A Review on Start-Up Phase Optimization of Kitchen Waste Anaerobic Digestion
Abstract
:1. Introduction
2. Factors Affecting Anaerobic Digestion Start-Up
2.1. Temperature
2.2. pH and Substrate
2.3. Organic Loading Rate
2.4. Inoculum and Inoculation Ratio
2.5. Trace Elements
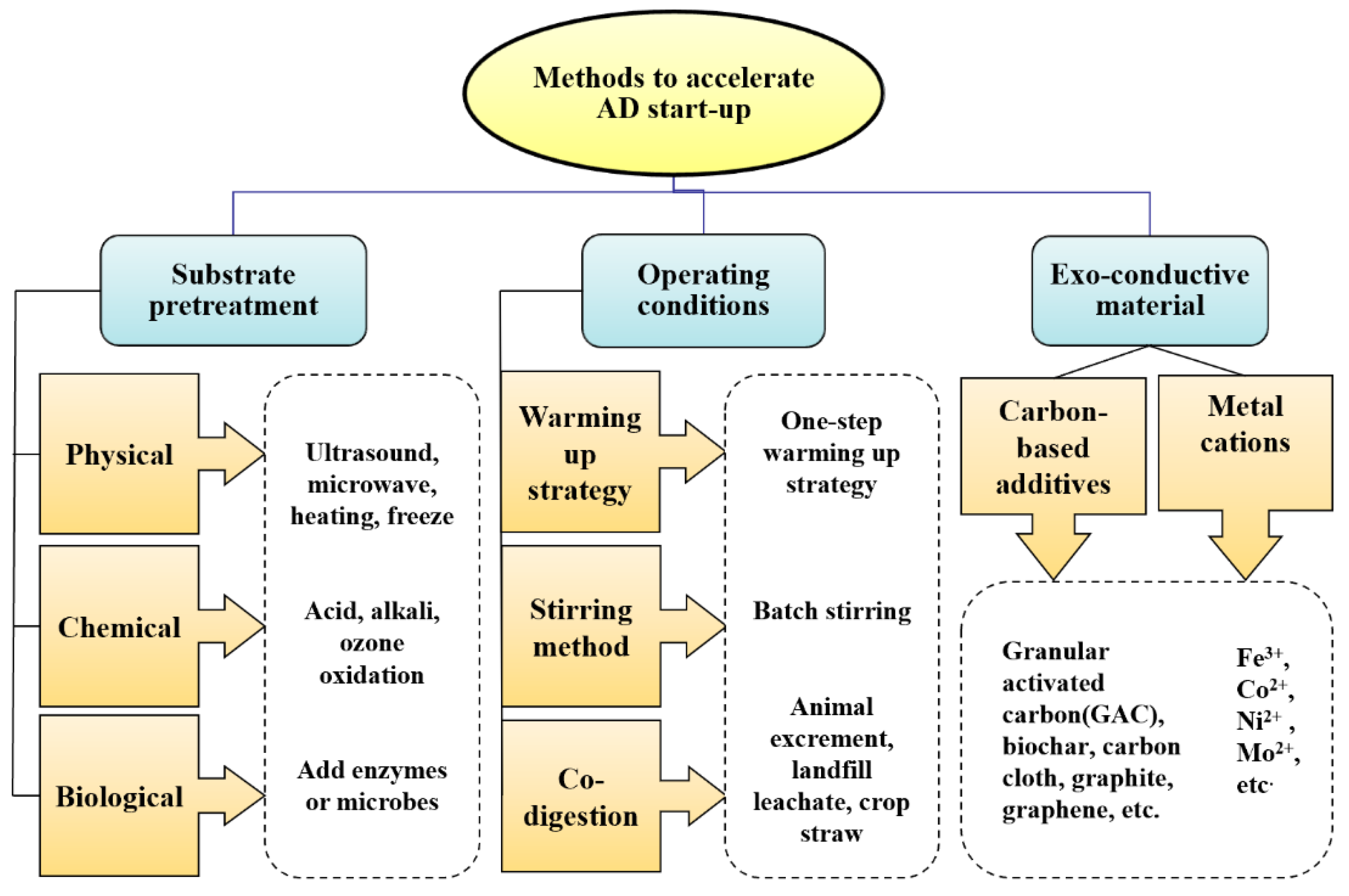
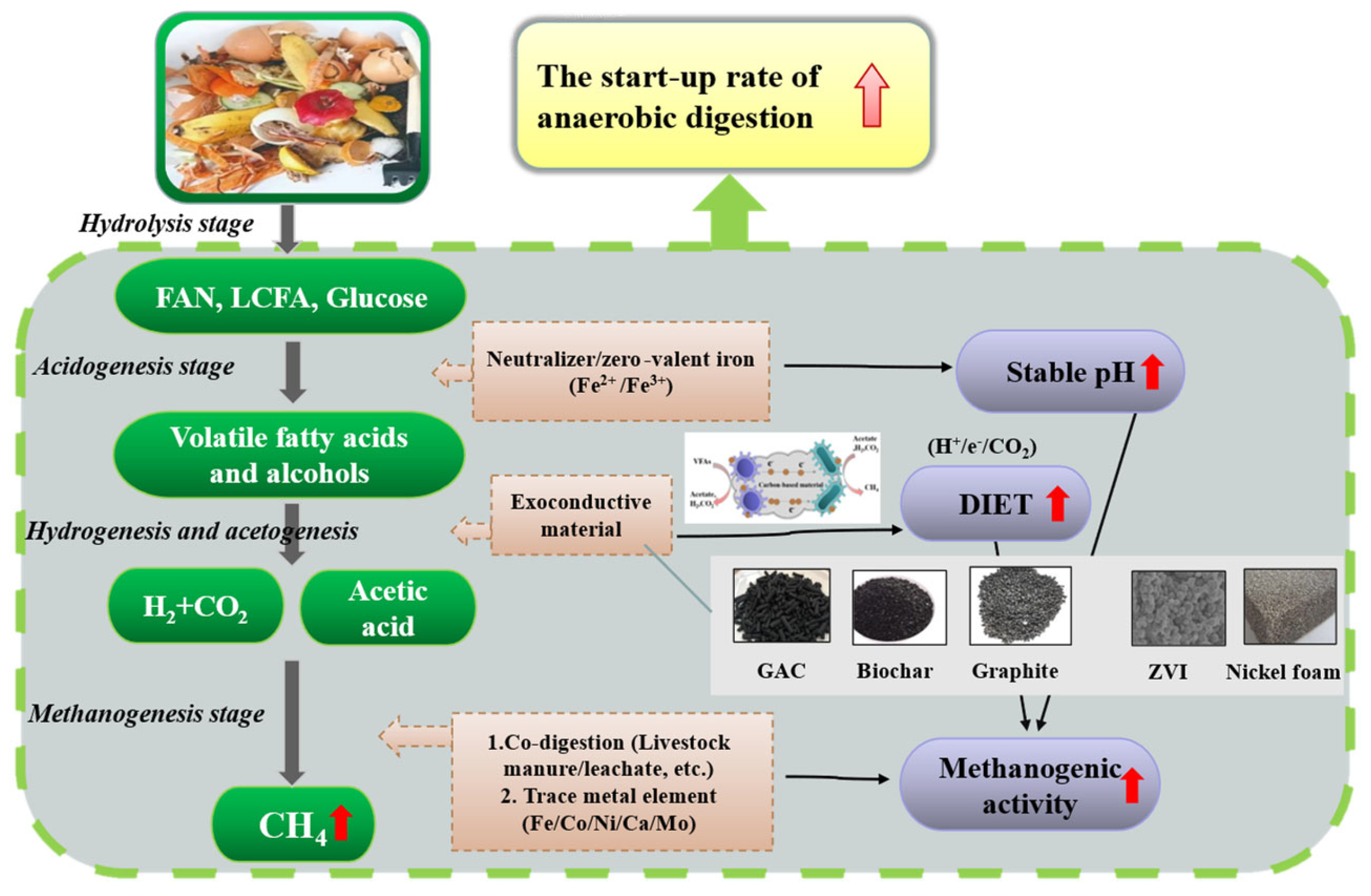
3. Methods to Accelerate the Start-Up Phase
- A short lag time before methane production.
- A significant methane production rate (>0.2 L/L/d) after the lag time.
- The presence of acetate-utilizing or hydrogen-utilizing MPA.
3.1. Substrate Pretreatment
- (i)
- to improve surface performance for the sake of achieving better microbial interaction reaction efficiency;
- (ii)
- to reduce/remove toxic and harmful compounds that are detrimental to the process;
- (iii)
- to improve the kinetics of the rate of hydrolysis of proteins and lipids;
- (iv)
3.1.1. Single Pretreatment
| Pretreatment Type | Pretreatment Conditions | Temperature | pH | Treatment Effect | Start-Up Time (d) | Reference |
|---|---|---|---|---|---|---|
| Freeze/thaw | Freeze for 24 h; thaw 12 h; the duration is 0.5 to 30 min | −20 °C/25 °C | 7.2 | Methane production increased by 6.7%; batch operation time is reduced by 42%; hydrogen production rate increased by 127% | 8 | [69] |
| Hydrothermal | 6.2 bar | 160 °C | 7.3~7.6 | Methane production is increased by 5–10% | 10 | [70] |
| Hydrothermal | 140 °C for 60 min | 140 °C | 7.96 | Methane yield increased by 27.78% | 11 | [71] |
| Ultrasonic | Sonication time is 24 min/d; HRT is 2 d; 20 kHz | 37 °C | 6.9~7.2 | The removal rate of VS was 67%; Methane production rate increased by 100% | 15 | [72] |
| Ultrasonic | 250 W for 40 min; 50 d | 37 °C | 6.9~7.4 | Cumulative gas production increased by 42.6%; methane content increased to 58.8%; biodegradation rate increased to 73.5%. | 10 | [73] |
| Microwave | 3.9 and 1.9/min; 175 °C for 1 min | 33 ± 2 °C | - | Average gas production increased by 16% | 30 | [74] |
| Aeration | 37.5 mL O2/LR-d; 4 day; 5 L/h for 24 h | 35 °C | 5.2~7.0 | Cumulative methane production increased by 21%; methane production increased by 45.6% | 25 | [75] |
| Acid/alkali | 4 mol/L NaOH/HCl; pH (2–13) | 35 °C | - | The hydrogen production rate of the acid pretreatment and alkali pretreatment increased by 21% and 480%, respectively | - | [76] |
| Alkali | 121 °C for 15 min; 4 mol/L NaOH/HCl | 35 ± 1 °C | 7.5 | The hydrogen production rate increased by 266% when the pH was 13 | 20 | [77] |
| Bio-enzyme | 45 °C for 24 h; 6 days at 30 °C | 60 °C | 4.0~4.5 | The rate of methane production increased by 350%; VS removal rate was 80.5% | 24 | [78] |
3.1.2. Combined Pretreatment
3.2. Type of Inoculum
3.3. Optimization of Operating Conditions
3.3.1. Warming-Up Strategy
3.3.2. Stirring Method
3.3.3. Co-Digestion
| Co-Digestible Mass Ratio | Operation Mode | Temperature (°C) | pH | Maximum Methane Yield (mL CH4/g VS) | Start-Up Duration (d) | Reference |
|---|---|---|---|---|---|---|
| Sewage sludge: KW = 1:1 | Batch assay | 35 ± 1 | 7.07~7.27 | 251 | 12 | [103] |
| Cow manure: KW = 1:2.5 | Semi-continuous | 39 | 7.63~7.67 | 441 | 13 | [100] |
| Chicken manure: KW = 1:1 | Semicontinuous | 55 | - | 136 | - | [104] |
| KW: Yard waste: Waste activated sludge = 0.8:1.7:0.5 | Semi-continuous | 35 | 6.74~6.98 | 149 | 28 | [105] |
| Maize straw silage: KW = 1:9 | Semi-continuous | 35 | 7.25~7.55 | 613 | 30 | [106] |
| Livestock manure: KW = 3:1 | Batch assay | 37 | 6.50~7.00 | 250 | 18 | [107] |
| Excess sludge: KW = 1:4 | Continuous | 35 | 7.17~7.77 | 274 | 12 | [108] |
| Sewage sludge: KW = 1:1 | Semi-continuous | 37 ± 1 | 7.00~7.50 | 402 | 6 | [109] |
| Cattle manure: KW = 1:3 | Batch assay | 35 ± 1 | 7.20~7.30 | 233 | 7 | [110] |
| Wastewater sludge: KW = 1:1 | Semi-continuous | 35 ± 1 | 6.00~7.00 | - | 10 | [111] |
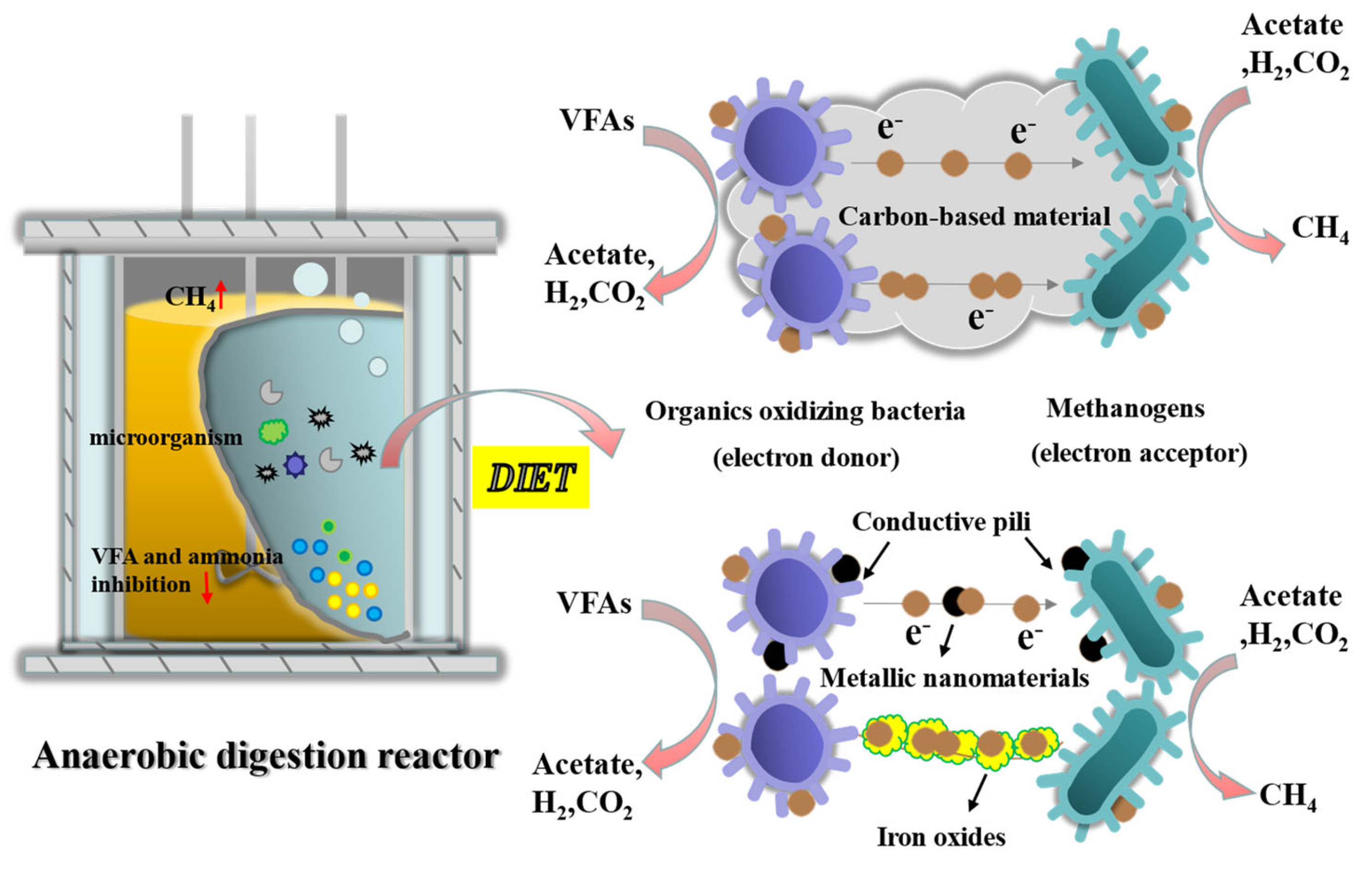
3.4. Exo-Conductive Material
3.4.1. Mechanisms of Electron Transfer
3.4.2. Carbon-Based Additives
3.4.3. Metal Cations
3.5. Combination of Methods
4. Conclusions
- (1)
- In general, a KW AD system starts faster and more stably under the following parameters: under mesophilic conditions, pH is controlled around 7.0, OLR ranges from 2.0 to 4.5 kg COD/m3·d and the inoculation ratio is higher than 1.5. In addition, the metal ion concentration should be strictly controlled to maintain the stability of the bioreactor.
- (2)
- On the other hand, utilizing KW as the only substrate for long-term AD reactor operation may generate a slow start-up, so it can be considered to add livestock manure or residual sludge for co-digestion, or to add exogenous conductive materials to enhance the DIET and microbial methanogenic activity to accelerate the start-up phase.
5. Prospects and Challenges
- (1)
- A dry AD reactor for FW biodegradation has been shown to exhibit better performance under thermophilic conditions. However, the optimal start-up temperature for wet AD has not been concluded yet, which needs further studies.
- (2)
- The addition of multiple metal elements can reduce the lag time of the AD start-up phase. Yet, it is unconclusive to the effect of co-precipitation and adsorption between different metals on DIET.
- (3)
- The AD process of KW can be affected by diverse factors, and a variety of methods are derived to accelerate the start-up phase of the reaction. Nevertheless, which method is the most efficient and economical remains to be explored.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, H.; Xu, J.; Sheng, L. Study on the comprehensive utilization of city kitchen waste as a resource in China. Energy 2019, 173, 263–277. [Google Scholar] [CrossRef]
- Halloran, A.; Clement, J.; Kornum, N.; Bucatariu, C.; Magid, J. Addressing food waste reduction in Denmark. Food Policy 2014, 49, 294–301. [Google Scholar] [CrossRef]
- Ul Saqib, N.; Sharma, H.B.; Baroutian, S.; Dubey, B.; Sarmah, A.K. Valorisation of food waste via hydrothermal carbonisation and techno-economic feasibility assessment. Sci. Total Environ. 2019, 690, 261–276. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Gaur, V.K.; Kim, S.-H.; Pandey, A. Microbial strategies for bio-transforming food waste into resources. Bioresour. Technol. 2020, 299, 122580. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Jiang, J. Effects of activated carbon on the in-situ control of odorous gases emitted from anaerobic digestion of food waste and the microbial community response. Environ. Technol. Innov. 2021, 21, 101170. [Google Scholar] [CrossRef]
- Girotto, F.; Alibardi, L.; Cossu, R. Food waste generation and industrial uses: A review. Waste Manag. 2015, 45, 32–41. [Google Scholar] [CrossRef]
- Jeganathan, J.; Nakhla, G.; Bassi, A. Long-term performance of high-rate anaerobic reactors for the treatment of oily wastewater. Environ. Sci. Technol. 2006, 40, 6466–6472. [Google Scholar] [CrossRef]
- Wu, B.; Wang, X.; Deng, Y.Y.; He, X.L.; Li, Z.W.; Li, Q.; Qin, H.; Chen, J.T.; He, M.X.; Zhang, M. Adaption of microbial community during the start-up stage of a thermophilic anaerobic digester treating food waste. Biosci. Biotechnol. Biochem. 2016, 80, 2025–2032. [Google Scholar] [CrossRef] [Green Version]
- Lim, E.Y.; Tian, H.L.; Chen, Y.Y.; Ni, K.W.; Zhang, J.X.; Tong, Y.W. Methanogenic pathway and microbial succession during start-up and stabilization of thermophilic food waste anaerobic digestion with biochar. Bioresour. Technol. 2020, 314, 123751. [Google Scholar] [CrossRef]
- Coelho, N.M.G.; Droste, R.L.; Kennedy, K.J. Evaluation of continuous mesophilic, thermophilic and temperature phased anaerobic digestion of microwaved activated sludge. Water Res. 2011, 45, 2822–2834. [Google Scholar] [CrossRef]
- Chiu, S.L.H.; Lo, I.M.C. Reviewing the anaerobic digestion and co-digestion process of food waste from the perspectives on biogas production performance and environmental impacts. Environ. Sci. Pollut. Res. 2016, 23, 24435–24450. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.-g.; Imai, T.; Ukita, M.; Sekine, M. Start-up performances of dry anaerobic mesophilic and thermophilic digestions of organic solid wastes. J. Environ. Sci. 2007, 19, 416–420. [Google Scholar] [CrossRef] [PubMed]
- Dinh Duc, N.; Chang, S.W.; Cha, J.H.; Jeong, S.Y.; Yoon, Y.S.; Lee, S.J.; Minh Chi, T.; Ngo, H.H. Dry semi-continuous anaerobic digestion of food waste in the mesophilic and thermophilic modes: New aspects of sustainable management and energy recovery in South Korea. Energy Convers. Manag. 2017, 135, 445–452. [Google Scholar] [CrossRef]
- Jang, H.M.; Ha, J.H.; Kim, M.-S.; Kim, J.-O.; Kim, Y.M.; Park, J.M. Effect of increased load of high-strength food wastewater in thermophilic and mesophilic anaerobic co-digestion of waste activated sludge on bacterial community structure. Water Res. 2016, 99, 140–148. [Google Scholar] [CrossRef]
- Jang, H.M.; Ha, J.H.; Kim, M.-S.; Kim, J.-O.; Kim, Y.M.; Park, J.M. Evaluating the impact of Iron Oxide nanoparticles (IO-NPs) and IO-NPs doped granular activated carbon on the anaerobic digestion of food waste at mesophilic and thermophilic temperature. J. Environ. Chem. Eng. 2022, 10, 107388. [Google Scholar] [CrossRef]
- Kim, M.; Speece, R.E. Waste Activated Sludge for Start-up Seed of Thermophilic Anaerobic Digestion. J. Korean Soc. Water Environ. 2005, 21, 490–495. [Google Scholar]
- Li, X.; Yang, Y.; Lu, C.-S.; Kobayashi, T.; Kong, Z.; Hu, Y. Oleate Impacts on Acetoclastic and Hydrogenotrophic Methanogenesis under Mesophilic and Thermophilic Conditions. Int. J. Environ. Res. Public Health 2023, 20, 3423. [Google Scholar] [CrossRef]
- Avery, G.B.; Shannon, R.D.; White, J.R.; Martens, C.S.; Alperin, M.J. Controls on methane production in a tidal freshwater estuary and a peatland: Methane production via acetate fermentation and CO2 reduction. Biogeochemistry 2003, 62, 19–37. [Google Scholar] [CrossRef]
- Yu, H.Q.; Fang, H.H.P. Acidogenesis of gelatin-rich wastewater in an upflow anaerobic reactor: Influence of pH and temperature. Water Res. 2003, 37, 55–66. [Google Scholar] [CrossRef]
- Horiuchi, J.I.; Shimizu, T.; Tada, K.; Kanno, T.; Kobayashi, M. Selective production of organic acids in anaerobic acid reactor by pH control. Bioresour. Technol. 2002, 82, 209–213. [Google Scholar] [CrossRef]
- Xu, Y.; Jiang, J.; Liu, N.; Yang, M.; Meng, Y. Effects of pretreatments on anaerobic co-digestion of kitchen waste and other organic wastes. J. Tsinghua Univ. Sci. Technol. 2019, 59, 558–566. [Google Scholar]
- Ghangrekar, M.M.; Asolekar, S.R.; Joshi, S.G. Characteristics of sludge developed under different loading conditions during UASB reactor start-up and granulation. Water Res. 2005, 39, 1123–1133. [Google Scholar] [CrossRef]
- Song, Y.-C.; Sik, S.H.; Chae, S.-R.; Oh, S.-E. Effects of Organic Loading Rate and Dilution Rate on Acidogenesis of Food Wastes. J. Korean Soc. Waste Manag. 2002, 19, 722–729. [Google Scholar]
- Meng, Y.; Shen, F.; Yuan, H.; Zou, D.; Liu, Y.; Zhu, B.; Chufo, A.; Jaffar, M.; Li, X. Start-up and operation strategies on the liquefied food waste anaerobic digestion and a full-scale case application. Bioprocess Biosyst. Eng. 2014, 37, 2333–2341. [Google Scholar] [CrossRef]
- Moset, V.; Al-zohairi, N.; Moller, H.B. The impact of inoculum source, inoculum to substrate ratio and sample preservation on methane potential from different substrates. Biomass Bioenergy 2015, 83, 474–482. [Google Scholar] [CrossRef]
- Moestedt, J.; Westerholm, M.; Isaksson, S.; Schnurer, A. Inoculum Source Determines Acetate and Lactate Production during Anaerobic Digestion of Sewage Sludge and Food Waste. Bioengineering 2020, 7, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajput, A.A.; Sheikh, Z. Effect of inoculum type and organic loading on biogas production of sunflower meal and wheat straw. Sustain. Environ. Res. 2019, 29, 4. [Google Scholar] [CrossRef] [Green Version]
- Córdoba, V.; Fernández, M.; Santalla, E. The effect of different inoculums on anaerobic digestion of swine wastewater. J. Environ. Chem. Eng. 2016, 4, 115–122. [Google Scholar] [CrossRef]
- Quintero, M.; Castro, L.; Ortiz, C.; Guzman, C.; Escalante, H. Enhancement of starting up anaerobic digestion of lignocellulosic substrate: Fique’s bagasse as an example. Bioresour. Technol. 2012, 108, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Suwannoppadol, S.; Ho, G.; Cord-Ruwisch, R. Rapid start-up of thermophilic anaerobic digestion with the turf fraction of MSW as inoculum. Bioresour. Technol. 2011, 102, 7762–7767. [Google Scholar] [CrossRef] [Green Version]
- Zheng, X.; Li, B.; Li, Y.; Chen, L. Effect of different inoculation ratios on start-up performances of dry anaerobic digestions for kitchen waste. Chin. J. Environ. Eng. 2014, 8, 1157–1162. [Google Scholar]
- Zheng, X.; Li, B.; Guo, D.; Fu, X.; Chen, L. Start-up performance and methane production efficiency on anaerobic digestion for kitchen waste. Environ. Eng. 2018, 36, 128–132. [Google Scholar]
- Bozym, M.; Florczak, I.; Zdanowska, P.; Wojdalski, J.; Klimkiewicz, M. An analysis of metal concentrations in food wastes for biogas production. Renew. Energy 2015, 77, 467–472. [Google Scholar] [CrossRef]
- Soto, M.; Mendez, R.; Lema, J.M. Methanogenic and non-methanogenic activity tests—Theoretical basis and experimental set-up. Water Res. 1993, 27, 1361–1376. [Google Scholar] [CrossRef]
- Huang, J.; Pinder, K.L. Effects of calcium on development of anaerobic acidogenic biofilms. Biotechnol. Bioeng. 1995, 45, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Jacksonmoss, C.A.; Duncan, J.R. The effect of aluminum on anaerobic-digestion. Biotechnol. Lett. 1991, 13, 143–148. [Google Scholar] [CrossRef]
- Schmidt, J.E.; Ahring, B.K. Effects of magnesium on thermophilic acetate-degrading granules in upflow anaerobic sludge blanket (uasb) reactors. Enzym. Microb. Technol. 1993, 15, 304–310. [Google Scholar] [CrossRef]
- Yuan, H.; Wen, J.; Xing, B.; Han, Y.; Cao, S.; Zhang, K.; Wang, X. Inhibition effects of sodium salinity on the high-rate mesophilic anaerobic codigestion of food waste with waste activated sludge. Acta Sci. Circumstantiae 2020, 40, 3331–3340. [Google Scholar]
- Jin, P.K.; Bhattacharya, S.K.; Williams, C.J.; Zhang, H.N. Effects of sulfide addition on copper inhibition in methanogenic systems. Water Res. 1998, 32, 977–988. [Google Scholar] [CrossRef]
- Liu, S.-H.; Zeng, G.-M.; Niu, Q.-Y.; Liu, Y.; Zhou, L.; Jiang, L.-H.; Tan, X.-f.; Xu, P.; Zhang, C.; Cheng, M. Bioremediation mechanisms of combined pollution of PAHs and heavy metals by bacteria and fungi: A mini review. Bioresour. Technol. 2017, 224, 25–33. [Google Scholar] [CrossRef]
- Mudhoo, A.; Kumar, S. Effects of heavy metals as stress factors on anaerobic digestion processes and biogas production from biomass. Int. J. Environ. Sci. Technol. 2013, 10, 1383–1398. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Zhang, L.; Li, A. Enhanced anaerobic digestion of food waste by trace metal elements supplementation and reduced metals dosage by green chelating agent S, S -EDDS via improving metals bioavailability. Water Res. 2015, 84, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Babich, H.; Stotzky, G. Toxicity of nickel to microbes—Environmental aspects. Adv. Appl. Microbiol. 1983, 29, 195–265. [Google Scholar] [CrossRef]
- Zhao, J.; Bello, M.O.; Meng, Y.; Prosser, J.I. Selective inhibition of ammonia oxidising archaea by simvastatin stimulates growth of ammonia oxidising bacteria. Soil Biol. Biochem. 2020, 141, 107673. [Google Scholar] [CrossRef]
- Guo, X.; Sun, C.; Lin, R.; Xia, A.; Huang, Y.; Zhu, X. Effects of foam nickel supplementation on anaerobic digestion: Direct interspecies electron transfer. J. Hazard. Mater. 2020, 399, 122830. [Google Scholar] [CrossRef] [PubMed]
- Quiroga, G.; Castrillon, L.; Fernandez-Nava, Y.; Maranon, E.; Negral, L. Effect of ultrasound pre-treatment in the anaerobic co-digestion of cattle manure with food waste and sludge. Bioresour. Technol. 2014, 154, 74–79. [Google Scholar] [CrossRef] [Green Version]
- Deepanraj, B.; Sivasubramanian, V.; Jayaraj, S. Effect of substrate pretreatment on biogas production through anaerobic digestion of food waste. Int. J. Hydrogen Energy 2017, 42, 26522–26528. [Google Scholar] [CrossRef]
- Ariunbaatar, J.; Panico, A.; Esposito, G.; Pirozzi, F.; Lens, P.N.L. Pretreatment methods to enhance anaerobic digestion of organic solid waste. Appl. Energy 2014, 123, 143–156. [Google Scholar] [CrossRef]
- Carlsson, M.; Lagerkvist, A.; Morgan-Sagastume, F. The effects of substrate pre-treatment on anaerobic digestion systems: A review. Waste Manag. 2012, 32, 1634–1650. [Google Scholar] [CrossRef]
- Rafique, R.; Poulsen, T.G.; Nizami, A.-S.; Asam, Z.-u.-Z.; Murphy, J.D.; Kiely, G. Effect of thermal, chemical and thermo-chemical pre-treatments to enhance methane production. Energy 2010, 35, 4556–4561. [Google Scholar] [CrossRef]
- Kurian, J.K.; Nair, G.R.; Hussain, A.; Raghavan, G.S.V. Feedstocks, logistics and pre-treatment processes for sustainable lignocellulosic biorefineries: A comprehensive review. Renew. Sustain. Energy Rev. 2013, 25, 205–219. [Google Scholar] [CrossRef]
- Monlau, F.; Sambusiti, C.; Barakat, A.; Guo, X.M.; Latrille, E.; Trably, E.; Steyer, J.-P.; Carrere, H. Predictive Models of Biohydrogen and Biomethane Production Based on the Compositional and Structural Features of Lignocellulosic Materials. Environ. Sci. Technol. 2012, 46, 12217–12225. [Google Scholar] [CrossRef]
- Szlachta, J.; Prask, H.; Fugol, M.; Luberanski, A. Effect of Mechanical Pre-Treatment of the Agricultural Substrates on Yield of Biogas and Kinetics of Anaerobic Digestion. Sustainability 2018, 10, 3669. [Google Scholar] [CrossRef] [Green Version]
- Fisgativa, H.; Tremier, A.; Dabert, P. Characterizing the variability of food waste quality: A need for efficient valorisation through anaerobic digestion. Waste Manag. 2016, 50, 264–274. [Google Scholar] [CrossRef]
- Zhang, C.; Su, H.; Baeyens, J.; Tan, T. Reviewing the anaerobic digestion of food waste for biogas production. Renew. Sustain. Energy Rev. 2014, 38, 383–392. [Google Scholar] [CrossRef]
- Kim, J.K.; Oh, B.R.; Chun, Y.N.; Kim, S.W. Effects of temperature and hydraulic retention time on anaerobic digestion of food waste. J. Biosci. Bioeng. 2006, 102, 328–332. [Google Scholar] [CrossRef]
- Jia, S.; Zhang, D.; Zhao, J.; Yu, S. Research on different pre-treatment methods for improving anaerobic digestion of primary/excess sludge of biogas production. Chem. Ind. Eng. Prog. 2013, 32, 193–198. [Google Scholar]
- Obulisamy, P.K.; Chakraborty, D.; Selvam, A.; Wong, J.W.C. Anaerobic co-digestion of food waste and chemically enhanced primary-treated sludge under mesophilic and thermophilic conditions. Environ. Technol. 2016, 37, 3200–3207. [Google Scholar] [CrossRef]
- Izumi, K.; Okishio, Y.-k.; Nagao, N.; Niwa, C.; Yamamoto, S.; Toda, T. Effects of particle size on anaerobic digestion of food waste. Int. Biodeterior. Biodegrad. 2010, 64, 601–608. [Google Scholar] [CrossRef]
- Gianico, A.; Gallipoli, A.; Gazzola, G.; Pastore, C.; Tonanzi, B.; Braguglia, C.M. A novel cascade biorefinery approach to transform food waste into valuable chemicals and biogas through thermal pretreatment integration. Bioresour. Technol. 2021, 338, 125517. [Google Scholar] [CrossRef]
- Parra-Orobio, B.A.; Giron-Bol, L.M.; Gomez-Munoz, D.F.; Marmolejo-Rebellon, L.F.; Torres-Lozada, P. Thermal pre-treatment as a tool for energy recovery from food waste through anaerobic digestion. Effect on kinetic and physicochemical characteristics of the substrate. Environ. Technol. Innov. 2021, 21, 101262. [Google Scholar] [CrossRef]
- Qin, L.; Liu, Z.-H.; Li, B.-Z.; Dale, B.E.; Yuan, Y.-J. Mass balance and transformation of corn stover by pretreatment with different dilute organic acids. Bioresour. Technol. 2012, 112, 319–326. [Google Scholar] [CrossRef]
- Ma, J.; Duong, T.H.; Smits, M.; Verstraete, W.; Carballa, M. Enhanced biomethanation of kitchen waste by different pre-treatments. Bioresour. Technol. 2011, 102, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S.; Fonoll, X.; Khanal, S.K.; Raskin, L. Biological strategies for enhanced hydrolysis of lignocellulosic biomass during anaerobic digestion: Current status and future perspectives. Bioresour. Technol. 2017, 245, 1245–1257. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Han, W.; Xu, X.; Chen, L.; Tang, J.; Hou, P. Ethanol production from waste pizza by enzymatic hydrolysis and fermentation. Biochem. Eng. J. 2020, 156, 107528. [Google Scholar] [CrossRef]
- Moon, H.C.; Song, I.S.; Kim, J.C.; Shirai, Y.; Lee, D.H.; Kim, J.K.; Chung, S.O.; Kim, D.H.; Oh, K.K. Enzymatic hydrolysis of food waste and ethanol fermentation. Int. J. Energy Res. 2009, 33, 164–172. [Google Scholar] [CrossRef]
- Duvernay, W.H.; Chinn, M.S.; Yencho, G.C. Hydrolysis and fermentation of sweetpotatoes for production of fermentable sugars and ethanol. Ind. Crops Prod. 2013, 42, 527–537. [Google Scholar] [CrossRef]
- Vavouraki, A.I.; Volioti, V.; Kornaros, M.E. Optimization of thermo-chemical pretreatment and enzymatic hydrolysis of kitchen wastes. Waste Manag. 2014, 34, 167–173. [Google Scholar] [CrossRef]
- Stabnikova, O.; Liu, X.Y.; Wang, J.Y. Digestion of frozen/thawed food waste in the hybrid anaerobic solid-liquid system. Waste Manag. 2008, 28, 1654–1659. [Google Scholar] [CrossRef]
- Tampio, E.; Ervasti, S.; Paavola, T.; Heaven, S.; Banks, C.; Rintala, J. Anaerobic digestion of autoclaved and untreated food waste. Waste Manag. 2014, 34, 370–377. [Google Scholar] [CrossRef] [Green Version]
- Ding, L.; Yang, M.; Areeprasert, C.; Cheng, X.; Chen, X.; Wang, F.; Yu, G. Analysis of micro-particle effect and methanogenic potential of food waste model compounds by hydrothermal pretreatment. Fuel 2023, 331, 125686. [Google Scholar] [CrossRef]
- Elbeshbishy, E.; Nakhla, G. Comparative study of the effect of ultrasonication on the anaerobic biodegradability of food waste in single and two-stage systems. Bioresour. Technol. 2011, 102, 6449–6457. [Google Scholar] [CrossRef]
- Feng, L.; Li, R. Efficiency of anaerobic digestion of kitchen waste by low intensity ultrasound pretreatment. Chin. J. Environ. Eng. 2012, 6, 3280–3286. [Google Scholar]
- Marin, J.; Kennedy, K.J.; Eskicioglu, C. Effect of microwave irradiation on anaerobic degradability of model kitchen waste. Waste Manag. 2010, 30, 1772–1779. [Google Scholar] [CrossRef]
- Lim, J.W.; Wang, J.-Y. Enhanced hydrolysis and methane yield by applying microaeration pretreatment to the anaerobic co-digestion of brown water and food waste. Waste Manag. 2013, 33, 813–819. [Google Scholar] [CrossRef]
- Zhao, M.X.; Yan, Q.; Ruan, W.Q.; Miao, H.F.; Ren, H.Y.; Xu, Y. Enhancement of substrate solubilization and hydrogen production from kitchen wastes by pH pretreatment. Environ. Technol. 2011, 32, 119–125. [Google Scholar] [CrossRef]
- Zhao, M.X.; Yan, Q.; Ruan, W.Q.; Xu, Y. Enhancement of hydrogen production from kitchen wastes by alkaline pretreatment. J. Saf. Environ. 2009, 9, 88–91. [Google Scholar]
- Kiran, E.U.; Liu, Y. Bioethanol production from mixed food waste by an effective enzymatic pretreatment. Fuel 2015, 159, 463–469. [Google Scholar] [CrossRef]
- Cheng, J.; Yue, L.; Hua, J.; Dong, H.; Zhou, J.; Li, Y.-Y. Hydrothermal alkali pretreatment contributes to fermentative methane production of a typical lipid from food waste through co-production of hydrogen with methane. Bioresour. Technol. 2020, 306, 123164. [Google Scholar] [CrossRef]
- Hafid, H.S.; Rahman, N.A.A.; Shah, U.K.M.; Baharudin, A.S. Enhanced fermentable sugar production from kitchen waste using various pretreatments. J. Environ. Manag. 2015, 156, 290–298. [Google Scholar] [CrossRef]
- laqa Kakar, F.; Purohit, N.; Okoye, F.; Liss, S.N.; Elbeshbishy, E. Combined hydrothermal and free nitrous acid, alkali and acid pretreatment for biomethane recovery from municipal sludge. Waste Manag. 2021, 131, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Simkins, S.; Alexander, M. Models for mineralization kinetics with the variables of substrate concentration and population-density. Appl. Environ. Microbiol. 1984, 47, 1299–1306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chudoba, P.; Capdeville, B.; Chudoba, J. Explanation of biological meaning of the s0/x0 ratio in batch cultivation. Water Sci. Technol. 1992, 26, 743–751. [Google Scholar] [CrossRef]
- Lim, J.W.; Wong, S.W.K.; Dai, Y.; Tong, Y.W. Effect of seed sludge source and start-up strategy on the performance and microbial communities of thermophilic anaerobic digestion of food waste. Energy 2020, 203, 117922. [Google Scholar] [CrossRef]
- Elbeshbishy, E.; Nakhla, G.; Hafez, H. Biochemical methane potential (BMP) of food waste and primary sludge: Influence of inoculum pre-incubation and inoculum source. Bioresour. Technol. 2012, 110, 18–25. [Google Scholar] [CrossRef]
- Wilkins, D.; Rao, S.; Lu, X.; Lee, P.K.H. Effects of sludge inoculum and organic feedstock on active microbial communities and methane yield during anaerobic digestion. Front. Microbiol. 2015, 6, 1114. [Google Scholar] [CrossRef] [Green Version]
- Ding, H.; Barlaz, M.A.; de los Reyes Iii, F.L.; Call, D.F. Influence of Inoculum Type on Volatile Fatty Acid and Methane Production in Short-Term Anaerobic Food Waste Digestion Tests. ACS Sustain. Chem. Eng. 2022, 10, 17071–17080. [Google Scholar] [CrossRef]
- Wang, K.; Yin, J.; Shen, D.; Li, N. Anaerobic digestion of food waste for volatile fatty acids (VFAs) production with different types of inoculum: Effect of pH. Bioresour. Technol. 2014, 161, 395–401. [Google Scholar] [CrossRef]
- Tian, Z.; Zhang, Y.; Li, Y.; Chi, Y.; Yang, M. Rapid establishment of thermophilic anaerobic microbial community during the one-step startup of thermophilic anaerobic digestion from a mesophilic digester. Water Res. 2015, 69, 9–19. [Google Scholar] [CrossRef]
- Palatsi, J.; Gimenez-Lorang, A.; Ferrer, I.; Flotats, X. Start-up strategies of thermophilic anaerobic digestion of sewage sludge. Water Sci. Technol. 2009, 59, 1777–1784. [Google Scholar] [CrossRef]
- Xu, R.; Yang, Z.-H.; Wang, Q.-P.; Bai, Y.; Liu, J.-B.; Zheng, Y.; Zhang, Y.-R.; Xiong, W.-P.; Ahmad, K.; Fan, C.-Z. Rapid startup of thermophilic anaerobic digester to remove tetracycline and sulfonamides resistance genes from sewage sludge. Sci. Total Environ. 2018, 612, 788–798. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Jang, H.M.; Shin, S.G.; Kim, Y.M. Thermophilic anaerobic digestion: Effect of start-up strategies on performance and microbial community. Sci. Total Environ. 2019, 687, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Wu, Y.P.; Xu, K.Q.; Li, Y.Y. Effect of Mixing Driven by Siphon Flow: Parallel Experiments Using the Anaerobic Reactors with Different Mixing Modes. Energies 2013, 6, 4207–4222. [Google Scholar] [CrossRef] [Green Version]
- Kariyama, I.D.; Zhai, X.; Wu, B. Influence of mixing on anaerobic digestion efficiency in stirred tank digesters: A review. Water Res. 2018, 143, 503–517. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Li, Y.; Wang, J.; Sheng, H.; Li, Q.; Zeng, Y.; Song, R. A comparative experimental study of the anaerobic treatment of food wastes using an anaerobic digester with a polyamide stirring rake or a stainless-steel stirring rake. J. Environ. Manag. 2018, 218, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, B.; Cai, W. Research Progress on Propionic Acid Accumulation and Control in Anaerobic Digestion System. China Water Wastewater 2005, 21, 25–27. [Google Scholar]
- Srisowmeya, G.; Chakravarthy, M.; Devi, G.N. Critical considerations in two-stage anaerobic digestion of food waste—A review. Renew. Sustain. Energy Rev. 2020, 119, 109587. [Google Scholar] [CrossRef]
- Wang, Q.; Yamabe, K.; Narita, J.; Morishita, M.; Ohsumi, Y.; Kusano, K.; Shirai, Y.; Ogawa, H.I. Suppression of growth of putrefactive and food poisoning bacteria by lactic acid fermentation of kitchen waste. Process Biochem. 2001, 37, 351–357. [Google Scholar] [CrossRef]
- Zhang, B.; Shi, H.; Zhang, L.; Cai, W. The influence of pH on hydrolysis and acidogenesis of kitchen wastes in two-phase anaerobic digestion. Acta Sci. Circumstantiae 2005, 25, 665–669. [Google Scholar] [CrossRef]
- Xing, B.-S.; Cao, S.; Han, Y.; Wen, J.; Zhang, K.; Wang, X.C. Stable and high-rate anaerobic co-digestion of food waste and cow manure: Optimisation of start-up conditions. Bioresour. Technol. 2020, 307, 123195. [Google Scholar] [CrossRef]
- Liao, X.; Zhu, S.; Zhong, D.; Zhu, J.; Liao, L. Anaerobic co-digestion of food waste and landfill leachate in single-phase batch reactors. Waste Manag. 2014, 34, 2278–2284. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Xiao, G.; Peng, L.; Su, H.; Tan, T. The anaerobic co-digestion of food waste and cattle manure. Bioresour. Technol. 2013, 129, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y. Start-up strategy of co-digestion system of sewage sludge and food waste. Environ. Eng. 2018, 36, 137–140. [Google Scholar]
- Zhang, J.; Qi, Q.; Mao, L.; He, Y.; Loh, K.-C.; Tong, Y.W. Mixing strategies? Activated carbon nexus: Rapid start-up of thermophilic anaerobic digestion with the mesophilic anaerobic sludge as inoculum. Bioresour. Technol. 2020, 310, 123401. [Google Scholar] [CrossRef]
- Lee, E.; Bittencourt, P.; Casimir, L.; Jimenez, E.; Wang, M.; Zhang, Q.; Ergas, S.J. Biogas production from high solids anaerobic co-digestion of food waste, yard waste and waste activated sludge. Waste Manag. 2019, 95, 432–439. [Google Scholar] [CrossRef]
- Yong, Z.H.; Dong, Y.L.; Zhang, X.; Tan, T.W. Anaerobic co-digestion of food waste and straw for biogas production. Renew. Energy 2015, 78, 527–530. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, Q.; Jiang, Y.; Che, Z.; Zhang, H.; Wang, X.; Cao, Y.; Kou, W. Analysis of microorganism at starting stage of anaerobic fermentation of vegetable waste and restaurant garbage. Chin. J. Environ. Eng. 2014, 8, 310–316. [Google Scholar]
- Chang, C.; Ming, L.; Mu, Y.; Hua, Z.; Li, X.; Zhang, D. Synergistic effect of kitchen waste and sludge anaerobic fermentation for methane production. China Environ. Sci. 2022, 42, 1259–1266. [Google Scholar]
- Varsha, S.S.V.; Soomro, A.F.; Baig, Z.T.; Vuppaladadiyam, A.K.; Murugavelh, S.; Antunes, E. Methane production from anaerobic mono- and co-digestion of kitchen waste and sewage sludge: Synergy study on cumulative methane production and biodegradability. Biomass Convers. Biorefin. 2022, 12, 3911–3919. [Google Scholar] [CrossRef]
- Li, R.; Chen, S.; Li, X. Anaerobic Co-digestion of Kitchen Waste and Cattle Manure for Methane Production. Energy Sources Part A Recovery Util. Environ. Eff. 2009, 31, 1848–1856. [Google Scholar] [CrossRef]
- Antony, D.; Murugavelh, S. Anaerobic co-digestion of kitchen waste and wastewater sludge: Biogas-based power generation. Biofuels 2018, 9, 157–162. [Google Scholar] [CrossRef]
- Schink, B. Energetics of syntrophic cooperation in methanogenic degradation. Microbiol. Mol. Biol. Rev. 1997, 61, 262–280. [Google Scholar] [CrossRef] [Green Version]
- Morris, B.E.L.; Henneberger, R.; Huber, H.; Moissl-Eichinger, C. Microbial syntrophy: Interaction for the common good. Fems Microbiol. Rev. 2013, 37, 384–406. [Google Scholar] [CrossRef] [Green Version]
- Shen, L.; Zhao, Q.; Wu, X.; Li, X.; Li, Q.; Wang, Y. Interspecies electron transfer in syntrophic methanogenic consortia: From cultures to bioreactors. Renew. Sustain. Energy Rev. 2016, 54, 1358–1367. [Google Scholar] [CrossRef]
- Li, J.; Zhang, B.; Liu, Q.; Han, Y. Research progress on enhancement of methane production through direct interspecific electron transfer by conductive materials. Acta Microbiol. Sin. 2021, 61, 1507–1524. [Google Scholar]
- Stams, A.J.M. Metabolic interactions between anaerobic-bacteria in methanogenic environments. Antonie Leeuwenhoek Int. J. Gen. Mol. Microbiol. 1994, 66, 271–294. [Google Scholar] [CrossRef]
- Cheng, Q.; Call, D.F. Hardwiring microbes via direct interspecies electron transfer: Mechanisms and applications. Environ. Sci. Process. Impacts 2016, 18, 968–980. [Google Scholar] [CrossRef]
- Rotaru, A.-E.; Shrestha, P.M.; Liu, F.; Markovaite, B.; Chen, S.; Nevin, K.P.; Lovley, D.R. Direct Interspecies Electron Transfer between Geobacter metallireducens and Methanosarcina barkeri. Appl. Environ. Microbiol. 2014, 80, 4599–4605. [Google Scholar] [CrossRef] [Green Version]
- Achinas, S.; Achinas, V.; Euverink, G.J.W. A Technological Overview of Biogas Production from Biowaste. Engineering 2017, 3, 299–307. [Google Scholar] [CrossRef]
- Johnravindar, D.; Liang, B.; Fu, R.; Luo, G.; Meruvu, H.; Yang, S.; Yuan, B.; Fei, Q. Supplementing granular activated carbon for enhanced methane production in anaerobic co-digestion of post-consumer substrates. Biomass Bioenergy 2020, 136, 105543. [Google Scholar] [CrossRef]
- Li, Y.; Liu, M.; Che, X.; Li, C.; Liang, D.; Zhou, H.; Liu, L.; Zhao, Z.; Zhang, Y. Biochar stimulates growth of novel species capable of direct interspecies electron transfer in anaerobic digestion via ethanol-type fermentation. Environ. Res. 2020, 189, 109983. [Google Scholar] [CrossRef] [PubMed]
- Aulenta, F.; Rossetti, S.; Amalfitano, S.; Majone, M.; Tandoi, V. Conductive Magnetite Nanoparticles Accelerate the Microbial Reductive Dechlorination of Trichloroethene by Promoting Interspecies Electron Transfer Processes. Chemsuschem 2013, 6, 433–436. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Qian, G.; Liu, J.; Xu, Z.P. Anaerobic methanogenesis of fresh leachate from municipal solid waste: A brief review on current progress. Renew. Sustain. Energy Rev. 2015, 49, 21–28. [Google Scholar] [CrossRef]
- Chen, S.; Tao, Z.; Yao, F.; Wu, B.; He, L.; Hou, K.; Pi, Z.; Fu, J.; Yin, H.; Huang, Q. Enhanced anaerobic co-digestion of waste activated sludge and food waste by sulfidated microscale zerovalent iron: Insights in direct interspecies electron transfer mechanism. Bioresour. Technol. 2020, 316, 123901. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Wen, H.; Cheng, J. The effect of adding zero-valent iron on methane production from kitchen wastes by anaerobic digestion. Environ. Pollut. Control 2016, 38, 54. [Google Scholar]
- Gao, X.; Zhou, L.; Qin, J.; Xu, Q. Effect of iron dioxide addition modes on methane generation in food waste anaerobic digestion. Environ. Eng. 2017, 35, 101–105. [Google Scholar]
- Song, Z.; Tang, H.; Xu, D.; Xu, J.; Cai, C. Effect of trace Co on the anaerobic digestion of food wastes. J. Saf. Environ. 2014, 14, 164–167. [Google Scholar]
- Vintiloiu, A.; Boxriker, M.; Lemmer, A.; Oechsner, H.; Jungbluth, T.; Mathies, E.; Ramhold, D. Effect of ethylenediaminetetraacetic acid (EDTA) on the bioavailability of trace elements during anaerobic digestion. Chem. Eng. J. 2013, 223, 436–441. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Q.; Cai, W. Effect of Fe3+, Cu2+ and Zn2+ on the hydrolysis and acidification of two-phase anaerobic digesting kitchen wastes at different pH values. Chin. J. Environ. Eng. 2007, 1, 116–119. [Google Scholar]
- Ryue, J.; Lin, L.; Liu, Y.; Lu, W.; McCartney, D.; Dhar, B.R. Comparative effects of GAC addition on methane productivity and microbial community in mesophilic and thermophilic anaerobic digestion of food waste. Biochem. Eng. J. 2019, 146, 79–87. [Google Scholar] [CrossRef]
- Capson-Tojo, G.; Moscoviz, R.; Ruiz, D.; Santa-Catalina, G.; Trably, E.; Rouez, M.; Crest, M.; Steyer, J.-P.; Bernet, N. Addition of granular activated carbon and trace elements to favor volatile fatty acid consumption during anaerobic digestion of food waste. Bioresour. Technol. 2018, 260, 157–168. [Google Scholar] [CrossRef]
- Ko, J.H.; Wang, N.; Yuan, T.; Lu, F.; He, P.; Xu, Q. Effect of nickel-containing activated carbon on food waste anaerobic digestion. Bioresour. Technol. 2018, 266, 516–523. [Google Scholar] [CrossRef]
- Sunyoto, N.M.S.; Zhu, M.M.; Zhang, Z.Z.; Zhang, D.K. Effect of biochar addition on hydrogen and methane production in two-phase anaerobic digestion of aqueous carbohydrates food waste. Bioresour. Technol. 2016, 219, 29–36. [Google Scholar] [CrossRef]
- Ma, S.; Wu, D.; Wu, T.; Zhou, L.; Hu, Z.; Liu, S.; Liu, D.; Rune, B. Response surface optimization of coconut shell biochar’s promoting anaerobic digestion of kitchen wastes. Environ. Eng. 2019, 37, 142–146. [Google Scholar]
- Deng, G.Y.; Zhang, T.Y.; Wang, W.; Lv, Y.L.; Deng, H.C.; Lu, W.X.; Cheng, X.G. Enhancement from Anaerobic Digestion of Food Waste by Conductive Materials: Performance and Mechanism. ACS Omega 2022, 7, 40782–40788. [Google Scholar] [CrossRef]
- Li, Q.; Xu, M.J.; Wang, G.J.; Chen, R.; Qiao, W.; Wang, X.C. Biochar assisted thermophilic co-digestion of food waste and waste activated sludge under high feedstock to seed sludge ratio in batch experiment. Bioresour. Technol. 2018, 249, 1009–1016. [Google Scholar] [CrossRef]
- Chen, S.-J.; Yao, F.-B.; Pi, Z.-J.; Hou, K.-J.; He, L.; Li, X.-M.; Wang, D.-B.; Yang, Q. Enhancement Effects and Mechanisms of Microscale Zero Valent Iron on the Performance of Anaerobic Co-digestion of Waste Activated Sludge and Food Waste. Search Life Sci. Lit. 2021, 42, 891–899. [Google Scholar] [CrossRef]
- Wo, D.; Bi, G.; Li, L.; Kang, X.; Kong, X.; Jiang, E.; Xie, J. Effects of Iron Oxides on the Anaerobic Codigestion Performances of the Pennisetum Hybrid and Kitchen Waste. J. Environ. Eng. 2022, 148. [Google Scholar] [CrossRef]
- Chen, Y.; Li, L.; Liu, H.; Yang, D.; Liu, W.; Yang, D.; Dai, X.; Chen, Y. Regulating effects of Fe/C materials on thermophilic anaerobic digestion of kitchen waste: Digestive performances and methanogenic metabolism pathways. Fuel 2023, 332, 126140. [Google Scholar] [CrossRef]
- Ma, J.Y.; Wei, H.W.; Su, Y.L.; Gu, W.C.; Wang, B.H.; Xie, B. Powdered activated carbon facilitates methane productivity of anaerobic co-digestion via acidification alleviating: Microbial and metabolic insights. Bioresour. Technol. 2020, 313, 123706. [Google Scholar] [CrossRef]


| Exogenous Material | Concentration (g/L) | Substrate | Temperature (°C) | Increase in CH4 Production Rate (%) | Start-Up Duration (d) | Reduction in CH4 Lag Phase (%) | Reference |
|---|---|---|---|---|---|---|---|
| Granular activated carbon | 25.0 | KW | 37 | 26.10 | 12 | 29.40 | [130] |
| Granular activated carbon | 10.0 | KW | 37 | 10.10 | 15 | 80.00 | [131] |
| Nickel-containing activated carbon | 10.0 | KW | 35 | 50.00 | 20 | 67.00 | [132] |
| Sawdust biochar | 8.3 | KW | 35 | 41.60 | 16 | 45.00 | [133] |
| Coconut shell biochar | 22.1 | KW | 35 | 18.50 | 21 | 66.60 | [134] |
| Fe–metal organic frameworks (Fe–MOF) | 0.2 | KW | 36 | 44.27 | 18 | 49.20 | [135] |
| Sawdust biochar | 10.0 | KW and WAS | 55 | 21.20 | 13 | 95.70 | [136] |
| Microscale zero-valent iron | 10.0 | KW and WAS | 35 ± 1 | 20.05 | 15 | 46.44 | [137] |
| Fe2O3 | 0.2 | Pennisetum, KW | 37 ± 1 | 23.50 | 14 | - | [138] |
| Fe3O4 | 0.2 | Pennisetum, KW | 37 ± 1 | 37.90 | 10 | - | [138] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, Y.-J.; Li, X.; Lu, C.-S.; Kobayashi, T.; Zhen, G.-Y.; Hu, Y. A Review on Start-Up Phase Optimization of Kitchen Waste Anaerobic Digestion. Fermentation 2023, 9, 603. https://doi.org/10.3390/fermentation9070603
Yan Y-J, Li X, Lu C-S, Kobayashi T, Zhen G-Y, Hu Y. A Review on Start-Up Phase Optimization of Kitchen Waste Anaerobic Digestion. Fermentation. 2023; 9(7):603. https://doi.org/10.3390/fermentation9070603
Chicago/Turabian StyleYan, Yi-Juan, Xiang Li, Chen-Shun Lu, Takuro Kobayashi, Guang-Yin Zhen, and Yong Hu. 2023. "A Review on Start-Up Phase Optimization of Kitchen Waste Anaerobic Digestion" Fermentation 9, no. 7: 603. https://doi.org/10.3390/fermentation9070603
APA StyleYan, Y.-J., Li, X., Lu, C.-S., Kobayashi, T., Zhen, G.-Y., & Hu, Y. (2023). A Review on Start-Up Phase Optimization of Kitchen Waste Anaerobic Digestion. Fermentation, 9(7), 603. https://doi.org/10.3390/fermentation9070603






