Investigating the Anaerobic Digestion of Water Hyacinth (Eichhornia crassipes) Sourced from Hartbeespoort Dam in South Africa
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Preparation
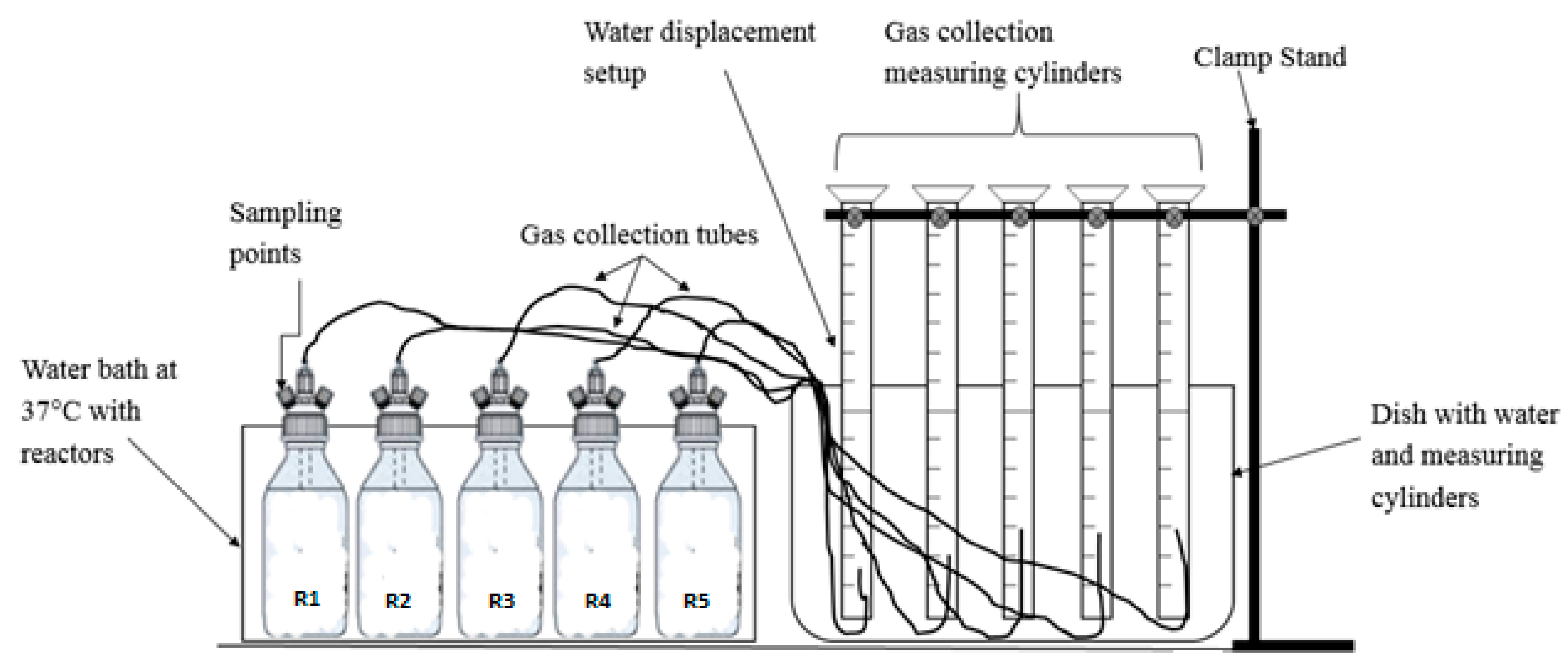
2.2. Experimental Set-Up for Biomethane Potential Assays
2.3. Analytical Methods and Data Analysis
2.3.1. Proximate and Ultimate Analyses
2.3.2. Biogas Analysis
2.3.3. Biomethane Potential Analysis
2.3.4. Microbial Analysis
3. Results and Discussion
3.1. Substrate and Inoculum Characteristics
3.2. Water Hyacinth Biomethanation and Codigestion
3.2.1. Biomethane Potential Tests
3.2.2. Biomethanation Kinetic Studies
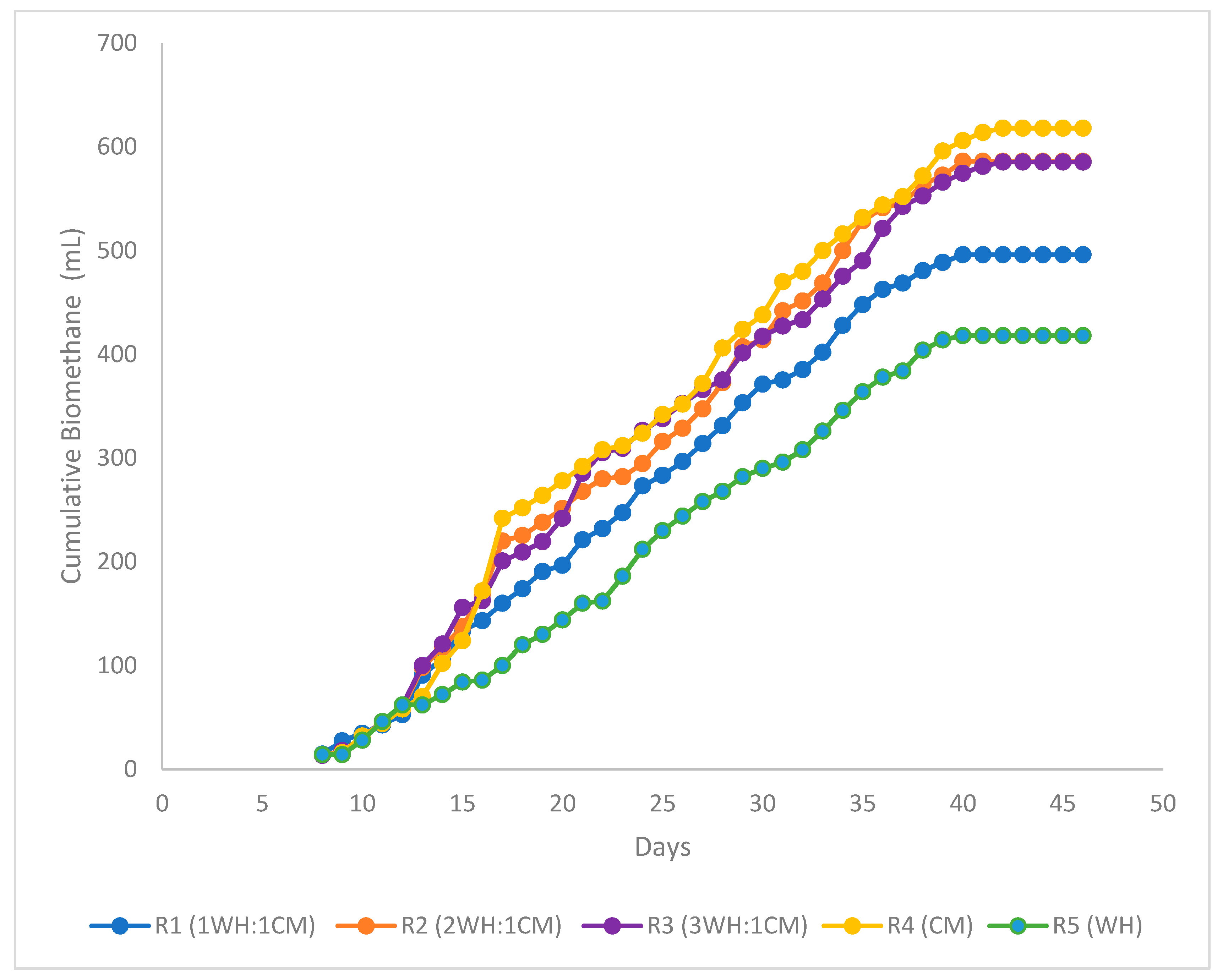
| Kinetic Model | Parameter | R1 | R2 | R3 | R4 | R5 |
|---|---|---|---|---|---|---|
| Modified Gompertz | B∞ (mLCH4) | 549.47 | 622.81 | 526.05 | 690.53 | 501.56 |
| Rm (mL/day) | 18.23 | 21.11 | 22.75 | 22.90 | 15.34 | |
| λ (days) | 5.24 | 5.15 | 5.28 | 5.47 | 6.68 | |
| R2 | 0.995 | 0.990 | 0.986 | 0.990 | 0.996 | |
| Logistic | B∞ (mLCH4) | 1377.81 | 1860.23 | 1763.66 | 1907.55 | 1333.72 |
| Rm (mL/day) | 60.77 | 69.06 | 67.46 | 73.34 | 51.51 | |
| λ (days) | 23.99 | 27.56 | 26.36 | 26.72 | 28.31 | |
| R2 | 0.987 | 0.985 | 0.987 | 0.983 | 0.995 |
3.3. Effects of Pretreatment Methods on Water Hyacinth
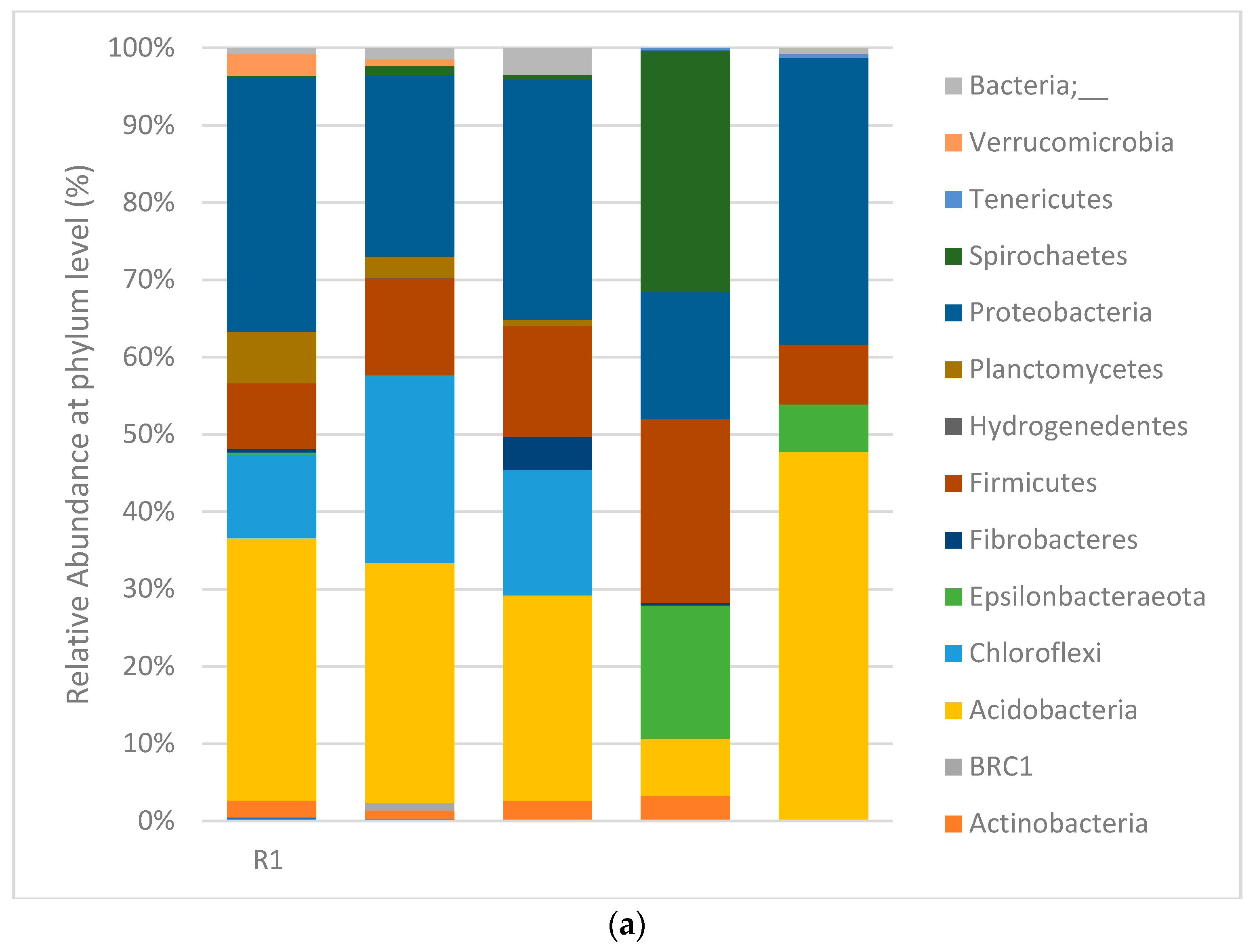
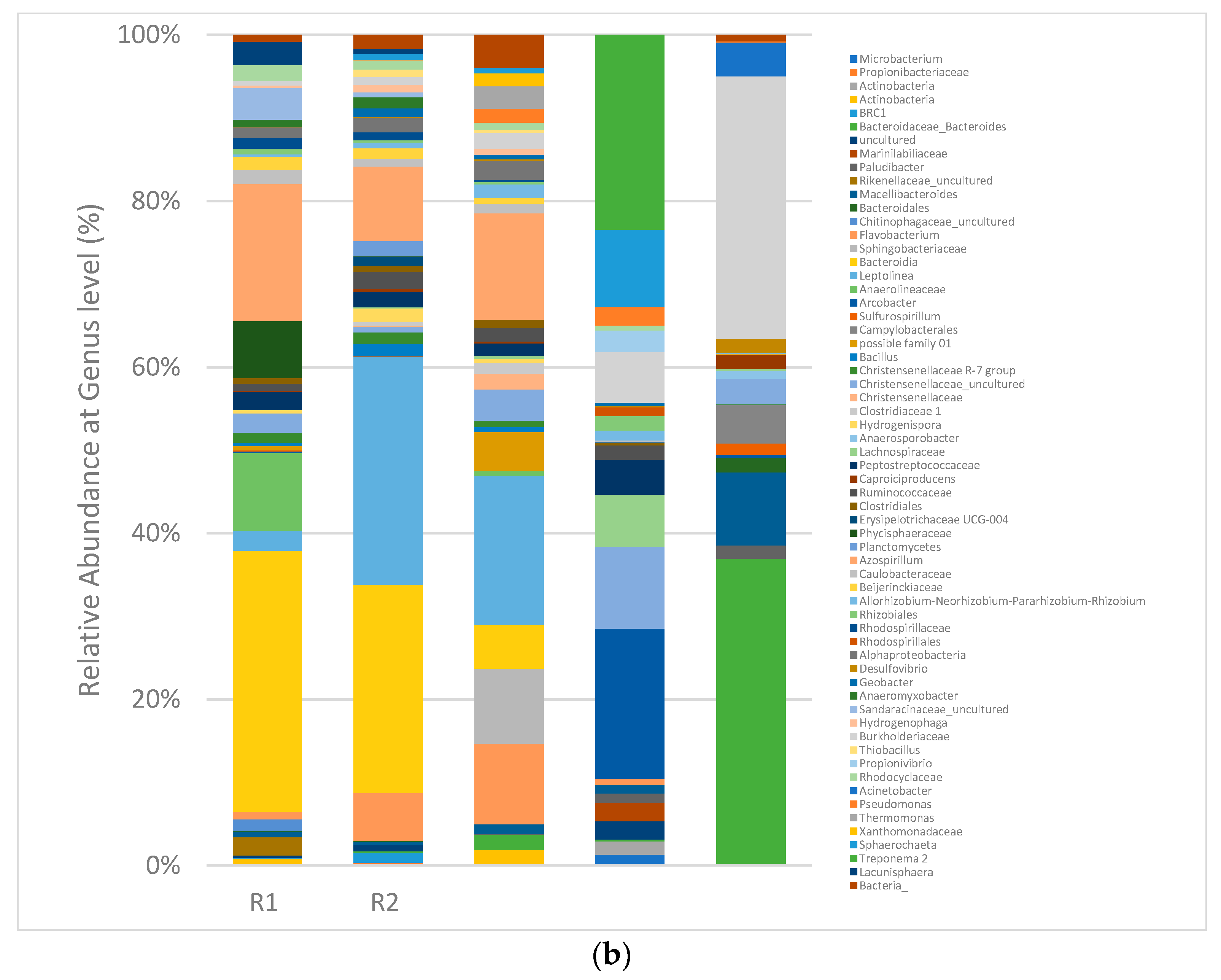
3.4. Digester Microbial Dynamics
3.5. Digestate Potential as Biofertilizer
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Yan, S.H.; Song, W.; Guo, J.Y. Advances in Management and Utilization of Invasive Water Hyacinth (Eichhornia crassipes) in Acquatic Ecosystems—A Review. Crit. Rev. Biotechnol. 2017, 37, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Ilo, O.P.; Simatele, M.D.; Nkomo, S.L.; Mkhize, N.M.; Prabhu, N.G. Methodological Approaches to Optimising Anaerobic Digestion of Water Hyacinth for Energy Efficiency in South Africa. Sustainability 2021, 13, 6746. [Google Scholar] [CrossRef]
- Adelodun, A.A.; Olajire, T.M.; Ojo, O.M. Biogas Generation from Codigestion Waste Systems: The Role of Water Hyacinth. In Sustainable Rural Development Perspective and Global Challenges; Orhan, Ö., Ed.; IntechOpen: London, UK, 2022. [Google Scholar]
- Ali, N.; Chaudhary, B.L.; Panwar, N.L. The Fungal Pre-Treatment of Maize Cob Heart and Water Hyacinth for Enhanced Bioremediation. Int. J. Green Energy 2014, 11, 40–49. [Google Scholar] [CrossRef]
- Barua, V.B.; Goud, V.V.; Kalamdhad, A.S. Microbial Pre-Treatment of Water Hyacinth for Enhanced Hydrolysis Followed by Biogas Production. Renew. Energy 2018, 126, 21–29. [Google Scholar] [CrossRef]
- Ali, S.S.; Elsamahy, T.; Abdelfattah, A.; Mustafa, A.M.; Khalil, M.A.; Mastropetros, S.G.; Kornaros, M.; Sun, J.; Azab, M. Exploring the Potential of Anaerobic Codigestion of Water Hyacinth and Cattle Dung for Enhanced Bioremediation and Techno-Economic Feasibility. Fuel 2022, 329, 125397. [Google Scholar] [CrossRef]
- Dolle, K.; Hughes, T. Biogas Production from Anaerobic Codigestion of Water Hyacinth (Eichhornia crassipes) and Cow. J. Energy Res. Rev. 2020, 5, 49–60. [Google Scholar] [CrossRef]
- Ofoefule, A.U.; Uzodinma, E.O.; Onukwuli, O.D. Comparative Study of the Effect of Different Pretreatment Methods on Biogas Yield from Water Hyacinth (Eichhornia crassipes). Int. J. Phys. Sci. 2009, 4, 535–539. [Google Scholar]
- Abraham, A.; Mathew, A.K.; Park, H.; Choi, O.; Sindhu, R.; Parameswaran, B.; Pandey, A.; Park, J.H.; Sang, B.-I. Pretreatment Strategies for Enhanced Biogas Production from Lignocellulosic Biomass. Bioresour. Technol. 2020, 301, 122725. [Google Scholar] [CrossRef]
- Panigrahi, S.; Dubey, B.K. A Critical Review on Operating Parameters and Strategies to Improve the Biogas Yield from Anaerobic Digestion of Organic Fraction of Municipal Solid Waste. Renew. Energy 2019, 143, 779–797. [Google Scholar] [CrossRef]
- Rashama, C.; Ijoma, G.N.; Matambo, T.S. Appraising Different Models for Predicting Biomethane Potential: The Case of Avocado Oil Processing by-Products. J. Mater. Cycles Waste Manag. 2020, 23, 409–415. [Google Scholar] [CrossRef]
- Holliger, C.; Alves, M.; Andrade, D.; Angelidaki, I.; Astals, S.; Baier, U.; Bougrier, C.; Buf, P.; Carballa, M.; De Wilde, V.; et al. Towards a Standardization of Biomethane Potential Tests. Water Sci. Technol. 2016, 74, 2515–2522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Auchterlonie, J.; Eden, C.L.; Sheridan, C. The Phytoremediation Potential of Water Hyacinth: A Case Study from Hartbeespoort Dam, South Africa. S. Afr. J. Chem. Eng. 2021, 37, 31–36. [Google Scholar] [CrossRef]
- Tham, H.T. Water hyacinth (Eichhornia crassipes)—Biomass Production, Ensilability and Feeding Value to Growing Cattle. Ph.D. Thesis, Swedish University of Agricultural Sciences, Uppsala, Sweden, 2012. [Google Scholar]
- Lareo, L.; Bressani, R. Possible Utilization of the Water Hyacinth in Nutrition and Industry. Food Nutr. Bull. 1982, 4, 1–6. [Google Scholar] [CrossRef]
- Hashemi, S.; Hashemi, S.E.; Lien, K.M.; Lamb, J.J. Molecular Microbial Community Analysis as an Analysis Tool for Optimal Biogas Production. Microorganisms 2021, 9, 1162. [Google Scholar] [CrossRef] [PubMed]
- Mirmohamadsadeghi, S.; Karimi, K.; Azarbaijani, R.; Parsa Yeganeh, L.; Angelidaki, I.; Nizami, A.S.; Bhat, R.; Dashora, K.; Vijay, V.K.; Aghbashlo, M.; et al. Pretreatment of Lignocelluloses for Enhanced Biogas Production: A Review on Influencing Mechanisms and the Importance of Microbial Diversity. Renew. Sustain. Energy Rev. 2021, 135, 110173. [Google Scholar] [CrossRef]
- Czekała, W.; Jasiński, T.; Grzelak, M.; Witaszek, K.; Dach, J. Biogas Plant Operation: Digestate as the Valuable Product. Energies 2022, 15, 8275. [Google Scholar] [CrossRef]
- Ansari, F.A.; Wahal, S.; Gupta, S.K.; Rawat, I.; Bux, F. A Comparative Study on Biochemical Methane Potential of Algal Substrates: Implications of Biomass Pre-Treatment and Product Extraction. Bioresour. Technol. 2017, 234, 320–326. [Google Scholar] [CrossRef]
- Rodriguez, C.; Alaswad, A.; Benyounis, K.Y.; Olabi, A.G. Pretreatment Techniques Used in Biogas Production from Grass. Renew. Sustain. Energy Rev. 2017, 68, 1193–1204. [Google Scholar] [CrossRef] [Green Version]
- Wagner, A.O.; Lackner, N.; Mutschlechner, M.; Prem, E.M.; Markt, R.; Illmer, P. Biological Pretreatment Strategies for Second-Generation Lignocellulosic Resources to Enhance Biogas Production. Energies 2018, 11, 1797. [Google Scholar] [CrossRef]
- APHA/AWWA/WEF. Standard Methods for the Examination of Water and Wastewater; Standard Methods; American Public Health Association, American Waterworks Association, Water Environment Federation: Washington, DC, USA, 2012; pp. 1–541. [Google Scholar]
- Selvankumar, T.; Sudhakar, C.; Govindaraju, M.; Selvam, K.; Aroulmoji, V.; Sivakumar, N.; Govarthanan, M. Process Optimization of Biogas Energy Production from Cow Dung with Alkali Pre-Treated Coffee Pulp. 3 Biotech 2017, 7, 254. [Google Scholar] [CrossRef]
- Jingura, R.M.; Kamusoko, R. Methods for Determination of Biomethane Potential of Feedstocks: A Review. Biofuel Res. J. 2017, 14, 573–586. [Google Scholar] [CrossRef]
- Paritosh, K.; Mathur, S.; Pareek, N.; Vivekanand, V. Feasibility Study of Waste (d) Potential: Co-Digestion of Organic Wastes, Synergistic Effect and Kinetics of Biogas Production. Int. J. Environ. Sci. Technol. 2018, 15, 1009–1018. [Google Scholar] [CrossRef]
- Sunwanee, J.; Chairat, S. Kinetic Model of Biogas Production from Co-Digestion of Thai Rice Noodle Wastewater (Khanomjeen) with Chicken Manure. Energy Procedia 2017, 138, 386–392. [Google Scholar]
- Ijoma, G.N.; Nkuna, R.; Mutungwazi, A.; Rashama, C.; Matambo, T.S. Applying PICRUSt and 16S RRNA Functional Characterisation to Predicting Co-Digestion Strategies of Various Animal Manures for Biogas Production. Sci. Rep. 2021, 11, 19913. [Google Scholar] [CrossRef]
- Zahan, Z.; Othman, M.Z.; Muster, T.H. Anaerobic Digestion/Co-Digestion Kinetic Potentials of Different Agro-Industrial Wastes: A Comparative Batch Study for C/N Optimisation. Waste Manag. 2018, 71, 663–674. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Zan, F.; Zhang, W.; Hao, T. Alleviating Nutrient Imbalance of Low Carbon-to-Nitrogen Ratio Food Waste in Anaerobic Digestion by Controlling the Inoculum-to-Substrate Ratio. Bioresour. Technol. 2022, 346, 126342. [Google Scholar] [CrossRef]
- Lüttge, U. Fat-Carbohydrate-Protein: Storage in Plant Seeds. Lipid Technol. 2013, 25, 79–81. [Google Scholar] [CrossRef]
- Ransom-Jones, E.; Jones, D.L.; McCarthy, A.J.; McDonald, J.E. The Fibrobacteres: An Important Phylum of Cellulose-Degrading Bacteria. Microb. Ecol. 2012, 63, 267–281. [Google Scholar] [CrossRef]
- Yang, Y.; Yu, K.; Xia, Y.; Lau, F.T.K.; Tang, D.T.W.; Fung, W.C.; Fang, H.H.P.; Zhang, T. Metagenomic Analysis of Sludge From Full-scale Anaerobic Digesters Operated in Municipal Wastewater Treatment Plants. Environ. Biotechnol. 2014, 98, 5709–5718. [Google Scholar] [CrossRef]
- Nelson, M.C.; Morrison, M.; Yu, Z. A Meta-analysis of the Microbial Diversity Observed in Anaerobic Digesters. Bioresour. Technol. 2011, 102, 3730–3739. [Google Scholar] [CrossRef]
- Chouari, R.; Le Paslier, D.; Daegelen, P.; Ginestet, P.; Weissenbach, J.; Sghir, A. Novel Predominant Archaeal and Bacterial Groups Revealed by Molecular Analysis of an Anaerobic Sludge Digester. Environ. Microbiol. 2005, 7, 1104–1115. [Google Scholar] [CrossRef] [PubMed]
- Baştabak, B.; Koçar, G. A Review of the Biogas Digestate in Agricultural Framework. J. Mater. Cycles Waste Manag. 2020, 22, 1318–1327. [Google Scholar] [CrossRef]
- Malhotra, M.; Aboudi, K.; Pisharody, L.; Singh, A.; Banu, J.R.; Bhatia, S.K.; Varjani, S.; Kumar, S.; González-Fernández, C.; Kumar, S.; et al. Biorefinery of Anaerobic Digestate in a Circular Bioeconomy: Opportunities, Challenges and Perspectives. Renew. Sustain. Energy Rev. 2022, 166, 112642. [Google Scholar] [CrossRef]
- Foster, P.; Prasad, M. Development of Quality Standards for Compost and Digestate in Ireland; Environmental Protection Agency: Wexford, Ireland, 2021.
- Monlau, F.; Sambusiti, C.; Ficara, E.; Aboulkas, A.; Barakat, A.; Carrère, H. New Opportunities for Agricultural Digestate Valorization: Current Situation and Perspectives. Energy Environ. Sci. 2015, 8, 2600–2621. [Google Scholar] [CrossRef]


| Parameter | Units | WH | CM |
|---|---|---|---|
| Moisture content | % of TS | 95 ± 2.3 | 83 ± 3 |
| Volatile solids (VS) | % of TS | 88 ± 2 | 77 ± 1.6 |
| Carbon (C) | % of TS | 31 ± 1 | 48 ± 1 |
| Hydrogen (H) | % of TS | 19 ± 0.7 | 7 ± 0.8 |
| Nitrogen (N) | % of TS | 3 ± 1 | 3 ± 0.6 |
| C:N | % of TS | 10 | 16 |
| Protein | % of TS | 1.76 ± 0.12 | 2.3 ± 0.09 |
| Fats | % of TS | 0 | 0.75 ± 0.01 |
| Cellulose | % of TS | 0.98 ± 0.03 | 3.34 ± 0.02 |
| Hemicellulose | % of TS | 1.33 ± 0.02 | 4.21 ± 0.04 |
| Lignin | % of TS | 0.6 ± 0.01 | 2.55 ± 0.02 |
| Degradables:VS | % | 2 | 4 |
| pH | - | 6 ± 0.1 | 7.8 ± 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simbayi, T.M.; Rashama, C.; Awosusi, A.A.; Nkuna, R.; Christian, R.; Matambo, T.S. Investigating the Anaerobic Digestion of Water Hyacinth (Eichhornia crassipes) Sourced from Hartbeespoort Dam in South Africa. Fermentation 2023, 9, 685. https://doi.org/10.3390/fermentation9070685
Simbayi TM, Rashama C, Awosusi AA, Nkuna R, Christian R, Matambo TS. Investigating the Anaerobic Digestion of Water Hyacinth (Eichhornia crassipes) Sourced from Hartbeespoort Dam in South Africa. Fermentation. 2023; 9(7):685. https://doi.org/10.3390/fermentation9070685
Chicago/Turabian StyleSimbayi, Trevor M., Charles Rashama, Ayo A. Awosusi, Rosina Nkuna, Riann Christian, and Tonderayi S. Matambo. 2023. "Investigating the Anaerobic Digestion of Water Hyacinth (Eichhornia crassipes) Sourced from Hartbeespoort Dam in South Africa" Fermentation 9, no. 7: 685. https://doi.org/10.3390/fermentation9070685
APA StyleSimbayi, T. M., Rashama, C., Awosusi, A. A., Nkuna, R., Christian, R., & Matambo, T. S. (2023). Investigating the Anaerobic Digestion of Water Hyacinth (Eichhornia crassipes) Sourced from Hartbeespoort Dam in South Africa. Fermentation, 9(7), 685. https://doi.org/10.3390/fermentation9070685







