Exploring the Possibility of Enriching Fermented Milks with Young Barley Leaves Powder Preparation
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
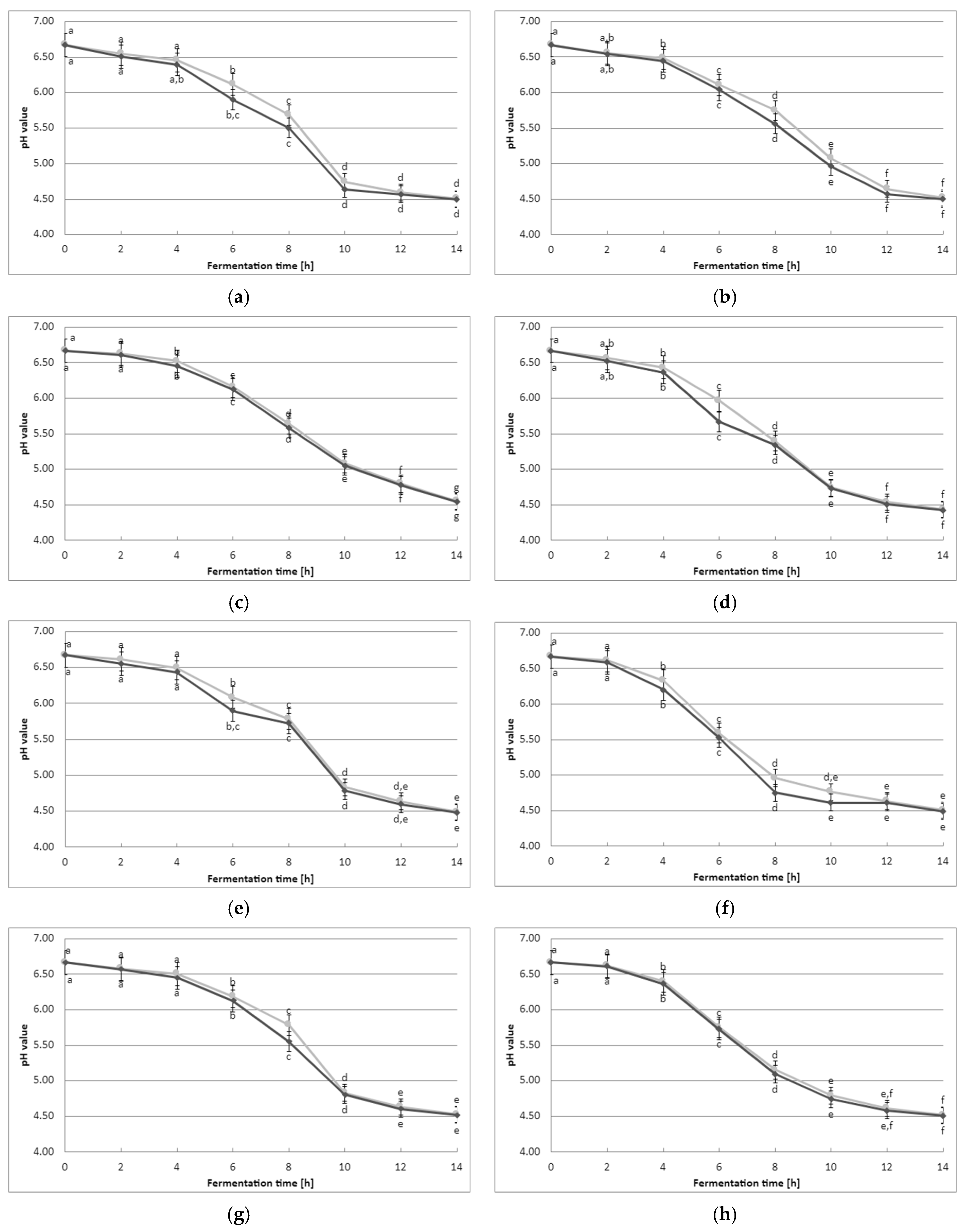
3.1. Acidification Curve
3.2. Change in pH during Refrigerated Storage of Fermented Milks
3.3. Change in Bacterial Cell Population during Refrigerated Storage of Fermented Milks
3.4. Change in Hardness and Adhesion during Refrigerated Storage of Fermented Milks
3.5. Change in Water-Holding Capacity during Refrigerated Storage of Fermented Milks
3.6. Antimicrobial Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yamaura, K.; Nakayama, N.; Shimada, M.; Bi, Y.; Fukata, H.; Ueno, K. Antidepressant-like Effects of Young Green Barley Leaf (Hordeum vulgare L.) in the Mouse Forced Swimming Test. Pharmacogn. Res. 2012, 4, 22–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saunders, J.A.; McClure, J.W. The Occurrence and Photoregulation of Flavonoids in Barley Plastids. Phytochemistry 1976, 15, 805–807. [Google Scholar] [CrossRef]
- Choe, J.-H.; Jang, A.; Choi, J.-H.; Choi, Y.-S.; Han, D.-J.; Kim, H.-Y.; Lee, M.-A.; Kim, H.-W.; Kim, C.-J. Antioxidant Activities of Lotus Leaves (Nelumbo nucifera) and Barley Leaves (Hordeum vulgare) Extracts. Food Sci. Biotechnol. 2010, 19, 831–836. [Google Scholar] [CrossRef]
- Choe, J.-H.; Jang, A.; Lee, E.-S.; Choi, J.-H.; Choi, Y.-S.; Han, D.-J.; Kim, H.-Y.; Lee, M.-A.; Shim, S.-Y.; Kim, C.-J. Oxidative and Color Stability of Cooked Ground Pork Containing Lotus Leaf (Nelumbo nucifera) and Barley Leaf (Hordeum vulgare) Powder during Refrigerated Storage. Meat Sci. 2011, 87, 12–18. [Google Scholar] [CrossRef]
- Madhujith, T.; Shahidi, F. Antioxidative and Antiproliferative Properties of Selected Barley (Hordeum vulgarae L.) Cultivars and Their Potential for Inhibition of Low-Density Lipoprotein (LDL) Cholesterol Oxidation. J. Agric. Food Chem. 2007, 55, 5018–5024. [Google Scholar] [CrossRef]
- Osawa, T.; Katsuzaki, H.; Hagiwara, Y.; Hagiwara, H.; Shibamoto, T. A Novel Antioxidant Isolated from Young Green Barley Leaves. J. Agric. Food Chem. 1992, 40, 1135–1138. [Google Scholar] [CrossRef]
- Benedet, J.A.; Umeda, H.; Shibamoto, T. Antioxidant Activity of Flavonoids Isolated from Young Green Barley Leaves toward Biological Lipid Samples. J. Agric. Food Chem. 2007, 55, 5499–5504. [Google Scholar] [CrossRef]
- Ohkawa, M.; Kinjo, J.; Hagiwara, Y.; Hagiwara, H.; Ueyama, H.; Nakamura, K.; Ishikawa, R.; Ono, M.; Nohara, T. Three New Anti-Oxidative Saponarin Analogs from Young Green Barley Leaves. Chem. Pharm. Bull. 1998, 46, 1887–1890. [Google Scholar] [CrossRef] [Green Version]
- Ikeguchi, M.; Tsubata, M.; Takano, A.; Kamiya, T.; Takagaki, K.; Ito, H.; Sugawa-Katayama, Y.; Tsuji, H. Effects of Young Barley Leaf Powder on Gastrointestinal Functions in Rats and Its Efficacy-Related Physicochemical Properties. Evid. Based Complement. Alternat. Med. 2014, 2014, e974840. [Google Scholar] [CrossRef]
- Škrbić, B.; Milovac, S.; Dodig, D.; Filipčev, B. Effects of Hull-Less Barley Flour and Flakes on Bread Nutritional Composition and Sensory Properties. Food Chem. 2009, 115, 982–988. [Google Scholar] [CrossRef]
- Kim, D.-C.; In, M.-J.; Chae, H.-J. Preparation of Mulberry Leaves Tea and Its Quality Characteristics. J. Appl. Biol. Chem. 2010, 53, 56–59. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.-M.; Wu, C.-H.; Tseng, Y.-H.; Tsai, C.E.; Chang, W.-C. Antioxidative and Hypolipidemic Effects of Barley Leaf Essence in a Rabbit Model of Atherosclerosis. Jpn. J. Pharmacol. 2002, 89, 142–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Y.-M.; Chang, W.-C.; Chang, C.-T.; Hsieh, C.-L.; Tsai, C.E. Effects of Young Barley Leaf Extract and Antioxidative Vitamins on LDL Oxidation and Free Radical Scavenging Activities in Type 2 Diabetes. Diabetes Metab. 2002, 28, 107–114. [Google Scholar] [PubMed]
- Han, H.-S.; Shin, J.-S.; Song, Y.-R.; Rhee, Y.K.; Cho, C.-W.; Ryu, J.H.; Inn, K.-S.; Hong, H.-D.; Lee, K.-T. Immunostimulatory Effects of Polysaccharides Isolated from Young Barley Leaves (Hordeum vulgare L.) with Dual Activation of Th1 and Th2 in Splenic T Cells and Cyclophosphamide-Induced Immunosuppressed Mice. Int. J. Biol. Macromol. 2020, 147, 954–964. [Google Scholar] [CrossRef]
- Fadaei, V.; Mohamadi-Alasti, F.; Khosravi-Darani, K. Influence of Spirulina Platensis Powder on the Starter Culture Viabilityin Probiotic Yoghurt Containing Spinach during Cold Storage. Eur. J. Exp. Biol. 2013, 3, 389–393. [Google Scholar]
- Yangmei, Q.; Jiang, W.; Hou, H.; Zhang, H. Application of Plant Green Juice Powder in Maintaining Stability of Viable Count of Yoghourt in Shelf Life. Patent No. CN 102106386 B, 22 March 1991. [Google Scholar]
- Ziarno, M.; Zaręba, D. The Effect of the Addition of Microbial Transglutaminase before the Fermentation Process on the Quality Characteristics of Three Types of Yogurt. Food Sci. Biotechnol. 2020, 29, 109–119. [Google Scholar] [CrossRef] [Green Version]
- Bezerra, M.F.; Souza, D.F.S.; Correia, R.T.P. Acidification Kinetics, Physicochemical Properties and Sensory Attributes of Yoghurts Prepared from Mixtures of Goat and Buffalo Milks. Int. J. Dairy Technol. 2012, 65, 437–443. [Google Scholar] [CrossRef]
- Varghese, K.S.; Mishra, H.N. Modelling of Acidification Kinetics and Textural Properties in Dahi (Indian Yogurt) Made from Buffalo Milk Using Response Surface Methodology. Int. J. Dairy Technol. 2008, 61, 284–289. [Google Scholar] [CrossRef]
- ISO 6887-5:2020; Microbiology of the Food Chain—Preparation of Test Samples, Initial Suspension and Decimal Dilutions for Microbiological Examination—Part 5: Specific Rules for the Preparation of Milk and Milk Products. International Organization for Standardization: Geneva, Switzerland, 2020.
- ISO 15214:1998; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Mesophilic Lactic Acid Bacteria—Colony–Count Technique at 30 Degrees. International Organization for Standardization: Geneva, Switzerland, 1998.
- Bonev, B.; Hooper, J.; Parisot, J. Principles of Assessing Bacterial Susceptibility to Antibiotics Using the Agar Diffusion Method. J. Antimicrob. Chemother. 2008, 61, 1295–1301. [Google Scholar] [CrossRef] [Green Version]
- Oh, N.S.; Lee, J.Y.; Joung, J.Y.; Kim, K.S.; Shin, Y.K.; Lee, K.-W.; Kim, S.H.; Oh, S.; Kim, Y. Microbiological Characterization and Functionality of Set-Type Yogurt Fermented with Potential Prebiotic Substrates Cudrania Tricuspidata and Morus alba L. Leaf Extracts. J. Dairy Sci. 2016, 99, 6014–6025. [Google Scholar] [CrossRef]
- Amirdivani, S.; Baba, A.S. Changes in Yogurt Fermentation Characteristics, and Antioxidant Potential and in Vitro Inhibition of Angiotensin-1 Converting Enzyme upon the Inclusion of Peppermint, Dill and Basil. LWT—Food Sci. Technol. 2011, 44, 1458–1464. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, R.P.S.; Florence, A.C.R.; Silva, R.C.; Perego, P.; Converti, A.; Gioielli, L.A.; Oliveira, M.N. Effect of Different Prebiotics on the Fermentation Kinetics, Probiotic Survival and Fatty Acids Profiles in Nonfat Symbiotic Fermented Milk. Int. J. Food Microbiol. 2009, 128, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Jaya, S.; Das, H. Effect of Maltodextrin, Glycerol Monostearate and Tricalcium Phosphate on Vacuum Dried Mango Powder Properties. J. Food Eng. 2004, 63, 125–134. [Google Scholar] [CrossRef]
- Mocanu, G.; Botez, E.; Nistor, O.; Georgeta, D.; Andronoiu, D.G.; Vlăsceanu, G. Influence of Spirulina Platensis Biomass over Some Starter Culture of Lactic Bacteria. J. Agroalim. Proc. Technol. 2013, 19, 474–479. [Google Scholar]
- Ziarno, M.; Zaręba, D.; Dryzek, W.; Hassaliu, R.; Florowski, T. Effect of the Addition of Soy Beverage and Propionic Bacteria on Selected Quality Characteristics of Cow’s Milk Yoghurt Products. Appl. Sci. 2022, 12, 12603. [Google Scholar] [CrossRef]
- Ścibisz, I.; Ziarno, M.; Mitek, M. Color Stability of Fruit Yogurt during Storage. J. Food Sci. Technol. 2019, 56, 1997–2009. [Google Scholar] [CrossRef] [Green Version]
- Derewiaka, D.; Stepnowska, N.; Bryś, J.; Ziarno, M.; Ciecierska, M.; Kowalska, J. Chia Seed Oil as an Additive to Yogurt. Grasas Aceites 2019, 70, e302. [Google Scholar] [CrossRef] [Green Version]
- Ásványi-Molnár, N.; Sipos-Kozma, Z.; Tóth, Á.; Ásványi, B.; Varga, L. Development of functional dairy food enriched in spirulina (Arthrospira platensis). Tejgazdaság 2009, 69, 15–22. [Google Scholar]
- Molnár, N.; Gyenis, B.; Varga, L. Influence of a Powdered Spirulina Platensis Biomass on Acid Production of Lactococci in Milk. Milchwissenschaft 2005, 60, 380–382. [Google Scholar]
- Cichońska, P.; Pudło, E.; Wojtczak, A.; Ziarno, M. Effect of the Addition of Whole and Milled Flaxseed on the Quality Characteristics of Yogurt. Foods 2021, 10, 2140. [Google Scholar] [CrossRef]
- Ziarno, M.; Kozłowska, M.; Ścibisz, I.; Kowalczyk, M.; Pawelec, S.; Stochmal, A.; Szleszyński, B. The Effect of Selected Herbal Extracts on Lactic Acid Bacteria Activity. Appl. Sci. 2021, 11, 3898. [Google Scholar] [CrossRef]
- Varga, L.; Szigeti, J.; Kovács, R.; Földes, T.; Buti, S. Influence of a Spirulina Platensis Biomass on the Microflora of Fermented ABT Milks During Storage (R1). J. Dairy Sci. 2002, 85, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Beheshtipour, H.; Mortazavian, A.M.; Haratian, P.; Darani, K.K. Effects of Chlorella Vulgaris and Arthrospira Platensis Addition on Viability of Probiotic Bacteria in Yogurt and Its Biochemical Properties. Eur. Food Res. Technol. 2012, 235, 719–728. [Google Scholar] [CrossRef]
- Zielke, H.; Kneifel, H.; Webb, L.E.; Soeder, C.J. Stimulation of Lactobacilli by an Aqueous Extract of the Green Alga Scenedesmus Acutus 276-3a. Eur. J. Appl. Microbiol. Biotechnol. 1978, 6, 79–86. [Google Scholar] [CrossRef]
- Webb, L.E. Detection by Warburg Manometry of Compounds Stimulatory to Lactic Acid Bacteria. J. Dairy Res. 1982, 49, 479–486. [Google Scholar] [CrossRef]
- Abdeldaiem, A.M.; Ali, A.H.; Shah, N.; Ayyash, M.; Mousa, A.H. Physicochemical Analysis, Rheological Properties, and Sensory Evaluation of Yogurt Drink Supplemented with Roasted Barley Powder. LWT 2023, 173, 114319. [Google Scholar] [CrossRef]
- Kaur, R.; Riar, C.S. Sensory, Rheological and Chemical Characteristics during Storage of Set Type Full Fat Yoghurt Fortified with Barley β-Glucan. J. Food Sci. Technol. 2020, 57, 41–51. [Google Scholar] [CrossRef]
- Havrlentová, M.; Petruláková, Z.; Burgárová, A.; Gago, F.; Hlinková, A.; Šturdík, E. β-Glucans and Their Significance for the Preparation of Functional Foods—A Review. Czech J. Food Sci. 2011, 29, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Qu, X.; Nazarenko, Y.; Yang, W.; Nie, Y.; Zhang, Y.; Li, B. Effect of Oat β-Glucan on the Rheological Characteristics and Microstructure of Set-Type Yogurt. Molecules 2021, 26, 4752. [Google Scholar] [CrossRef]
- Yan, J.-K.; Chen, T.-T.; Wang, Z.-W.; Wang, C.; Liu, C.; Li, L. Comparison of Physicochemical Characteristics and Biological Activities of Polysaccharides from Barley (Hordeum vulgare L.) Grass at Different Growth Stages. Food Chem. 2022, 389, 133083. [Google Scholar] [CrossRef]
- Qiu, L.; Zhang, M.; Mujumdar, A.S.; Chang, L. Effect of Edible Rose (Rosa Rugosa Cv. Plena) Flower Extract Addition on the Physicochemical, Rheological, Functional and Sensory Properties of Set-Type Yogurt. Food Biosci. 2021, 43, 101249. [Google Scholar] [CrossRef]
- Dönmez, Ö.; Mogol, B.A.; Gökmen, V. Syneresis and Rheological Behaviors of Set Yogurt Containing Green Tea and Green Coffee Powders. J. Dairy Sci. 2017, 100, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Mohamed Ahmed, I.A.; Alqah, H.A.S.; Saleh, A.; Al-Juhaimi, F.Y.; Babiker, E.E.; Ghafoor, K.; Hassan, A.B.; Osman, M.A.; Fickak, A. Physicochemical Quality Attributes and Antioxidant Properties of Set-Type Yogurt Fortified with Argel (Solenostemma argel Hayne) Leaf Extract. LWT 2021, 137, 110389. [Google Scholar] [CrossRef]
- Ardabilchi Marand, M.; Amjadi, S.; Ardabilchi Marand, M.; Roufegarinejad, L.; Jafari, S.M. Fortification of Yogurt with Flaxseed Powder and Evaluation of Its Fatty Acid Profile, Physicochemical, Antioxidant, and Sensory Properties. Powder Technol. 2020, 359, 76–84. [Google Scholar] [CrossRef]
- Kwon, H.C.; Bae, H.; Seo, H.G.; Han, S.G. Short Communication: Chia Seed Extract Enhances Physiochemical and Antioxidant Properties of Yogurt. J. Dairy Sci. 2019, 102, 4870–4876. [Google Scholar] [CrossRef]
- Almusallam, I.A.; Mohamed Ahmed, I.A.; Babiker, E.E.; Al-Juhaimi, F.Y.; Saleh, A.; Qasem, A.A.; Al Maiman, S.; Osman, M.A.; Ghafoor, K.; Hajji, H.A.; et al. Effect of Date Palm (Phoenix dactylifera L.) Spikelets Extract on the Physicochemical and Microbial Properties of Set-Type Yogurt during Cold Storage. LWT 2021, 148, 111762. [Google Scholar] [CrossRef]
- Zhang, T.; Jeong, C.H.; Cheng, W.N.; Bae, H.; Seo, H.G.; Petriello, M.C.; Han, S.G. Moringa Extract Enhances the Fermentative, Textural, and Bioactive Properties of Yogurt. LWT 2019, 101, 276–284. [Google Scholar] [CrossRef]
- Wang, X.; Kristo, E.; LaPointe, G. Adding Apple Pomace as a Functional Ingredient in Stirred-Type Yogurt and Yogurt Drinks. Food Hydrocoll. 2020, 100, 105453. [Google Scholar] [CrossRef]
- Duboc, P.; Mollet, B. Applications of Exopolysaccharides in the Dairy Industry. Int. Dairy J. 2001, 11, 759–768. [Google Scholar] [CrossRef]
- Hassan, A.N. ADSA Foundation Scholar Award: Possibilities and Challenges of Exopolysaccharide-Producing Lactic Cultures in Dairy Foods. J. Dairy Sci. 2008, 91, 1282–1298. [Google Scholar] [CrossRef]
- Prasanna, P.H.P.; Grandison, A.S.; Charalampopoulos, D. Screening Human Intestinal Bifidobacterium Strains for Growth, Acidification, EPS Production and Viscosity Potential in Low-Fat Milk. Int. Dairy J. 2012, 23, 36–44. [Google Scholar] [CrossRef]
- Corrieu, G.; Béal, C. Yogurt: The Product and Its Manufacture; Academic Press: Cambridge, MA, USA, 2016; Volume 5, p. 617. [Google Scholar]
- Bakirci, I. The Effects of Some Herbs on the Activities of Thermophilic Dairy Cultures. Food Nahr. 1999, 43, 333–335. [Google Scholar] [CrossRef]
- Kivanç, M.; Akgül, A.; Doǧan, A. Inhibitory and Stimulatory Effects of Cumin, Oregano and Their Essential Oils on Growth and Acid Production of Lactobacillus Plantarum and Leuconostoc Mesenteroides. Int. J. Food Microbiol. 1991, 13, 81–85. [Google Scholar] [CrossRef]
- Dunn, L.L.; Davidson, P.M.; Critzer, F.J. Antimicrobial Efficacy of an Array of Essential Oils Against Lactic Acid Bacteria. J. Food Sci. 2016, 81, M438–M444. [Google Scholar] [CrossRef]
- Zaika, L.L.; Kissinger, J.C.; Wasserman, A.E. Inhibition of Lactic Acid Bacteria by Herbs. J. Food Sci. 1983, 48, 1455–1459. [Google Scholar] [CrossRef]
- Kozłowska, M.; Ścibisz, I.; Zaręba, D.; Ziarno, M. Antioxidant Properties and Effect on Lactic Acid Bacterial Growth of Spice Extracts. CyTA—J. Food 2015, 13, 573–577. [Google Scholar] [CrossRef]
- Houle, J.-F.; Lafrance, M.; Julien, J.-P.; Brochu, E.; Champagne, C.P. Selection of Mixed Cultures for Meat Fermentation. J. Food Sci. 1989, 54, 839–842. [Google Scholar] [CrossRef]
- Lachowicz; Jones; Briggs; Bienvenu; Wan; Wilcock; Coventry. The Synergistic Preservative Effects of the Essential Oils of Sweet Basil (Ocimum basilicum L.) against Acid-tolerant Food Microflora. Lett. Appl. Microbiol. 1998, 26, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Verluyten, J.; Leroy, F.; de Vuyst, L. Effects of Different Spices Used in Production of Fermented Sausages on Growth of and Curvacin A Production by Lactobacillus Curvatus LTH 1174. Appl. Environ. Microbiol. 2004, 70, 4807–4813. [Google Scholar] [CrossRef] [Green Version]
- Robles-Escajeda, E.; Lerma, D.; Nyakeriga, A.M.; Ross, J.A.; Kirken, R.A.; Aguilera, R.J.; Varela-Ramirez, A. Searching in Mother Nature for Anti-Cancer Activity: Anti-Proliferative and Pro-Apoptotic Effect Elicited by Green Barley on Leukemia/Lymphoma Cells. PLoS ONE 2013, 8, e73508. [Google Scholar] [CrossRef] [Green Version]




| No. | Symbol | Manufacturer | Declared Microbiological Composition |
|---|---|---|---|
| 1 | Flora Danica | Chr. Hansen, Hørsholm, Denmark | Lactococcus lactis subsp. cremoris, Lactococcus lactis subsp. lactis, Lactococcus lactis subsp. diacetylactis, Leuconostoc mesenteroides subsp. cremoris |
| 2 | CHN-19 | Chr. Hansen, Hørsholm, Denmark | Lactococcus lactis subsp. cremoris, Lactococcus lactis subsp. lactis, Lactococcus lactis subsp. diacetylactis, Leuconostoc mesenteroides subsp. cremoris |
| 3 | XPL-1 | Chr. Hansen, Hørsholm, Denmark | Lactococcus lactis subsp. cremoris, Lactococcus lactis subsp. lactis, Leuconostoc sp., Lactococcus lactis subsp. lactis biovar diacetylactis, Streptococcus thermophilus |
| 4 | CHOOZIT TP 05 | DuPont™ Danisco®, Copenhagen, Denmark | Lactococcus lactis subsp. cremoris, Lactococcus lactis subsp. lactis, Lactococcus lactis subsp. lactis biovar diacetylactis, Leuconostoc mesenteroides subsp. mesenteroides |
| 5 | CHOOZIT MTM 2 | DuPont™ Danisco®, Copenhagen, Denmark | Lactococcus lactis subsp. cremoris, Lactococcus lactis subsp. lactis, Lactococcus lactis subsp. lactis biovar diacetylactis, Streptococcus thermophilus |
| 6 | Probat 222 | DuPont™ Danisco®, Copenhagen, Denmark | Lactococcus lactis subsp. lactis, Lactococcus lactis subsp. cremoris, Lactococcus lactis subsp. lactis biovar. diacetylactis, Leuconostoc mesenteroides subsp. cremoris |
| 7 | Kefirferment | Brouwland bv, Beverlo, Belgium | Lactococcus cremoris, L. lactis, L. lactic subsp. diacetilactis, Leuconostoc cremoris |
| 8 | Dickmilch bioferment | IP Ingredients GmbH, Süderlügum, Germany | Lactococcus lactis subsp. lactis, Lactococcus lactis subsp. cremoris, Lactococcus lactis subsp. lactis biovar. diacetylactis, Leuconostoc mesenteroides subsp. cremoris |
| Starter Cultures | Samples of Milk | Vmax Maximum Acidification Rate | Tmax Time at Which the Maximum Acidification Rate Was Obtained | Te Time at Which pH 4.5 Was Reached |
|---|---|---|---|---|
| Flora Danica | control | 0.47 c ± 0.11 | 10.0 c ± 0.5 | 11.0 a ± 0.4 |
| with YBLP | 0.43 c ± 0.20 | 10.0 c ± 0.4 | 10.6 a ± 0.2 | |
| CHN-19 | control | 0.34 a,b ± 0.06 | 10.0 c ± 0.6 | 12.4 b ± 0.3 |
| with YBLP | 0.30 a,b ± 0.07 | 10.0 c ± 0.5 | 10.9 a ± 0.2 | |
| XPL-1 | control | 0.28 a ± 0.09 | 9.0 b ± 0.5 | 12.8 b ± 0.1 |
| with YBLP | 0.27 a ± 0.08 | 9.0 b ± 0.4 | 12.8 b ± 0.2 | |
| CHOOZIT TP 05 | control | 0.33 a,b ± 0.07 | 9.0 b ± 0.5 | 10.7 a ± 0.3 |
| with YBLP | 0.35 b ± 0.06 | 9.0 b ± 0.4 | 10.6 a ± 0.2 | |
| CHOOZIT MTM 2 | control | 0.48 c ± 0.11 | 10.0 c ± 0.5 | 11.2 a ± 0.1 |
| with YBLP | 0.47 c ± 0.12 | 10.0 c ± 0.6 | 12.0 b ± 0.2 | |
| Probat 222 | control | 0.37 b,c ± 0.08 | 7.0 a ± 0.4 | 12.3 b ± 0.3 |
| with YBLP | 0.39 b,c ± 0.11 | 7.0 a ± 0.3 | 12.1 b ± 0.4 | |
| Kefirferment | control | 0.48 c ± 0.20 | 10.0 c ± 0.5 | 12.3 b ± 0.3 |
| with YBLP | 0.37 b,c ± 0.19 | 10.0 c ± 0.4 | 12.0 b ± 0.2 | |
| Dickmilch bioferment | control | 0.32 a,b ± 0.05 | 7.0 a ± 0.3 | 12.1 b ± 0.3 |
| with YBLP | 0.32 a,b ± 0.07 | 7.0 a ± 0.4 | 10.9 a ± 0.4 |
| Storage Time [Days] | 0 | 7 | 14 | 21 | 28 | |
|---|---|---|---|---|---|---|
| Starter Cultures | Samples | |||||
| Flora Danica | control | 4.51 a ± 0.03 | 4.49 a ± 0.03 | 4.47 a ± 0.03 | 4.35 c ± 0.05 | 4.34 c ± 0.03 |
| with YBLP | 4.50 a ± 0.04 | 4.45 a,b ± 0.04 | 4.42 a,b ± 0.02 | 4.40 a,b ± 0.03 | 4.41 a,b ± 0.03 | |
| CHN-19 | control | 4.52 a ± 0.02 | 4.49 a,b ± 0.02 | 4.49 a,b ± 0.02 | 4.44 b ± 0.02 | 4.42 b,c ± 0.02 |
| with YBLP | 4.50 a ± 0.02 | 4.46 b ± 0.02 | 4.45 b ± 0.01 | 4.44 b,c ± 0.02 | 4.41 c ± 0.02 | |
| XPL-1 | control | 4.55 a ± 0.05 | 4.51 a ± 0.06 | 4.49 a ± 0.06 | 4.45 b ± 0.05 | 4.44 b ± 0.04 |
| with YBLP | 4.54 a ± 0.05 | 4.52 a ± 0.05 | 4.50 a,b ± 0.05 | 4.50 b ± 0.06 | 4.44 b ± 0.06 | |
| CHOOZIT TP 05 | control | 4.44 a ± 0.06 | 4.42 a ± 0.06 | 4.42 a ± 0.06 | 4.41 a ± 0.06 | 4.40 a ± 0.06 |
| with YBLP | 4.45 a ± 0.06 | 4.42 a ± 0.06 | 4.40 a ± 0.06 | 4.39 a ± 0.06 | 4.38 a ± 0.10 | |
| CHOOZIT MTM 2 | control | 4.49 a ± 0.04 | 4.45 a,b ± 0.03 | 4.42 b ± 0.04 | 4.39 b,c ± 0.05 | 4.38 c ± 0.04 |
| with YBLP | 4.48 a ± 0.04 | 4.42 b ± 0.07 | 4.41 b ± 0.04 | 4.35 c ± 0.04 | 4.33 c ± 0.04 | |
| Probat 222 | control | 4.51 a ± 0.03 | 4.47 a,b ± 0.03 | 4.46 b ± 0.03 | 4.42 c ± 0.03 | 4.41 c ± 0.05 |
| with YBLP | 4.49 a,b ± 0.03 | 4.46 b ± 0.05 | 4.45 b ± 0.03 | 4.44 c ± 0.04 | 4.42 c ± 0.03 | |
| Kefirferment | control | 4.53 a ± 0.03 | 4.50 a,b ± 0.03 | 4.50 a,b ± 0.03 | 4.49 a,b ± 0.03 | 4.48 b ± 0.04 |
| with YBLP | 4.52 a ± 0.03 | 4.52 a,b ± 0.05 | 4.51 a,b ± 0.03 | 4.50 a,b ± 0.03 | 4.47 b ± 0.08 | |
| Dickmilch bioferment | control | 4.52 a ± 0.02 | 4.47 a,b ± 0.02 | 4.45 b ± 0.02 | 4.28 d ± 0.04 | 4.28 d ± 0.03 |
| with YBLP | 4.51 a ± 0.02 | 4.45 b ± 0.02 | 4.41 b ± 0.02 | 4.40 b ± 0.02 | 4.34 c ± 0.02 | |
| Storage Time [Days] | 0 | 7 | 14 | 21 | 28 | |
|---|---|---|---|---|---|---|
| Starter Cultures | Samples | |||||
| Flora Danica | control | 26.0 c,d ± 0.9 | 26.0 c,d ± 0.9 | 26.9 d ± 1.0 | 30.0 e ± 1.1 | 30.6 e ± 1.1 |
| with YBLP | 23.9 a,b ± 0.7 | 21.9 a ± 0.7 | 26.4 d ± 1.1 | 24.5 b,c ± 0.4 | 25.0 c ± 0.4 | |
| CHN-19 | control | 31.0 d ± 1.1 | 28.9 c ± 1.0 | 22.9 a ± 0.8 | 24.0 a ± 0.8 | 23.9 a ± 1.2 |
| with YBLP | 30.5 d ± 0.4 | 27.5 c ± 0.4 | 25.5 b ± 0.4 | 25.9 b ± 0.7 | 25.6 b ± 0.4 | |
| XPL-1 | control | 28.9 b,c ± 1.0 | 28.0 b ± 1.0 | 28.9 b,c ± 1.0 | 29.0 c ± 1.0 | 29.3 c ± 1.1 |
| with YBLP | 27.3 a ± 0.4 | 27.0 a ± 0.4 | 29.0 c ± 0.4 | 28.3 b ± 0.8 | 29.3 c ± 0.8 | |
| CHOOZIT TP 05 | control | 30.9 c ± 1.1 | 26.9 b,c ± 1.0 | 26.0 b ± 0.9 | 24.0 a ± 0.8 | 25.0 a,b ± 0.7 |
| with YBLP | 30.0 c ± 0.4 | 25.1 a,b ± 0.7 | 26.6 b ± 1.1 | 23.5 a ± 0.4 | 24.3 a ± 0.4 | |
| CHOOZIT MTM 2 | control | 34.7 b ± 1.2 | 33.6 a,b ± 1.2 | 34.7 b ± 1.2 | 34.7 b ± 1.2 | 35.1 c ± 1.3 |
| with YBLP | 32.8 a ± 0.5 | 32.4 a ± 0.5 | 34.8 b ± 0.5 | 33.9 a,b ± 1.0 | 35.1 c ± 1.0 | |
| Probat 222 | control | 34.0 d ± 1.2 | 31.8 c ± 1.1 | 25.2 a ± 0.9 | 26.4 a ± 1.0 | 26.3 a ± 1.4 |
| with YBLP | 33.5 d ± 0.4 | 30.2 b,c ± 0.4 | 28.1 b ± 0.4 | 28.5 b ± 0.8 | 28.2 b ± 0.5 | |
| Kefirferment | control | 29.3 d ± 1.0 | 25.5 b,c ± 0.9 | 24.7 b ± 0.9 | 22.8 a ± 0.8 | 23.7 a ± 0.7 |
| with YBLP | 28.5 d ± 0.7 | 23.8 b ± 0.7 | 25.2 b ± 1.0 | 22.3 a ± 0.4 | 23.0 a,b ± 0.4 | |
| Dickmilch bioferment | control | 27.8 b ± 1.0 | 27.8 b ± 1.0 | 28.7 b,c ± 1.0 | 32.1 d ± 1.2 | 32.7 d ± 1.2 |
| with YBLP | 25.5 a,b ± 0.8 | 23.4 a ± 0.8 | 28.2 b,c ± 1.2 | 26.1 b ± 0.4 | 26.7 b ± 0.4 | |
| Storage Time [Days] | 0 | 7 | 14 | 21 | 28 | |
|---|---|---|---|---|---|---|
| Starter Cultures | Samples | |||||
| Flora Danica | control | 9.0 a,b ± 0.3 | 8.0 a ± 0.3 | 10.0 b ± 0.4 | 10.0 b ± 0.4 | 10.7 b ± 0.4 |
| with YBLP | 8.3 a ± 0.4 | 8.4 a ± 1.9 | 8.5 a ± 1.6 | 8.8 a ± 1.5 | 9.9 a,b ± 1.3 | |
| CHN-19 | control | 9.0 a ± 0.3 | 8.0 a ± 0.3 | 11.0 b ± 0.4 | 9.0 a ± 0.3 | 10.1 b ± 0.7 |
| with YBLP | 9.3 a ± 0.4 | 9.0 a ± 0.8 | 9.9 a,b ± 1.3 | 9.7 a,b ± 2.0 | 10.7 b ± 2.0 | |
| XPL-1 | control | 7.0 a ± 0.3 | 8.0 a ± 0.3 | 9.0 a ± 0.3 | 8.0 a ± 0.3 | 9.3 a ± 0.5 |
| with YBLP | 8.6 a ± 0.4 | 9.0 a ± 0.4 | 8.9 a ± 1.3 | 8.6 a ± 1.6 | 8.2 a ± 1.0 | |
| CHOOZIT TP 05 | control | 9.0 a,b ± 0.3 | 10.0 c ± 0.4 | 9.0 a,b ± 0.3 | 10.0 c ± 0.4 | 10.4 c ± 0.4 |
| with YBLP | 8.6 a ± 0.9 | 9.0 a,b ± 0.8 | 8.6 a ± 0.9 | 9.6 b ± 0.4 | 10.4 c ± 0.4 | |
| CHOOZIT MTM 2 | control | 7.8 a ± 0.3 | 9.0 a ± 0.3 | 10.0 a ± 0.9 | 9.0 a ± 0.4 | 10.5 a ± 0.7 |
| with YBLP | 9.9 a ± 0.3 | 10.0 a ± 0.3 | 9.9 a ± 0.4 | 9.6 a ± 0.4 | 9.2 a ± 0.6 | |
| Probat 222 | control | 9.9 a,b ± 0.9 | 8.8 a ± 0.9 | 11.0 b,c ± 1.5 | 11.0 b,c ± 0.7 | 12.0 c ± 0.9 |
| with YBLP | 9.0 a ± 0.4 | 9.3 a ± 0.4 | 9.4 a ± 0.4 | 9.7 a,b ± 1.9 | 10.9 a,b ± 1.5 | |
| Kefirferment | control | 10.1 b ± 0.5 | 9.0 a ± 0.3 | 11.2 b ± 2.1 | 11.2 b ± 0.4 | 11.9 b ± 0.5 |
| with YBLP | 9.3 a ± 0.5 | 9.5 a ± 2.1 | 9.7 a,b ± 1.8 | 10.1 a ± 1.7 | 11.2 a,b ± 1.5 | |
| Dickmilch bioferment | control | 9.1 a ± 0.3 | 10.3 b ± 0.4 | 9.1 a ± 0.3 | 10.2 b ± 0.5 | 10.6 b,c ± 0.4 |
| with YBLP | 8.7 a ± 0.5 | 9.1 a ± 0.9 | 8.7 a ± 1.0 | 9.7 a,b ± 0.5 | 10.7 b,c ± 0.7 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozłowska, M.; Ziarno, M.; Zaręba, D.; Ścibisz, I. Exploring the Possibility of Enriching Fermented Milks with Young Barley Leaves Powder Preparation. Fermentation 2023, 9, 731. https://doi.org/10.3390/fermentation9080731
Kozłowska M, Ziarno M, Zaręba D, Ścibisz I. Exploring the Possibility of Enriching Fermented Milks with Young Barley Leaves Powder Preparation. Fermentation. 2023; 9(8):731. https://doi.org/10.3390/fermentation9080731
Chicago/Turabian StyleKozłowska, Mariola, Małgorzata Ziarno, Dorota Zaręba, and Iwona Ścibisz. 2023. "Exploring the Possibility of Enriching Fermented Milks with Young Barley Leaves Powder Preparation" Fermentation 9, no. 8: 731. https://doi.org/10.3390/fermentation9080731
APA StyleKozłowska, M., Ziarno, M., Zaręba, D., & Ścibisz, I. (2023). Exploring the Possibility of Enriching Fermented Milks with Young Barley Leaves Powder Preparation. Fermentation, 9(8), 731. https://doi.org/10.3390/fermentation9080731









