Biotransformation of Chinese Jujube with Cordyceps militaris to Enhance the Antioxidant Activity In Vitro and the Protective Effect against Ethanol-Induced Oxidative Stress in Zebrafish
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganism and Materials
2.2. Solid-State Fermentation and Biomass Measurements
2.3. Content of Total Phenolic and Flavonoids
2.4. Extraction of Free, Free/Conjugated, and Bound Phenolics
2.5. Measurements of Antioxidant Activities In Vitro
2.6. Measurements of the Antioxidant Responses In Vivo
2.6.1. Zebrafish Husbandry and Embryo Collection
2.6.2. Exposure and Antioxidant Responses of Adult Zebrafish
2.6.3. Exposure and Antioxidant Responses of Zebrafish Larvae
2.7. Quantitative Real-Time PCR (qRT-PCR) Analysis
2.8. Ethics Statement
3. Results
3.1. Biomass and Content of Total Phenolic and Flavonoids
3.2. Measurement of Free, Free/Conjugated, and Bound Phenolics
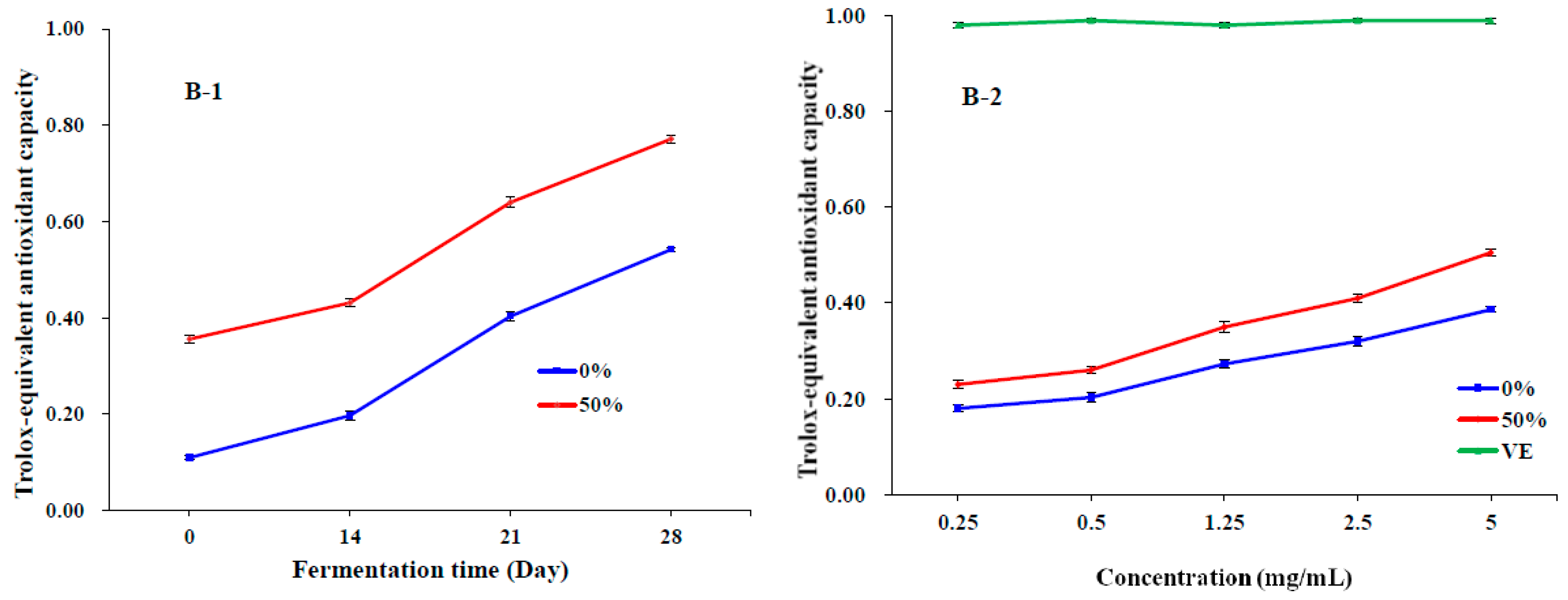
3.3. Antioxidant Activities In Vitro
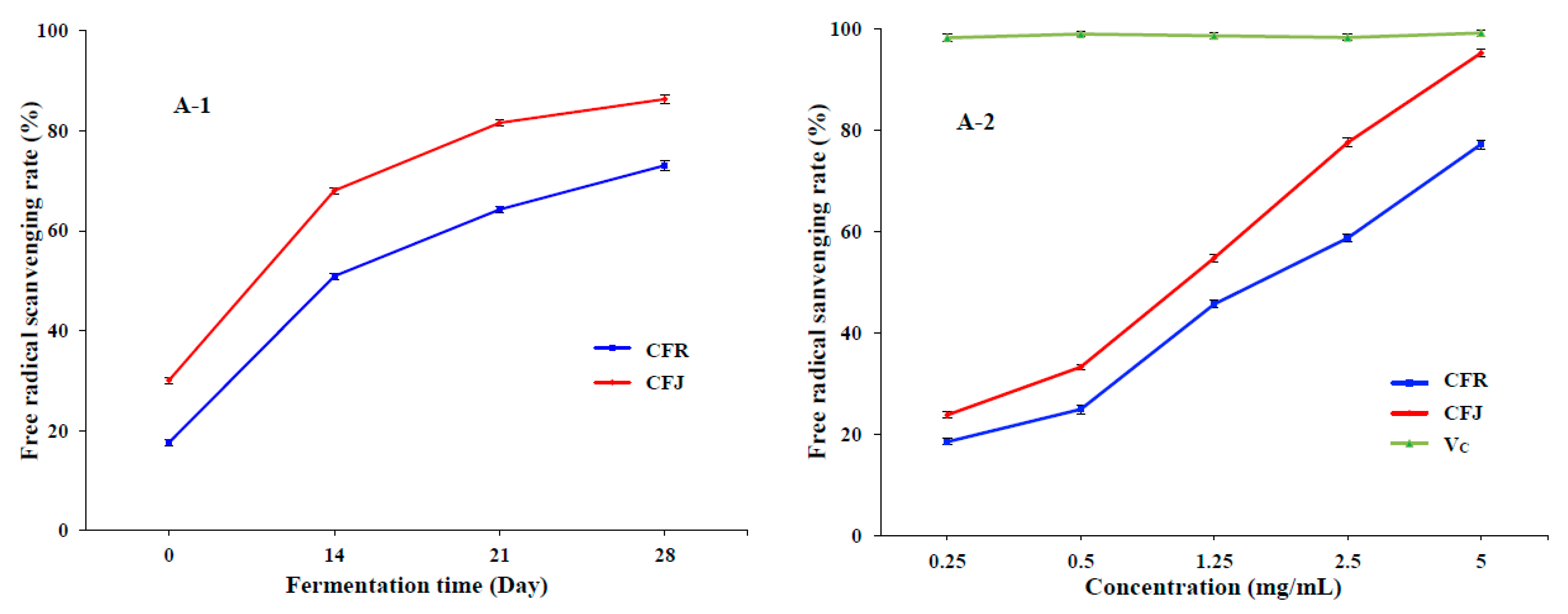
3.3.1. Scavenging Activity on DPPH and ABTS Radicals
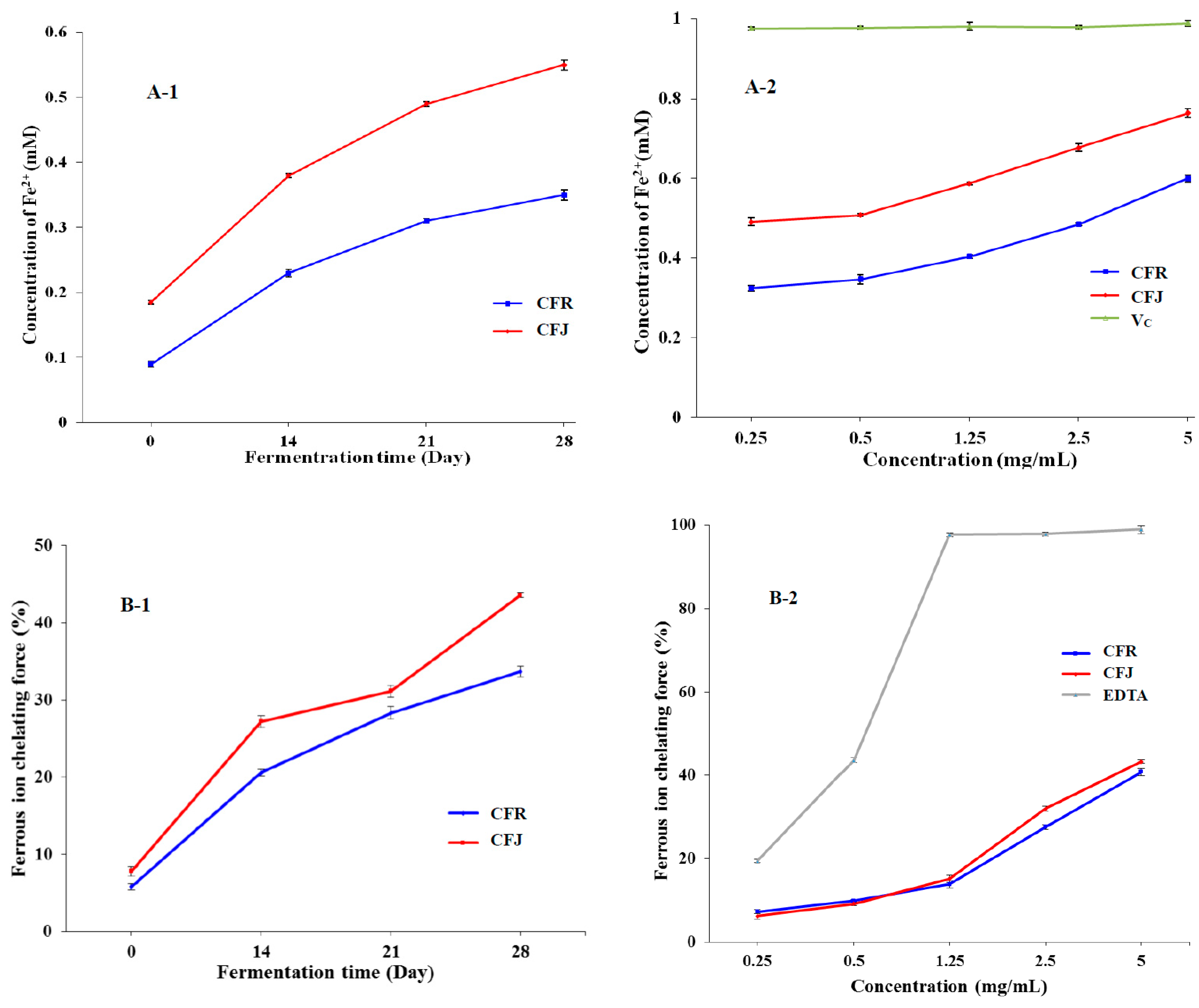
3.3.2. Ferric Reducing Antioxidant Power (FRAP) and Ferrous Ion-Chelating Ability
3.4. Antioxidant Activities In Vivo
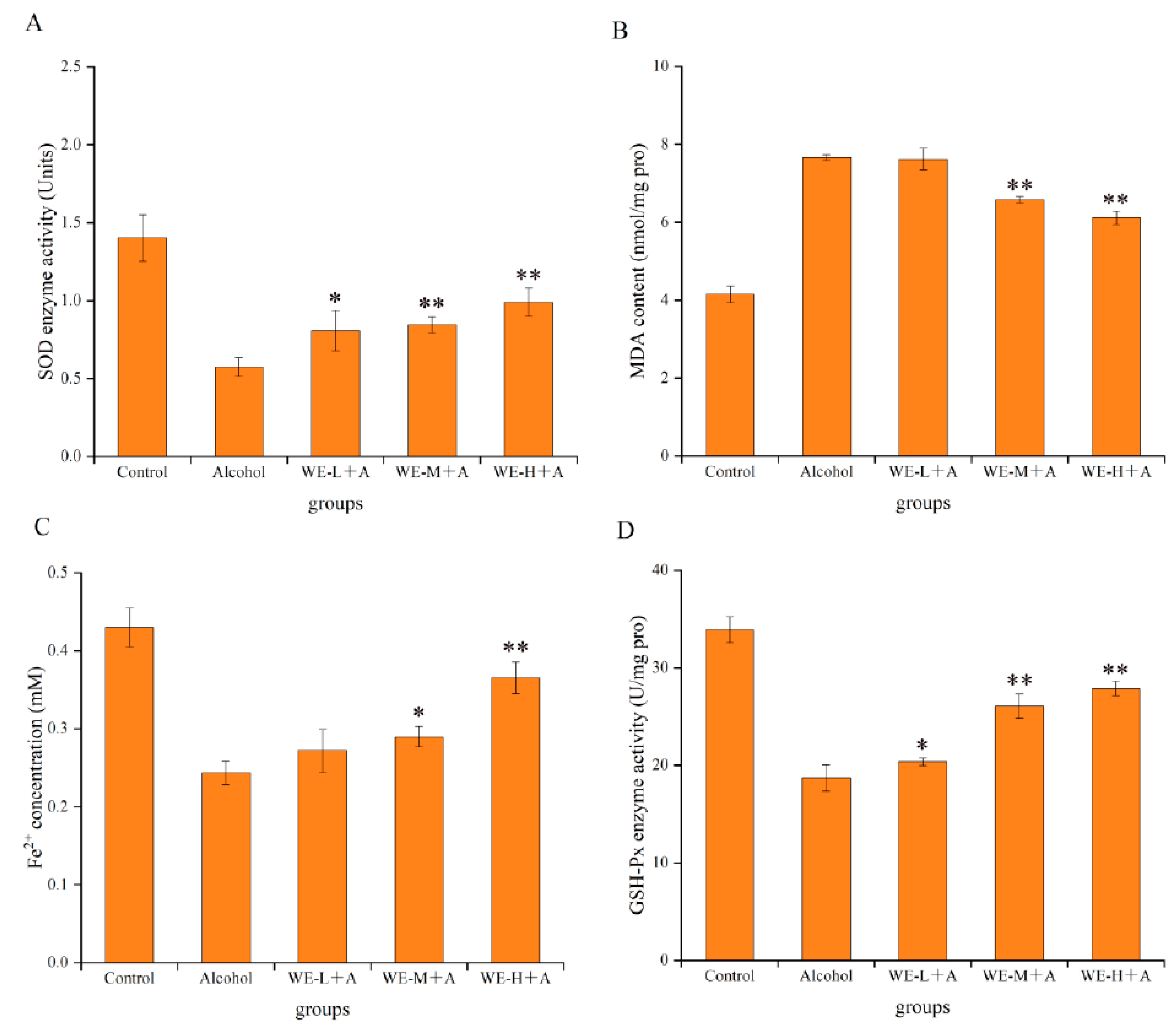
3.4.1. Protective Effects of SSF-Jujube in Ethanol-Induced Adult Zebrafish
3.4.2. Protective Effect of SSF-Jujube in Ethanol-Induced Larval Zebrafish
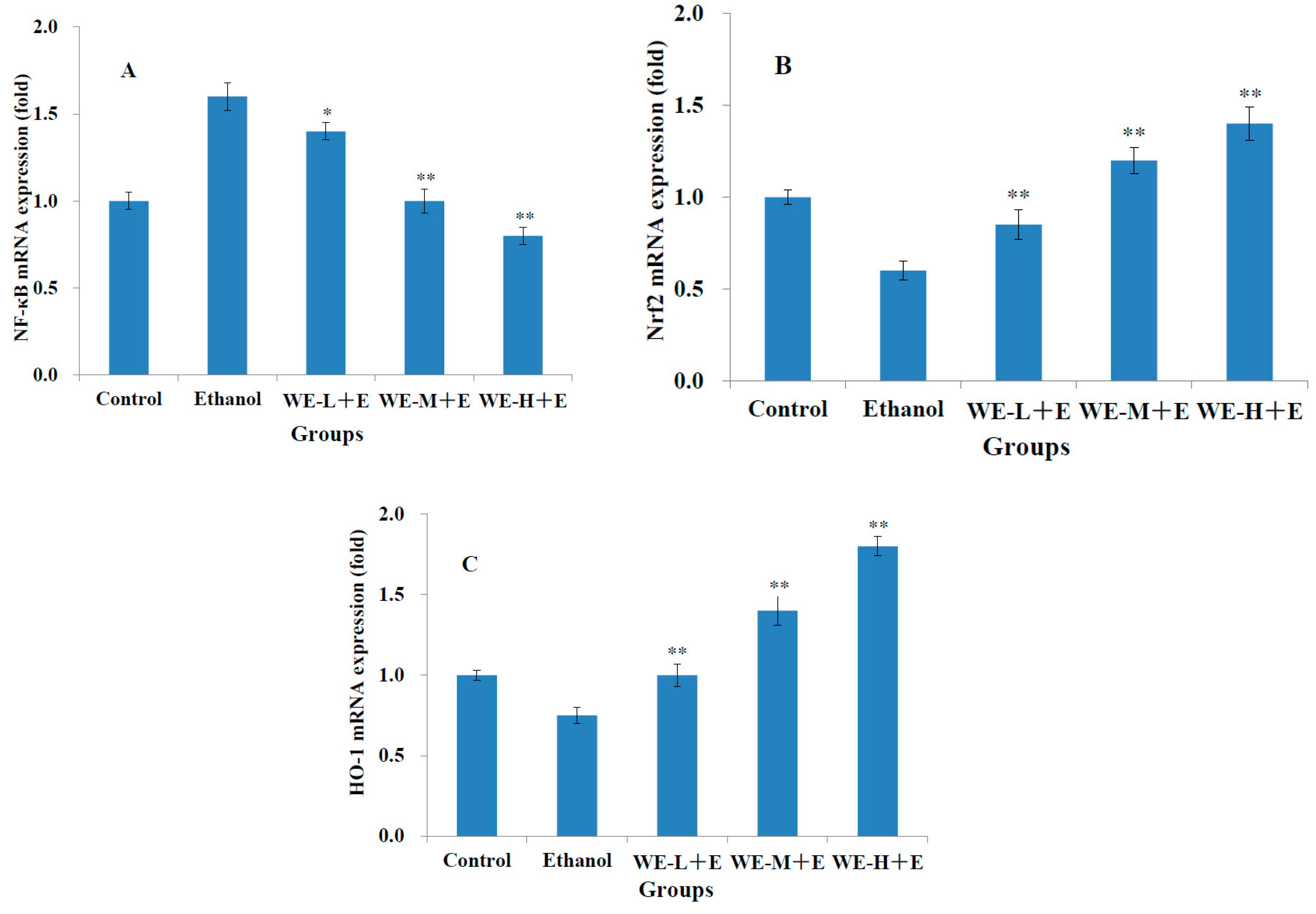
3.5. Gene Expression
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, T.L.; Jiang, T.; Liu, N.; Wu, C.Y.; Xu, H.D.; Lei, H.J. Biotransformation of phenolic profiles and improvement of antioxidant capacities in jujube juice by select lactic acid bacteria. Food Chem. 2021, 339, 127859–127869. [Google Scholar] [CrossRef] [PubMed]
- National Bureau of Statistics of China. National Data. 2019. Available online: https://data.stats.gov.cn (accessed on 26 May 2022).
- Reche, J.; Almansa, M.S.; Hernández, F.; Amorós, A.; Legua, P. Physicochemical and antioxidant capacity of jujube (Ziziphus jujuba Mill.) at different maturation stages. Agronomy 2021, 11, 132. [Google Scholar] [CrossRef]
- Arslan, M.; Zareef, M.; Tahir, H.E.; Ali, S.; Huang, X.W.; Rakha, A.; Zou, X.B. Comparative analyses of phenolic compounds and antioxidant properties of Chinese jujube as affected by geographical region and drying methods (Puff-drying and convective hot air-drying systems). J. Food Meas. Charact. 2021, 15, 933–943. [Google Scholar] [CrossRef]
- National Health Commission of the People’s Republic of China. 2014. Available online: https://www.nhc.gov.cn/sps/s7890201406/8268613682e44b1cb2098e0b9c9143d7 (accessed on 26 May 2022).
- Song, Q.Y.; Zhu, Z.Y. Using Cordyceps militaris extracellular polysaccharides to prevent Pb2+-induced liver and kidney toxicity by activating Nrf2 signals and modulating gut microbiota. Food Funct. 2020, 11, 9226–9239. [Google Scholar] [CrossRef] [PubMed]
- Ueda, Y.; Mori, K.; Satoh, S.; Dansako, H.; Ikeda, M.; Kato, N. Anti-HCV activity of the Chinese medicinal fungus Cordyceps militaris. Biochem. Biophys. Res. Commun. 2014, 447, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.Y.; Cho, H.D.; Cho, Y.S. Anti-oxidant and anti-hyperlipidemic effects of cordycepin-rich Cordyceps militaris in a Sprague-Dawley rat model of alcohol-induced hyperlipidemia and oxidative stress. Bioresour. Bioprocess. 2020, 7, 33. [Google Scholar] [CrossRef]
- Gong, X.B.; Li, T.J.; Wan, R.Z.; Sha, L. Cordycepin attenuates high-fat diet-induced non-alcoholic fatty liver disease via down-regulation of lipid metabolism and inflammatory responses. Int. Immunopharmacol. 2021, 91, 107173. [Google Scholar] [CrossRef]
- Dulf, F.V.; Vodnar, D.C.; Toşa, M.I.; Dulf, E.H. Simultaneous enrichment of grape pomace with γ-linolenic acid and carotenoids by solid-state fermentation with Zygomycetes fungi and antioxidant potential of the bioprocessed substrates. Food Chem. 2020, 310, 125927. [Google Scholar] [CrossRef]
- Lin, F.J.; Li, H.; Wu, D.T.; Zhuang, Q.G.; Li, H.B.; Geng, F.; Gan, R.Y. Recent development in zebrafish model for bioactivity and safety evaluation of natural products. Crit. Rev. Food Sci. 2022, 62, 8646–8674. [Google Scholar] [CrossRef]
- Xie, M.X.; Xie, Y.D.; Li, Y.; Zhou, W.; Zhang, Z.; Yang, Y.L.; Zhou, Z.G. Stabilized fermentation product of Cetobacterium somerae improves gut and liver health and antiviral immunity of zebrafish. Fish Shellfish Immun. 2022, 120, 56–66. [Google Scholar] [CrossRef]
- Bei, Q.; Liu, Y.; Wang, L.; Chen, G.; Wu, Z.Q. Improving free, conjugated, and bound phenolic fractions in fermented oats (Avena sativa L.) with Monascus anka and their antioxidant activity. J. Funct. Foods 2017, 32, 185–194. [Google Scholar] [CrossRef]
- Liu, R.H. Whole Grain Phytochemicals and Health. J. Cereal Sci. 2007, 46, 207–219. [Google Scholar] [CrossRef]
- Juan, M.Y.; Chou, C.C. Enhancement of antioxidant activity, total phenolic and flavonoid content of black soybeans by solid state fermentation with Bacillus subtilis BCRC 14715. Food Microbiol. 2010, 27, 586–591. [Google Scholar] [CrossRef]
- Shao, Y.F.; Xu, F.F.; Sun, X.; Bao, J.S.; Beta, T. Phenolic acids, anthocyanins, and antioxidant capacity in rice (Oryza sativa L.) grains at four stages of development after flowering. Food Chem. 2014, 143, 90–96. [Google Scholar] [CrossRef]
- Zhang, M.W.; Zhang, R.F.; Zhang, F.X.; Liu, R.H. Phenolic profiles and antioxidant activity of black rice bran of different commercially available varieties. J. Agric. Food Chem. 2010, 58, 7580–7587. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Zhang, Q.Q.; Miao, J.Q.; Rui, X.; Li, T.; Dong, M.S. Antioxidant activity and DNA damage protection of mung beans processed by solid state fermentation with Cordyceps militaris SN-18. Innov. Food Sci. Emerg. 2015, 31, 216–225. [Google Scholar] [CrossRef]
- Tran, S.; Nowicki, M.; Chatterjee, D.; Gerlai, R. Acute and chronic ethanol exposure differentially alters alcohol dehydrogenase and aldehyde dehydrogenase activity in the zebrafish liver. Prog. Neuro-Psychopharmacology 2015, 56, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Howarth, D.L.; Passeri, M.; Sadler, K.C. Drinks like a fish: Using zebrafish to understand alcoholic liver disease. Alcohol. Clin. Exp. Res. 2011, 35, 826–829. [Google Scholar] [CrossRef] [Green Version]
- Dey, T.B.; Chakraborty, S.; Jain, K.K.; Sharma, A.; Kuhad, R.C. Antioxidant phenolics and their microbial production by submerged and solid state fermentation process: A review. Trends Food Sci. Technol. 2016, 53, 60–74. [Google Scholar] [CrossRef]
- Zhang, Z.; Lv, G.; Pan, H.; Fan, L.; Soccol, C.R.; Pandey, A. Production of powerful antioxidant supplements via solid-state fermentation of wheat (Triticum aestivum Linn.) by Cordyceps militaris. Food Technol. Biotechnol. 2012, 50, 32–39. [Google Scholar] [CrossRef]
- Gao, L.; Zhou, Y.C.; Zhong, W.C.; Zhao, X.H.; Chen, C.; Chen, X.M.; Shen, J.G. Caveolin-1 is essential for protecting against binge drinking-induced liver damage through inhibiting reactive nitrogen species. Hepatology 2014, 60, 687–699. [Google Scholar] [CrossRef]
- Ren, W.K.; Yin, Y.L.; Liu, G.; Yu, X.L.; Li, Y.H.; Yang, G.; Wu, G.Y. Effect of dietary arginine supplementation on reproductive performance of mice with porcine circovirus type 2 infection. Amino Acids 2012, 42, 2089–2094. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.P.; Liu, H.L.; Wang, C.G.; Yang, P.; Sun, C.B.; Chen, S.M. Effect of oxidized fish oil on growth performance and oxidative stress of Litopenaeus vannamei. Aquacult. Nutr. 2015, 21, 121–127. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, X.; Du, Y.; Ji, H.; Li, S.; Li, L.; Cao, X. Leonurine protects brain injury by increased activities of UCP4, SOD, CAT and Bcl-2, decreased levels of MDA and Bax, and ameliorated ultrastructure of mitochondria in experimental stroke. Brain Res. 2012, 1474, 73–81. [Google Scholar] [CrossRef]
- Lin, T.; Zhou, D.; Dong, J.; Jiang, F.; Chen, W. Acute toxicity of dichloroacetonitrile (DCAN), a typical nitrogenous disinfection by-product (N-DBP), on zebrafish (Danio rerio). Ecotoxicol. Environ. Saf. 2016, 133, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.N.; Li, W.; Mehmood, S.; Pan, W.J.; Wang, Y.; Meng, F.J.; Chen, Y. Structural characterization, in vitro and in vivo antioxidant activities of a heteropolysaccharide from the fruiting bodies of Morchella esculenta. Carbohyd. Polym. 2018, 195, 29–38. [Google Scholar] [CrossRef]
- Jaeschke, H. Reactive oxygen and mechanisms of inflammatory liver injury. J. Gastroenterol. Hepatol. 2000, 15, 718–724. [Google Scholar] [CrossRef]
- Cadena, P.G.; Cadena, M.R.S.; Sarmah, S.; Marrs, J.A. Folic acid reduces the ethanol-induced morphological and behavioral defects in embryonic and larval zebrafish (Danio rerio) as a model for fetal alcohol spectrum disorder (FASD). Reprod. Toxicol. 2020, 96, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Dorval, J.; Leblond, V.S.; Hontela, A. Oxidative stress and loss of cortisol secretion in adrenocortical cells of rainbow trout (Oncorhynchus mykiss) exposed in vitro to endosulfan, an organochlorine pesticide. Aquat. Toxicol. 2003, 63, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Li, B.; Zhang, J.; Liu, X.; Liu, J.; Li, K.; Diao, Y. Protective effect of phenolic acids from Chebulae Fructus immaturus on carbon tetrachloride induced acute liver injury via suppressing oxidative stress, inflammation and apoptosis in mouse. Nat. Prod. Res. 2020, 34, 3249–3252. [Google Scholar] [CrossRef]
- Rocco, A.; Compare, D.; Angrisani, D. Alcoholic disease: Liver and beyond. World J. Gastroenterol. 2014, 20, 14652–14659. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, S.M.U.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 signaling pathway: Pivotal roles in inflammation. BBA-Mol. Basis Dis. 2017, 1863, 585–597. [Google Scholar] [CrossRef]
- Lu, Q.X.; Shu, Y.Y.; Wang, L.; Li, G.X.; Zhang, S.Y.; Gu, W.Q.; Tang, L. The protective effect of Veronica ciliata Fisch extracts on relieving oxidative stress-induced liver injury via activating AMPK/p62/Nrf2 pathway. J. Ethnopharmacol. 2021, 270, 113775. [Google Scholar] [CrossRef]
- Mann, G.E.; Rowlands, D.J.; Li, F.Y.L.; De Winter, P.; Siow, R.C.M. Activation of endothelial nitric oxide synthase by dietary isoflavones: Role of NO in Nrf2-mediated antioxidant gene expression. Cardiovasc. Res. 2007, 75, 261–274. [Google Scholar] [CrossRef]
- Tak, P.P.; Firestein, G.S. NF-kappaB: A key role in inflammatory diseases. J. Clin. Investig. 2001, 107, 7–11. [Google Scholar] [CrossRef] [Green Version]
- Staal, F.J.T.; Roederer, M.; Herzenberg, L.A.; Herzenberg, L.A. Intracellular thiols regulate activation of nuclear factor kappa B and transcription of human immunodeficiency virus. Proc. Natl. Acad. Sci. USA 1990, 87, 9943–9947. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.J.; Li, Y.; Liu, J.L.; Lin, Y.; Jiao, J.; Chen, B.; Li, C.Y. Photothermal therapy with regulated Nrf 2/NF-κB signaling pathway for treating bacteria-induced periodontitis. Bioact. Mater. 2022, 9, 428–445. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.B.; Wang, L.J.; Wang, T.T.; Li, Z.G.; Gao, Y.X.; Cui, S.W.; Qiu, J. Comparison of quercetin and rutin inhibitory influence on Tartary buckwheat starch digestion in vitro and their differences in binding sites with the digestive enzyme. Food Chem. 2022, 367, 130762. [Google Scholar] [CrossRef] [PubMed]

| Content | Fermentation Time (days) | Fermentation Product of Jujube Medium | |||
|---|---|---|---|---|---|
| 0% (Control, CFR) | 10% | 30% | 50% (CFJ) | ||
| Glucosamine (mg/g d.w.) | 0 | 7.07 ± 0.21 | 8.06 ± 0.16 | 8.97 ± 0.06 | 9.97 ± 0.32 |
| 14 | 34.13 ± 0.07 | 32.15 ± 0.64 | 30.37 ± 0.16 | 27.17 ± 0.35 | |
| 21 | 39.07 ± 0.10 | 37.58 ± 0.51 | 36.23 ± 0.54 | 33.25 ± 0.11 | |
| 28 | 50.85 ± 0.38 | 40.45 ± 0.32 | 36.42 ± 0.06 | 33.79 ± 0.06 | |
| TPC (mg GAE/g d.w.) | 0 | 0.27 ± 0.01 | 0.85 ± 0.03 | 2.35 ± 0.13 | 3.31 ± 0.12 |
| 14 | 0.66 ± 0.03 | 1.34 ± 0.04 | 3.30 ± 0.09 | 4.33 ± 0.12 | |
| 21 | 0.74 ± 0.07 | 1.55 ± 0.02 | 3.45 ± 0.09 | 4.70 ± 0.15 | |
| 28 | 1.11 ± 0.07 | 1.75 ± 0.02 | 3.75 ± 0.21 | 4.90 ± 0.05 | |
| TFC (mg RE/g d.w.) | 0 | 0.14 ± 0.02 | 0.28 ± 0.02 | 0.49 ± 0.01 | 0.74 ± 0.02 |
| 14 | 0.42 ± 0.03 | 0.62 ± 0.03 | 0.77 ± 0.03 | 0.94 ± 0.02 | |
| 21 | 0.55 ± 0.01 | 0.74 ± 0.01 | 0.94 ± 0.03 | 1.02 ± 0.04 | |
| 28 | 0.84 ± 0.03 | 0.85 ± 0.02 | 1.00 ± 0.06 | 1.20 ± 0.04 | |
| Free phenolics (mg GAE/g d.w.) | 0 | 0.081 ± 0.004 | NT | NT | 0.330 ± 0.002 |
| 14 | 0.086 ± 0.002 | 0.374 ± 0.001 | |||
| 21 | 0.096 ± 0.003 | 0.389 ± 0.013 | |||
| 28 | 0.133 ± 0.003 | 0.476 ± 0.020 | |||
| Free/conjugated phenolics (mg GAE /g d.w.) | 0 | 0.044 ± 0.002 | NT | NT | 0.183 ± 0.005 |
| 14 | 0.096 ± 0.001 | 0.220 ± 0.002 | |||
| 21 | 0.126 ± 0.001 | 0.262 ± 0.002 | |||
| 28 | 0.113 ± 0.002 | 0.227 ± 0.006 | |||
| Bound phenolics (mg GAE/g d.w.) | 0 | 0.111 ± 0.001 | NT | NT | 0.154 ± 0.002 |
| 14 | 0.159 ± 0.002 | 0.192 ± 0.002 | |||
| 21 | 0.119 ± 0.001 | 0.286 ± 0.002 | |||
| 28 | 0.156 ± 0.002 | 0.326 ± 0.004 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Dun, M.; Liu, X.; Zhang, G.; Ling, J. Biotransformation of Chinese Jujube with Cordyceps militaris to Enhance the Antioxidant Activity In Vitro and the Protective Effect against Ethanol-Induced Oxidative Stress in Zebrafish. Fermentation 2023, 9, 656. https://doi.org/10.3390/fermentation9070656
Wang M, Dun M, Liu X, Zhang G, Ling J. Biotransformation of Chinese Jujube with Cordyceps militaris to Enhance the Antioxidant Activity In Vitro and the Protective Effect against Ethanol-Induced Oxidative Stress in Zebrafish. Fermentation. 2023; 9(7):656. https://doi.org/10.3390/fermentation9070656
Chicago/Turabian StyleWang, Manman, Mengqian Dun, Xinyuan Liu, Guoying Zhang, and Jianya Ling. 2023. "Biotransformation of Chinese Jujube with Cordyceps militaris to Enhance the Antioxidant Activity In Vitro and the Protective Effect against Ethanol-Induced Oxidative Stress in Zebrafish" Fermentation 9, no. 7: 656. https://doi.org/10.3390/fermentation9070656
APA StyleWang, M., Dun, M., Liu, X., Zhang, G., & Ling, J. (2023). Biotransformation of Chinese Jujube with Cordyceps militaris to Enhance the Antioxidant Activity In Vitro and the Protective Effect against Ethanol-Induced Oxidative Stress in Zebrafish. Fermentation, 9(7), 656. https://doi.org/10.3390/fermentation9070656





