Effects of Cynanchum bungei Decne Addition on the Physicochemical Properties and Antioxidant Activity of Rice Wine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Brewing Method
2.3. General Component Measurements
2.4. Total Phenolic and Total Flavonoid Measurements
2.5. γ-Aminobutyric Acid (GABA) Content Measurements
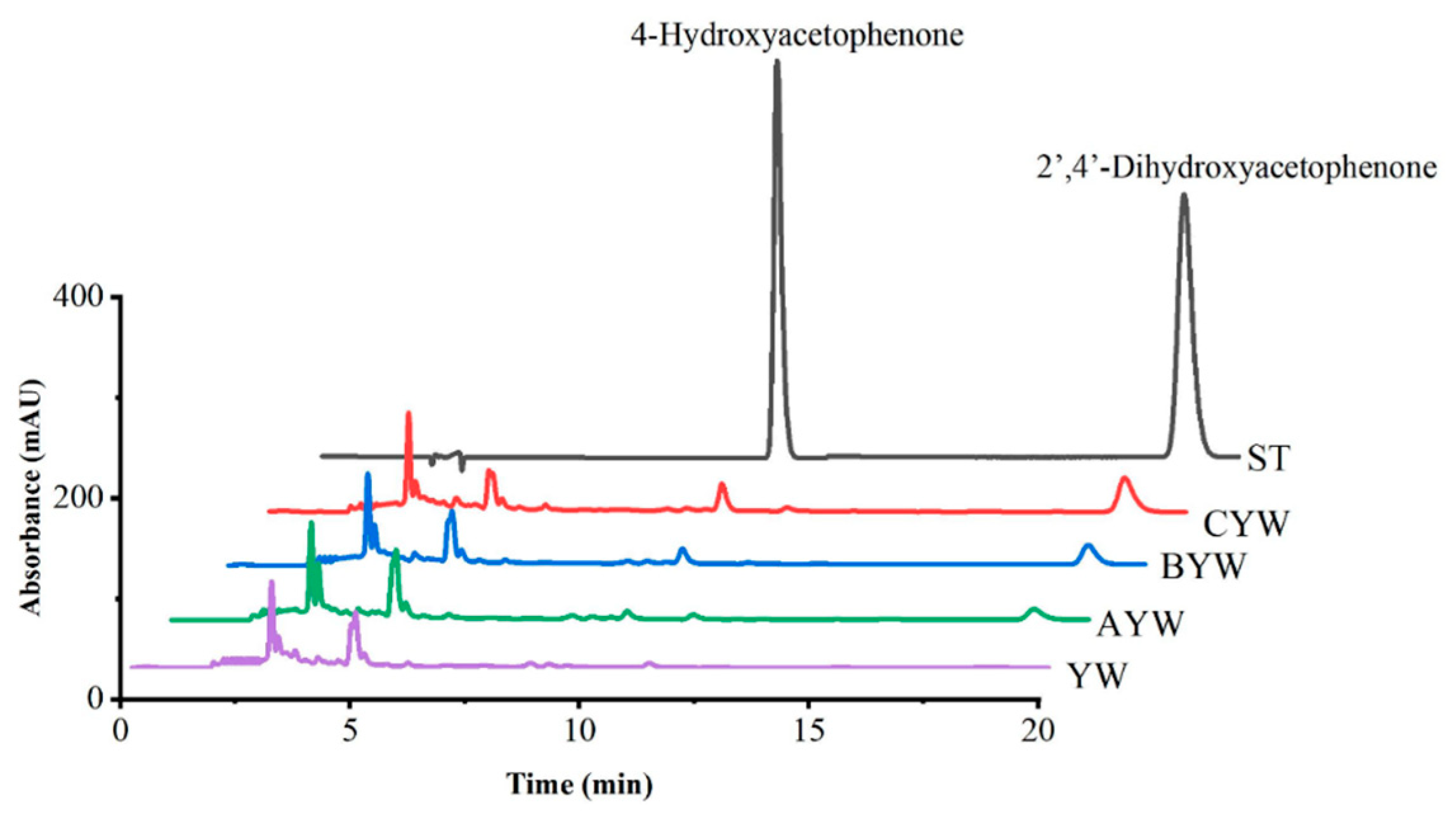
2.6. Acetophenone Content Measurements
2.7. Antioxidant Capacity Measurement
2.8. Sensory Assessment
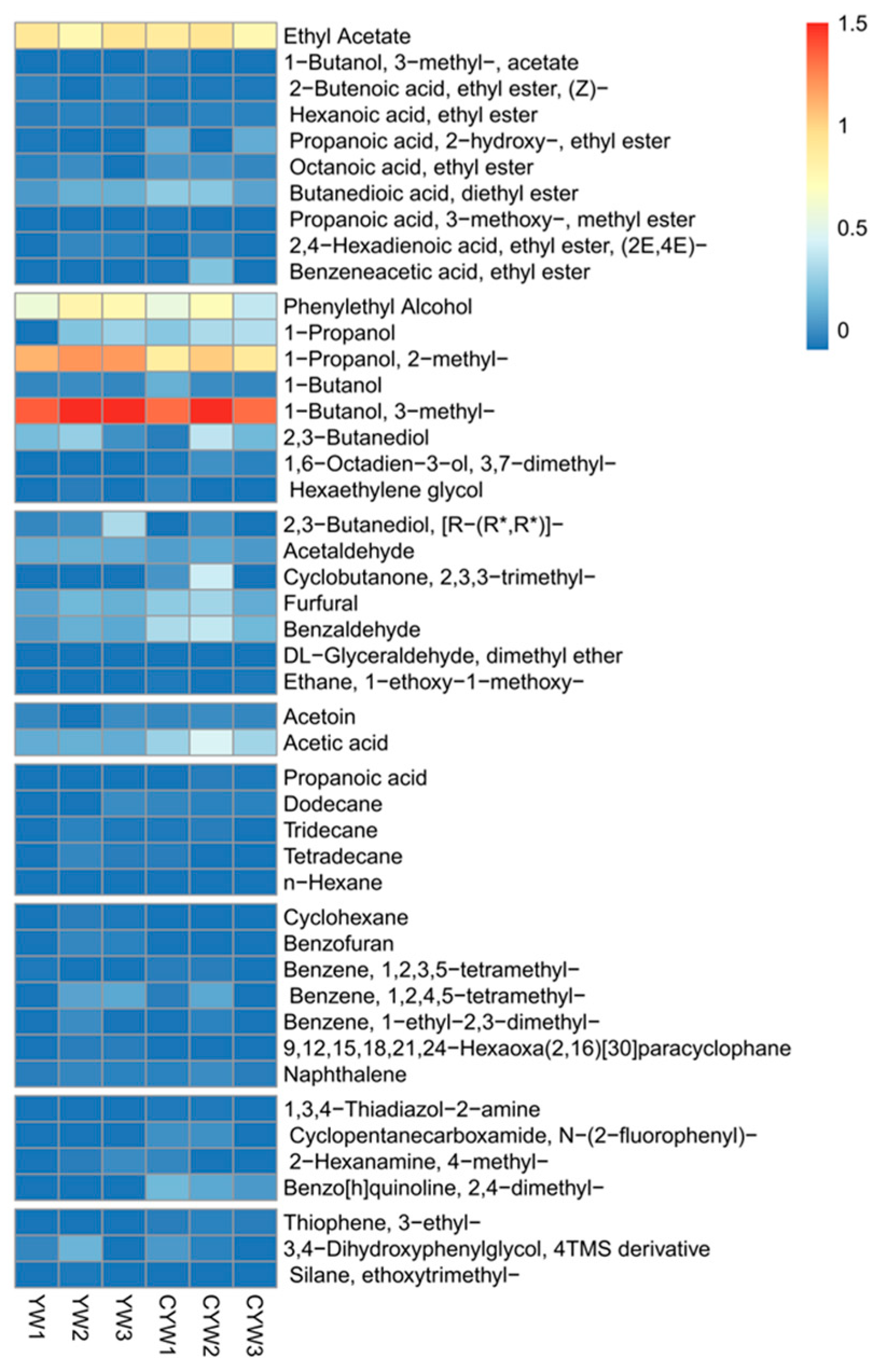
2.9. Volatile Compound Identification
2.10. Measurement of the Glucose Uptake of Yeast Cells
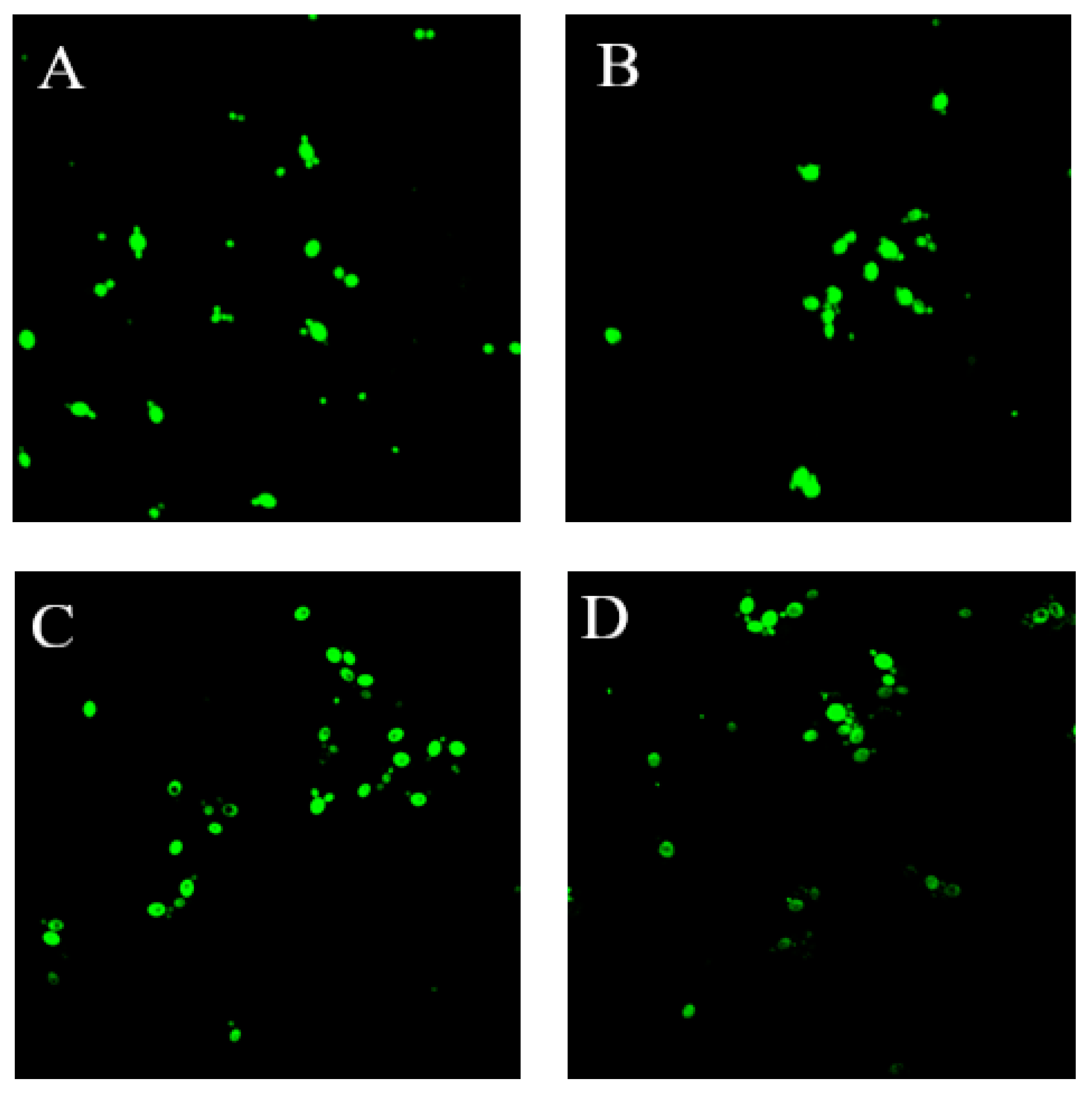
2.11. Measurement of the Viability of Yeast Cells
2.12. Determination of Alcohol Dehydrogenase (ADH) Activity
2.13. Data Analysis
3. Results and Discussion
3.1. Basic Physical and Chemical Characteristics of C. bungei Decne Rice Wine
3.2. Total Phenolic and Flavonoid Contents of Rice Wine
3.3. GABA Content in Rice Wine
3.4. Acetophenone Content in Rice Wine
3.5. Antioxidant Capacity of Rice Wine
3.6. Volatile Compound Identification
3.7. Effects of C. bungei Decne Extract on Yeast Vitality
3.8. Sensory Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jiao, A.; Xu, X.; Jin, Z. Research progress on the brewing techniques of new-type rice wine. Food Chem. 2017, 215, 508–515. [Google Scholar] [CrossRef]
- Guo, H.; Liu, L.; Shi, Y.; Sun, A.; Xu, F.; Chi, J.; Huang, D. Chinese yellow wine and red wine inhibit matrix metalloproteinase-2 and improve atherosclerotic plaque in LDL receptor knockout mice. Cardiovasc. Ther. 2010, 28, 161–168. [Google Scholar] [CrossRef]
- Liu, R.; Fu, Z.; Zhang, F.; Mao, Q.; Luan, C.; Han, X.; Xue, J.; Wang, D.; Qin, S.; Hao, F. Effect of yellow rice wine on anti-aging ability in aged mice induced by d-galactose. Food Sci. Hum. Wellness 2020, 9, 184–191. [Google Scholar] [CrossRef]
- Zhou, W.; Hu, J.; Zhang, X.; Chen, Q. Application of bamboo leaves extract to Chinese yellow rice wine brewing for ethyl carbamate regulation and its mitigation mechanism. Food Chem. 2020, 319, 126554. [Google Scholar] [CrossRef]
- Ren, N.; Gong, W.; Zhao, Y.; Zhao, D.G.; Xu, Y. Innovation in sweet rice wine with high antioxidant activity: Eucommia ulmoides leaf sweet rice wine. Front. Nutr. 2022, 9, 1108843. [Google Scholar] [CrossRef]
- Chen, L.; Liu, B.; Li, D.; Wang, S.; Ma, X.; Zhang, Y. Effects of fermentation on flavor and antioxidant activity in ginkgo rice wine. Food Biosci. 2023, 53, 102652. [Google Scholar] [CrossRef]
- Miao, M.; Jiang, H.; Jiang, B.; Cui, S.W.; Jin, Z.; Zhang, T. Functional characteristics of starches from the root of Cynanchum auriculatum Royle ex Wight grown in China. Carbohydr. Polym. 2012, 88, 568–575. [Google Scholar] [CrossRef]
- Bailly, C. Anticancer properties of caudatin and related C-21 steroidal glycosides from Cynanchum plants. Steroids 2021, 172, 108855. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, D.; Li, B. Neuroprotection of two C21 steroidal glycosides from Cynanchum auriculatum against H2O2-induced damage on PC12 cells. Nat. Prod. Res. 2021, 35, 1752–1755. [Google Scholar] [CrossRef]
- Wang, X.J.; Li, Z.L.; Lv, X.H.; Zuo, Q.Y.; Zhao, Y.M.; Ding, Y.F.; Pu, S.B.; Qian, S.H.; Peng, Y.R. Antitumor evaluation and multiple analysis on different extracted fractions of the root of Cynanchum auriculatum Royle ex Wight. J. Sep. Sci. 2017, 40, 3054–3063. [Google Scholar] [CrossRef]
- Zhou, W.; Fang, R.; Chen, Q. Effect of gallic and protocatechuic acids on the metabolism of ethyl carbamate in Chinese yellow rice wine brewing. Food Chem. 2017, 233, 174–181. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- GB/T 13662-2018; National Standard of the People’s Republic of China-Huangjiu. State Administration for Market Regulation, China National Committee for Standardization: Beijing, China, 2018; pp. 1–25.
- Gasinski, A.; Kawa-Rygielska, J.; Szumny, A.; Czubaszek, A.; Gasior, J.; Pietrzak, W. Volatile compounds content, physicochemical parameters, and antioxidant activity of beers with addition of mango fruit (Mangifera Indica). Molecules 2020, 25, 3033. [Google Scholar] [CrossRef] [PubMed]
- Chu, X.; Li, R.; Wei, H.; Liu, H.; Mu, Y.; Jiang, H.; Ma, Z. Determination of total flavonoid and polysaccharide content in Anoectochilus formosanus in response to different light qualities using hyperspectral imaging. Infrared Phys. Technol. 2022, 122, 104098. [Google Scholar] [CrossRef]
- Zhang, Q.; Sun, Q.; Tan, X.; Zhang, S.; Zeng, L.; Tang, J.; Xiang, W. Characterization of γ-aminobutyric acid (GABA)-producing Saccharomyces cerevisiae and coculture with Lactobacillus plantarum for mulberry beverage brewing. J. Biosci. Bioeng. 2020, 129, 447–453. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, Z.; Wang, J.; Yang, S.; Li, B.; Xu, N. Aqueous ionic liquid based ultrasonic assisted extraction of four acetophenones from the Chinese medicinal plant Cynanchum bungei Decne. Ultrason. Sonochem. 2013, 20, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Pinteus, S.; Silva, J.; Alves, C.; Horta, A.; Fino, N.; Rodrigues, A.I.; Mendes, S.; Pedrosa, R. Cytoprotective effect of seaweeds with high antioxidant activity from the Peniche coast (Portugal). Food Chem. 2017, 218, 591–599. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, X.; Liu, H.; Gong, D.; Li, X. Comparison of the nutritional and phytochemical composition and antioxidant activities of Aralia elata (Miq.) Seem fruits in Northeast China. Arab. J. Chem. 2021, 14, 103448. [Google Scholar] [CrossRef]
- Yu, H.; Guo, W.; Xie, T.; Ai, L.; Tian, H.; Chen, C. Aroma characteristics of traditional Huangjiu produced around Winter Solstice revealed by sensory evaluation, gas chromatography–mass spectrometry and gas chromatography–ion mobility spectrometry. Food Res. Int. 2021, 145, 110421. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, Y.; Wang, J.; Zhu, H.; Zhou, H.; Hu, J.; Wang, J. Preparative isolation and purification of acetophenones from the Chinese medicinal plant Cynanchum bungei Decne. by high-speed counter-current chromatography. Sep. Purif. Technol. 2008, 64, 247–252. [Google Scholar] [CrossRef]
- Chukwuma, C.I.; Matsabisa, M.G.; Erukainure, O.L.; Ibeji, C.U.; Islam, M.S. D-mannitol modulates glucose uptake ex vivo; suppresses intestinal glucose absorption in normal and type 2 diabetic rats. Food Biosci. 2019, 29, 30–36. [Google Scholar] [CrossRef]
- Tanaka, J.; Kiyoshi, K.; Kadokura, T.; Suzuki, K.-i.; Nakayama, S. Elucidation of the enzyme involved in 2,3,5-triphenyl tetrazolium chloride (TTC) staining activity and the relationship between TTC staining activity and fermentation profiles in Saccharomyces cerevisiae. J. Biosci. Bioeng. 2021, 131, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Kwolek-Mirek, M.; Bednarska, S.; Zadrąg-Tȩcza, R.; Bartosz, G. The hydrolytic activity of esterases in the yeast Saccharomyces cerevisiae is strain dependent. Cell Biol. Int. 2011, 35, 1111–1119. [Google Scholar] [CrossRef]
- Chen, X.; Li, L.; Yang, H.; Zhou, H. Effects of the addition of Dendrobium officinale on beer yeast fermentation. Fermentation 2022, 8, 595. [Google Scholar] [CrossRef]
- Li, S.Y.; Zhang, M.M.; Song, Y.Q.; Hu, X.Y.; Liang, N. Optimization of fermentation conditions of alcohol dehydrogenase from Acetobacter pomorum. China Brew. 2018, 37, 61–66. [Google Scholar] [CrossRef]
- Wang, X.; Yang, H.; Tian, R.; Mo, Y.; Dong, L.; Shen, C.; Han, X. Effect of the joint fermentation of pyracantha powder and glutinous rice on the physicochemical characterization and functional evaluation of rice wine. Food Sci. Nutr. 2021, 9, 6099–6108. [Google Scholar] [CrossRef] [PubMed]
- Hao, R.; Li, M.; Li, F.; Sun-Waterhouse, D.; Li, D. Protective effects of the phenolic compounds from mung bean hull against H2O2-induced skin aging through alleviating oxidative injury and autophagy in HaCaT cells and HSF cells. Sci. Total Environ. 2022, 841, 156669. [Google Scholar] [CrossRef]
- Yang, Y.; Xia, Y.; Wang, G.; Yu, J.; Ai, L. Effect of mixed yeast starter on volatile flavor compounds in Chinese rice wine during different brewing stages. LWT Food Sci. Technol. 2017, 78, 373–381. [Google Scholar] [CrossRef]
- Lu, Q.-Y.; Lee, R.-P.; Huang, J.; Yang, J.; Henning, S.M.; Hong, X.; Heber, D.; Li, Z. Quantification of bioactive constituents and antioxidant activity of Chinese yellow wine. J. Food Compos. Anal. 2015, 44, 86–92. [Google Scholar] [CrossRef]
- Shen, D.; Labreche, F.; Wu, C.; Fan, G.; Li, T.; Shi, H.; Ye, C. Preparation and aroma analysis of flavonoid-rich ginkgo seeds fermented using rice wine starter. Food Biosci. 2021, 44, 101459. [Google Scholar] [CrossRef]
- Chen, X.; Jia, X.; Yang, S.; Zhang, G.; Li, A.; Du, P.; Liu, L.; Li, C. Optimization of ultrasonic-assisted extraction of flavonoids, polysaccharides, and eleutherosides from Acanthopanax senticosus using response surface methodology in development of health wine. LWT Food Sci. Technol. 2022, 165, 113725. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, P.; Pan, D.; Zeng, X.; Guo, Y.; Zhao, G. Effect of adzuki bean sprout fermented milk enriched in γ-aminobutyric acid on mild depression in a mouse model. J. Dairy Sci. 2021, 104, 78–91. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, Y.; Yin, P.; Zhang, K.; Liu, Y.; Gao, Y.; Li, Y.; Wang, T.; Lu, S.; Li, B. Probiotic potential of γ-aminobutyric acid (GABA)–producing yeast and its influence on the quality of cheese. J. Dairy Sci. 2021, 104, 6559–6576. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Shah, N.P. High γ-aminobutyric acid production from lactic acid bacteria: Emphasis on Lactobacillus brevis as a functional dairy starter. Crit. Rev. Food Sci. Nutr. 2017, 57, 3661–3672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, J.; Ma, Z.; Wang, Y.; Wang, Y.; Sun, L.; Liu, X. Effects of dandelion addition on antioxidant property, sensory characteristics and inhibitory activity against xanthine oxidase of beer. Curr. Res. Food Sci. 2022, 5, 927–939. [Google Scholar] [CrossRef]
- Li, W.; Zhao, L.C.; Sun, Y.S.; Lei, F.J.; Wang, Z.; Gui, X.B.; Wang, H. Optimization of pressurized liquid extraction of three major acetophenones from Cynanchum bungei using a Box-Behnken design. Int. J. Mol. Sci. 2012, 13, 14533–14544. [Google Scholar] [CrossRef] [Green Version]
- Que, F.; Mao, L.; Zhu, C.; Xie, G. Antioxidant properties of Chinese yellow wine, its concentrate and volatiles. LWT Food Sci. Technol. 2006, 39, 111–117. [Google Scholar] [CrossRef]
- Leskosek-Cukalovic, I.; Despotovic, S.; Lakic, N.; Niksic, M.; Nedovic, V.; Tesevic, V. Ganoderma lucidum—Medical mushroom as a raw material for beer with enhanced functional properties. Food Res. Int. 2010, 43, 2262–2269. [Google Scholar] [CrossRef]
- Zhang, H.J.; Wang, Y.; Feng, X.J.; Iftikhar, M.; Meng, X.Y.; Wang, J. The analysis of changes in nutritional components and flavor characteristics of Wazu rice wine during fermentation process. Food Anal. Methods 2022, 15, 1132–1142. [Google Scholar] [CrossRef]
- Xiao, Z.B.; Dai, X.; Zhu, J.C.; Yu, H.Y. Classification of Chinese rice wine according to geographic origin and wine age based on chemometric methods and SBSE-TD-GC-MS analysis of volatile compounds. Food Sci. Technol. Res. 2015, 21, 371–380. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Zhang, C.; Qi, Y.; Dai, L.; Ma, H.; Guo, X.; Xiao, D. Acetate ester production by Chinese yellow rice wine yeast overexpressing the alcohol acetyltransferase-encoding gene ATF2. Genet. Mol. Res. 2014, 13, 9735–9746. [Google Scholar] [CrossRef]
- Sá, F.A.D.S.; Silva, T.C.; Andrade, W.M.; de Ávila, R.I.; Valadares, M.C.; Costa, C.R.; Santos, A.S.; Feitas, V.A.Q.; de Paula, J.R.; Silva, M.d.R.R. Antifungal activity of the ethanolic extract and flavonoid avicularin from Myrcia tomentosa (Aubl.) DC. on virulence factors of Candida species. J. Herb. Med. 2023, 38, 100643. [Google Scholar] [CrossRef]
- Riešutė, R.; Šalomskienė, J.; Šalaševičienė, A.; Mačionienė, I. Combined impacts of various plant derivative extracts and lactic acid bacteria on yeasts to develop a nutritional bar with antifungal properties. Food Biosci. 2022, 47, 101718. [Google Scholar] [CrossRef]
- Wu, C.D.; Zhang, M.; He, M.T.; Gu, M.F.; Lin, M.; Zhang, G. Selection of solvent for extraction of antioxidant components from Cynanchum auriculatum, Cynanchum bungei and Cynanchum wilfordii roots. Food Sci. Nutr. 2019, 7, 1337–1343. [Google Scholar] [CrossRef] [Green Version]

| Characteristic | YW | AYW | BYW | CYW |
|---|---|---|---|---|
| Reducing sugar (mg mL−1) | 7.92 ± 0.66 d | 11.36 ± 1.02 c | 13.19 ± 0.35 b | 17.70 ± 0.12 a |
| Alcohol content (%, v/v) | 16.35 ± 0.24 a | 13.9 ± 0.43 b | 14.49 ± 0.60 b | 14.69 ± 1.09 b |
| Total acid (mg mL−1) | 4.83 ± 0.26 d | 7.29 ± 0.09 c | 7.50 ± 0.09 b | 8.53 ± 0.05 a |
| Amino acid nitrogen (mg mL−1) | 0.72 ± 0.00 d | 0.96 ± 0.02 c | 1.25 ± 0.03 b | 1.32 ± 0.01 a |
| Protein content (μgBSE mL−1) | 61.50 ± 1.07 d | 74.19 ± 3.38 c | 88.83 ± 0.54 b | 98.14 ± 0.45 a |
| Assays | YW | AYW | BYW | CYW |
|---|---|---|---|---|
| Total phenolic content (mg GAE mL−1) | 0.65 ± 0.00 b | 0.63 ± 0.03 b | 0.66 ± 0.02 b | 0.74 ± 0.03 a |
| Total flavonoid content (μg RE mL−1) | 50.76 ± 1.36 d | 94.09 ± 2.41 c | 125.00 ± 4.17 b | 197.42 ± 1.05 a |
| GABA content (μg mL−1) | 29.97 ± 0.53 d | 44.87 ± 0.98 c | 56.09 ± 1.22 b | 73.28 ± 2.87 a |
| 2′,4′-Dihydroxyacetophenone (μg mL−1) | ND | 8.64 ± 0.11 c | 20.14 ± 0.14 b | 38.39 ± 0.21 a |
| 4-Hydroxyacetophenone (μg mL−1) | ND | 2.88 ± 0.09 c | 6.15 ± 0.09 b | 9.75 ± 0.03 a |
| DPPH radical scavenging activity (μg VCE mL−1) | 6.80 ± 0.23 c | 8.98 ± 0.30 b | 9.82 ± 1.11 b | 23.45 ± 0.77 a |
| Fe3+-reducing antioxidant power (FRAP) (mg TRE mL−1) | 0.03 ± 0.00 d | 0.07 ± 0.00 c | 0.08 ± 0.01 b | 0.14 ± 0.01 a |
| ABTS radical scavenging activity (μg VCE mL−1) | 139.61 ± 3.99 b | 143.10 ± 3.21 b | 166.78 ± 2.05 a | 171.98 ± 0.48 a |
| Total Phenolics | Total Flavonoids | DPPH• Scavenging Activity | FRAP | ABTS• Scavenging Activity | |
|---|---|---|---|---|---|
| Total phenolics | 1 | 0.8704 | 0.9559 | 0.8500 | 0.7813 |
| Total flavonoids | 1 | 0.9396 | 0.9932 | 0.9067 | |
| DPPH• scavenging activity | 1 | 0.9470 | 0.7647 | ||
| FRAP | 1 | 0.8537 | |||
| ABTS• scavenging activity | 1 |
| Time (d) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
|---|---|---|---|---|---|---|---|---|
| Content | ||||||||
| Glucose uptake (mg mL−1 min−1) | YW | 0.70 ± 0.01 b | 0.69 ± 0.02 b | 0.70 ± 0.01 c | 0.78 ± 0.05 a | 0.77 ± 0.02 a | 0.90 ± 0.03 a | 0.92 ± 0.04 a |
| AYW | 0.75 ± 0.01 a | 0.75 ± 0.03 a | 0.74 ± 0.02 b | 0.75 ± 0.00 a | 0.77 ± 0.00 ab | 0.81 ± 0.03 bc | 0.80 ± 0.01 b | |
| BYW | 0.71 ± 0.01 ab | 0.70 ± 0.03 b | 0.78 ± 0.02 a | 0.78 ± 0.03 a | 0.76 ± 0.01 b | 0.82 ± 0.03 b | 0.76 ± 0.02 bc | |
| CYW | 0.75 ± 0.04 a | 0.73 ± 0.01 ab | 0.79 ± 0.00 a | 0.77 ± 0.02 a | 0.80 ± 0.04 a | 0.77 ± 0.02 c | 0.74 ± 0.01 c | |
| Cell viability (A485 nm) | YW | 0.32 ± 0.00 c | 0.41 ± 0.01 c | 0.41 ± 0.02 c | 0.68 ± 0.02 a | 0.75 ± 0.02 a | 0.80 ± 0.00 a | 0.89 ± 0.02 a |
| AYW | 0.36 ± 0.00 b | 0.44 ± 0.00 b | 0.63 ± 0.01 b | 0.58 ± 0.01 c | 0.69 ± 0.03 b | 0.77 ± 0.03 ab | 0.73 ± 0.01 b | |
| BYW | 0.35 ± 0.00 b | 0.44 ± 0.01 b | 0.65 ± 0.00 b | 0.57 ± 0.01 c | 0.71 ± 0.01 b | 0.74 ± 0.04 b | 0.74 ± 0.03 b | |
| CYW | 0.44 ± 0.02 a | 0.46 ± 0.00 a | 0.73 ± 0.03 a | 0.61 ± 0.02 b | 0.68 ± 0.02 b | 0.73 ± 0.01 b | 0.74 ± 0.04 b | |
| ADH enzyme activity (U mg−1) | YW | 4317.36 ± 58.34 a | 4599.01 ± 32.92 a | 4326.09 ± 78.98 a | 3998.69 ± 102.34 a | 3760.45 ± 46.43 a | 3627.53 ± 65.45 a | 3366.24 ± 21.92 a |
| AYW | 3858 ± 252.62 b | 4417.46 ± 80.65 b | 4294.26 ± 30.48 a | 3744.78 ± 27.84 b | 3385.19 ± 132.02 b | 3411.96 ± 42.12 b | 3380.69 ± 60.28 a | |
| BYW | 3913.20 ± 50.81 b | 4281.82 ± 128.79 bc | 4262.79 ± 50.15 a | 3772.68 ± 82.71 b | 3486.03 ± 89.96 b | 3422.98 ± 40.75 b | 3368.65 ± 102.55 a | |
| CYW | 4069.12 ± 55.74 ab | 4209.22 ± 26.80 c | 4131.19 ± 27.31 b | 3804 ± 44.76 b | 3536.62 ± 63.81 b | 3290.31 ± 57.99 c | 3262.30 ± 60.28 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, G.; Dong, H.; Liu, S.; Zhou, H.; Yang, H. Effects of Cynanchum bungei Decne Addition on the Physicochemical Properties and Antioxidant Activity of Rice Wine. Fermentation 2023, 9, 700. https://doi.org/10.3390/fermentation9080700
Cai G, Dong H, Liu S, Zhou H, Yang H. Effects of Cynanchum bungei Decne Addition on the Physicochemical Properties and Antioxidant Activity of Rice Wine. Fermentation. 2023; 9(8):700. https://doi.org/10.3390/fermentation9080700
Chicago/Turabian StyleCai, Gonglin, Hangmeng Dong, Shoulong Liu, Huabin Zhou, and Hailong Yang. 2023. "Effects of Cynanchum bungei Decne Addition on the Physicochemical Properties and Antioxidant Activity of Rice Wine" Fermentation 9, no. 8: 700. https://doi.org/10.3390/fermentation9080700





