Disinfection of Digestate Effluents Using Photocatalytic Nanofiltration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Liquid Digestate Origin, Photocatalyst and Pure Cultures
2.2. Determination of Physical and Chemical Parameters
2.3. Microbiological Analyses of Indicator Pathogens
2.4. Pre-Treatment of LD
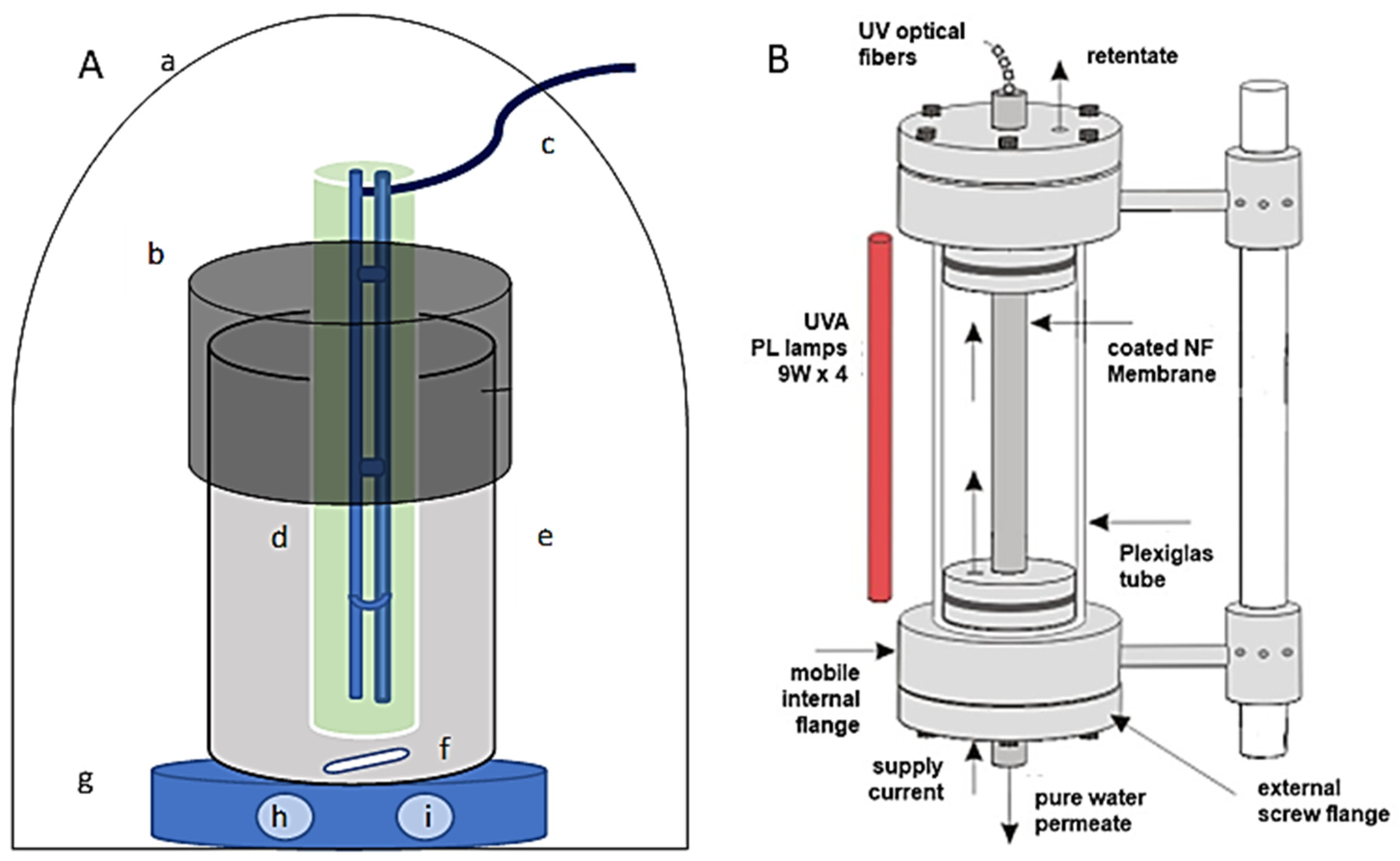
2.5. Photocatalysis Experiments
3. Results and Discussion
3.1. Characterization of LD
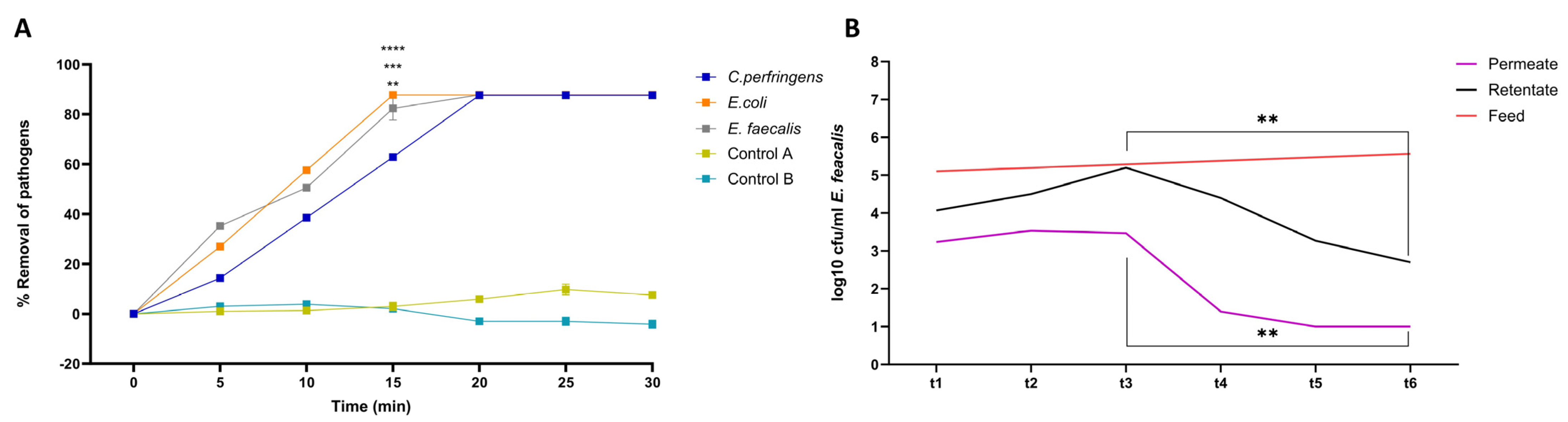
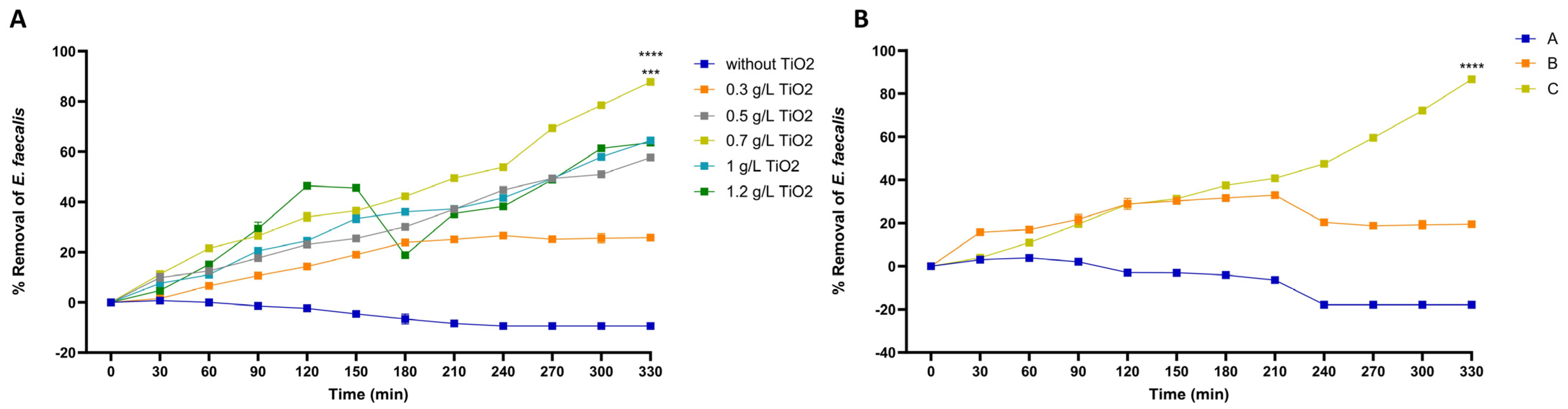
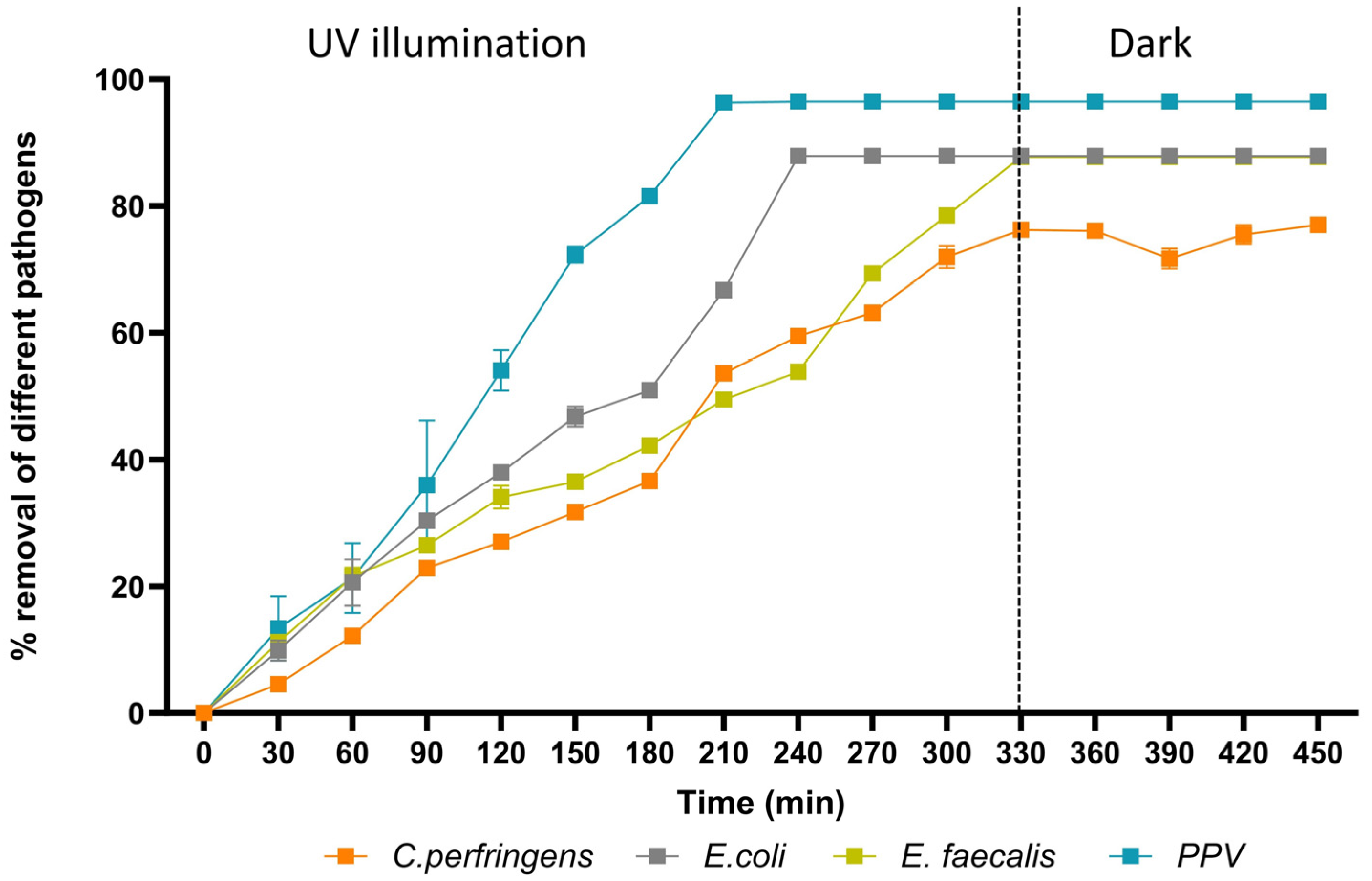
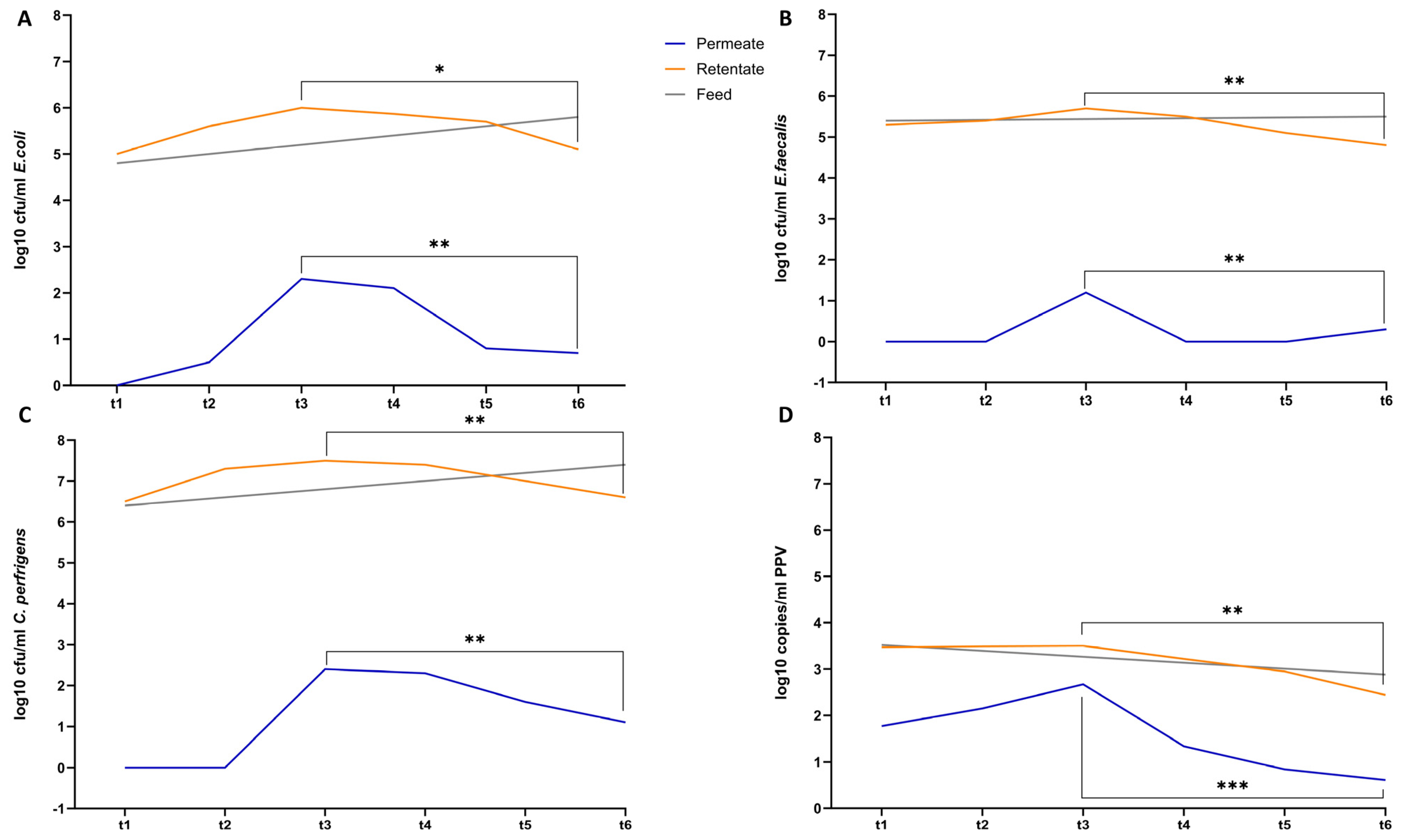
3.2. Configuration of Photocatalytic Disinfection
3.3. PNFR Implementation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saravanan, A.; Kumar, P.S.; Nhung, T.C.; Ramesh, B.; Srinivasan, S.; Rangasamy, G. A review on biological methodologies in municipal solid waste management and landfilling: Resource and energy recovery. Chemosphere 2022, 309, 136630. [Google Scholar] [CrossRef] [PubMed]
- Pasalari, H.; Gholami, M.; Rezaee, A.; Esrafili, A.; Farzadkia, M. Perspectives on microbial community in anaerobic digestion with emphasis on environmental parameters: A systematic review. Chemosphere 2021, 270, 128618. [Google Scholar] [CrossRef]
- Uzinger, N.; Szecsy, O.; Szucs-Vasarhelyi, N.; Padra, I.; Sandor, D.B.; Loncaric, Z.; Draskovits, E.; Rekasi, M. Short-Term Decomposition and Nutrient-Supplying Ability of Sewage Sludge Digestate, Digestate Compost, and Vermicompost on Acidic Sandy and Calcareous Loamy Soils. Agronomy 2021, 11, 2249. [Google Scholar] [CrossRef]
- Kovacic, D.; Loncaric, Z.; Jovic, J.; Samac, D.; Popovic, B.; Tisma, M. Digestate Management and Processing Practices: A Review. Appl. Sci. 2022, 12, 9216. [Google Scholar] [CrossRef]
- Peng, W.; Lu, F.; Hao, L.P.; Zhang, H.; Shao, L.M.; He, P.J. Digestate management for high-solid anaerobic digestion of organic wastes: A review. Bioresour. Technol. 2020, 297, 122485. [Google Scholar] [CrossRef]
- Hutchison, M.L.; Walters, L.D.; Avery, S.M.; Synge, B.A.; Moore, A. Levels of zoonotic agents in British livestock manures. Lett. Appl. Microbiol. 2004, 39, 207–214. [Google Scholar] [CrossRef]
- Johansson, M.; Emmoth, E.; Salomonsson, A.C.; Albihn, A. Potential risks when spreading anaerobic digestion residues on grass silage crops—Survival of bacteria, moulds and viruses. Grass Forage Sci. 2005, 60, 175–185. [Google Scholar] [CrossRef]
- Goberna, M.; Podmirseg, S.M.; Waldhuber, S.; Knapp, B.A.; Garcia, C.; Insam, H. Pathogenic bacteria and mineral N in soils following the land spreading of biogas digestates and fresh manure. Appl. Soil Ecol. 2011, 49, 18–25. [Google Scholar] [CrossRef]
- Smith, S.R.; Lang, N.L.; Cheung, K.H.M.; Spanoudaki, K. Factors controlling pathogen destruction during anaerobic digestion of biowastes. Waste Manag. 2005, 25, 417–425. [Google Scholar] [CrossRef]
- Sfetsas, T.; Patsatzis, S.; Chioti, A.; Kopteropoulos, A.; Dimitropoulou, G.; Tsioni, V.; Kotsopoulos, T. A review of advances in valorization and post-treatment of anaerobic digestion liquid fraction effluent. Waste Manag. Res. 2022, 40, 1093–1109. [Google Scholar] [CrossRef]
- Erickson, M.C.; Ortega, Y.R. Inactivation of protozoan parasites in food, water, and environmental systems. J. Food Prot. 2006, 69, 2786–2808. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.J.; Lendormi, T.; Lanoiselle, J.L. Conventional and Innovative Hygienization of Feedstock for Biogas Production: Resistance of Indicator Bacteria to Thermal Pasteurization, Pulsed Electric Field Treatment, and Anaerobic Digestion. Energies 2021, 14, 1938. [Google Scholar] [CrossRef]
- Seruga, P.; Krzywonos, M.; Paluszak, Z.; Urbanowska, A.; Pawlak-Kruczek, H.; Niedzwiecki, L.; Pinkowska, H. Pathogen Reduction Potential in Anaerobic Digestion of Organic Fraction of Municipal Solid Waste and Food Waste. Molecules 2020, 25, 275. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.J.; Lendormi, T.; Lanoiselle, J.L. Overview of hygienization pretreatment for pasteurization and methane potential enhancement of biowaste: Challenges, state of the art and alternative technologies. J. Clean. Prod. 2019, 236, 117525. [Google Scholar] [CrossRef]
- Froschle, B.; Heiermann, M.; Lebuhn, M.; Messelhausser, U.; Plochl, M. Hygiene and Sanitation in Biogas Plants. In Biogas Science and Technology; Guebitz, G.M., Bauer, A., Bochmann, G., Gronauer, A., Weiss, S., Eds.; (Advances in Biochemical Engineering-Biotechnology); Springer: Berlin/Heidelberg, Germany, 2015; Volume 151, pp. 63–99. [Google Scholar]
- Golovko, O.; Ahrens, L.; Schelin, J.; Sorengard, M.; Bergstrand, K.J.; Asp, H.; Hultberg, M.; Wiberg, K. Organic micropollutants, heavy metals and pathogens in anaerobic digestate based on food waste. J. Environ. Manag. 2022, 313, 114997. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Wang, A.J.; Ren, L.J.; Qiao, W.; Wandera, S.M.; Dong, R.J. Challenges of pathogen inactivation in animal manure through anaerobic digestion: A short review. Bioengineered 2022, 13, 1149–1161. [Google Scholar] [CrossRef]
- Bagge, E.; Persson, M.; Johansson, K.E. Diversity of spore-forming bacteria in cattle manure, slaughterhouse waste and samples from biogas plants. J. Appl. Microbiol. 2010, 109, 1549–1565. [Google Scholar] [CrossRef]
- Le Marechal, C.; Druilhe, C.; Reperant, E.; Boscher, E.; Rouxel, S.; Le Roux, S.; Poezevara, T.; Ziebal, C.; Houdayer, C.; Nagard, B.; et al. Evaluation of the occurrence of sporulating and nonsporulating pathogenic bacteria in manure and in digestate of five agricultural biogas plants. Microbiologyopen 2019, 8, e872. [Google Scholar] [CrossRef] [Green Version]
- Pulvirenti, A.; Ronga, D.; Zaghi, M.; Tomasselli, A.R.; Mannella, L.; Pecchioni, N. Pelleting is a successful method to eliminate the presence of Clostridium spp. from the digestate of biogas plants. Biomass Bioenergy 2015, 81, 479–482. [Google Scholar] [CrossRef]
- Galedari, M.; Ghazi, M.M.; Mirmasoomi, S.R. Photocatalytic process for the tetracycline removal under visible light: Presenting a degradation model and optimization using response surface methodology (RSM). Chem. Eng. Res. Des. 2019, 145, 323–333. [Google Scholar] [CrossRef]
- Al-Hamdi, A.M.; Rinner, U.; Sillanpaa, M. Tin dioxide as a photocatalyst for water treatment: A review. Process Saf. Environ. Prot. 2017, 107, 190–205. [Google Scholar] [CrossRef]
- Rivera-Utrilla, J.; Sanchez-Polo, M.; Ferro-Garcia, M.A.; Prados-Joya, G.; Ocampo-Perez, R. Pharmaceuticals as emerging contaminants and their removal from water. A review. Chemosphere 2013, 93, 1268–1287. [Google Scholar] [CrossRef]
- Rimoldi, L.; Meroni, D.; Cappelletti, G.; Ardizzone, S. Green and low cost tetracycline degradation processes by nanometric and immobilized TiO2 systems. Catal. Today 2017, 281, 38–44. [Google Scholar] [CrossRef]
- Liu, Y.Q.; He, X.X.; Duan, X.D.; Fu, Y.S.; Fatta-Kassinos, D.; Dionysiou, D.D. Significant role of UV and carbonate radical on the degradation of oxytetracycline in UV-AOPs: Kinetics and mechanism. Water Res. 2016, 95, 195–204. [Google Scholar] [CrossRef]
- M’Arimi, M.M.; Mecha, C.A.; Kiprop, A.K.; Ramkat, R. Recent trends in applications of advanced oxidation processes (AOPs) in bioenergy production: Review. Renew. Sustain. Energy Rev. 2020, 121, 109669. [Google Scholar] [CrossRef]
- Wu, S.Q.; Hu, H.Y.; Lin, Y.; Zhang, J.L.; Hu, Y.H. Visible light photocatalytic degradation of tetracycline over TiO2. Chem. Eng. J. 2020, 382, 122842. [Google Scholar] [CrossRef]
- Wang, H.; Wang, H.L.; Jiang, W.F. Solar photocatalytic degradation of 2,6-dinitro-p-cresol (DNPC) using multi-walled carbon nanotubes (MWCNTs)-TiO2 composite photocatalysts. Chemosphere 2009, 75, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.P.; Yuan, Q.X. Photocatalytic degradation of tetracyclines in liquid digestate: Optimization, kinetics and correlation studies. Chem. Eng. J. 2021, 410, 128327. [Google Scholar] [CrossRef]
- Wang, P.P.; Xu, C.; Zhang, X.; Yuan, Q.X.; Shan, S.D. Effect of photocatalysis on the physicochemical properties of liquid digestate. Environ. Res. 2023, 223, 115467. [Google Scholar] [CrossRef] [PubMed]
- Yin, F.B.; Lin, S.Y.; Zhou, X.Q.; Dong, H.M.; Zhan, Y.H. Fate of antibiotics during membrane separation followed by physical-chemical treatment processes. Sci. Total Environ. 2021, 759, 143520. [Google Scholar] [CrossRef]
- Jin, X.D.; Zhou, X.Q.; Sun, P.; Lin, S.Y.; Cao, W.B.; Li, Z.F.; Liu, W.X. Photocatalytic degradation of norfloxacin using N-doped TiO2: Optimization, mechanism, identification of intermediates and toxicity evaluation. Chemosphere 2019, 237, 124433. [Google Scholar] [CrossRef]
- Hu, X.; Hu, X.J.; Peng, Q.Q.; Zhou, L.; Tan, X.F.; Jiang, L.H.; Tang, C.F.; Wang, H.; Liu, S.H.; Wang, Y.Q.; et al. Mechanisms underlying the photocatalytic degradation pathway of ciprofloxacin with heterogeneous TiO2. Chem. Eng. J. 2020, 380, 122366. [Google Scholar] [CrossRef]
- Huang, J.X.; Li, D.G.; Li, R.B.; Chen, P.; Zhang, Q.X.; Liu, H.J.; Lv, W.Y.; Liu, G.G.; Feng, Y.P. One-step synthesis of phosphorus/oxygen co-doped g-C3N4/anatase TiO2 Z-scheme photocatalyst for significantly enhanced visible-light photocatalysis degradation of enrofloxacin. J. Hazard. Mater. 2020, 386, 121634. [Google Scholar] [CrossRef] [PubMed]
- Espindola, J.C.; Cristovao, R.O.; Mendes, A.; Boaventura, R.A.R.; Vilar, V.J.P. Photocatalytic membrane reactor performance towards oxytetracycline removal from synthetic and real matrices: Suspended vs. immobilized TiO2-P25. Chem. Eng. J. 2019, 378, 122114. [Google Scholar] [CrossRef]
- Devi, M.; Praharaj, S.; Rout, D. 20—Industrial problems and solution towards visible light photocatalysis. In Nanostructured Materials for Visible Light Photocatalysis; Nayak, A.K., Sahu, N.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 535–567. [Google Scholar]
- He, J.H.; Kumar, A.; Khan, M.; Lo, I.M.C. Critical review of photocatalytic disinfection of bacteria: From noble metals-and carbon nanomaterials-TiO2 composites to challenges of water characteristics and strategic solutions. Sci. Total Environ. 2021, 758, 143953. [Google Scholar] [CrossRef]
- Lofrano, G.; Ubaldi, F.; Albarano, L.; Carotenuto, M.; Vaiano, V.; Valeriani, F.; Libralato, G.; Gianfranceschi, G.; Fratoddi, I.; Meric, S.; et al. Antimicrobial Effectiveness of Innovative Photocatalysts: A Review. Nanomaterials 2022, 12, 2831. [Google Scholar] [CrossRef]
- Alrousan, D.M.A.; Dunlop, P.S.M.; McMurray, T.A.; Byrne, J.A. Photocatalytic inactivation of E. coli in surface water using immobilised nanoparticle TiO2 films. Water Res. 2009, 43, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, F.; Silkina, A.; Fuentes-Grunewald, C.; Wood, E.E.; Ndovela, V.L.S.; Oatley-Radcliffe, D.L.; Lovitt, R.W.; Llewellyn, C.A. Valorising nutrient-rich digestate: Dilution, settlement and membrane filtration processing for optimisation as a waste-based media for microalgal cultivation. Waste Manag. 2020, 118, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Camilleri-Rumbau, M.S.; Soler-Cabezas, J.L.; Christensen, K.V.; Norddahl, B.; Mendoza-Roca, J.A.; Vincent-Vela, M.C. Application of aquaporin-based forward osmosis membranes for processing of digestate liquid fractions. Chem. Eng. J. 2019, 371, 583–592. [Google Scholar] [CrossRef]
- Camilleri-Rumbau, M.S.; Norddahl, B.; Wei, J.; Christensen, K.V.; Sotoft, L.F. Microfiltration and ultrafiltration as a post-treatment of biogas plant digestates for producing concentrated fertilizers. Desalination Water Treat. 2015, 55, 1639–1653. [Google Scholar] [CrossRef]
- Swiatczak, P.; Cydzik-Kwiatkowska, A.; Zielinska, M. Treatment of Liquid Phase of Digestate from Agricultural Biogas Plant in a System with Aerobic Granules and Ultrafiltration. Water 2019, 11, 104. [Google Scholar] [CrossRef] [Green Version]
- Yue, C.D.; Dong, H.M.; Chen, Y.X.; Shang, B.; Wang, Y.; Wang, S.L.; Zhu, Z.P. Direct Purification of Digestate Using Ultrafiltration Membranes: Influence of Pore Size on Filtration Behavior and Fouling Characteristics. Membranes 2021, 11, 179. [Google Scholar] [CrossRef]
- Adam, G.; Mottet, A.; Lemaigre, S.; Tsachidou, B.; Trouve, E.; Delfosse, P. Fractionation of anaerobic digestates by dynamic nanofiltration and reverse osmosis: An industrial pilot case evaluation for nutrient recovery. J. Environ. Chem. Eng. 2018, 6, 6723–6732. [Google Scholar] [CrossRef]
- Zhan, Y.H.; Yin, F.B.; Yue, C.D.; Zhu, J.; Zhu, Z.P.; Zou, M.Y.; Dong, H.M. Effect of Pretreatment on Hydraulic Performance of the Integrated Membrane Process for Concentrating Nutrient in Biogas Digestate from Swine Manure. Membranes 2020, 10, 249. [Google Scholar] [CrossRef] [PubMed]
- Oller, I.; Malato, S.; Sanchez-Perez, J.A. Combination of Advanced Oxidation Processes and biological treatments for wastewater decontamination-A review. Sci. Total Environ. 2011, 409, 4141–4166. [Google Scholar] [CrossRef] [PubMed]
- Falaras, P.; Romanos, G.; Aloupogiannis, P. Photocatalytic purification device. Application number EP10275076.7, 26 January 2012. [Google Scholar]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.M.; Hamilton, J.W.J.; Byrne, J.A.; O’Shea, K.; et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B-Environ. 2012, 125, 331–349. [Google Scholar] [CrossRef] [Green Version]
- Athanasekou, C.P.; Romanos, G.E.; Katsaros, F.K.; Kordatos, K.; Likodimos, V.; Falaras, P. Very efficient composite titania membranes in hybrid ultrafiltration/photocatalysis water treatment processes. J. Membr. Sci. 2012, 392, 192–203. [Google Scholar] [CrossRef]
- Theodorakopoulos, G.V.; Arfanis, M.K.; Perez, J.A.S.; Aguera, A.; Aponte, F.X.C.; Markellou, E.; Romanos, G.E.; Falaras, P. Novel Pilot-Scale Photocatalytic Nanofiltration Reactor for Agricultural Wastewater Treatment. Membranes 2023, 13, 202. [Google Scholar] [CrossRef] [PubMed]
- 2540 SOLIDS. In Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2017.
- 5220 CHEMICAL OXYGEN DEMAND (COD). In Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2017.
- 4500-H+ pH. In Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2017.
- 4500-NO3− NITROGEN (NITRATE). In Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2017.
- 4500-NH3 NITROGEN (AMMONIA). In Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2017.
- British Standards Institution. BS ISO 17294-1. Water Quality. Application of Inductively Coupled Plasma Mass Spectrometry (ICP-MS); British Standards Institution: London, UK, 2022. [Google Scholar]
- Rice, E.W.; Baird, R.B. Methods for the Examination of Water and Wastewater. Am. Public Heal. Assoc. Am. Water Work. 2017, 23, 3–48. [Google Scholar]
- Bojinova, A.; Kralchevska, R.; Poulios, I.; Dushkin, C. Anatase/rutile TiO2 composites: Influence of the mixing ratio on the photocatalytic degradation of Malachite Green and Orange II in slurry. Mater. Chem. Phys. 2007, 106, 187–192. [Google Scholar] [CrossRef]
- Moustakas, N.G.; Katsaros, F.K.; Kontos, A.G.; Romanos, G.E.; Dionysiou, D.D.; Falaras, P. Visible light active TiO2 photocatalytic filtration membranes with improved permeability and low energy consumption. Catal. Today 2014, 224, 56–69. [Google Scholar] [CrossRef]
- Papageorgiou, S.K.; Katsaros, F.K.; Favvas, E.P.; Romanos, G.E.; Athanasekou, C.P.; Beltsios, K.G.; Tzialla, O.I.; Falaras, P. Alginate fibers as photocatalyst immobilizing agents applied in hybrid photocatalytic/ultrafiltration water treatment processes. Water Res. 2012, 46, 1858–1872. [Google Scholar] [CrossRef]
- Du, P.; Cameiro, J.T.; Moulijn, J.A.; Mul, G. A novel photocatalytic monolith reactor for multiphase heterogeneous photocatalysis. Appl. Catal. Gen. 2008, 334, 119–128. [Google Scholar] [CrossRef]
- Pelaez, M.; Falaras, P.; Kontos, A.G.; de la Cruz, A.A.; O’Shea, K.; Dunlop, P.S.M.; Byrne, J.A.; Dionysiou, D.D. A comparative study on the removal of cylindrospermopsin and microcystins from water with NF-TiO2-P25 composite films with visible and UV-vis light photocatalytic activity. Appl. Catal. B-Environ. 2012, 121, 30–39. [Google Scholar] [CrossRef]
- Hardcastle, F.D. Raman Spectroscopy of Titania (TiO2) Nanotubular Water-Splitting Catalysts. J. Ark. Acad. Sci. 2011, 65, 43–48. [Google Scholar] [CrossRef]
- Likodimos, V.; Chrysi, A.; Calamiotou, M.; Fernandez-Rodriguez, C.; Dona-Rodriguez, J.M.; Dionysiou, D.D.; Falaras, P. Microstructure and charge trapping assessment in highly reactive mixed phase TiO2 photocatalysts. Appl. Catal. B-Environ. 2016, 192, 242–252. [Google Scholar] [CrossRef]
- Bagge, E.; Sahlstrom, L.; Albihn, A. The effect of hygienic treatment on the microbial flora of biowaste at biogas plants. Water Res. 2005, 39, 4879–4886. [Google Scholar] [CrossRef]
- Mecha, A.C.; Onyango, M.S.; Ochieng, A.; Momba, M.N.B. UV and solar photocatalytic disinfection of municipal wastewater: Inactivation, reactivation and regrowth of bacterial pathogens. Int. J. Environ. Sci. Technol. 2019, 16, 3687–3696. [Google Scholar] [CrossRef]
- Wang, W.; Huang, G.; Yu, J.C.; Wong, P.K. Advances in photocatalytic disinfection of bacteria: Development of photocatalysts and mechanisms. J. Environ. Sci. 2015, 34, 232–247. [Google Scholar] [CrossRef]
- Reddy, P.V.L.; Kavitha, B.; Reddy, P.A.K.; Kim, K.-H. TiO2-based photocatalytic disinfection of microbes in aqueous media: A review. Environ. Res. 2017, 154, 296–303. [Google Scholar] [CrossRef]
- McCullagh, C.; Robertson, J.M.C.; Bahnemann, D.W.; Robertson, P.K.J. The application of TiO2 photocatalysis for disinfection of water contaminated with pathogenic micro-organisms: A review. Res. Chem. Intermed. 2007, 33, 359–375. [Google Scholar] [CrossRef]
- Mecha, A.C.; Onyango, M.S.; Ochieng, A.; Momba, M.N.B. Evaluation of synergy and bacterial regrowth in photocatalytic ozonation disinfection of municipal wastewater. Sci. Total Environ. 2017, 601, 626–635. [Google Scholar] [CrossRef]
- Liu, N.; Ming, J.; Sharma, A.; Sun, X.; Kawazoe, N.; Chen, G.P.; Yang, Y.N. Sustainable photocatalytic disinfection of four representative pathogenic bacteria isolated from real water environment by immobilized TiO2-based composite and its mechanism. Chem. Eng. J. 2021, 426, 131217. [Google Scholar] [CrossRef]
- Subirats, J.; Sharpe, H.; Topp, E. Fate of Clostridia and other spore-forming Firmicute bacteria during feedstock anaerobic digestion and aerobic composting. J. Environ. Manag. 2022, 309, 114643. [Google Scholar] [CrossRef]

| Parameter | Unit | Min-Max Values | Average | Standard Deviation |
|---|---|---|---|---|
| EC | ms/m | 318–1880 | 650 | 626 |
| pH | - | 7.9–8.6 | 8.2 | 0.2 |
| TS | % | 1.32–5.83 | 3.22 | 1.6 |
| VS | % | 0.65–5.72 | 1.9 | 1.6 |
| COD | mg/L | 21,333–42,615 | 33,800 | 809 |
| TN | % | 0.2–0.44 | 0.33 | 0.08 |
| TP | % | 0.01–0.14 | 0.04 | 0.05 |
| N-NO3 | % | 0.002─0.01 | 0.006 | 0.003 |
| N-NH4 | % | 0.13–0.36 | 0.22 | 0.06 |
| Ca | % | 0.014–0.14 | 0.044 | 0.05 |
| K | % | 0.12–0.35 | 0.24 | 0.08 |
| Mg | % | 0.007–5.1 | 0.04 | 1.9 |
| Fe | % | 0.004–0.022 | 0.008 | 0.007 |
| Mn | % | 0.00014–0.002 | 0.0006 | 0.0007 |
| Na | % | 0.07–0.16 | 0.09 | 0.03 |
| Cl | % | 0.14–1.19 | 0.35 | 0.39 |
| Cd | mg/kg TS | <0.3–0.4 | 0.34 | 0.05 |
| Cr | mg/kg TS | <8.3–14.6 | 7.5 | 3.8 |
| Pb | mg/kg TS | <1–2.6 | <1 | - |
| Zn | mg/kg TS | 209–1220 | 504 | 374 |
| As | mg/kg TS | <3–15.15 | <3 | - |
| Cu | mg/kg TS | 59–449 | 197 | 135 |
| Hg | mg/kg TS | - | <0.02 | - |
| Ni | mg/kg TS | 11–30.3 | 17.1 | 7.1 |
| E. coli | cfu/g | <10–13,000 | - | - |
| E. faecalis | cfu/g | <10–250,000 | - | - |
| A: Untreated | B: Centrifuged | C: μFiltration | Di: nFiltration | Dii: Photocatalysis | Diii: PNFR | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | Unit | Average | SD | Average | SD | Average | SD | Average | SD | Average | SD | Average | SD |
| pH | - | 8.14 | 0.11 | 8.20 | 0.10 | 8.50 | 0.12 | 8.50 | 0.10 | 8.30 | 0.10 | 8.20 | 0.10 |
| EC | mS/m | 534 | 18 | 550 | 26 | 645 | 30 | 415 | 41 | 520 | 28 | 284 | 27 |
| TS | % | 3.94 | 0.21 | 1.91 | 0.01 | 0.36 | 0.00 | 0.17 | 0.00 | 0.20 | 0.00 | 0.11 | 0.00 |
| N-NH4 | mg N/L | 2881 | 267 | 2151 | 250 | <0.1 | n.a. | <0.1 | n.a. | <0.1 | n.a. | <0.1 | n.a. |
| Ν | % | 0.42 | 0.02 | 0.30 | 0.00 | 0.08 | 0.00 | 0.02 | 0.00 | 0.02 | 0.00 | 0.02 | 0.00 |
| Cl | mg/L | 2941 | 310 | 3900 | 607 | 745 | 99 | 672 | 192 | 520 | 144 | 481 | 151 |
| Salinity | ‰ | 3.22 | 0.11 | 3.26 | 0.17 | 4.40 | 0.10 | 2.50 | 0.10 | 3.15 | 0.10 | 2.50 | 0.10 |
| Ν-ΝO3 | mg N/L | 60.93 | 12.95 | 43.50 | 8.00 | <0.01 | n.a. | <0.01 | n.a. | <0.01 | n.a. | <0.01 | n.a. |
| VS | % | 2.43 | 0.16 | 0.92 | 0.03 | 0.10 | n.a. | <0.1 | n.a. | <0.1 | n.a. | <0.1 | n.a. |
| P | g/kg | 0.61 | 0.00 | 0.11 | 0.00 | <0.1 | n.a. | <0.1 | n.a. | <0.1 | n.a. | <0.1 | n.a. |
| COD | mg O2/L | 33,750 | 4431 | 14,280 | 1966 | 1190 | 190 | 732 | 35 | 417 | 20 | 298 | 22 |
| TSS | mg/L | 28,000 | 9528 | 1775 | 159 | 180 | 33 | 61 | 1.00 | 33 | 1.22 | 4 | 0.01 |
| Turbidity | NTU | n.a. * | n.a. | 8000 | 244 | 1687 | 321 | 1100 | 208 | 477 | 132 | 248 | 75 |
| E. coli | cfu/g | 3250 | 3718 | 743 | 405 | <10 | n.a. | <10 | n.a. | <10 | n.a. | <10 | n.a. |
| E. faecalis | cfu/g | 3173 | 3217 | 2390 | 2492 | 410 | 53 | <10 | n.a. | <10 | n.a. | <10 | n.a. |
| C. perfrigens | cfu/g | 4440 | 3526 | 3270 | 2873 | 300 | 130 | <10 | n.a. | <10 | n.a. | <10 | n.a. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chioti, A.G.; Sarikaki, G.; Tsioni, V.; Kostopoulou, E.; Romanos, G.E.; Falaras, P.; Sfetsas, T. Disinfection of Digestate Effluents Using Photocatalytic Nanofiltration. Fermentation 2023, 9, 662. https://doi.org/10.3390/fermentation9070662
Chioti AG, Sarikaki G, Tsioni V, Kostopoulou E, Romanos GE, Falaras P, Sfetsas T. Disinfection of Digestate Effluents Using Photocatalytic Nanofiltration. Fermentation. 2023; 9(7):662. https://doi.org/10.3390/fermentation9070662
Chicago/Turabian StyleChioti, Afroditi G., Georgia Sarikaki, Vasiliki Tsioni, Eleni Kostopoulou, George Em. Romanos, Polycarpos Falaras, and Themistoklis Sfetsas. 2023. "Disinfection of Digestate Effluents Using Photocatalytic Nanofiltration" Fermentation 9, no. 7: 662. https://doi.org/10.3390/fermentation9070662
APA StyleChioti, A. G., Sarikaki, G., Tsioni, V., Kostopoulou, E., Romanos, G. E., Falaras, P., & Sfetsas, T. (2023). Disinfection of Digestate Effluents Using Photocatalytic Nanofiltration. Fermentation, 9(7), 662. https://doi.org/10.3390/fermentation9070662







