Purification and Characterization of Xylanase Produced by Aspergillus fumigatus Isolated from the Northern Border Region of Saudi Arabia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal Strains and Their Xylanolytic Activity
2.2. Molecular Identification of the Aspergillus Isolates
2.3. Optimization of Xylanase Production in Submerged Fermentation (SmF)
2.4. Xylanase Production by A. fumigatus KSA-2 from Lignocellulosic Biomass in Solid-State Fermentation (SSF)
2.5. Xylanase Assay and Protein Determination
2.6. Procedures for the Extraction and Purification of Xylanase from A. fumigatus KSA-2
2.7. Production of Xylanase from Wheat Bran by A. fumigatus KSA-2 in SSF
2.8. Impact of pH, Temperature, Ions, and Inhibitors on the Xylanase Activity
2.9. Substrate Specificity
2.10. Statistical Analysis
3. Results
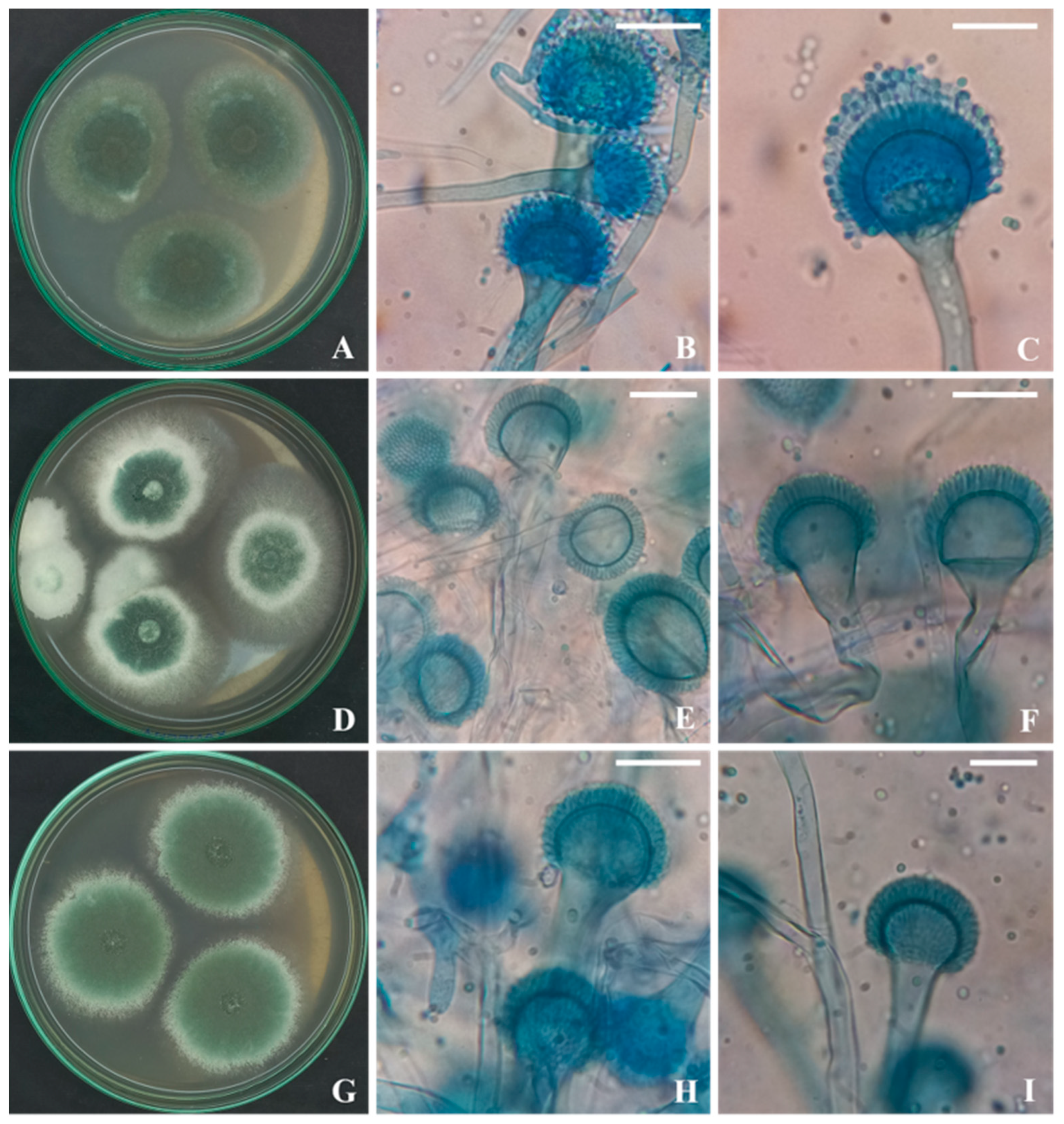
3.1. Strain’s Isolation and Identification
3.2. Optimization of Xylanase Production Conditions
3.3. Molecular Identification of the Aspergillus Strains
3.4. Screening Xylanolytic Activity of A. fumigatus KSA-2 on Lignocellulosic Wastes under SSF
3.5. Production of Xylanase by A. fumigatus KSA-2 from Wheat Bran in SSF
3.6. Effect of pH and Temperature on Xylanase Activity
3.7. Substrate Specificity
3.8. Effect of Ions and Inhibitors on Xylanase Activity
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rastogi, M.; Shrivastava, S. Recent advances in second generation bioethanol production: An insight to pretreatment, saccharification and fermentation processes. Renew. Sustain. Energy Rev. 2017, 80, 330–340. [Google Scholar] [CrossRef]
- Rastogi, M.; Shrivastava, S.; Shukla, P. Bioprospecting of xylanase producing fungal strains: Multilocus phylogenetic analysis and enzyme activity profiling. J. Basic Microbiol. 2022, 62, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Knob, A.; Fortkamp, D.; X Prolo, T.; Izidoro, S.C.; Almeida, J.M. Agro-residues as alternative for xylanase production by filamentous fungi. BioResources 2014, 9, 5738–5773. [Google Scholar]
- Fasiku, S.A.; Bello, M.A.; Odeniyi, O.A. Production of xylanase by Aspergillus niger Gio and Bacillus sp. (BA) through solid-state fermentation. Access Microbiol. 2022, 5, 000506. [Google Scholar]
- AL-Kolaibe, A.M.; Moharram, A.M.; Al-Bedak, O.A. Worthwhile enzyme production and eco-friendly bioconversion of three agricultural residues by Aspergillus curvatus and Aspergillus gaarensis, promising enzyme-producers isolated from extreme environment. J. Basic Appl. Mycol. (Egypt) 2021, 12, 1–14. [Google Scholar]
- Ismail, M.A.; Moubasher, A.H.; Mohamed, R.A.; Al-Bedak, O.A. Agro–industrial residues as alternative sources for cellulases and xylanases production and purification of xylanase produced by Aspergillus flavus AUMC 10331 isolated from extreme habitat. Curr. Res. Environ. Appl. Mycol. 2018, 8, 313–322. [Google Scholar] [CrossRef]
- Korkmaz, M.N.; Ozdemir, S.C.; Uzel, A. Xylanase production from marine derived Trichoderma pleuroticola 08ÇK001 strain isolated from Mediterranean coastal sediments. J. Basic Microbiol. 2017, 57, 839–851. [Google Scholar] [CrossRef]
- Shrivastava, S. Introduction to glycoside hydrolases: Classification, identification and occurrence. In Industrial Applications of Glycoside Hydrolases; Springer: Singapore, 2020; pp. 3–84. [Google Scholar]
- Dias, L.M.; dos Santos, B.V.; Albuquerque, C.J.B.; Baeta, B.E.L.; Pasquini, D.; Baffi, M.A. Biomass sorghum as a novel substrate in solid-state fermentation for the production of hemicellulases and cellulases by Aspergillus niger and A. fumigatus. J. Appl. Microbiol. 2018, 124, 708–718. [Google Scholar] [CrossRef]
- Rastogi, M.; Shrivastava, S. Glycosyl Hydrolases and biofuel. In Industrial Applications of Glycoside Hydrolases; Springer: Singapore, 2020; pp. 167–190. [Google Scholar]
- Warcup, J. The soil-plate method for isolation of fungi from soil. Nature 1950, 166, 117–118. [Google Scholar] [CrossRef]
- Smith, D.; Onions, A.H. The Preservation and Maintenance of Living Fungi; CAB International: Wallingford, UK, 1994.
- Moubasher, A.H.; Ismail, M.A.; Mohamed, R.A.; Al-Bedak, O.A. Ramophialophora chlamydospora, a new species from an alkaline lake of Wadi-El-Natron, Egypt. Asian J. Mycol. 2019, 2, 110–117. [Google Scholar] [CrossRef]
- Al-Bedak, O.A.; Moubasher, A.H. Aspergillus gaarensis, a new addition to section Circumdati from soil of Lake El-Gaar in Wadi-El-Natron, Egypt. Stud. Fungi 2020, 5, 59–65. [Google Scholar] [CrossRef]
- White, T.J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Academic Press, Inc.: Cambridge, MA, USA, 1990; Volume 18, pp. 315–322. [Google Scholar]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Criscuolo, A.; Gribaldo, S. BMGE (Block Mapping and Gathering with Entropy): A new software for selection of phylogenetic informative regions from multiple sequence alignments. BMC Evol. Biol. 2010, 10, 210. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Posada, D.; Crandall, K.A. Modeltest: Testing the model of DNA substitution. Bioinformatics 1998, 14, 817–818. [Google Scholar] [CrossRef] [Green Version]
- Al-Bedak, O.A.; Teama, E.A.; Ali, E.; Said, M.; Shalaby, E.; Moharram, Z.A. Impact of fumigation with phosphine on viability of wheat grains stored for six months at two levels of moisture content, in addition to description of four new records of associated fungi and assessment of their potential for enzymatic production. J. Basic Appl. Mycol. (Egypt) 2020, 11, 77–97. [Google Scholar]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Gaffney, M.; Carberry, S.; Doyle, S.; Murphy, R. Purification and characterisation of a xylanase from Thermomyces lanuginosus and its functional expression by Pichia pastoris. Enzym. Microb. Technol. 2009, 45, 348–354. [Google Scholar] [CrossRef]
- St, L.; Wold, S. Analysis of variance (ANOVA). Chemom. Intell. Lab. Syst. 1989, 6, 259–272. [Google Scholar]
- Uhoraningoga, A.; Kinsella, G.K.; Henehan, G.T.; Ryan, B.J. The goldilocks approach: A review of employing design of experiments in prokaryotic recombinant protein production. Bioengineering 2018, 5, 89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brandelli, A.; Daroit, D.J.; Riffel, A. Biochemical features of microbial keratinases and their production and applications. Appl. Microbiol. Biotechnol. 2010, 85, 1735–1750. [Google Scholar] [CrossRef]
- Muthezhilan, R.; Ashok, R.; Jayalakshmi, S. Production and optimization of thermostable alkaline xylanase by Penicillium oxalicum in solid state fermentation. Afr. J. Microbiol. Res. 2007, 1, 20–28. [Google Scholar]
- Ding, C.; Li, M.; Hu, Y. High-activity production of xylanase by Pichia stipitis: Purification, characterization, kinetic evaluation and xylooligosaccharides production. Int. J. Biol. Macromol. 2018, 117, 72–77. [Google Scholar] [CrossRef]
- Ajijolakewu, A.K.; Leh, C.P.; Abdullah, W.N.W.; Lee, C.K. Optimization of production conditions for xylanase production by newly isolated strain Aspergillus niger through solid state fermentation of oil palm empty fruit bunches. Biocatal. Agric. Biotechnol. 2017, 11, 239–247. [Google Scholar] [CrossRef]
- Pithadiya, D.; Nandha, D.; Thakkar, A. Partial purification and optimization of xylanase from Bacillus circulans. Arch. Appl. Sci. Res. 2016, 8, 1–10. [Google Scholar]
- da Silva, P.O.; Guimarães, N.C.A.; Peixoto-Nogueira, S.C.; Betini, J.H.; Marchetti, C.R.; Zanoelo, F.F.; Polizeli, M.L.T.M.; Marques, M.R.; Giannesi, G.C. Production of cellulase-free xylanase by Aspergillus flavus: Effect of polyols on the thermostability and its application on cellulose pulp biobleaching. Afr. J. Biotechnol. 2015, 14, 3368–3373. [Google Scholar]
- Nair, S.G.; Shashidhar, S. Fungal xylanase production under solid state and submerged fermentation conditions. Afr. J. Microbiol. Res. 2008, 2, 82–86. [Google Scholar]
- Pirota, R.D.P.B.; Tonelotto, M.; Delabona, P.D.S.; Fonseca, R.F.; Paixão, D.A.A.; Baleeiro, F.C.F.; Neto, V.B.; Farinas, C.S. Enhancing xylanases production by a new Amazon Forest strain of Aspergillus oryzae using solid-state fermentation under controlled operation conditions. Ind. Crops Prod. 2013, 45, 465–471. [Google Scholar] [CrossRef]
- Mostafa, F.A.; El Aty, A.; Wehaidy, H.R. Improved Xylanase production by mixing low cost wastes and novel co-culture of three marine-derived fungi in solid state fermentation. Int. J. Curr. Microbiol. App. Sci. 2014, 3, 336–349. [Google Scholar]
- Oliveira, A.C.; Amorim, G.M.; Azevêdo, J.A.G.; Godoy, M.G.; Freire, D.M.G. Solid-state fermentation of co-products from palm oil processing: Production of lipase and xylanase and effects on chemical composition. Biocatal. Biotransform. 2018, 36, 381–388. [Google Scholar] [CrossRef]
- Meilany, D.; Anugeraheni, D.; Aziz, A.; Kresnowati, M.T.A.P.; Setiadi, T. The Effects of Operational Conditions in Scaling Up of Xylanase Enzyme Production for Xylitol Production. Reaktor 2020, 20, 32–37. [Google Scholar] [CrossRef]
- Chapla, D.; Patel, H.; Singh, A.; Madamwar, D.; Shah, A. Production, purification and properties of a cellulase-free thermostable endoxylanase from newly isolated Paenibacillus sp. ASCD2. Ann. Microbiol. 2012, 62, 825–834. [Google Scholar] [CrossRef]
- Dhivahar, J.; Khusro, A.; Paray, B.A.; Rehman, M.U.; Agastian, P. Production and partial purification of extracellular xylanase from Pseudomonas nitroreducens using frugivorous bat (Pteropus giganteus) faeces as ideal substrate and its role in poultry feed digestion. J. King Saud Univ.-Sci. 2020, 32, 2474–2479. [Google Scholar] [CrossRef]
- Seemakram, W.; Boonrung, S.; Aimi, T.; Ekprasert, J.; Lumyong, S.; Boonlue, S. Purification, characterization and partial amino acid sequences of thermo-alkali-stable and mercury ion-tolerant xylanase from Thermomyces dupontii KKU–CLD–E2–3. Sci. Rep. 2020, 10, 21663. [Google Scholar] [CrossRef]
- Walia, A.; Mehta, P.; Chauhan, A.; Kulshrestha, S.; Shirkot, C.K. Purification and characterization of cellulase-free low molecular weight endo β-1, 4 xylanase from an alkalophilic Cellulosimicrobium cellulans CKMX1 isolated from mushroom compost. World J. Microbiol. Biotechnol. 2014, 30, 2597–2608. [Google Scholar] [CrossRef]
- Roy, S.; Dutta, T.; Sarkar, T.S.; Ghosh, S. Novel xylanases from Simplicillium obclavatum MTCC 9604: Comparative analysis of production, purification and characterization of enzyme from submerged and solid state fermentation. SpringerPlus 2013, 2, 382. [Google Scholar] [CrossRef] [Green Version]
- Kamble, R.D.; Jadhav, A.R. Isolation, purification, and characterization of xylanase produced by a new species of Bacillus in solid state fermentation. Int. J. Microbiol. 2012, 2012, 683193. [Google Scholar] [CrossRef] [Green Version]
- Moharram, A.M.; Zohri, A.A.; Hesham, A.E.; Abdel-Raheam, H.E.F.; Maher, M.A.; Al-Bedak, O.A. Production of cold-active pectinases by three novel Cladosporium species isolated from Egypt and application of the most active enzyme. Sci. Rep. 2022, 12, 15599. [Google Scholar] [CrossRef]
- Seemakram, W.; Boonrung, S.; Katekaew, S.; Aimi, T.; Boonlue, S. Purification and characterization of low molecular weight alkaline xylanase from Neosartorya tatenoi KKU-CLB-3-2-4-1. Mycoscience 2016, 57, 326–333. [Google Scholar] [CrossRef]
- Gupta, P.K.; Choudhary, S.; Chandrananthi, C.; Eveline, J.S.; Sushmitha, S.P.; Hiremath, L.; Srivastava, A.K.; Kumar, S.N. Fungal Biodiversity Producing Xylanase Enzymes Involved in Efficient Uses of Xylanolysis. In Mycodegradation of Lignocelluloses; Springer: Cham, Switzerland, 2019; pp. 51–63. [Google Scholar]
- Bhardwaj, N.; Kumar, B.; Agarwal, K.; Chaturvedi, V.; Verma, P. Purification and characterization of a thermo-acid/alkali stable xylanases from Aspergillus oryzae LC1 and its application in Xylo-oligosaccharides production from lignocellulosic agricultural wastes. Int. J. Biol. Macromol. 2019, 122, 1191–1202. [Google Scholar] [CrossRef]
- Ang, S.K.; Shaza, E.M.; Adibah, Y.; Suraini, A.A.; Madihah, M.S. Production of cellulases and xylanase by Aspergillus fumigatus SK1 using untreated oil palm trunk through solid state fermentation. Process Biochem. 2013, 48, 1293–1302. [Google Scholar] [CrossRef] [Green Version]
- Ferraz, J.L.d.A.A.; Souza, L.O.; Soares, G.A.; Coutinho, J.P.; de Oliveira, J.R.; Aguiar-Oliveira, E.; Franco, M. Enzymatic saccharification of lignocellulosic residues using cellulolytic enzyme extract produced by Penicillium roqueforti ATCC 10110 cultivated on residue of yellow mombin fruit. Bioresour. Technol. 2018, 248, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Souza, L.O.; de Brito, A.R.; Bonomo, R.C.F.; Santana, N.B.; Ferraz, J.L.d.A.; Aguiar-Oliveira, E.; Fernandes, A.G.d.A.; Ferreira, M.L.O.; de Oliveira, J.R.; Franco, M. Comparison of the biochemical properties between the xylanases of Thermomyces lanuginosus (Sigma®) and excreted by Penicillium roqueforti ATCC 10110 during the solid state fermentation of sugarcane bagasse. Biocatal. Agric. Biotechnol. 2018, 16, 277–284. [Google Scholar] [CrossRef]
- Ferraz, J.L.d.A.; Souza, L.O.; Fernandes, A.G.d.A.; Oliveira, M.L.F.; de Oliveira, J.R.; Franco, M. Optimization of the solid-state fermentation conditions and characterization of xylanase produced by Penicillium roqueforti ATCC 10110 using yellow mombin residue (Spondias mombin L.). Chem. Eng. Commun. 2020, 207, 31–42. [Google Scholar] [CrossRef]
- Simanjuntak, B.; Julian, H.; Kresnowati, M. Downstream Process of Xylanase Production from Oil Palm Empty Fruit Bunches: A Review. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2022. [Google Scholar]








| Substrate Type | Relative Activity (U/mL/min) | Specific Activity (U/mg) |
|---|---|---|
| Oat spelt xylan | 2.48 ± 0.4 | 11.81 ± 1.9 |
| Birchwood xylan | 4.311 ± 0.36 | 20.53 ± 1.714 |
| Corn cob xylan | 0.7 ± 0.15 | 3.33 ± 0.7 |
| Wheat bran xylan | 4.2 ± 0.32 | 20.0 ± 1.524 |
| Maize stalk xylan | 1.72 ± 0.28 | 8.2 ± 1.3 |
| Carboxymethyl cellulose | 0.84 ± 0.1 | 4.0 ± 0.47 |
| Microcrystalline cellulose | 0.52 ± 0.1 | 2.47 ± 0.47 |
| Ions and Inhibitors | Activity (U/mg) | Residual Activity (%) |
|---|---|---|
| Control | 20.52 ± 1.714 b | 100 |
| Na+ | 7.52 ± 0.94 g | 36.64 |
| K+ | 8 ± 0.88 g | 38.98 |
| Ca2+ | 14.95 ± 1.1 d | 72.85 |
| Mg2+ | 13.2 ± 1 e | 64.32 |
| Mn2+ | 26.3 ± 1.5 a | 128.16 |
| Zn2+ | 11.43 ± 0.74 f | 55.7 |
| Fe2+ | 16.3 ± 1 c | 79.43 |
| Cu2+ | 20.95 ± 1.7 b | 102.1 |
| Co2+ | 17.24 ± 0.9 c | 84 |
| Ni2+ | 15 ± 0.86 d | 73.1 |
| EDTA | 12 ± 1.1 ef | 58.48 |
| SDS | 8.62 ± 0.75 g | 42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ameen, F. Purification and Characterization of Xylanase Produced by Aspergillus fumigatus Isolated from the Northern Border Region of Saudi Arabia. Fermentation 2023, 9, 595. https://doi.org/10.3390/fermentation9070595
Ameen F. Purification and Characterization of Xylanase Produced by Aspergillus fumigatus Isolated from the Northern Border Region of Saudi Arabia. Fermentation. 2023; 9(7):595. https://doi.org/10.3390/fermentation9070595
Chicago/Turabian StyleAmeen, Fuad. 2023. "Purification and Characterization of Xylanase Produced by Aspergillus fumigatus Isolated from the Northern Border Region of Saudi Arabia" Fermentation 9, no. 7: 595. https://doi.org/10.3390/fermentation9070595
APA StyleAmeen, F. (2023). Purification and Characterization of Xylanase Produced by Aspergillus fumigatus Isolated from the Northern Border Region of Saudi Arabia. Fermentation, 9(7), 595. https://doi.org/10.3390/fermentation9070595





