Screening of Ultraviolet-Induced Thermotolerant Yeast Mutants and Their Performance
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Strain
2.1.2. Media
2.2. Ultraviolet Mutagenesis
2.3. Survival Screening and Separation of the Thermotolerant Yeast
2.4. Fermentation and SSF Process
2.5. Analytical Methods
2.6. Calculation
3. Results and Discussion
3.1. Ultraviolet-Induced Mutagenesis and Screening
3.1.1. Determination of Mutagenesis Time
3.1.2. Survival Screening of Thermotolerant Strains
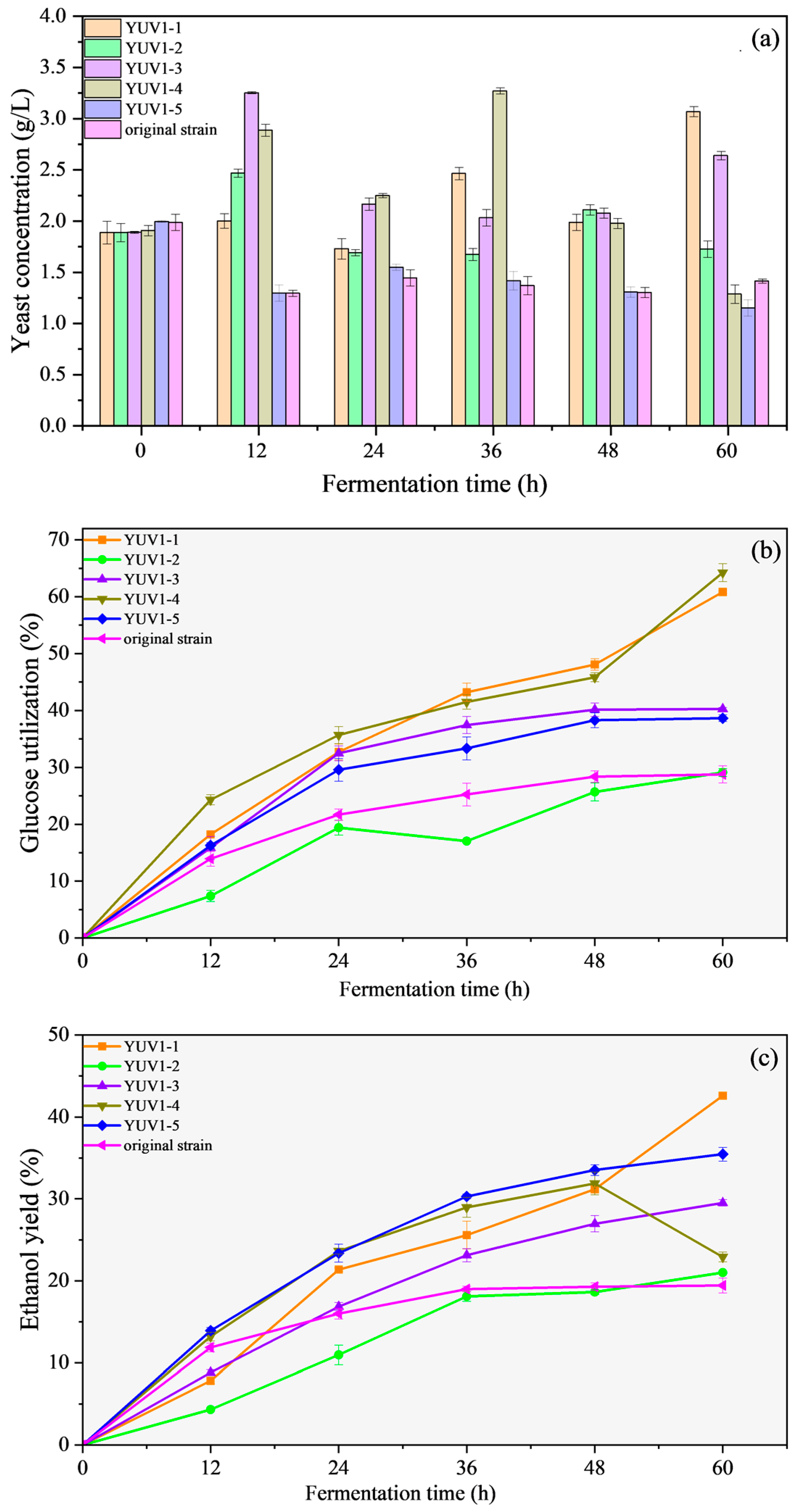
3.1.3. Fermentation Screening of Thermotolerant Strains
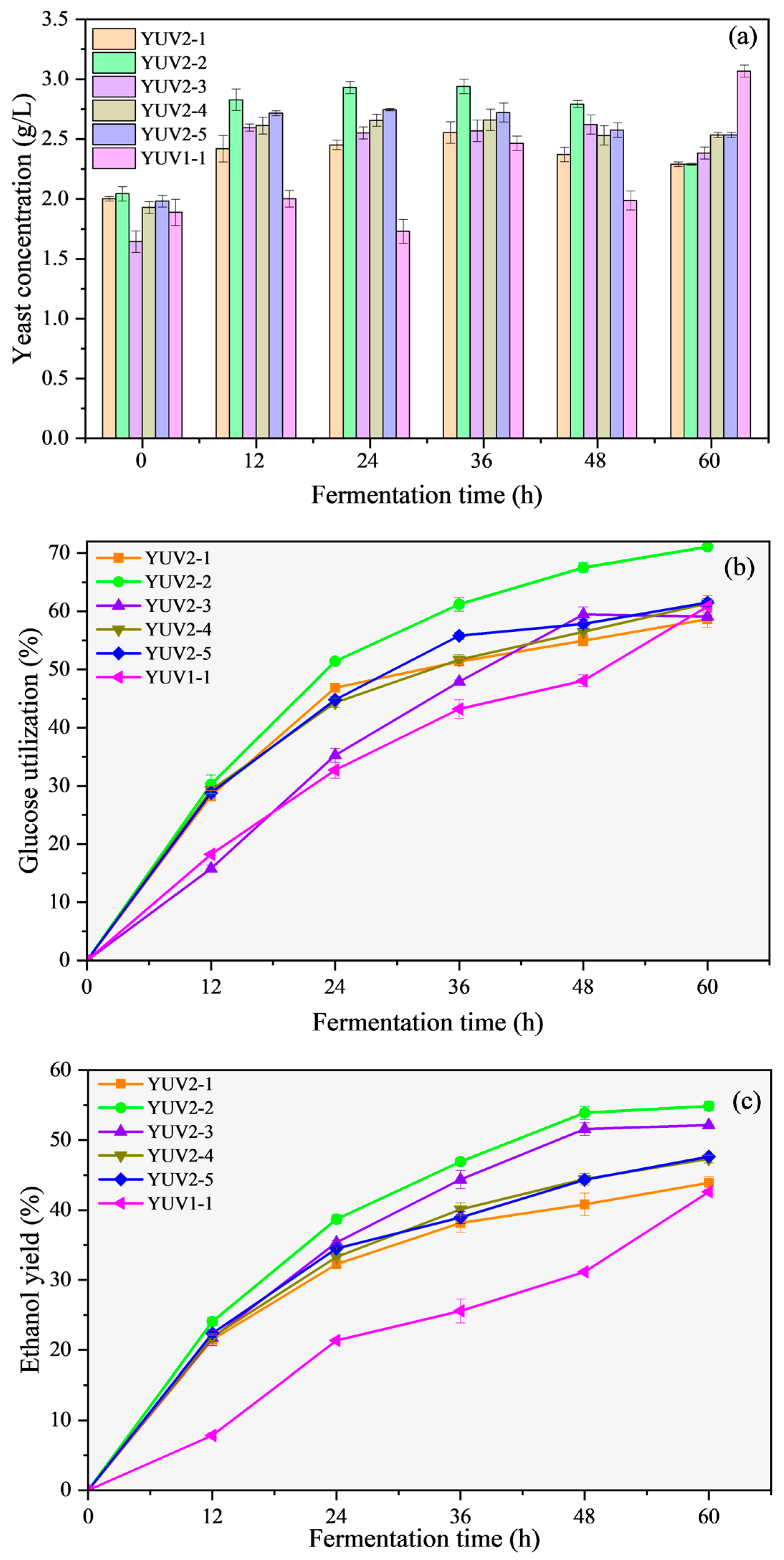
3.2. Fermentation Performance of Mutated Strains
3.3. Performance of Mutated Strains in Simultaneous Saccharification and Fermentation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mathimani, T.; Mallick, N. A review on the hydrothermal processing of microalgal biomass to bio-oil-knowledge gaps and recent advances. J. Clean. Prod. 2019, 217, 69–84. [Google Scholar] [CrossRef]
- Zabed, H.; Sultana, S.; Sahu, J.N.; Qi, X. An overview on the application of ligninolytic microorganisms and enzymes for pretreatment of lignocellulosic biomass. In Recent Advancements in Biofuels and Bioenergy Utilization; Sarangi, P.K., Nanda, S., Mohanty, P., Eds.; Springer: Singapore, 2018; pp. 53–72. [Google Scholar]
- Toor, M.; Kumar, S.S.; Malyan, S.K.; Bishnoi, N.R.; Mathimani, T.; Rajendran, K.; Pugazhendhi, A. An overview on bioethanol production from lignocellulosic feedstocks. Chemosphere 2020, 242, 125080. [Google Scholar] [CrossRef]
- Liu, C.-G.; Xiao, Y.; Xia, X.-X.; Zhao, X.-Q.; Peng, L.; Srinophakun, P.; Bai, F.-W. Cellulosic ethanol production: Progress, challenges and strategies for solutions. Biotechnol. Adv. 2019, 37, 491–504. [Google Scholar] [CrossRef]
- Limayem, A.; Ricke, S.C. Lignocellulosic biomass for bioethanol production: Current perspectives, potential issues and future prospects. Prog. Energ. Combust. Sci. 2012, 38, 449–467. [Google Scholar] [CrossRef]
- Carrillo-Nieves, D.; Rostro Alanis, M.J.; de la Cruz Quiroz, R.; Ruiz, H.A.; Iqbal, H.M.N.; Parra-Saldivar, R. Current status and future trends of bioethanol production from agro-industrial wastes in Mexico. Renew. Sust. Energ. Rev. 2019, 102, 63–74. [Google Scholar] [CrossRef]
- Zhao, J.; Xia, L. Simultaneous saccharification and fermentation of alkaline-pretreated corn stover to ethanol using a recombinant yeast strain. Fuel Process. Technol. 2009, 90, 1193–1197. [Google Scholar] [CrossRef]
- Tan, H.; Yu, Y.; Zhu, Y.; Liu, T.; Miao, R.; Hu, R.; Peng, W.; Chen, J. Impacts of size reduction and alkaline-soaking pretreatments on microbial community and organic matter decomposition during wheat straw composting. Bioresour. Technol. 2022, 360, 127549. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Lin, Y.; Zhang, Q.; Wang, X.; Wu, D.; Kong, H. Optimisation of simultaneous saccharification and fermentation of wheat straw for ethanol production. Fuel 2013, 112, 331–337. [Google Scholar] [CrossRef]
- Rodionova, M.V.; Bozieva, A.M.; Zharmukhamedov, S.K.; Leong, Y.K.; Lan, J.C.-W.; Veziroglu, A.; Veziroglu, T.N.; Tomo, T.; Chang, J.-S.; Allakhverdiev, S.I. A comprehensive review on lignocellulosic biomass biorefinery for sustainable biofuel production. Int. J. Hydrog. Energ. 2022, 47, 1481–1498. [Google Scholar] [CrossRef]
- Zhao, F.; Fang, S.; Gao, Y.; Bi, J. Removal of aqueous pharmaceuticals by magnetically functionalized Zr-MOFs: Adsorption kinetics, isotherms, and regeneration. J. Colloid. Interf. Sci. 2022, 615, 876–886. [Google Scholar] [CrossRef]
- Hoffman, S.M.; Alvarez, M.; Alfassi, G.; Rein, D.M.; Garcia-Echauri, S.; Cohen, Y.; Avalos, J.L. Cellulosic biofuel production using emulsified simultaneous saccharification and fermentation (eSSF) with conventional and thermotolerant yeasts. Biotechnol. Biofuels 2021, 14, 1–17. [Google Scholar] [CrossRef]
- Saratale, R.G.; Cho, S.K.; Bharagava, R.N.; Patel, A.K.; Varjani, S.; Mulla, S.I.; Kim, D.S.; Bhatia, S.K.; Ferreira, L.F.R.; Shin, H.S.; et al. A critical review on biomass-based sustainable biorefineries using nanobiocatalysts: Opportunities, challenges, and future perspectives. Bioresour. Technol. 2022, 363, 127926. [Google Scholar] [CrossRef]
- Patel, A.; Patel, H.; Divecha, J.; Shah, A.R. Enhanced production of ethanol from enzymatic hydrolysate of microwave-treated wheat straw by statistical optimization and mass balance analysis of bioconversion process. Biofuels 2021, 12, 1251–1258. [Google Scholar] [CrossRef]
- Cheng, G.; Zhao, Y.; Pan, S.; Wang, X.; Dong, C. A comparative life cycle analysis of wheat straw utilization modes in China. Energy 2020, 194, 116914. [Google Scholar] [CrossRef]
- Xu, X.; Wang, X.; He, Y. The crucial problems and solutions on producing fuel ethanol by straw. Food Ferment. Ind. 2010, 36, 108–113. [Google Scholar]
- Arnoult, S.; Obeuf, A.; Bethencourt, L.; Mansard, M.-C.; Brancourt-Hulmel, M. Miscanthus clones for cellulosic bioethanol production: Relationships between biomass production, biomass production components, and biomass chemical composition. Ind. Crop Prod. 2015, 63, 316–328. [Google Scholar] [CrossRef]
- Olofsson, K.; Bertilsson, M.; Liden, G. A short review on SSF-an interesting process option for ethanol production from lignocellulosic feedstocks. Biotechnol. Biofuels 2008, 1, 7. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Juneja, A.; Singh, V. Fermentation technology to improve productivity in dry grind corn process for bioethanol production. Fuel Process. Technol. 2018, 173, 66–74. [Google Scholar] [CrossRef]
- Park, I.; Kim, I.; Kang, K.; Sohn, H.; Rhee, I.; Jin, I.; Jang, H. Cellulose ethanol production from waste newsprint by simultaneous saccharification and fermentation using Saccharomyces cerevisiae KNU5377. Process. Biochem. 2010, 45, 487–492. [Google Scholar] [CrossRef]
- Menon, V.; Divate, R.; Rao, M. Bioethanol production from renewable polymer lichenan using lichenase from an alkalothermophilic Thermomonospora sp. and thermotolerant yeast. Fuel Process. Technol. 2011, 92, 401–406. [Google Scholar] [CrossRef]
- Liu, K.; Jiang, B.; Wang, Q.; Yang, L.; Lu, B.; Li, X.; Yuan, H. Study on the conditions of pretreating vinegar residue with sodium hydroxide for simultaneous saccharification and fermentation to produce alcohol and xylose. Food Sci. Technol. Res. 2020, 26, 381–388. [Google Scholar] [CrossRef]
- Carrillo-Nieves, D.; Ruiz, H.A.; Aguilar, C.N.; Ilyina, A.; Parra-Saldivar, R.; Antonio Torres, J.; Martinez Hernandez, J.L. Process alternatives for bioethanol production from mango stem bark residues. Bioresour. Technol. 2017, 239, 430–436. [Google Scholar] [CrossRef]
- Sassner, P.; Galbe, M.; Zacchi, G. Techno-economic evaluation of bioethanol production from three different lignocellulosic materials. Biomass Bioenerg. 2008, 32, 422–430. [Google Scholar] [CrossRef]
- Rudolf, A.; Alkasrawi, M.; Zacchi, G.; Liden, G. A comparison between batch and fed-batch simultaneous saccharification and fermentation of steam pretreated spruce. Enzym. Microb. Technol. 2005, 37, 195–204. [Google Scholar] [CrossRef]
- Claassen, P.A.M.; van Lier, J.B.; Contreras, A.M.L.; van Niel, E.W.J.; Sijtsma, L.; Stams, A.J.M.; de Vries, S.S.; Weusthuis, R.A. Utilisation of biomass for the supply of energy carriers. Appl. Microbiol. Biotechnol. 1999, 52, 741–755. [Google Scholar] [CrossRef]
- Eklund, R.; Zacchi, G. Simultaneous saccharification and fermentation of steam-pretreated willow. Enzym. Microb. Technol. 1995, 17, 255–259. [Google Scholar] [CrossRef]
- Hasunuma, T.; Kondo, A. Consolidated bioprocessing and simultaneous saccharification and fermentation of lignocellulose to ethanol with thermotolerant yeast strains. Process. Biochem. 2012, 47, 1287–1294. [Google Scholar] [CrossRef]
- Sridhar, M.; Sree, N.K.; Rao, L.V. Effect of UV radiation on thermotolerance, ethanol tolerance and osmotolerance of Saccharomyces cerevisiae VS1 and VS3 strains. Bioresour. Technol. 2002, 83, 199–202. [Google Scholar] [CrossRef]
- De Melo, A.H.F.; Lopes, A.M.M.; Dezotti, N.; Santos, I.L.; Teixeira, G.S.; Goldbeck, R. Evolutionary engineering of two robust brazilian industrial yeast strains for thermotolerance and second-generation biofuels. Ind. Biotechnol. 2020, 16, 91–98. [Google Scholar] [CrossRef]
- Li, Y.J.; Wei, H.B.; Wang, T.; Xu, Q.Y.; Zhang, C.L.; Fan, X.G.; Ma, Q.; Chen, N.; Xie, X.X. Current status on metabolic engineering for the production of L-aspartate family amino acids and derivatives. Bioresour. Technol. 2017, 245, 1588–1602. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, C.; Zhou, Q.Q.; Zhang, X.F.; Wang, L.Y.; Chang, H.B.; Li, H.P.; Oda, Y.; Xing, X.H. Quantitative evaluation of DNA damage and mutation rate by atmospheric and room-temperature plasma (ARTP) and conventional mutagenesis. Appl. Microbiol. Biotechnol. 2015, 99, 5639–5646. [Google Scholar] [CrossRef]
- Zheng, D.Q.; Zhang, K.; Gao, K.H.; Liu, Z.W.; Zhang, X.; Li, O.; Sun, J.G.; Zhang, X.Y.; Du, F.G.; Sun, P.Y.; et al. Construction of novel Saccharomyces cerevisiae strains for bioethanol active dry yeast (ADY) production. PLoS ONE 2013, 8, e85022. [Google Scholar] [CrossRef]
- Zhang, B.; Geberekidan, M.; Yan, Z.; Yi, X.; Bao, J. Very high thermotolerance of an adaptive evolved Saccharomyces cerevisiae in cellulosic ethanol fermentation. Fermentation 2023, 9, 393. [Google Scholar] [CrossRef]
- Dodd, M.C. Potential impacts of disinfection processes on elimination and deactivation of antibiotic resistance genes during water and wastewater treatment. J. Environ. Monit. 2012, 14, 1754–1771. [Google Scholar] [CrossRef]
- Boonchuay, P.; Techapun, C.; Leksawasdi, N.; Seesuriyachan, P.; Hanmoungjai, P.; Watanabe, M.; Srisupa, S.; Chaiyaso, T. Bioethanol production from cellulose-rich corncob residue by the thermotolerant Saccharomyces cerevisiae TC-5. J. Fungi 2021, 7, 547. [Google Scholar] [CrossRef] [PubMed]
- Pessani, N.K.; Atiyeh, H.K.; Wilkins, M.R.; Bellmer, D.D.; Banat, I.M. Simultaneous saccharification and fermentation of Kanlow switchgrass by thermotolerant Kluyveromyces marxianus IMB3: The effect of enzyme loading, temperature and higher solid loadings. Bioresour. Technol. 2011, 102, 10618–10624. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wu, S.; Fan, C.; Zheng, X.; Zhang, W.; Wu, D.; Wang, X.; Kong, H. Optimisation of enzymatic saccharification of wheat straw pre-treated with sodium hydroxide. Sci. Rep. 2021, 11, 23234. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, D.; Lin, Y.; Wang, X.; Kong, H.; Tanaka, S. Substrate and product inhibition on yeast performance in ethanol fermentation. Energy Fuels 2015, 29, 1019–1027. [Google Scholar] [CrossRef]
- Yi, Y.I. Mutation screening of Saccharomyces cerevisiae strains with alcohol tolerance. J. Anhui Agric. Sci. 2009, 37, 3. [Google Scholar]
- Yao, Z.; Zhou, P.; Su, B.; Su, S.; Ye, L.; Yu, H. Enhanced Isoprene production by reconstruction of metabolic balance between strengthened precursor supply and improved isoprene synthase in Saccharomyces cerevisiae. ACS Synth. Biol. 2018, 7, 2308–2316. [Google Scholar] [CrossRef]
- Lin, Y.; Zhang, W.; Li, C.; Sakakibara, K.; Tanaka, S.; Kong, H. Factors affecting ethanol fermentation using Saccharomyces cerevisiae BY4742. Biomass Bioenerg. 2012, 47, 395–401. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, B.; Liu, B.; Yi, Y.; Shan, Y.; Zhou, Y.; Wang, X.; Lu, X. Full components conversion of lignocellulose via a closed-circuit biorefinery process on a pilot scale. Environ. Res. 2022, 214 Pt 2, 113946. [Google Scholar] [CrossRef]
- Nuanpeng, S.; Thanonkeo, S.; Yamada, M.; Thanonkeo, P. Ethanol production from sweet sorghum juice at high temperatures using a newly isolated thermotolerant Yeast Saccharomyces cerevisiae DBKKU Y-53. Energies 2016, 9, 253. [Google Scholar] [CrossRef]
- Liu, Q.G.; Cheng, H.; Wu, J.L.; Chen, X.C.; Ying, H.J.; Zhou, P.; Chen, Y. Long-term production of fuel ethanol by immobilized yeast in repeated-batch simultaneous saccharification and fermentation of Cassava. Energy Fuels 2015, 29, 185–190. [Google Scholar] [CrossRef]
- Liu, K.; Lin, X.H.; Yue, J.; Li, X.Z.; Fang, X.; Zhu, M.T.; Lin, J.Q.; Qu, Y.B.; Xiao, L. High concentration ethanol production from corncob residues by fed-batch strategy. Bioresour. Technol. 2010, 101, 4952–4958. [Google Scholar] [CrossRef] [PubMed]
- Mei, X.Y.; Liu, R.H.; Shen, F.; Wu, H.J. Optimization of fermentation conditions for the production of ethanol from stalk juice of sweet sorghum by immobilized yeast using response surface methodology. Energy Fuels 2009, 23, 487–491. [Google Scholar] [CrossRef]
- Sassner, P.; Galbe, M.; Zacchi, G. Bioethanol production based on simultaneous saccharification and fermentation of steam-pretreated Salix at high dry-matter content. Enzym. Microb. Technol. 2006, 39, 756–762. [Google Scholar] [CrossRef]



| Condition | Strain Name | CY, g/L | CE, g/L | YCE, g/g | VPE, g/L/h |
|---|---|---|---|---|---|
| Fermentation at 40 °C for 60 h | S. cerevisiae BY4742 | 2.52 | 16.82 | 0.34 | 0.28 |
| YUV1-1 | 3.07 | 22.85 | 0.39 | 0.38 | |
| YUV2-2 | 3.03 | 23.44 | 0.39 | 0.39 | |
| SSF at 40 °C for 120 h | S. cerevisiae BY4742 | - | 33.01 | 0.21 | 0.28 |
| YUV1-1 | - | 41.31 | 0.26 | 0.34 | |
| YUV2-2 | - | 41.69 | 0.26 | 0.35 | |
| SSF at 38 °C for 120 h | S. cerevisiae BY4742 | - | 38.33 | 0.24 | 0.32 |
| YUV1-1 | - | 39.68 | 0.25 | 0.33 | |
| YUV2-2 | - | 40.41 | 0.25 | 0.34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Lin, Y.; Kong, H.; Wang, Z. Screening of Ultraviolet-Induced Thermotolerant Yeast Mutants and Their Performance. Fermentation 2023, 9, 608. https://doi.org/10.3390/fermentation9070608
Li X, Lin Y, Kong H, Wang Z. Screening of Ultraviolet-Induced Thermotolerant Yeast Mutants and Their Performance. Fermentation. 2023; 9(7):608. https://doi.org/10.3390/fermentation9070608
Chicago/Turabian StyleLi, Xiaodi, Yan Lin, Hainan Kong, and Zhiquan Wang. 2023. "Screening of Ultraviolet-Induced Thermotolerant Yeast Mutants and Their Performance" Fermentation 9, no. 7: 608. https://doi.org/10.3390/fermentation9070608
APA StyleLi, X., Lin, Y., Kong, H., & Wang, Z. (2023). Screening of Ultraviolet-Induced Thermotolerant Yeast Mutants and Their Performance. Fermentation, 9(7), 608. https://doi.org/10.3390/fermentation9070608





