicinal plants. Saudi J. Biol. Sci. 2021, 28, 6069–6076. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-H.; Hsueh, Y.-H.; Kuo, J.-M.; Liu, S.-J. Characterization of a potential probiotic Lactobacillus brevis RK03 and efficient production of γ-aminobutyric acid in batch fermentation. Int. J. Mol. Sci. 2018, 19, 143. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.-H.; Dong, C.-D.; Patel, A.K.; Singhania, R.R.; Yang, M.-J.; Guo, H.-R.; Kuo, J.-M. Characterization of waste cell biomass derived glutamate decarboxylase for in vitro γ-aminobutyric acid production and value-addition. Bioresour. Technol. 2021, 337, 125423. [Google Scholar] [CrossRef]
- Razola-Díaz, M.d.C.; De Montijo-Prieto, S.; Aznar-Ramos, M.J.; Jiménez-Valera, M.; Ruiz-Bravo, A.; Verardo, V.; Gómez-Caravaca, A.M. Effect of Lactic Acid Bacteria Fermentation on the Polar Compounds Content with Antioxidant and Antidiabetic Activity of Avocado Seed Extracts. Fermentation 2023, 9, 420. [Google Scholar] [CrossRef]
- Negrete-Romero, B.; Valencia-Olivares, C.; Baños-Dossetti, G.A.; Pérez-Armendáriz, B.; Cardoso-Ugarte, G.A. Nutritional contributions and health associations of traditional fermented foods. Fermentation 2021, 7, 289. [Google Scholar] [CrossRef]
- Wang, G.; Si, Q.; Yang, S.; Jiao, T.; Zhu, H.; Tian, P.; Wang, L.; Li, X.; Gong, L.; Zhao, J. Lactic acid bacteria reduce diabetes symptoms in mice by alleviating gut microbiota dysbiosis and inflammation in different manners. Food Funct. 2020, 11, 5898–5914. [Google Scholar] [CrossRef]
- Chen, P.; Zhang, Q.; Dang, H.; Liu, X.; Tian, F.; Zhao, J.; Chen, Y.; Zhang, H.; Chen, W. Screening for potential new probiotic based on probiotic properties and α-glucosidase inhibitory activity. Food Control 2014, 35, 65–72. [Google Scholar] [CrossRef]
- Meena, K.K.; Taneja, N.K.; Jain, D.; Ojha, A.; Kumawat, D.; Mishra, V. In Vitro Assessment of Probiotic and Technological Properties of Lactic Acid Bacteria Isolated from Indigenously Fermented Cereal-Based Food Products. Fermentation 2022, 8, 529. [Google Scholar] [CrossRef]
- Masi, C.; Gemechu, G.; Tafesse, M. Isolation, screening, characterization, and identification of alkaline protease-producing bacteria from leather industry effluent. Ann. Microbiol. 2021, 71, 24. [Google Scholar] [CrossRef]
- Zheng, Q.; Jia, R.-B.; Ou, Z.-R.; Li, Z.-R.; Zhao, M.; Luo, D.; Lin, L. Comparative study on the structural characterization and α-glucosidase inhibitory activity of polysaccharide fractions extracted from Sargassum fusiforme at different pH conditions. Int. J. Biol. Macromol. 2022, 194, 602–610. [Google Scholar] [CrossRef]
- Tsunoda, T.; Samadi, A.; Burade, S.; Mahmud, T. Complete biosynthetic pathway to the antidiabetic drug acarbose. Nat. Commun. 2022, 13, 3455. [Google Scholar] [CrossRef]
- Liu, W.; Chen, M.; Duo, L.; Wang, J.; Guo, S.; Sun, H.; Menghe, B.; Zhang, H. Characterization of potentially probiotic lactic acid bacteria and bifidobacteria isolated from human colostrum. J. Dairy Sci. 2020, 103, 4013–4025. [Google Scholar] [CrossRef]
- Markkinen, N.; Laaksonen, O.; Yang, B. Impact of malolactic fermentation with Lactobacillus plantarum on volatile compounds of sea buckthorn juice. Eur. Food Res. Technol. 2021, 247, 719–736. [Google Scholar] [CrossRef]
- Cai, T.; Wu, H.; Qin, J.; Qiao, J.; Yang, Y.; Wu, Y.; Qiao, D.; Xu, H.; Cao, Y. In vitro evaluation by PCA and AHP of potential antidiabetic properties of lactic acid bacteria isolated from traditional fermented food. Lwt 2019, 115, 108455. [Google Scholar] [CrossRef]
- Kwun, S.Y.; Bae, Y.W.; Yoon, J.A.; Park, E.H.; Kim, M.D. Isolation of acid tolerant lactic acid bacteria and evaluation of α-glucosidase inhibitory activity. Food Sci. Biotechnol. Rep. 2020, 29, 1125–1130. [Google Scholar] [CrossRef]
- Zeng, Z.; Luo, J.; Zuo, F.; Zhang, Y.; Ma, H.; Chen, S. Screening for potential novel probiotic Lactobacillus strains based on high dipeptidyl peptidase IV and α-glucosidase inhibitory activity. J. Funct. Foods 2016, 20, 486–495. [Google Scholar] [CrossRef]
- Wang, H.; Li, L. Comprehensive evaluation of probiotic property, hypoglycemic ability and antioxidant activity of lactic acid bacteria. Foods 2022, 11, 1363. [Google Scholar] [CrossRef]
- Jeong, Y.; Kim, H.; Lee, J.Y.; Won, G.; Choi, S.-I.; Kim, G.-H.; Kang, C.-H. The antioxidant, anti-diabetic, and anti-adipogenesis potential and probiotic properties of lactic acid bacteria isolated from human and fermented foods. Fermentation 2021, 7, 123. [Google Scholar] [CrossRef]
- Filailla, E.; Mulyani, H.; Maryati, Y.; Budiarti, S. The Effect of Fermentation Conditions on Chemicals Content And α-Glucosidase Inhibition Activity Of Red Ginger Kombucha (ù hw.). J. Kim. Terap. Indones. 2022, 24, 1–8. [Google Scholar]
- Jafarpour, D.; Hashemi, S.M.B. Pure and Co-Fermentation of Quinoa Seeds by Limosilactobacillus fermentum and Lacticaseibacillus rhamnosus: Bioactive Content, Antidiabetic and Antioxidant Activities. Fermentation 2023, 9, 80. [Google Scholar] [CrossRef]
- Cázares-Vásquez, M.L.; Rodríguez-Herrera, R.; Aguilar-González, C.N.; Sáenz-Galindo, A.; Solanilla-Duque, J.F.; Contreras-Esquivel, J.C.; Flores-Gallegos, A.C. Microbial exopolysaccharides in traditional Mexican fermented beverages. Fermentation 2021, 7, 249. [Google Scholar] [CrossRef]
- Guérin, M.; Silva, C.R.-D.; Garcia, C.; Remize, F. Lactic acid bacterial production of exopolysaccharides from fruit and vegetables and associated benefits. Fermentation 2020, 6, 115. [Google Scholar] [CrossRef]
- Yang, X.; Ren, Y.; Li, L. The relationship between charge intensity and bioactivities/processing characteristics of exopolysaccharides from lactic acid bacteria. LWT 2022, 153, 112345. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Rather, I.A.; Park, Y.H. Partially Purified Exo-Polysaccharide from Lactobacillus Sakei Probio 65 with Antioxidant, α-Glucosidase and Tyrosinase Inhibitory Potential. J. Food Biochem. 2016, 40, 264–274. [Google Scholar] [CrossRef]
- Sasikumar, K.; Vaikkath, D.K.; Devendra, L.; Nampoothiri, K.M. An exopolysaccharide (EPS) from a Lactobacillus plantarum BR2 with potential benefits for making functional foods. Bioresour. Technol. 2017, 241, 1152–1156. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Cui, Y.; Qu, X. Exopolysaccharides of lactic acid bacteria: Structure, bioactivity and associations: A review. Carbohydr. Polym. 2019, 207, 317–332. [Google Scholar] [CrossRef] [PubMed]
- Dilna, S.V.; Surya, H.; Aswathy, R.G.; Varsha, K.K.; Sakthikumar, D.N.; Pandey, A.; Nampoothiri, K.M. Characterization of an exopolysaccharide with potential health-benefit properties from a probiotic Lactobacillus plantarum RJF4. LWT-Food Sci. Technol. 2015, 64, 1179–1186. [Google Scholar] [CrossRef]
- Al-Nabulsi, A.A.; Jaradat, Z.W.; Al Qudsi, F.R.; Elsalem, L.; Osaili, T.M.; Olaimat, A.N.; Esposito, G.; Liu, S.-Q.; Ayyash, M.M. Characterization and bioactive properties of exopolysaccharides produced by Streptococcus thermophilus and Lactobacillus bulgaricus isolated from labaneh. LWT 2022, 167, 113817. [Google Scholar] [CrossRef]
- Nagarani, G.; Abirami, A.; Siddhuraju, P. Food prospects and nutraceutical attributes of Momordica species: A potential tropical bioresources–a review. Food Sci. Hum. Wellness 2014, 3, 117–126. [Google Scholar] [CrossRef] [Green Version]
- Cui, S.; Zhao, N.; Lu, W.; Zhao, F.; Zheng, S.; Wang, W.; Chen, W. Effect of different Lactobacillus species on volatile and nonvolatile flavor compounds in juices fermentation. Food Sci. Nutr. 2019, 7, 2214–2223. [Google Scholar] [CrossRef] [Green Version]
- Algboory, H.L.; Muhialdin, B.J. Novel peptides contribute to the antimicrobial activity of camel milk fermented with Lactobacillus plantarum IS10. Food Control 2021, 126, 108057. [Google Scholar] [CrossRef]
- Won, G.; Choi, S.-I.; Park, N.; Kim, J.-E.; Kang, C.-H.; Kim, G.-H. In vitro antidiabetic, antioxidant activity, and probiotic activities of Lactiplantibacillus plantarum and Lacticaseibacillus paracasei strains. Curr. Microbiol. 2021, 78, 3181–3191. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-Y.; Tsai, Y.-C.; Wang, S.-Y.; Chen, Y.-P.; Chen, M.-J. Coculture Strategy for Developing Lactobacillus paracasei PS23 Fermented Milk with Anti-Colitis Effect. Foods 2021, 10, 2337. [Google Scholar] [CrossRef]
- Ondee, T.; Pongpirul, K.; Visitchanakun, P.; Saisorn, W.; Kanacharoen, S.; Wongsaroj, L.; Kullapanich, C.; Ngamwongsatit, N.; Settachaimongkon, S.; Somboonna, N. Lactobacillus acidophilus LA5 improves saturated fat-induced obesity mouse model through the enhanced intestinal Akkermansia muciniphila. Sci. Rep. 2021, 11, 6367. [Google Scholar] [CrossRef] [PubMed]
- Ayyash, M.; Al-Dhaheri, A.S.; Al Mahadin, S.; Kizhakkayil, J.; Abushelaibi, A. In vitro investigation of anticancer, antihypertensive, antidiabetic, and antioxidant activities of camel milk fermented with camel milk probiotic: A comparative study with fermented bovine milk. J. Dairy Sci. 2018, 101, 900–911. [Google Scholar] [CrossRef] [Green Version]
- Begunova, A.V.; Rozhkova, I.V.; Glazunova, O.A.; Moiseenko, K.V.; Savinova, O.S.; Fedorova, T.V. Fermentation Profile and Probiotic-Related Characteristics of Bifidobacterium longum MC-42. Fermentation 2021, 7, 101. [Google Scholar] [CrossRef]
- Frediansyah, A.; Romadhoni, F.; Nurhayati, R.; Wibowo, A.T. Fermentation of Jamaican cherries juice using Lactobacillus plantarum elevates antioxidant potential and inhibitory activity against Type II diabetes-related enzymes. Molecules 2021, 26, 2868. [Google Scholar] [CrossRef]
- Li, C.; Ding, Q.; Nie, S.-P.; Zhang, Y.-S.; Xiong, T.; Xie, M.-Y. Carrot juice fermented with Lactobacillus plantarum NCU116 ameliorates type 2 diabetes in rats. J. Agric. Food Chem. 2014, 62, 11884–11891. [Google Scholar] [CrossRef]
- Gao, H.; Wen, J.-J.; Hu, J.-L.; Nie, Q.-X.; Chen, H.-H.; Xiong, T.; Nie, S.-P.; Xie, M.-Y. Polysaccharide from fermented Momordica charantia L. with Lactobacillus plantarum NCU116 ameliorates type 2 diabetes in rats. Carbohydr. Polym. 2018, 201, 624–633. [Google Scholar] [CrossRef]
- Wang, X.; Han, M.; Zhang, M.; Wang, Y.; Ren, Y.; Yue, T.; Gao, Z. In vitro evaluation of the hypoglycemic properties of lactic acid bacteria and its fermentation adaptability in apple juice. Lwt 2021, 136, 110363. [Google Scholar] [CrossRef]
- Liu, T.H.; Lin, W.J.; Cheng, M.C.; Tsai, T.Y. Lactobacillus plantarum TWK10-fermented soymilk improves cognitive function in type 2 diabetic rats. J. Sci. Food Agric. 2020, 100, 5152–5161. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, W.; Wei, Z.; Yin, B.; Man, C.; Jiang, Y. Enhancement of functional characteristics of blueberry juice fermented by Lactobacillus plantarum. Lwt 2021, 139, 110590. [Google Scholar] [CrossRef]
- Klongklaew, A.; Banwo, K.; Soodsawaeng, P.; Christopher, A.; Khanongnuch, C.; Sarkar, D.; Shetty, K. Lactic acid bacteria based fermentation strategy to improve phenolic bioactive-linked functional qualities of select chickpea (Cicer arietinum L.) varieties. NFS J. 2022, 27, 36–46. [Google Scholar] [CrossRef]
- Béal, C.; Fonseca, F.; Corrieu, G. Resistance to freezing and frozen storage of Streptococcus thermophilus is related to membrane fatty acid composition. J. Dairy Sci. 2001, 84, 2347–2356. [Google Scholar] [CrossRef]
- Wang, Y.; Corrieu, G.; Béal, C. Fermentation pH and temperature influence the cryotolerance of Lactobacillus acidophilus RD758. J. Dairy Sci. 2005, 88, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.J.; Kim, T.S.; Moon, H.W.; Lee, S.Y.; Lee, S.Y.; Ji, G.E.; Hwang, K.T. Lactobacillus plantarum PMO 08 as a probiotic starter culture for plant-based fermented beverages. Molecules 2020, 25, 5056. [Google Scholar] [CrossRef] [PubMed]
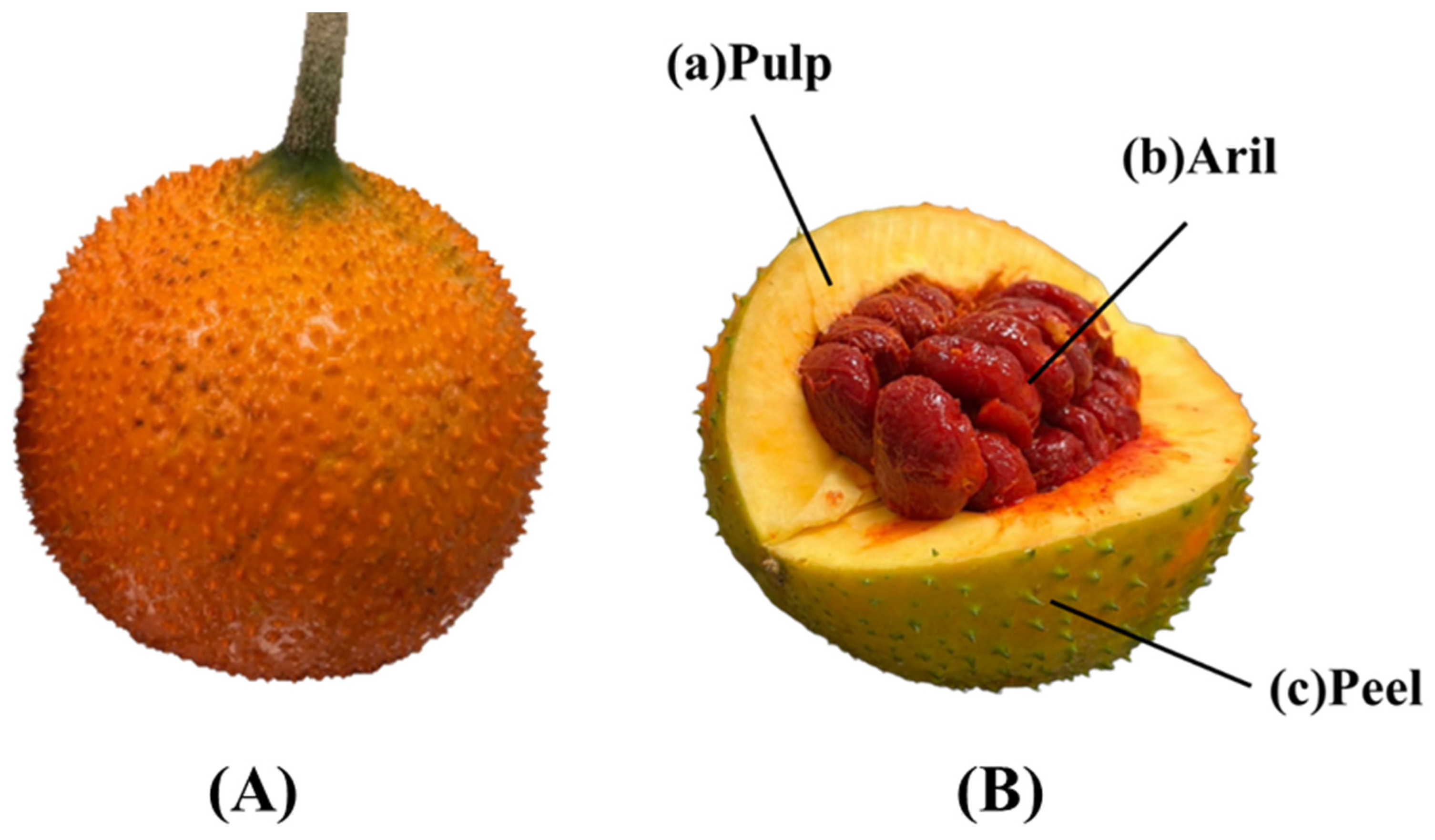
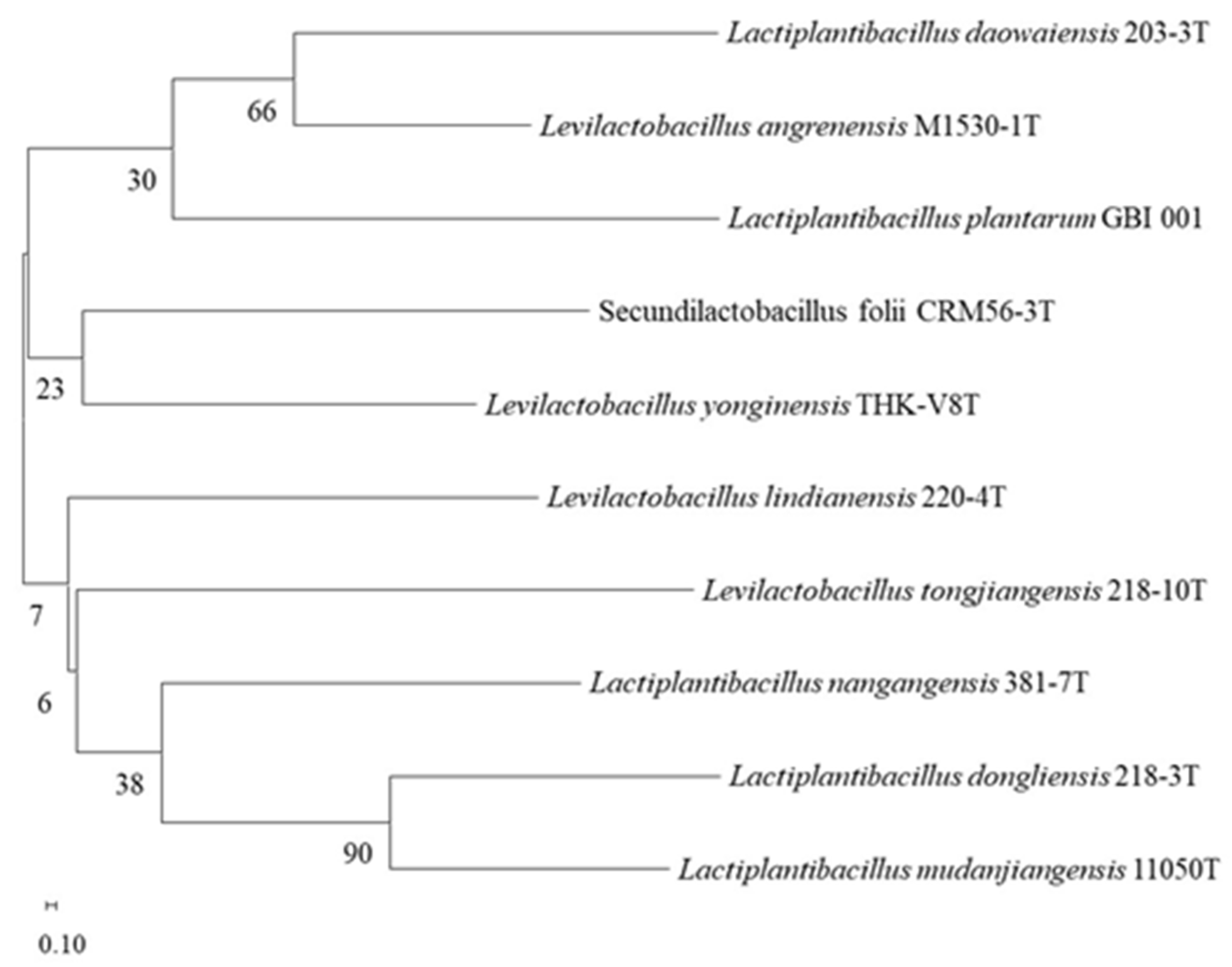


| Stain No. | Gram Test | Oxidase Test | Catalase Test | α-Glucosidase-Inhibitory Activity (%) |
|---|---|---|---|---|
| Acarbose (10 mg/mL) | N.D | N.D | N.D | 79.58% |
| MC1 | + | - | - | 11.52 |
| MC2 | + | - | - | 3.238 |
| MC3 | + | - | - | 20.38 |
| MC4 | + | - | - | 22.59 |
| MC5 | + | - | - | 14.75 |
| MC6 | + | - | - | 11.14 |
| MC7 | + | - | - | 49.59 |
| MC8 | + | - | - | 10.22 |
| MC9 | + | - | - | 64.86 |
| MC10 | + | - | - | 55.35 |
| MC11 | + | - | - | 74.23 |
| MC12 | + | - | - | 57.55 |
| MC13 | + | - | - | 15.01 |
| MC14 | + | - | - | 35.33 |
| MC15 | + | - | - | 58.21. |
| MC16 | + | - | - | 56.37 |
| MC17 | + | - | - | 14.85 |
| MC18 | + | - | - | 63.87 |
| MC19 | + | - | - | 5.42 |
| MC20 | + | - | - | 5.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, P.-H.; Guo, H.-R.; Liu, Y.-A.; Wu, C.-H.; Huang, C.-C.; Lin, J.-A.; Hsieh, C.-W. Development of Blood Sugar Regulatory Products from Momordica cochininensis via Probiotic Fermentation. Fermentation 2023, 9, 578. https://doi.org/10.3390/fermentation9060578
Wu P-H, Guo H-R, Liu Y-A, Wu C-H, Huang C-C, Lin J-A, Hsieh C-W. Development of Blood Sugar Regulatory Products from Momordica cochininensis via Probiotic Fermentation. Fermentation. 2023; 9(6):578. https://doi.org/10.3390/fermentation9060578
Chicago/Turabian StyleWu, Po-Hua, Huei-Rong Guo, Yi-An Liu, Chien-Hui Wu, Chun-Chen Huang, Jer-An Lin, and Chang-Wei Hsieh. 2023. "Development of Blood Sugar Regulatory Products from Momordica cochininensis via Probiotic Fermentation" Fermentation 9, no. 6: 578. https://doi.org/10.3390/fermentation9060578
APA StyleWu, P.-H., Guo, H.-R., Liu, Y.-A., Wu, C.-H., Huang, C.-C., Lin, J.-A., & Hsieh, C.-W. (2023). Development of Blood Sugar Regulatory Products from Momordica cochininensis via Probiotic Fermentation. Fermentation, 9(6), 578. https://doi.org/10.3390/fermentation9060578









