Effects of Main Nutrient Sources on Improving Monascus Pigments and Saccharifying Power of Monascus purpureus in Submerged Fermentation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strain and Culture Conditions
2.2. Metabolite Detection
2.3. Effects of Carbon and Nitrogen Sources on MPs and SP by M. purpureus G11 in SmF
2.4. Effect of Inorganic Salt Sources on MPs and SP by M. purpureus G11 in SmF
2.5. Effect of Vitamins on MPs and SP by M. purpureus G11 in SmF
2.6. Optimization of Medium Composition Using RSM
2.7. Statistical Analysis
3. Results and Discussion
3.1. Effects of Carbon Source on MPs and SP by M. purpureus G11
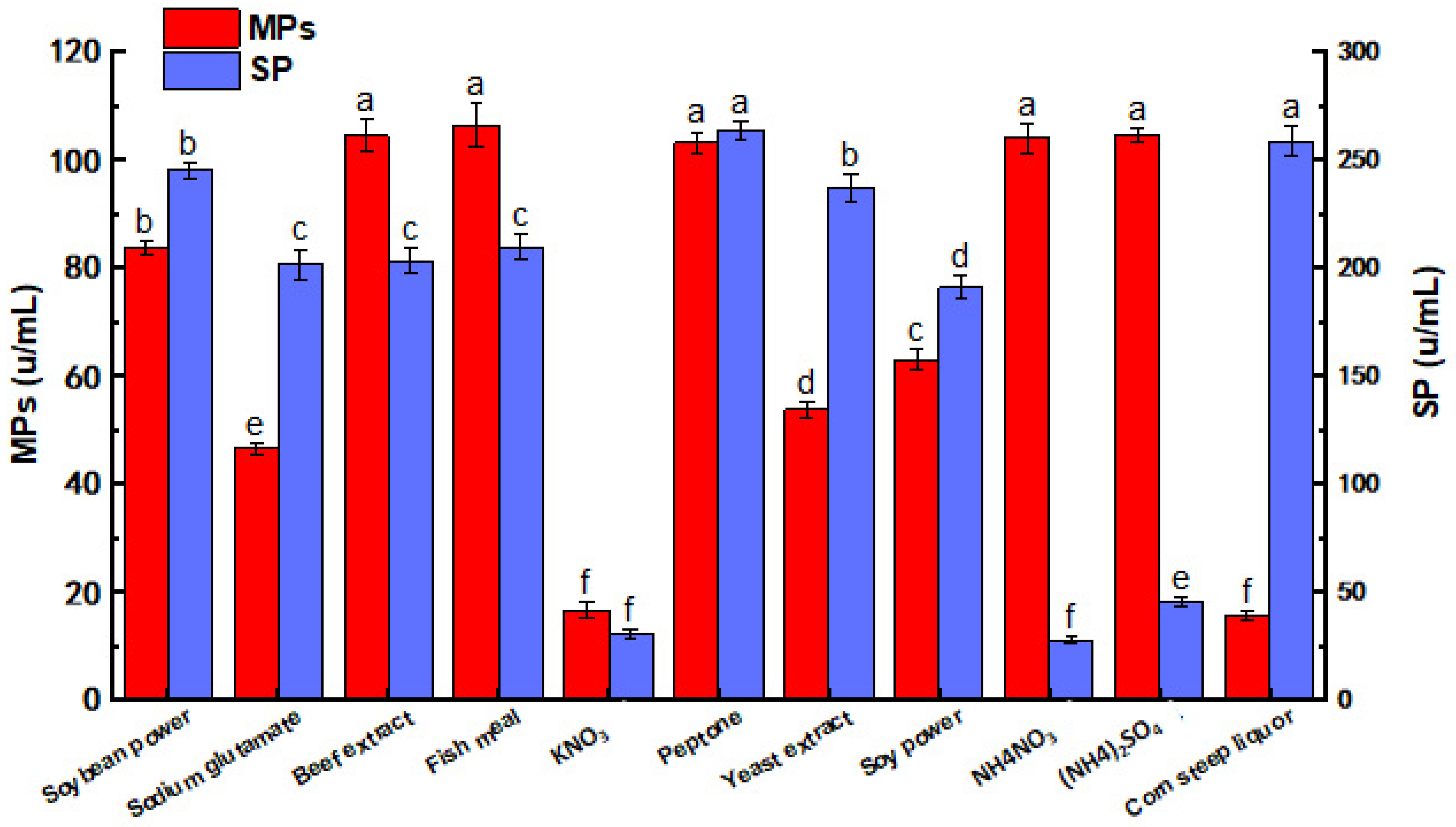
3.2. Effects of Nitrogen Source on MPs and SP by M. purpureus G11
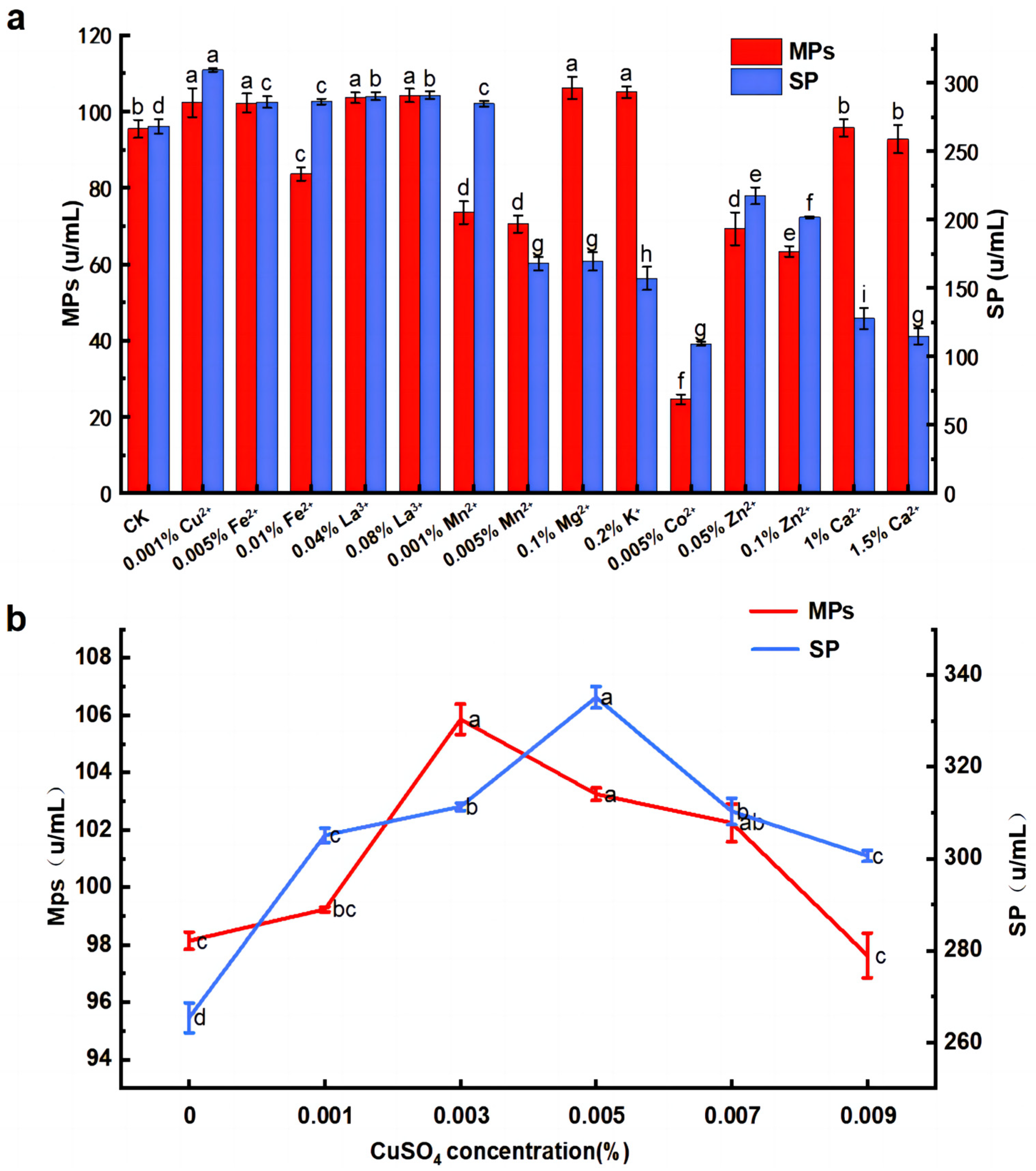
3.3. Effects of Inorganic Salts on MPs and SP by M. purpureus G11
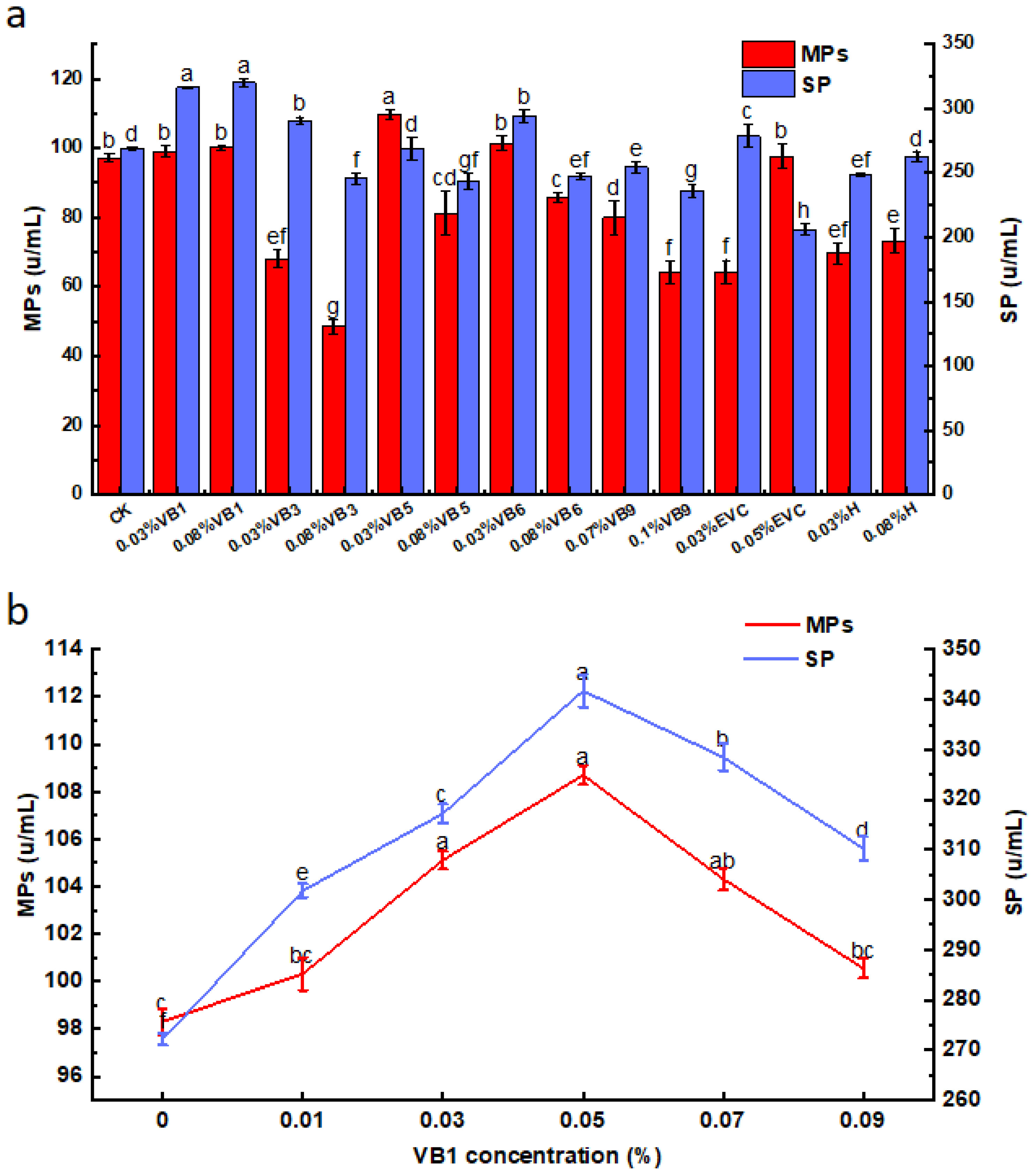
3.4. Effects of Vitamins on MPs and SP by M. purpureus G11
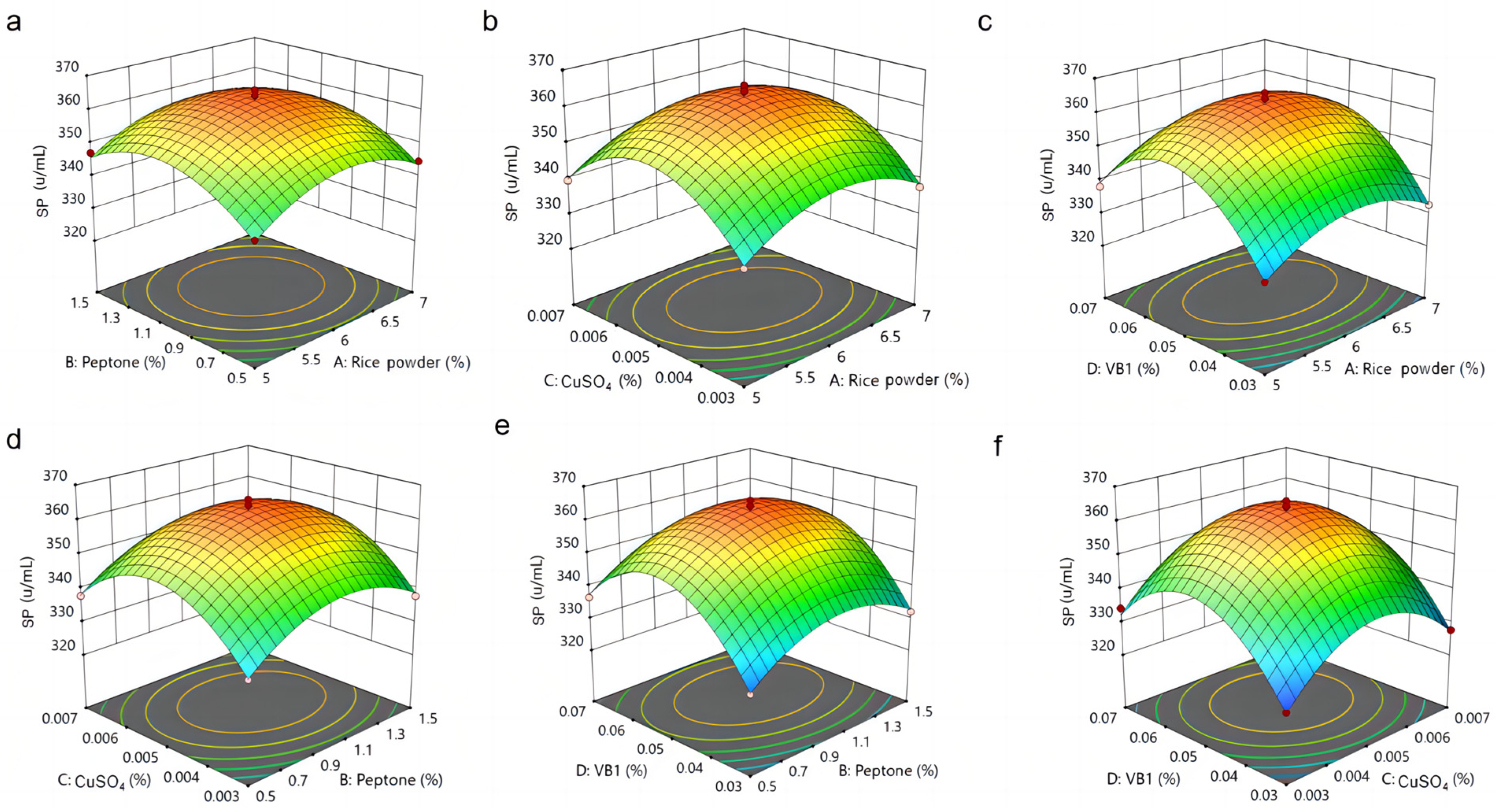
3.5. Analysis of RSM Results for MPs and SP by M. purpureus G11
3.6. Model Validation and Confirmation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, Y.Y.; Liang, Z.C.; Lin, X.Z.; He, Z.G.; Ren, X.Y.; Li, W.X.; Molnar, I. Fungal community diversity and fermentation characteristics in regional varieties of traditional fermentation starters for Hong Qu glutinous rice wine. Food Res. Int. 2021, 141, 110146. [Google Scholar] [CrossRef]
- Shao, Y.; Lei, M.; Mao, Z.; Zhou, Y.; Chen, F. Insights into Monascus biology at the genetic level. Appl. Microbiol. Biotechnol. 2014, 98, 3911–3922. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.M.; Lim, J.W.; Shin, C.G.; Shin, C.S. Comparative characteristics of rice wine fermentations using Monascus koji and rice nuruk. Food Sci. Biotechnol. 2017, 26, 1349–1355. [Google Scholar] [CrossRef]
- Chen, J.Z.; Lin, J.; Ye, X.Y.; Xu, M.A.; Li, W.J.; Deng, X.Z. Study on the liquid fermentation conditions of glucoamylase by Monascus purpureus. Sci. Technol. Food Ind. 2017, 38, 146–150. [Google Scholar]
- Yang, C.L.; Wu, X.P.; Chen, B.; Deng, S.S.; Chen, Z.E.; Huang, Y.Y.; Jin, S.S. Comparative analysis of genetic polymorphisms among Monascus strains by ISSR and RAPD markers. J. Sci. Food Agric. 2017, 97, 636–640. [Google Scholar] [CrossRef] [PubMed]
- Agboyibor, C.; Kong, W.; Chen, D.; Zhang, A.; Niu, S. Monascus pigments production, composition, bioactivity and its application: A review. Biocatal. Agric. Biotechnol. 2018, 16, 433–447. [Google Scholar] [CrossRef]
- Zhao, W.; Qian, M.; Dong, H.; Liu, X.; Bai, W.; Liu, G.; Lv, X. Effect of Hong Qu on the flavor and quality of Hakka yellow rice wine (Huangjiu) produced in Southern China. LWT 2022, 160, 113264. [Google Scholar] [CrossRef]
- Lin, X.; Ren, X.; Huang, Y.; Liang, Z.; Li, W.; Su, H.; He, Z. Regional characteristics and discrimination of the fermentation starter Hong Qu in traditional rice wine brewing. Int. J. Food Sci. Technol. 2021, 56, 5664–5673. [Google Scholar] [CrossRef]
- Chen, S.; Huang, Y.Y.; Yang, C.L. Research status and prospect of brewing Hongqu. China Brew. 2022, 41, 8–12. [Google Scholar]
- Huang, Z.R.; Guo, W.L.; Zhou, W.B.; Li, L.; Xu, J.X.; Hong, J.L.; Liu, H.P.; Zeng, F.; Bai, W.D.; Liu, B.; et al. Microbial communities and volatile metabolites in different traditional fermentation starters used for Hong Qu glutinous rice wine. Food Res. Int. 2019, 121, 593–603. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, Z.; Lv, X.; Zhu, X.; Chen, L.; Ni, L. Comparison study of the volatile profiles and microbial communities of Wuyi Qu and Gutian Qu, two major types of traditional fermentation starters of Hong Qu glutinous rice wine. Food Microbiol. 2018, 69, 105–115. [Google Scholar] [CrossRef]
- Chen, G.M.; Huang, Z.R.; Wu, L.; Wu, Q.; Guo, W.L.; Zhao, W.H.; Liu, B.; Zhang, W.; Rao, P.F.; Lv, X.C.; et al. Microbial diversity and flavor of Chinese rice wine (Huangjiu): An overview of current research and future prospects. Curr. Opin. Food Sci. 2021, 42, 37–50. [Google Scholar] [CrossRef]
- Padmavathi, T.; Rashmi, D.; Anusha, J.; Umme, S. Partial purification and characterization of amylase enzyme under solid state fermentation from Monascus sanguineus. J. Genet. Eng. Biotechnol. 2017, 15, 95–101. [Google Scholar]
- Li, M.; Yang, L.R.; Xu, G.; Wu, J.P. Screening, purification and characterization of a novel cold-active and organic solvent-tolerant lipase from Stenotrophomonas maltophilia CGMCC 4254. Bioresour. Technol. 2013, 148, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Yumiko, Y.; Tomoka, S.; Kazunori, T.; Hisanori, T.; Kiyoshi, I.; Yoshihiro, S. Characterization of glucoamylase and α-amylase from Monascus anka: Enhanced production of α-amylase in red koji. J. Biosci. Bioeng. 2010, 110, 670–674. [Google Scholar]
- Xu, Y.; Wang, X.; Liu, X.; Li, X.; Zhang, C.; Li, W.; Sun, X.; Wang, W.; Sun, B. Discovery and development of a novel short-chain fatty acid ester synthetic biocatalyst under aqueous phase from Monascus purpureus isolated from Baijiu. Food Chem. 2021, 338, 128025. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, Y.; Shao, Y.; Chen, W.; Chen, F.; Li, M. Cloning, expression and characterization of a novel cold-active and organic solvent-tolerant esterase from Monascus ruber M7. Extrem. Life Under Extrem. Cond. 2016, 20, 451–459. [Google Scholar] [CrossRef]
- Yasuda, M.; Tachibana, S.; Kuba-Miyara, M. Biochemical aspects of red koji and tofuyo prepared using Monascus fungi. Appl. Microbiol. Biot. 2012, 96, 49–60. [Google Scholar] [CrossRef]
- Tachibana, S.; Yasuda, M. Purification and characterization of heterogeneous glucoamylases from Monascus purpureus. Biosci. Biotechnol. Biochem. 2007, 71, 2573–2576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, C.; Yoshizaki, Y.; Yin, X.; Wang, Z.; Okutsu, K.; Futagami, T.; Tamaki, H.; Takamine, K. Additional moisture during koji preparation contributes to the pigment production of red koji (Monascus-fermented rice) by influencing gene expression. J. Food Sci. 2021, 86, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Raveendran, S.; Parameswaran, B.; Ummalyma, S.B.; Abraham, A.; Mathew, A.K.; Madhavan, A.; Rebello, S.; Pandey, A. Applications of microbial enzymes in food industry. Food Technol. Biotech. 2018, 56, 16. [Google Scholar] [CrossRef] [PubMed]
- Tong, A.; Lu, J.; Huang, Z.; Huang, Q.; Zhang, Y.; Farag, M.A.; Liu, B.; Zhao, C. Comparative transcriptomics discloses the regulatory impact of carbon/nitrogen fermentation on the biosynthesis of Monascus kaoliang pigments. Food Chem. X 2022, 13, 100250. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.Y.; Chen, S.; Yang, C.L.; Lu, D.H. A review on metabolism regulation methods of pigment and citrinin in Monascus. China Condiment 2018, 43, 188–194. [Google Scholar]
- Yang, D.C.; Qiang, X.H.; Huang, Y.Q.; Wang, W.P. Effect of Cu2+, Fe2+ and Fe3+ on the yield of Monascus pigments. China Food Addit. 2017, 4, 100–102. [Google Scholar]
- Desai, K.M.; Survase, S.A.; Saudagar, P.S.; Lele, S.S.; Singhal, R.S. Comparison of artificial neural network (ANN) and response surface methodology (RSM) in fermentation media optimization: Case study of fermentative production of scleroglucan. Biochem. Eng. J. 2008, 41, 266–273. [Google Scholar] [CrossRef]
- Daud, N.S.; Azam, Z.M.; Othman, N.Z. Optimization of medium compositions and functional characteristics of exopolysaccharide from Paenibacillus polymyxa ATCC 824. Biocatal. Agric. Biotechnol. 2023, 49, 102656. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, C.; Molnar, I.; Chen, S. Comparative transcriptomic analysis of key genes involved in citrinin biosynthesis in Monascus purpureus. J. Fungi 2023, 9, 200. [Google Scholar] [CrossRef]
- Shang, X.P.; Xu, S.J.; Chen, L.H.Z.; Chen, L.J. Research progress and prospect of submerged fermentation of functional Monascus. Food Ferment. Ind. 2022, 48, 320–327. [Google Scholar]
- Jiang, X.; Qiu, X.; Yang, J.; Zhang, S.; Liu, J.; Ren, J.; Lu, D.; Zhou, X.; Zhou, B. A mutant of Monascus purpureus obtained by carbon ion beam irradiation yielded yellow pigments using various nitrogen sources. Enzym. Microb. Technol. 2023, 162, 110121. [Google Scholar] [CrossRef]
- Embaby, A.M.; Hussein, M.N.; Hussein, A. Monascus orange and red pigments production by Monascus purpureus ATCC16436 through co-solid state fermentation of corn cob and glycerol: An eco-friendly environmental low cost approach. PLoS ONE 2018, 13, e207755. [Google Scholar] [CrossRef] [Green Version]
- Said, F.M.; Brooks, J.; Chisti, Y. Optimal C: N ratio for the production of red pigments by Monascus ruber. World J. Microbiol. Biotechnol. 2014, 30, 2471–2479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.; Chen, R.; Liu, Q.; He, Y.; He, K.; Ding, X.; Kang, L.; Guo, X.; Xie, N.; Zhou, Y.; et al. Orange, red, yellow: Biosynthesis of azaphilone pigments in Monascus fungi. Chem. Sci. 2017, 8, 4917–4925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patrovsky, M.; Sinovska, K.; Branska, B.; Patakova, P. Effect of initial pH, different nitrogen sources, and cultivation time on the production of yellow or orange Monascus purpureus pigments and the mycotoxin citrinin. Food Sci. Nutr. 2019, 7, 3494–3500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guang, H.W.; Liu, T.T.; Chen, L.; Xu, G.R.; Zhang, B.B. Optimization of conditions for producing natural yellow pigment by submerged fermentation of Monascus sp. Food Ferment. Ind. 2020, 46, 150–156. [Google Scholar]
- Shi, K.; Song, D.; Chen, G.; Pistolozzi, M.; Wu, Z.; Quan, L. Controlling composition and color characteristics of Monascus pigments by pH and nitrogen sources in submerged fermentation. J. Biosci. Bioeng. 2015, 120, 145–154. [Google Scholar] [CrossRef]
- Huang, S.N.; Gao, M.X.; Liu, Y.B. Regulatory effect of 5 metal lons on the spectrum of major secondary metabolites of Monascus purpureus. Sci. Technol. Food Ind. 2021, 42, 89–99. [Google Scholar]
- Liu, H.H.; Yang, F.; Li, J.Z.; Wang, X.F.; Xue, Y.B.; Chen, M.H.; Wang, C.L. Screening and identification of high-yield esterase Monascus and the enzymatic property. China Brew. 2019, 38, 49–53. [Google Scholar]
- Kalil, S.J.; Maugeri, F.; Rodrigues, M.I. Response surface analysis and simulation as a tool for bioprocess design and optimization. Process Biochem. 2000, 35, 539. [Google Scholar] [CrossRef]

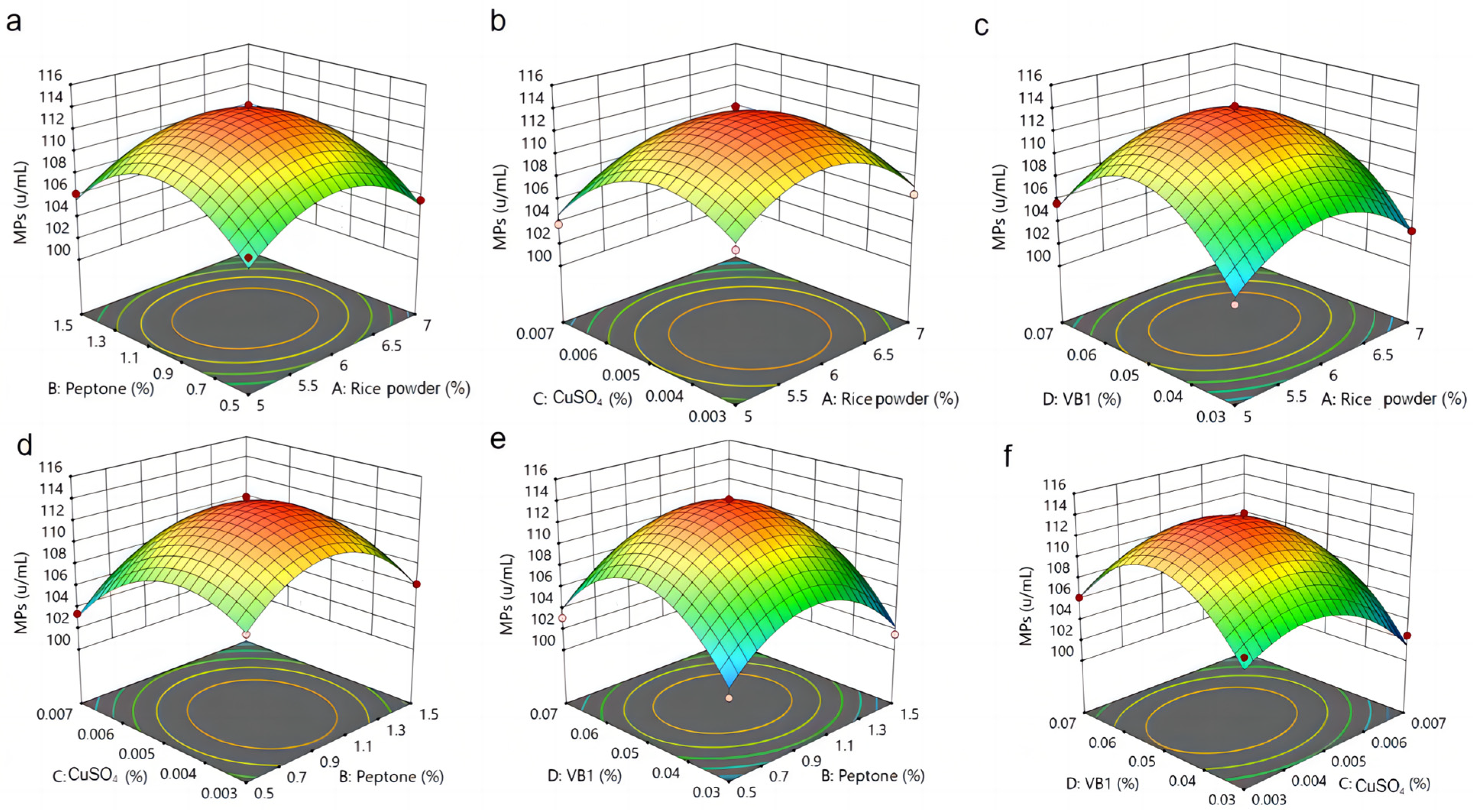
| Independent Factors | Symbol | Range and Code Levels | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| Rice powder (%) | A | 5 | 6 | 7 |
| Peptone (%) | B | 0.5 | 1 | 1.5 |
| Cu2+ (%) | C | 0.003 | 0.005 | 0.007 |
| VB1 (%) | D | 0.03 | 0.05 | 0.07 |
| No. | Rice Powder/% | Peptone/% | Cu2+/% | VB1/% | Observed MP Yield/(u/mL) | Observed SP Activity/(u/mL) |
|---|---|---|---|---|---|---|
| 1 | 5 | 0.5 | 0.005 | 0.05 | 106.4 | 339.56 |
| 2 | 7 | 0.5 | 0.005 | 0.05 | 105.6 | 344.63 |
| 3 | 5 | 1.5 | 0.005 | 0.05 | 106.2 | 347.17 |
| 4 | 7 | 1.5 | 0.005 | 0.05 | 104.4 | 349.78 |
| 5 | 6 | 1 | 0.003 | 0.03 | 106.5 | 323.66 |
| 6 | 6 | 1 | 0.007 | 0.03 | 102.5 | 327.63 |
| 7 | 6 | 1 | 0.003 | 0.07 | 106.2 | 334.25 |
| 8 | 6 | 1 | 0.007 | 0.07 | 103.2 | 340.14 |
| 9 | 5 | 1 | 0.005 | 0.03 | 103.1 | 329.45 |
| 10 | 7 | 1 | 0.005 | 0.03 | 103.2 | 332.64 |
| 11 | 5 | 1 | 0.005 | 0.07 | 105.7 | 338.16 |
| 12 | 7 | 1 | 0.005 | 0.07 | 105.2 | 341.57 |
| 13 | 6 | 0.5 | 0.003 | 0.05 | 107.5 | 332.72 |
| 14 | 6 | 1.5 | 0.003 | 0.05 | 106.2 | 337.80 |
| 15 | 6 | 0.5 | 0.007 | 0.05 | 103.4 | 337.84 |
| 16 | 6 | 1.5 | 0.007 | 0.05 | 104.4 | 342.25 |
| 17 | 5 | 1 | 0.003 | 0.05 | 107.5 | 334.42 |
| 18 | 7 | 1 | 0.003 | 0.05 | 106.5 | 337.80 |
| 19 | 5 | 1 | 0.007 | 0.05 | 103.8 | 339.63 |
| 20 | 7 | 1 | 0.007 | 0.05 | 102.9 | 342.76 |
| 21 | 6 | 0.5 | 0.005 | 0.03 | 102.1 | 327.04 |
| 22 | 6 | 1.5 | 0.005 | 0.03 | 101.5 | 332.16 |
| 23 | 6 | 0.5 | 0.005 | 0.07 | 103.1 | 336.59 |
| 24 | 6 | 1.5 | 0.005 | 0.07 | 103.2 | 341.77 |
| 25 | 6 | 1 | 0.005 | 0.05 | 113.42 | 364.15 |
| 26 | 6 | 1 | 0.005 | 0.05 | 114.2 | 365.86 |
| 27 | 6 | 1 | 0.005 | 0.05 | 113.5 | 360.85 |
| 28 | 6 | 1 | 0.005 | 0.05 | 113.2 | 364.26 |
| 29 | 6 | 1 | 0.005 | 0.05 | 114.2 | 358.13 |
| Source | Sum Sq | Df | Mean Sq | F Value | p Value | Significant |
|---|---|---|---|---|---|---|
| Model | 405.76 | 14 | 28.98 | 34.93 | <0.0001 | ** |
| A | 2 | 1 | 2 | 2.41 | 0.1428 | |
| B | 0.4033 | 1 | 0.4033 | 0.4861 | 0.4971 | |
| C | 34 | 1 | 34 | 40.98 | <0.0001 | ** |
| D | 4.94 | 1 | 4.94 | 5.95 | 0.0286 | * |
| AB | 0.25 | 1 | 0.25 | 0.3013 | 0.5917 | |
| AC | 0.0025 | 1 | 0.0025 | 0.003 | 0.957 | |
| AD | 0.09 | 1 | 0.09 | 0.1085 | 0.7468 | |
| BC | 1.32 | 1 | 1.32 | 1.59 | 0.2274 | |
| BD | 0.1225 | 1 | 0.1225 | 0.1476 | 0.7066 | |
| CD | 0.25 | 1 | 0.25 | 0.3013 | 0.5917 | |
| A2 | 97.92 | 1 | 97.92 | 118.02 | <0.0001 | ** |
| B2 | 143.15 | 1 | 143.15 | 172.54 | <0.0001 | ** |
| C2 | 97.29 | 1 | 97.29 | 117.26 | <0.0001 | ** |
| D2 | 215.23 | 1 | 215.23 | 259.41 | <0.0001 | ** |
| Residual | 11.62 | 14 | 0.8297 | |||
| Lack-of-fit | 10.75 | 10 | 1.07 | 4.95 | 0.0685 | |
| Pure error | 0.8683 | 4 | 0.2171 | |||
| Cor. total | 417.38 | 28 |
| Source | Sum Sq | DF | Mean Sq | F Value | p Value | Significant |
|---|---|---|---|---|---|---|
| Model | 3621.32 | 14 | 258.67 | 58.41 | <0.0001 | ** |
| A | 36.01 | 1 | 36.01 | 8.13 | 0.0128 | * |
| B | 88.23 | 1 | 88.23 | 19.92 | 0.0005 | ** |
| C | 73.08 | 1 | 73.08 | 16.5 | 0.0012 | ** |
| D | 298.98 | 1 | 298.98 | 67.51 | <0.0001 | ** |
| AB | 1.51 | 1 | 1.51 | 0.3405 | 0.5688 | |
| AC | 0.016 | 1 | 0.016 | 0.0036 | 0.9529 | |
| AD | 0.0125 | 1 | 0.0125 | 0.0028 | 0.9583 | |
| BC | 0.1112 | 1 | 0.1112 | 0.0251 | 0.8763 | |
| BD | 0.0011 | 1 | 0.0011 | 0.0002 | 0.9877 | |
| CD | 0.9254 | 1 | 0.9254 | 0.209 | 0.6546 | |
| A2 | 498.87 | 1 | 498.87 | 112.65 | <0.0001 | ** |
| B2 | 623.57 | 1 | 623.57 | 140.81 | <0.0001 | ** |
| C2 | 1383.4 | 1 | 1383.4 | 312.39 | <0.0001 | ** |
| D2 | 2063.08 | 1 | 2063.08 | 465.88 | <0.0001 | ** |
| Residual | 62 | 14 | 4.43 | |||
| Lack-of-fit | 23.23 | 10 | 2.32 | 0.2396 | 0.9696 | |
| Pure error | 38.77 | 4 | 9.69 | |||
| Cor. total | 3683.32 | 28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Chen, J.; Chen, Q.; Yang, C. Effects of Main Nutrient Sources on Improving Monascus Pigments and Saccharifying Power of Monascus purpureus in Submerged Fermentation. Fermentation 2023, 9, 696. https://doi.org/10.3390/fermentation9070696
Huang Y, Chen J, Chen Q, Yang C. Effects of Main Nutrient Sources on Improving Monascus Pigments and Saccharifying Power of Monascus purpureus in Submerged Fermentation. Fermentation. 2023; 9(7):696. https://doi.org/10.3390/fermentation9070696
Chicago/Turabian StyleHuang, Yingying, Jiashi Chen, Qing Chen, and Chenglong Yang. 2023. "Effects of Main Nutrient Sources on Improving Monascus Pigments and Saccharifying Power of Monascus purpureus in Submerged Fermentation" Fermentation 9, no. 7: 696. https://doi.org/10.3390/fermentation9070696
APA StyleHuang, Y., Chen, J., Chen, Q., & Yang, C. (2023). Effects of Main Nutrient Sources on Improving Monascus Pigments and Saccharifying Power of Monascus purpureus in Submerged Fermentation. Fermentation, 9(7), 696. https://doi.org/10.3390/fermentation9070696





