Preparation and Application of Carbon-Based Materials in the Production of Medium-Chain Carboxylic Acids by Anaerobic Digestion: A Review
Abstract
:1. Introduction
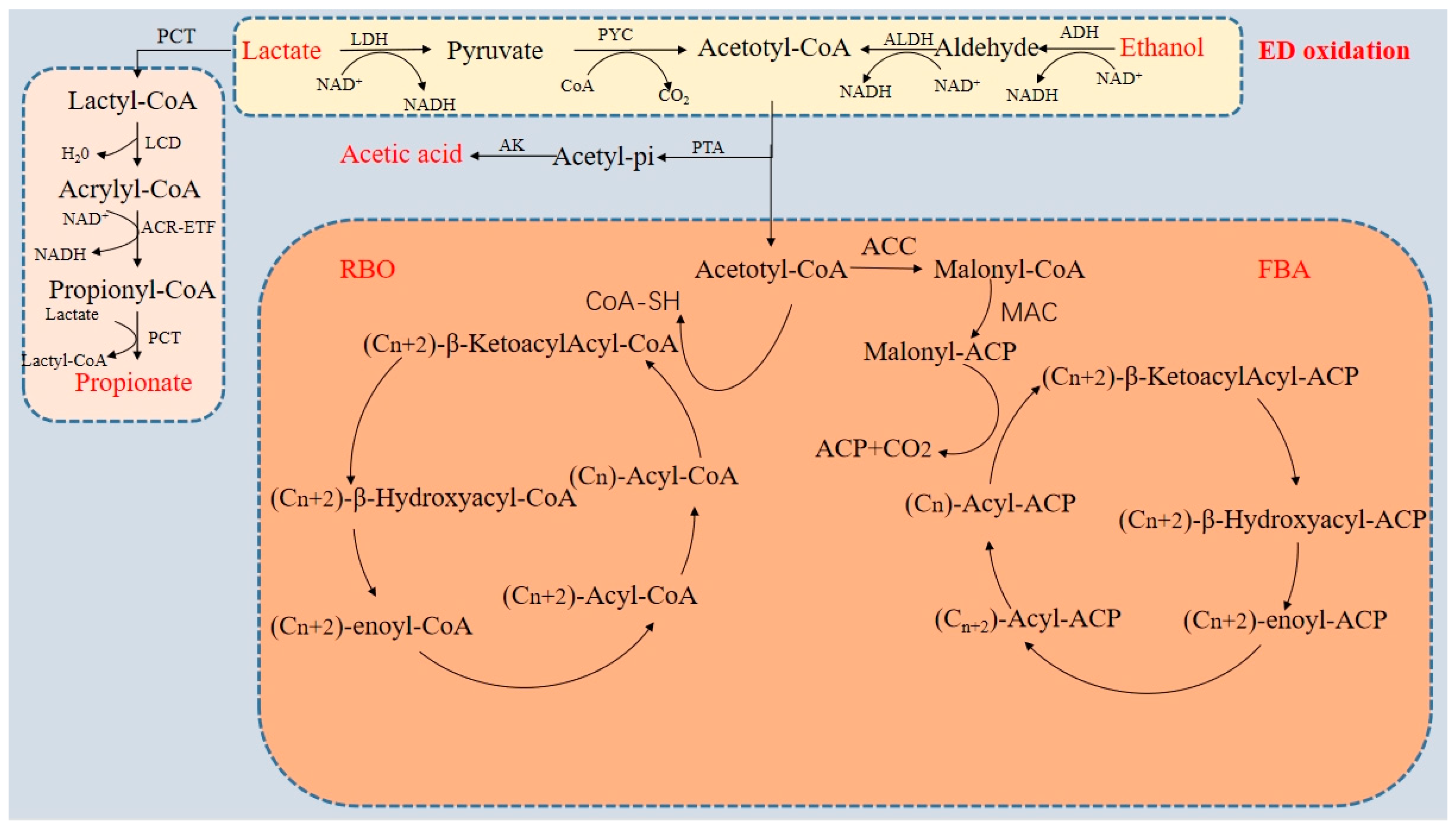
2. Chain Elongation Technology
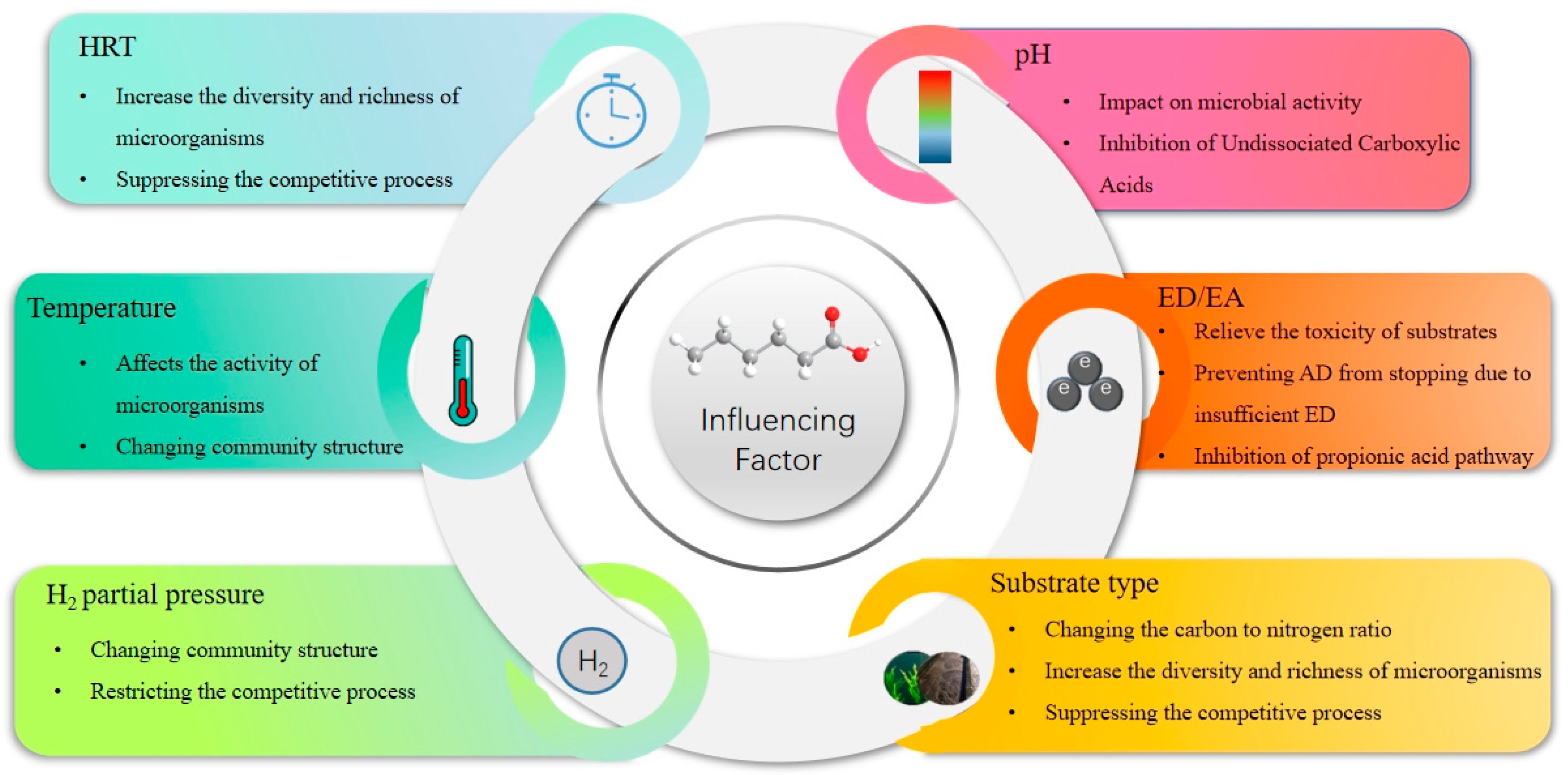
3. Factors Affecting the AD of Organic Waste to Produce Intermediate-Chain Carboxylic Acids
3.1. Temperature
3.2. pH
3.3. Ratio of ED/EA
3.4. Substrate Type
3.5. Other Factors
4. Preparation, Characteristics, and Application of Carbon-Based Materials in AD
4.1. Porous Carbon Materials
4.1.1. Preparation of Porous Carbon Materials
4.1.2. Characteristics of Porous Carbon
4.1.3. Application of Porous Carbon Materials
4.2. Graphene
4.2.1. Preparation of Graphene
4.2.2. Characteristics of Graphene
4.2.3. Application of Graphene
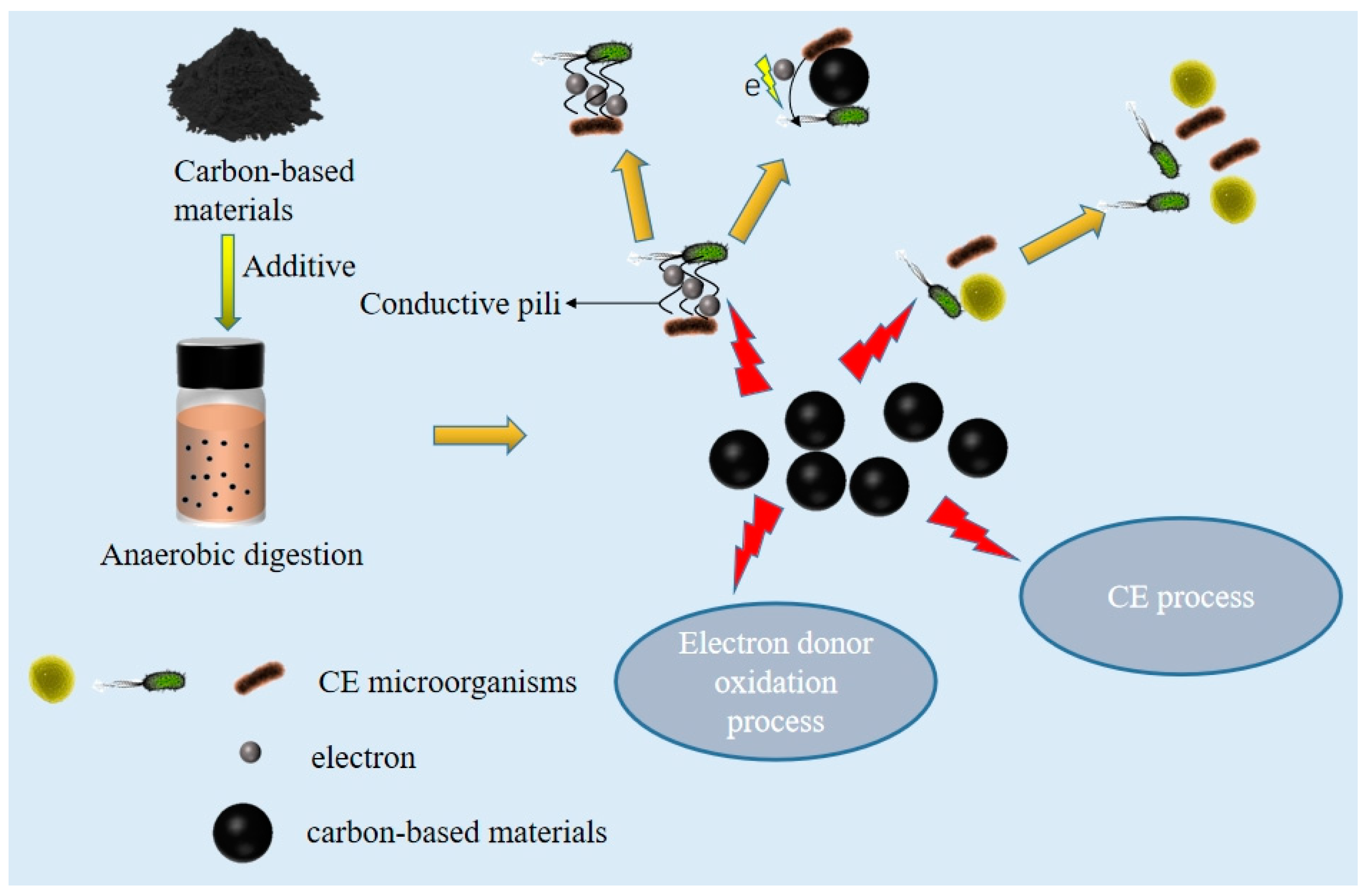
5. Mechanism Analysis of Carbon-Based Materials in AD
5.1. Optimization of Microbial Community Structure Using Carbon-Based Materials
5.2. Reduce the Toxicity of Substrates and Products
5.3. The Role of Promoting CE
6. Technical and Economic Assessment
7. Prospect
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Patel, A.K.; Singhania, R.R.; Albarico, F.; Pssandey, A.; Chen, C.W.; Dong, C.D. Organic wastes bioremediation and its changing prospects. Sci. Total Environ. 2022, 824, 153889. [Google Scholar] [CrossRef] [PubMed]
- Galán-Martín, Á.; Tulus, V.; Díaz, I.; Pozo, C.; Pérez-Ramírez, J.; Guillén-Gosálbez, G. Sustainability footprints of a renewable carbon transition for the petrochemical sector within planetary boundaries. One Earth 2021, 4, 565–583. [Google Scholar] [CrossRef]
- Fang, K.; Zhou, Y.; Wang, S.; Ye, R.; Guo, S. Assessing national renewable energy competitiveness of the G20: A revised Porter′s Diamond Model. Renew. Sustain. Energy Rev. 2018, 93, 719–731. [Google Scholar] [CrossRef]
- Qu, X.; Zeng, H.; Gao, Y.; Mo, T.; Li, Y. Bio-hydrogen production by dark anaerobic fermentation of organic wastewater. Front. Chem. 2022, 10, 978907. [Google Scholar] [CrossRef]
- Wang, H.; Xu, J.; Sheng, L.; Liu, X. Effect of addition of biogas slurry for anaerobic fermentation of deer manure on biogas production. Energy 2018, 165, 411–418. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Zheng, K.; Guo, H.; Tian, L.; Zhu, T.; Liu, Y. Ultrasound-sodium percarbonate effectively promotes short-chain carboxylic acids production from sewage sludge through anaerobic fermentation. Bioresour. Technol. 2022, 364, 128024. [Google Scholar] [CrossRef]
- Li, L.; Liu, C.; Xu, L.; Zhuang, H.; He, J.; He, Q.; Zhang, J. Acclimation of anaerobic fermentation microbiome with acetate and ethanol for chain elongation and the biochemical response. Chemosphere 2023, 320, 138083. [Google Scholar] [CrossRef]
- Wu, S.-L.; Luo, G.; Sun, J.; Wei, W.; Song, L.; Ni, B.-J. Medium chain fatty acids production from anaerobic fermentation of waste activated sludge. J. Clean. Prod. 2021, 279, 123482. [Google Scholar] [CrossRef]
- De Groof, V.; Coma, M.; Arnot, T.; Leak, D.J.; Lanham, A.B. Medium Chain Carboxylic Acids from Complex Organic Feedstocks by Mixed Culture Fermentation. Molecules 2019, 24, 398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, W.; Yang, Y.; Liu, C.; Zhang, J.; Pan, J.; Luo, L.; Wu, G.; Awasthi, M.K.; Yan, B. Caproic acid production from anaerobic fermentation of organic waste—Pathways and microbial perspective. Renew. Sustain. Energy Rev. 2023, 175, 113181. [Google Scholar] [CrossRef]
- Li, Z.; Gu, J.; Ding, J.; Ren, N.; Xing, D. Molecular mechanism of ethanol-H(2) co-production fermentation in anaerobic acidogenesis: Challenges and perspectives. Biotechnol. Adv. 2021, 46, 107679. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yin, Y. Biological production of medium-chain carboxylates through chain elongation: An overview. Biotechnol. Adv. 2022, 55, 107882. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, X.; Chen, Y.; Zhang, B.; Xu, H.; Li, C.; Zhou, Y. Medium-chain fatty acid production from thermal hydrolysed sludge without external electron donor supplementation. Bioresour. Technol. 2023, 374, 128805. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, S.; Yin, F.; Dong, H.; Cao, Q.; Lian, T.; Zhu, J. Produce individual medium chain carboxylic acids (MCCA) from swine manure: Performance evaluation and economic analysis. Waste Manag. 2022, 144, 255–262. [Google Scholar] [CrossRef]
- Ge, S.; Usack, J.G.; Spirito, C.M.; Angenent, L.T. Long-Term n-Caproic Acid Production from Yeast-Fermentation Beer in an Anaerobic Bioreactor with Continuous Product Extraction. Environ. Sci. Technol. 2015, 49, 8012–8021. [Google Scholar] [CrossRef]
- Xiang, S.; Wu, Q.; Ren, W.; Guo, W.; Ren, N. Mechanism of powdered activated carbon enhancing caproate production. Chin. Chem. Lett. 2022, 34, 107714. [Google Scholar] [CrossRef]
- Xu, J.; Hao, J.; Guzman, J.J.L.; Spirito, C.M.; Harroff, L.A.; Angenent, L.T. Temperature-Phased Conversion of Acid Whey Waste Into Medium-Chain Carboxylic Acids via Lactic Acid: No External e-Donor. Joule 2018, 2, 280–295. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Wang, S.; Yin, F.; Cao, Q.; Lian, T.; Zhang, H.; Zhu, Z.; Dong, H. Medium-chain carboxylates production from co-fermentation of swine manure and corn stalk silage via lactic acid: Without external electron donors. Chem. Eng. J. 2022, 439, 135751. [Google Scholar] [CrossRef]
- Yin, Y.; Wang, J. Medium-chain carboxylates production by co-fermentation of sewage sludge and macroalgae. Bioresour. Technol. 2022, 347, 126718. [Google Scholar] [CrossRef]
- Luo, T.; Xu, Q.; Wei, W.; Sun, J.; Dai, X.; Ni, B.J. Performance and Mechanism of Fe3O4 Improving Biotransformation of Waste Activated Sludge into Liquid High-Value Products. Environ. Sci. Technol. 2022, 56, 3658–3668. [Google Scholar] [CrossRef]
- Liu, Y.; He, P.; Shao, L.; Zhang, H.; Lu, F. Significant enhancement by biochar of caproate production via chain elongation. Water Res. 2017, 119, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.N.; Dissanayake, P.D.; Masek, O.; Priya, A.; Ki Lin, C.S.; Ok, Y.S.; Kim, S.-H. Recent trends in biochar integration with anaerobic fermentation: Win-win strategies in a closed-loop. Renew. Sustain. Energy Rev. 2021, 149, 111371. [Google Scholar] [CrossRef]
- Cheng, Q.; Call, D.F. Hardwiring microbes via direct interspecies electron transfer: Mechanisms and applications. Environ. Sci. Process Impacts 2016, 18, 968–980. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Dávila, C.A.; Nadal Alemany, N.; Garcia-Saravia Ortiz-de-Montellano, C.; Bao, Z.; Buisman, C.J.N.; Strik, D.P.B.T.B. Designing a Selective n-Caproate Adsorption–Recovery Process with Granular Activated Carbon and Screening of Conductive Materials in Chain Elongation. ACS EST Eng. 2021, 2, 54–64. [Google Scholar] [CrossRef]
- Liu, Y.; He, P.; Han, W.; Shao, L.; Lü, F. Outstanding reinforcement on chain elongation through five-micrometer-sized biochar. Renew. Energy 2020, 161, 230–239. [Google Scholar] [CrossRef]
- Ghysels, S.; Buffel, S.; Rabaey, K.; Ronsse, F.; Ganigue, R. Biochar and activated carbon enhance ethanol conversion and selectivity to caproic acid by Clostridium kluyveri. Bioresour. Technol. 2021, 319, 124236. [Google Scholar] [CrossRef]
- Wu, S.-L.; Wei, W.; Xu, Q.; Huang, X.; Sun, J.; Dai, X.; Ni, B.-J. Revealing the Mechanism of Biochar Enhancing the Production of Medium Chain Fatty Acids from Waste Activated Sludge Alkaline Fermentation Liquor. ACS ES&T Water 2021, 1, 1014–1024. [Google Scholar] [CrossRef]
- Güleç, F.; Williams, O.; Kostas, E.T.; Samson, A.; Lester, E. A comprehensive comparative study on the energy application of chars produced from different biomass feedstocks via hydrothermal conversion, pyrolysis, and torrefaction. Energy Convers. Manag. 2022, 270, 116260. [Google Scholar] [CrossRef]
- Zhang, Y.; Bai, J.; Zuo, J. Performance and mechanisms of medium-chain fatty acid production by anaerobic fermentation of food waste without external electron donors. Bioresour. Technol. 2023, 374, 128735. [Google Scholar] [CrossRef]
- Zhou, M.; Yan, B.; Wong, J.W.C.; Zhang, Y. Enhanced volatile fatty acids production from anaerobic fermentation of food waste: A mini-review focusing on acidogenic metabolic pathways. Bioresour. Technol. 2018, 248 Pt A, 68–78. [Google Scholar] [CrossRef]
- Wu, L.; Wei, W.; Chen, Z.; Ni, B.-J. Medium-chain carboxylate productions through open-culture fermentation of organic wastes. J. Clean. Prod. 2022, 373, 133911. [Google Scholar] [CrossRef]
- Kim, H.; Kang, S.; Sang, B.I. Metabolic cascade of complex organic wastes to medium-chain carboxylic acids: A review on the state-of-the-art multi-omics analysis for anaerobic chain elongation pathways. Bioresour. Technol. 2022, 344, 126211. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, W.d.A.; Leitão, R.C.; Gehring, T.A.; Angenent, L.T.; Santaella, S.T. Anaerobic fermentation for n-caproic acid production: A review. Process Biochem. 2017, 54, 106–119. [Google Scholar] [CrossRef]
- Wu, Q.; Bao, X.; Guo, W.; Wang, B.; Li, Y.; Luo, H.; Wang, H.; Ren, N. Medium chain carboxylic acids production from waste biomass: Current advances and perspectives. Biotechnol. Adv. 2019, 37, 599–615. [Google Scholar] [CrossRef]
- Zhu, X.; Tao, Y.; Liang, C.; Li, X.; Wei, N.; Zhang, W.; Zhou, Y.; Yang, Y.; Bo, T. The synthesis of n-caproate from lactate: A new efficient process for medium-chain carboxylates production. Sci. Rep. 2015, 5, 14360. [Google Scholar] [CrossRef] [Green Version]
- Xie, S.; Ma, J.; Li, L.; He, Q.; Xu, P.; Ke, S.; Shi, Z. Anaerobic caproate production on carbon chain elongation: Effect of lactate/butyrate ratio, concentration and operation mode. Bioresour. Technol. 2021, 329, 124893. [Google Scholar] [CrossRef]
- Tang, J.; Pu, Y.; Huang, J.; Pan, S.; Wang, X.C.; Hu, Y.; Ngo, H.H.; Li, Y.; Abomohra, A. Caproic acid production through lactate-based chain elongation: Effect of lactate-to-acetate ratio and substrate loading. Environ. Technol. Innov. 2022, 28, 102918. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, F.; Shao, L.; He, P. Alcohol-to-acid ratio and substrate concentration affect product structure in chain elongation reactions initiated by unacclimatized inoculum. Bioresour. Technol. 2016, 218, 1140–1150. [Google Scholar] [CrossRef]
- Spirito, C.M.; Richter, H.; Rabaey, K.; Stams, A.J.; Angenent, L.T. Chain elongation in anaerobic reactor microbiomes to recover resources from waste. Curr. Opin. Biotechnol. 2014, 27, 115–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Q.; Jiang, Y.; Chen, Y.; Liu, M.; Bao, X.; Guo, W. Opportunities and challenges in microbial medium chain fatty acids production from waste biomass. Bioresour. Technol. 2021, 340, 125633. [Google Scholar] [CrossRef] [PubMed]
- Ryue, J.; Lin, L.; Kakar, F.L.; Elbeshbishy, E.; Al-Mamun, A.; Dhar, B.R. A critical review of conventional and emerging methods for improving process stability in thermophilic anaerobic digestion. Energy Sustain. Dev. 2020, 54, 72–84. [Google Scholar] [CrossRef]
- Wu, Q.; Feng, X.; Guo, W.; Bao, X.; Ren, N. Long-term medium chain carboxylic acids production from liquor-making wastewater: Parameters optimization and toxicity mitigation. Chem. Eng. J. 2020, 388, 124218. [Google Scholar] [CrossRef]
- Zhu, X.; Zhou, Y.; Wang, Y.; Wu, T.; Li, X.; Li, D.; Tao, Y. Production of high-concentration n-caproic acid from lactate through fermentation using a newly isolated Ruminococcaceae bacterium CPB6. Biotechnol. Biofuels. 2017, 10, 102. [Google Scholar] [CrossRef] [Green Version]
- Sakarika, M.; Regueira, A.; Rabaey, K.; Ganigue, R. Thermophilic caproic acid production from grass juice by sugar-based chain elongation. Sci. Total Environ. 2023, 860, 160501. [Google Scholar] [CrossRef]
- Kim, M.; Gomec, C.Y.; Ahn, Y.; Speece, R.E. Hydrolysis and acidogenesis of particulate organic material in mesophilic and thermophilic anaerobic digestion. Environ. Technol. 2003, 24, 1183–1190. [Google Scholar] [CrossRef]
- Wu, S.L.; Wei, W.; Wang, Y.; Song, L.; Ni, B.J. Transforming waste activated sludge into medium chain fatty acids in continuous two-stage anaerobic fermentation: Demonstration at different pH levels. Chemosphere 2022, 288 Pt 1, 132474. [Google Scholar] [CrossRef]
- Candry, P.; Radic, L.; Favere, J.; Carvajal-Arroyo, J.M.; Rabaey, K.; Ganigue, R. Mildly acidic pH selects for chain elongation to caproic acid over alternative pathways during lactic acid fermentation. Water Res. 2020, 186, 116396. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Huang, Z.; Wu, P.; Zhao, M.; Miao, H.; Liu, C.; Ruan, W. Performance and microbial characterization of two-stage caproate fermentation from fruit and vegetable waste via anaerobic microbial consortia. Bioresour. Technol. 2019, 284, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Bao, S.; Wang, Q.; Zhang, P.; Zhang, Q.; Wu, Y.; Li, F.; Tao, X.; Wang, S.; Nabi, M.; Zhou, Y. Effect of Acid/Ethanol Ratio on Medium Chain Carboxylate Production with Different VFAs as the Electron Acceptor: Insight into Carbon Balance and Microbial Community. Energies 2019, 12, 3720. [Google Scholar] [CrossRef] [Green Version]
- Contreras-Davila, C.A.; Carrion, V.J.; Vonk, V.R.; Buisman, C.N.J.; Strik, D. Consecutive lactate formation and chain elongation to reduce exogenous chemicals input in repeated-batch food waste fermentation. Water Res. 2020, 169, 115215. [Google Scholar] [CrossRef]
- Wu, Q.; Ren, W.; Guo, W.; Ren, N. Effect of substrate structure on medium chain fatty acids production and reactor microbiome. Environ Res. 2022, 204 Pt A, 111947. [Google Scholar] [CrossRef]
- Yin, Y.; Wang, J. Production of medium-chain fatty acids by co-fermentation of antibiotic fermentation residue with fallen Ginkgo leaves. Bioresour. Technol. 2022, 360, 127607. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Huang, H.; He, Y.; Wang, X.; Jia, J.; Feng, X.; Li, D.; Li, H. A preliminary study on the feasibility of industrialization for n-caproic acid recovery from food wastewater: From lab to pilot. Bioresour. Technol. 2022, 366, 128154. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Hu, Y.; Wang, J. Co-fermentation of sewage sludge and lignocellulosic biomass for production of medium-chain fatty acids. Bioresour. Technol. 2022, 361, 127665. [Google Scholar] [CrossRef]
- Fernando-Foncillas, C.; Varrone, C. Effect of reactor operating conditions on carboxylate production and chain elongation from co-fermented sludge and food waste. J. Clean. Prod. 2021, 292, 126009. [Google Scholar] [CrossRef]
- Baleeiro, F.C.F.; Kleinsteuber, S.; Sträuber, H. Recirculation of H2, CO2, and Ethylene Improves Carbon Fixation and Carboxylate Yields in Anaerobic Fermentation. ACS Sustain. Chem. Eng. 2022, 10, 4073–4081. [Google Scholar] [CrossRef]
- Grootscholten, T.I.M.; Strik, D.P.B.T.B.; Steinbusch, K.J.J.; Buisman, C.J.N.; Hamelers, H.V.M. Two-stage medium chain fatty acid (MCFA) production from municipal solid waste and ethanol. Appl. Energy 2014, 116, 223–229. [Google Scholar] [CrossRef]
- Coelho, M.M.H.; Morais, N.W.S.; Pereira, E.L.; Leitão, R.C.; dos Santos, A.B. Potential assessment and kinetic modeling of carboxylic acids production using dairy wastewater as substrate. Biochem. Eng. J. 2020, 156, 107502. [Google Scholar] [CrossRef]
- Coelho, M.M.H.; Morais, N.W.S.; Ferreira, T.J.T.; Silva, F.S.S.; Pereira, E.L.; dos Santos, A.B. Carboxylic acids production using residual glycerol as a substrate in anaerobic fermentation: A kinetic modeling study. Biomass Bioenergy 2020, 143, 105874. [Google Scholar] [CrossRef]
- Long, F.; Fan, J.; Xu, W.; Liu, H. Predicting the performance of medium-chain carboxylic acid (MCCA) production using machine learning algorithms and microbial community data. J. Clean. Prod. 2022, 377, 134223. [Google Scholar] [CrossRef]
- Hu, J.; Zhao, L.; Luo, J.; Gong, H.; Zhu, N. A sustainable reuse strategy of converting waste activated sludge into biochar for contaminants removal from water: Modifications, applications and perspectives. J. Hazard. Mater. 2022, 438, 129437. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Chen, Y.; Yan, Y.; Feng, L.; Chen, Y.; Zhou, Q. New method for algae comprehensive utilization: Algae-derived biochar enhances algae anaerobic fermentation for short-chain fatty acids production. Bioresour. Technol. 2019, 289, 121637. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhao, X.; Zhu, S.; Yuan, L.; Li, X.; Feng, Z.; Yang, X.; Luo, L.; Xiao, Y.; Liu, Y.; et al. Conversion of swine manure into biochar for soil amendment: Efficacy and underlying mechanism of dissipating antibiotic resistance genes. Sci. Total Environ. 2023, 871, 162046. [Google Scholar] [CrossRef] [PubMed]
- Van Nguyen, T.T.; Phan, A.N.; Nguyen, T.A.; Nguyen, T.K.; Nguyen, S.T.; Pugazhendhi, A.; Ky Phuong, H.H. Valorization of agriculture waste biomass as biochar: As first-rate biosorbent for remediation of contaminated soil. Chemosphere 2022, 307 Pt 3, 135834. [Google Scholar] [CrossRef]
- Bhattacharya, R. A review on production and application of activated carbon from discarded plastics in the context of ‘waste treats waste’. J. Environ. Manag. 2023, 325, 116613. [Google Scholar] [CrossRef]
- Krysanova, K.; Krylova, A.; Kulikova, M.; Kulikov, A.; Rusakova, O. Biochar characteristics produced via hydrothermal carbonization and torrefaction of peat and sawdust. Fuel 2022, 328, 125220. [Google Scholar] [CrossRef]
- Güleç, F.; Samson, A.; Williams, O.; Kostas, E.T.; Lester, E. Biofuel characteristics of chars produced from rapeseed, whitewood, and seaweed via thermal conversion technologies—Impacts of feedstocks and process conditions. Fuel Process. Technol. 2022, 238, 107492. [Google Scholar] [CrossRef]
- Leng, L.; Huang, H. An overview of the effect of pyrolysis process parameters on biochar stability. Bioresour. Technol. 2018, 270, 627–642. [Google Scholar] [CrossRef]
- Weber, K.; Quicker, P. Properties of biochar. Fuel 2018, 217, 240–261. [Google Scholar] [CrossRef]
- Osman, A.I.; Farghali, M.; Ihara, I.; Elgarahy, A.M.; Ayyad, A.; Mehta, N.; Ng, K.H.; Abd El-Monaem, E.M.; Eltaweil, A.S.; Hosny, M.; et al. Materials, fuels, upgrading, economy, and life cycle assessment of the pyrolysis of algal and lignocellulosic biomass: A review. Environ. Chem. Lett. 2023, 21, 1419–1476. [Google Scholar] [CrossRef]
- Cong, H.; Meng, H.; Mašek, O.; Yao, Z.; Li, L.; Yu, B.; Qin, C.; Zhao, L. Comprehensive analysis of industrial-scale heating plants based on different biomass slow pyrolysis technologies: Product property, energy balance, and ecological impact. Clean. Eng. Technol. 2022, 6, 100391. [Google Scholar] [CrossRef]
- Yang, Y.; Brammer, J.G.; Wright, D.G.; Scott, J.A.; Serrano, C.; Bridgwater, A.V. Combined heat and power from the intermediate pyrolysis of biomass materials: Performance, economics and environmental impact. Appl. Energy 2017, 191, 639–652. [Google Scholar] [CrossRef] [Green Version]
- Tan, H.; Lee, C.T.; Ong, P.Y.; Wong, K.Y.; Bong, C.P.C.; Li, C.; Gao, Y. A Review On The Comparison Between Slow Pyrolysis And Fast Pyrolysis On The Quality Of Lignocellulosic And Lignin-Based Biochar. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1051, 012075. [Google Scholar] [CrossRef]
- Watson, C.; Schlösser, C.; Vögerl, J.; Wichern, F. Hydrochar, digestate, and process water impacts on a soil′s microbial community, processes, and metal bioavailability. Soil Sci. Soc. Am. J. 2021, 85, 717–731. [Google Scholar] [CrossRef]
- González-Arias, J.; Sánchez, M.E.; Cara-Jiménez, J.; Baena-Moreno, F.M.; Zhang, Z. Hydrothermal carbonization of biomass and waste: A review. Environ. Chem. Lett. 2021, 20, 211–221. [Google Scholar] [CrossRef]
- Malini, K.; Selvakumar, D.; Kumar, N.S. Activated carbon from biomass: Preparation, factors improving basicity and surface properties for enhanced CO2 capture capacity—A review. J. CO2 Util. 2023, 67, 102318. [Google Scholar] [CrossRef]
- Razmi, B.; Ghasemi-Fasaei, R.; Ronaghi, A.; Mostowfizadeh-Ghalamfarsa, R. Application of Taguchi optimization for evaluating the capability of hydrochar, biochar, and activated carbon prepared from different wastes in multi-elements bioadsorption. J. Clean. Prod. 2022, 347, 131292. [Google Scholar] [CrossRef]
- Heidarinejad, Z.; Dehghani, M.H.; Heidari, M.; Javedan, G.; Ali, I.; Sillanpää, M. Methods for preparation and activation of activated carbon: A review. Environ. Chem. Lett. 2020, 18, 393–415. [Google Scholar] [CrossRef]
- Hu, B.; Zhang, Z.-x.; Xie, W.-l.; Liu, J.; Li, Y.; Zhang, W.-m.; Fu, H.; Lu, Q. Advances on the fast pyrolysis of biomass for the selective preparation of phenolic compounds. Fuel Process. Technol. 2022, 237, 107465. [Google Scholar] [CrossRef]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar physicochemical properties: Pyrolysis temperature and feedstock kind effects. Rev. Environ. Sci. Bio. Technol. 2020, 19, 191–215. [Google Scholar] [CrossRef] [Green Version]
- Das, S.K.; Ghosh, G.K.; Avasthe, R.K.; Sinha, K. Compositional heterogeneity of different biochar: Effect of pyrolysis temperature and feedstocks. J. Environ. Manag. 2021, 278 Pt 2, 111501. [Google Scholar] [CrossRef]
- Qiu, B.; Shao, Q.; Shi, J.; Yang, C.; Chu, H. Application of biochar for the adsorption of organic pollutants from wastewater: Modification strategies, mechanisms and challenges. Sep. Purif. Technol. 2022, 300, 121925. [Google Scholar] [CrossRef]
- Masoumi, S.; Dalai, A.K. Optimized production and characterization of highly porous activated carbon from algal-derived hydrochar. J. Clean. Prod. 2020, 263, 121427. [Google Scholar] [CrossRef]
- Harindintwali, J.D.; He, C.; Xiang, L.; Dou, Q.; Liu, Y.; Wang, M.; Wen, X.; Fu, Y.; Islam, M.U.; Chang, S.X.; et al. Effects of ball milling on biochar adsorption of contaminants in water: A meta-analysis. Sci. Total Environ. 2023, 882, 163643. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Wang, L.; Li, H.; Westholm, L.J.; Carvalho, L.; Thorin, E.; Yu, Z.; Yu, X.; Skreiberg, Ø. A critical review on production, modification and utilization of biochar. J. Anal. Appl. Pyrolysis 2022, 161, 105405. [Google Scholar] [CrossRef]
- Patel, A.K.; Singhania, R.R.; Pal, A.; Chen, C.-W.; Pandey, A.; Dong, C.-D. Advances on tailored biochar for bioremediation of antibiotics, pesticides and polycyclic aromatic hydrocarbon pollutants from aqueous and solid phases. Sci. Total Environ. 2022, 817, 153054. [Google Scholar] [CrossRef] [PubMed]
- Vakili, A.; Zinatizadeh, A.A.; Rahimi, Z.; Zinadini, S.; Mohammadi, P.; Azizi, S.; Karami, A.; Abdulgader, M. The impact of activation temperature and time on the characteristics and performance of agricultural waste-based activated carbons for removing dye and residual COD from wastewater. J. Clean. Prod. 2023, 382, 134899. [Google Scholar] [CrossRef]
- Wu, B.; Lin, R.; Ning, X.; Kang, X.; Deng, C.; Dobson, A.D.W.; Murphy, J.D. An assessment of how the properties of pyrochar and process thermodynamics impact pyrochar mediated microbial chain elongation in steering the production of medium-chain fatty acids towards n-caproate. Bioresour. Technol. 2022, 358, 127294. [Google Scholar] [CrossRef]
- Karthik, V.; Selvakumar, P.; Senthil Kumar, P.; Vo, D.-V.N.; Gokulakrishnan, M.; Keerthana, P.; Tamil Elakkiya, V.; Rajeswari, R. Graphene-based materials for environmental applications: A review. Environ. Chem. Lett. 2021, 19, 3631–3644. [Google Scholar] [CrossRef]
- Shen, C.; Oyadiji, S.O. The processing and analysis of graphene and the strength enhancement effect of graphene-based filler materials: A review. Mater. Today Phys. 2020, 15, 100257. [Google Scholar] [CrossRef]
- Reina, G.; Gonzalez-Dominguez, J.M.; Criado, A.; Vazquez, E.; Bianco, A.; Prato, M. Promises, facts and challenges for graphene in biomedical applications. Chem. Soc. Rev. 2017, 46, 4400–4416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, C.; Zhang, K.; Sun, X.; Zhao, X.; Zheng, K.; Mi, J.; Qing, F.; Wen, Q.; Li, X. Porous Graphene Produced by Carbothermal Shock for Green Electromagnetic Interference Shielding in Both Microwave and Terahertz Bands. Small Methods 2023, 7, e2201493. [Google Scholar] [CrossRef]
- Nanjundappa, V.S.; Ramakrishnappa, T.; Sureshkumar, K.; Prakash, H.R.; Praveen, B.M. Efficient strategies to produce Graphene and functionalized graphene materials: A review. Appl. Surf. Sci. Adv. 2023, 14, 100386. [Google Scholar] [CrossRef]
- Sheng, G.P.; Yu, H.Q.; Li, X.Y. Extracellular polymeric substances (EPS) of microbial aggregates in biological wastewater treatment systems: A review. Biotechnol. Adv. 2010, 28, 882–894. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, Y.; Woodard, T.L.; Nevin, K.P.; Lovley, D.R. Enhancing syntrophic metabolism in up-flow anaerobic sludge blanket reactors with conductive carbon materials. Bioresour. Technol. 2015, 191, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gupta, R.; Zhang, Q.; You, S. Review of biochar production via crop residue pyrolysis: Development and perspectives. Bioresour. Technol. 2023, 369, 128423. [Google Scholar] [CrossRef] [PubMed]
| Related Factors Affecting MCCA | Substrate Type | Temperature | pH | OLR | HRT | ED/EA | Hydrogen Partial Pressure |
|---|---|---|---|---|---|---|---|
| Number | 81 | 87 | 71 | 11 | 5 | 32 | 6 |
| Carbon-Based Materials | Character | Substrate | Effect | Reference |
|---|---|---|---|---|
| biochar (20 g·L−1) | pH: 9.03, BET: 8.92 m2/g, diameter: 0.5–1.0 mm | ethanol acetate | The concentration of caproic acid was 21.1 g L−1, which increased by 1.46 times and shortened the lag period by 21 d. | [21] |
| biochar (20 g·L−1) | granularity < 5 mm, BET: 221±25 m2/g, total pore volume: 0.1 ± 3 × 10−3 cm3/g | ethanol acetate (3:1) | The concentration of caproic acid is 56 mmol L−1, which is 2.8–3.8 times that of the other groups. | [25] |
| biochar (26.4 g·L−1) | length dimension < 150 um, PH: 8.95–9.22, BET: 161.5 m2/g | ethanol acetate (3:1) | The concentration of caproic acid increased by 1.15 times compared to the control group. | [88] |
| graphene powder | granularity: 2 mm, length dimension < 2 um | ethanol acetate | Extension of the lag period. | [88] |
| biochar (20 g·L−1) | BET: 221 ± 25 m2/g, total pore volume: 0.1 ± 3 × 10−3 cm3/g | ethanol, waste activated sludge | The concentration of caproic acid is 5.7 g COD·L−1, which is 35.1 ± 4.9% of the control group. | [27] |
| powdered activated carbon (15 g·L−1) | - | ethanol acetate | The concentration of caproic acid is 154.6 ± 8.76 mmol·L−1, which is 2.04 times higher than the control. | [16] |
| granular activated carbon (15 g·L−1) | alkaline pH, granularity: 0.71–3.15 mm, BET: 875 m2·g−1, pore volume: 0.55 cm3·g−1, average pore diameter: 2.7 nm | lactic acid, acetic acid | GAC reduces the lag time of CE to 2 days. | [24] |
| activated carbon (10 g·L−1) | - | ethanol acetate | The concentration of caproic acid is 10.61 g·L−1, which is 1.1 times that of the control group. | [26] |
| biochar (10 g·L−1) | - | ethanol acetate | The concentration of caproic acid is 10.08 g·L−1, which is 1.05 times that of the control group. | [26] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiao, L.; Liu, Y.; Cao, C.; Bu, Q.; Liu, M.; Xi, Y. Preparation and Application of Carbon-Based Materials in the Production of Medium-Chain Carboxylic Acids by Anaerobic Digestion: A Review. Fermentation 2023, 9, 586. https://doi.org/10.3390/fermentation9070586
Jiao L, Liu Y, Cao C, Bu Q, Liu M, Xi Y. Preparation and Application of Carbon-Based Materials in the Production of Medium-Chain Carboxylic Acids by Anaerobic Digestion: A Review. Fermentation. 2023; 9(7):586. https://doi.org/10.3390/fermentation9070586
Chicago/Turabian StyleJiao, Lihua, Yang Liu, Chunhui Cao, Quan Bu, Mingqing Liu, and Yonglan Xi. 2023. "Preparation and Application of Carbon-Based Materials in the Production of Medium-Chain Carboxylic Acids by Anaerobic Digestion: A Review" Fermentation 9, no. 7: 586. https://doi.org/10.3390/fermentation9070586
APA StyleJiao, L., Liu, Y., Cao, C., Bu, Q., Liu, M., & Xi, Y. (2023). Preparation and Application of Carbon-Based Materials in the Production of Medium-Chain Carboxylic Acids by Anaerobic Digestion: A Review. Fermentation, 9(7), 586. https://doi.org/10.3390/fermentation9070586







