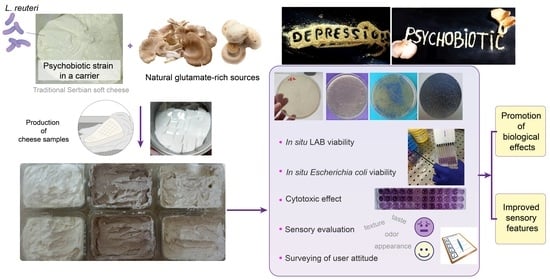
Cheese Fermented with Human-Derived Limosilactobacillus reuteri DSM 17938 and Mushroom Powders: A Novel Psychobiotic Food with Enhanced Bioactivity and Sensory Acceptability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Production of Acid-Coagulated Cheese Samples at Laboratory-Scale Level
2.3. In Situ LAB Viability
2.4. In Situ Escherichia coli Viability
2.5. Cytotoxicity
2.6. Sensory Analysis
2.7. Consumer and Patient Attitudes towards Psychobiotics: A Survey of Mental Health and Functional Foods
2.8. Statistical Analysis
3. Results and Discussion
3.1. The impact of L. reuteri on the Bioactivity of Acid-Coagulated Cheese
3.2. Sensory and Organoleptic Evaluation of Cheese Supplemented with Psychobiotics and Gourmet Mushroom Powders
3.3. Exploring Consumer Awareness and Preferences for Psychobiotic Foods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cavaillon, J.M.; Legout, S. Centenary of the death of Élie Metchnikoff: A visionary and an outstanding team leader. Microbes. Infect. 2016, 18, 577–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef]
- Nataraj, B.H.; Ali, S.A.; Behare, P.V.; Yadav, H. Postbiotics-parabiotics: The new horizons in microbial biotherapy and functional foods. Microb. Cell Factories 2020, 19, 1–22. [Google Scholar] [CrossRef]
- Dahiya, D.; Nigam, P.S. Clinical potential of microbial strains, used in fermentation for probiotic food, beverages and in synbiotic supplements, as psychobiotics for cognitive treatment through gut–brain signaling. Microorganisms 2022, 10, 1687. [Google Scholar] [CrossRef]
- Swanson, K.S.; Gibson, G.R.; Hutkins, R.; Reimer, R.A.; Reid, G.; Verbeke, K.; Scott, K.P.; Holscher, H.D.; Azad, M.B.; Delzenne, N.M.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 687–701. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [Green Version]
- Spinler, J.K.; Taweechotipatr, M.; Rognerud, C.L.; Ou, C.N.; Tumwasorn, S.; Versalovic, J. Human-derived probiotic Lactobacillus reuteri demonstrate antimicrobial activities targeting diverse enteric bacterial pathogens. Anaerobe 2008, 14, 166–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ross, K. Psychobiotics: Are they the future intervention for managing depression and anxiety? A literature review. EXPLORE 2023, in press. [CrossRef]
- Jones, K.M.; Donovan, M.; Mackey, C.S.; Lynch, M.D.; Platt, G.N.; Brown, A.N.; Washburn, B.K.; Trickey, D.J.; Curtis, J.T.; Liu, Y.; et al. Limosilactobacillus reuteri administration alters the gut-brain-behavior axis in a sex-dependent manner in socially monogamous prairie voles. Front. Microbiol. 2023, 14, 61. [Google Scholar]
- Bron, P.A.; Catalayud, M.; Marzorati, M.; Pane, M.; Kartal, E.; Dhir, R.; Reid, G. Delivery of metabolically neuroactive probiotics to the human gut. Int. J. Mol. Sci. 2021, 22, 9122. [Google Scholar] [CrossRef]
- Tyagi, A.; Shabbir, U.; Chelliah, R.; Daliri, E.B.M.; Chen, X.; Oh, D.H. Limosilactobacillus reuteri fermented brown rice: A product with enhanced bioactive compounds and antioxidant potential. Antioxidants 2021, 10, 1077. [Google Scholar] [CrossRef] [PubMed]
- Trifkovič, K.Č.; Mičetić-Turk, D.; Kmetec, S.; Strauss, M.; Dahlen, H.G.; Foster, J.P.; Fijan, S. Efficacy of Direct or Indirect Use of Probiotics for the Improvement of Maternal Depression during Pregnancy and in the Postnatal Period: A Systematic Review and Meta-Analysis. Healthcare 2022, 10, 970. [Google Scholar] [CrossRef]
- Yu, Z.; Chen, J.; Liu, Y.; Meng, Q.; Liu, H.; Yao, Q.; Song, W.; Ren, X.; Chen, X. The role of potential probiotic strains Lactobacillus reuteri in various intestinal diseases: New roles for an old player. Front. Microbiol. 2023, 14, 30. [Google Scholar] [CrossRef] [PubMed]
- Jovanović, M.; Vojvodić, P.; Petrović, M.; Radić, D.; Mitić-Ćulafić, D.; Kostić, M.; Veljović, S. Yogurt fortified with GABA-producing strain and Ganoderma lucidum industrial waste. Czech J. Food Sci. 2022, 40, 456–464. [Google Scholar] [CrossRef]
- Brenner, L.A.; Forster, J.E.; Stearns-Yoder, K.A.; Stamper, C.E.; Hoisington, A.J.; Brostow, D.P.; Mealer, M.; Wortzel, H.S.; Postolache, T.T.; Lowry, C.A. Evaluation of an immunomodulatory probiotic intervention for veterans with co-occurring mild traumatic brain injury and posttraumatic stress disorder: A pilot study. Front. Neurol. 2020, 11, 1015. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Huang, S.; Wang, Y.; Cai, S.; Yu, H.; Liu, H.; Zeng, X.; Zhang, G.; Qiao, S. Bridging intestinal immunity and gut microbiota by metabolites. Cell Mol. Life Sci. 2019, 76, 3917–3937. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.C.W.; Gorbovskaya, I.; Hahn, M.K.; Müller, D.J. The Gut Microbiome in Schizophrenia and the Potential Benefits of Prebiotic and Probiotic Treatment. Nutrients 2021, 13, 1152. [Google Scholar] [CrossRef]
- Ma, D.; Forsythe, P.; Bienenstock, J. Live Lactobacillus rhamnosus is essential for the inhibitory effect on tumor necrosis factor alpha-induced interleukin-8 expression. Infect. Immun. 2004, 72, 5308–5314. [Google Scholar] [CrossRef] [Green Version]
- Mu, Q.; Tavella, V.J.; Luo, X.M. Role of Lactobacillus reuteri in Human Health and Diseases. Front. Microbiol. 2018, 9, 757. [Google Scholar] [CrossRef] [Green Version]
- Erdman, S.E.; Poutahidis, T. Chapter Five—Microbes and Oxytocin: Benefits for Host Physiology and Behavior. In Gut Microbiome and Behavior; International Review of Neurobiology; Cryan, J.F., Clarke, G., Eds.; Academic Press: Cambridge, MA, USA, 2016; Volume 131, pp. 91–126. [Google Scholar]
- Poutahidis, T.; Kearney, S.M.; Levkovich, T.; Qi, P.; Varian, B.J.; Lakritz, J.R.; Ibrahim, Y.M.; Chatzigiagkos, A.; Alm, E.J.; Erdman, S.E. Microbial symbionts accelerate wound healing via the neuropeptide hormone oxytocin. PLoS ONE 2013, 8, e78898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Toro-Barbosa, M.; Hurtado-Romero, A.; Garcia-Amezquita, L.E.; García-Cayuela, T. Psychobiotics: Mechanisms of Action, Evaluation Methods and Effectiveness in Applications with Food Products. Nutrients 2020, 12, 3896. [Google Scholar] [CrossRef]
- Yogeswara, I.B.A.; Kusumawati, I.G.A.W.; Nursini, N.W.; Mariyatun, M.; Rahayu, E.S.; Haltrich, D. Health-Promoting Role of Fermented Pigeon Pea (Cajanus cajan L (Mill)) Milk Enriched with γ-aminobutyric acid (GABA) Using Probiotic Lactiplantibacillus plantarum Dad-13. Fermentation 2023, 9, 587. [Google Scholar] [CrossRef]
- Nishihira, J.; Kagami-Katsuyama, H.; Tanaka, A.; Nishimura, M. Elevation of natural killer cell activity and alleviation of mental stress by the consumption of yogurt containing Lactobacillus gasseri SBT2055 and Bifidobacterium longum SBT2928 in a double-blind, placebo-controlled clinical trial. J. Funct. Foods 2014, 11, 261–268. [Google Scholar] [CrossRef]
- Miloradovic, Z.; Miocinovic, J.; Kljajevic, N.; Tomasevic, I.; Pudja, P. The influence of milk heat treatment on composition, texture, colour and sensory characteristics of cows’ and goats’ Quark-type cheeses. Small Rumin. Res. 2018, 169, 154–159. [Google Scholar] [CrossRef]
- Rafiq, S.; Huma, N.; Gulzar, N.; Murtaza, M.A.; Hussain, I. Effect of cheddar cheese peptide extracts on growth inhibition, cell cycle arrest, and apoptosis induction in human lung cancer (H-1299) cell line. Int. J. Dairy Technol. 2018, 71, 975–980. [Google Scholar] [CrossRef]
- Han, S.K.; Kim, J.K.; Joo, M.K.; Lee, K.E.; Han, S.W.; Kim, D.H. Lactobacillus reuteri NK33 and Bifidobacterium adolescentis NK98 alleviate Escherichia coli-induced depression and gut dysbiosis in mice. J. Microbiol. Biotechnol. 2020, 30, 1222. [Google Scholar] [CrossRef]
- Diniz-Silva, H.T.; Brandão, L.R.; de Sousa Galvão, M.; Madruga, M.S.; Maciel, J.F.; de Souza, E.L.; Magnani, M. Survival of Lactobacillus acidophilus LA-5 and Escherichia coli O157: H7 in Minas Frescal cheese made with oregano and rosemary essential oils. Food Microbiol. 2020, 86, 103348. [Google Scholar] [CrossRef]
- Stromberg, Z.R.; Van Goor, A.; Redweik, G.A.; Wymore Brand, M.J.; Wannemuehler, M.J.; Mellata, M. Pathogenic and non-pathogenic Escherichia coli colonization and host inflammatory response in a defined microbiota mouse model. DMM Dis. Models Mech. 2018, 11, 035063. [Google Scholar] [CrossRef] [Green Version]
- Darwish, M.S.; Abou-Zeid, N.A.; Khojah, E.; Al Jumayi, H.A.; Alshehry, G.A.; Algarni, E.H.; Elawady, A.A. Supplementation of Labneh with Passion Fruit Peel Enhanced Survival of E. coli Nissle 1917 during Simulated Gastrointestinal Digestion and Adhesion to Caco-2 Cells. Foods 2022, 11, 1663. [Google Scholar] [CrossRef]
- Balakrishnan, K.; Dhanasekaran, D.; Krishnaraj, V.; Anbukumaran, A.; Ramasamy, T.; Manickam, M. Edible mushrooms: A promising bioresource for prebiotics. In Advances in Probiotics; Academic Press: Cambridge, MA, USA, 2021; pp. 81–97. [Google Scholar]
- Ramachandran, C.; Rani, R.S.; Usha, A. Evaluation of safety, antimicrobial activity and probiotic properties of Escherichia coli Nissle 1917 isolated from Idli batter. J. Biotechnol. 2016, 11, 7. [Google Scholar]
- Kumar, A.; Helmy, Y.A.; Fritts, Z.; Vlasova, A.; Saif, L.J.; Rajashekara, G. Anti-rotavirus properties and mechanisms of selected Gram-positive and Gram-negative probiotics on polarized human colonic (HT-29) cells. Probiotics Antimicrob. Proteins 2022, 15, 107–128. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, Y.; Ye, L.; Wang, C. The anti-cancer effects and mechanisms of lactic acid bacteria exopolysaccharides in vitro: A review. Carbohydr. Polym. 2021, 253, 117308. [Google Scholar] [CrossRef]
- Jovanović, M.; Zlatanović, S.; Micić, D.; Bacić, D.; Mitić-Ćulafić, D.; Đuriš, M.; Gorjanović, S. Functionality and palatability of yogurt produced using beetroot pomace flour granulated with lactic acid bacteria. Foods 2021, 10, 1696. [Google Scholar] [CrossRef] [PubMed]
- Sławińska, A.; Sołowiej, B.G.; Radzki, W.; Fornal, E. Wheat Bread Supplemented with Agaricus bisporus Powder: Effect on Bioactive Substances Content and Technological Quality. Foods 2022, 11, 3786. [Google Scholar] [CrossRef] [PubMed]
- Khider, M.; Seoudi, O.; Abdelaliem, Y.F. Functional processed cheese spreads with high nutritional value as supplemented with fresh and dried mushrooms. Int. J. Nutr. Food Sci. 2017, 6, 45–52. [Google Scholar] [CrossRef]
- Tupamahu, I.P.C.; Budiarso, T.Y. The effect of oyster mushroom (Pleurotus ostreatus) powder as prebiotic agent on yoghurt quality. AIP Conf. Proc. 2017, 1844, 030006. [Google Scholar]
- Stanczak, M.; Heuberger, R. Assessment of the knowledge and beliefs regarding probiotic use. Am. J. Health Educ. 2009, 40, 207–211. [Google Scholar] [CrossRef]

| Sample | LAB Viability (log CFU/mL) |
|---|---|
| control cheese | 8.10 ± 0.28 |
| cheese + L. reuteri | 7.67 ± 0.25 |
| cheese + A. bisporus | 8.06 ± 0.20 |
| cheese + L. reuteri + A. bisporus | 7.80 ± 0.34 |
| cheese + P. ostreatus | 7.64 ± 0.23 |
| cheese + L. reuteri + P. ostreatus | 7.67 ± 0.07 |
| Sample | E. coli Strain (log CFU/mL) | ||
|---|---|---|---|
| 0157:H7 | ATCC 35218 | Nissle 1917 | |
| control cheese | 4.91 ± 0.37 a | 5.20 ± 0.28 | 7.11 ± 0.26 |
| cheese + L. reuteri | 4.64 ± 0.39 bc | 5.06 ± 0.13 | 7.47 ± 0.13 |
| cheese + A. bisporus | 5.26 ± 0.64 d | 5.50 ± 0.46 | 7.24 ± 0.10 |
| cheese + L. reuteri + A. bisporus | 5.17 ± 0.33 e | 5.51 ± 0.37 | 7.23 ± 0.07 |
| cheese + P. ostreatus | 6.03 ± 0.04 b | 5.81 ± 0.06 | 7.25 ± 0.05 |
| cheese + L. reuteri + P. ostreatus | 6.70 ± 0.39 acde | 5.81 ± 0.04 | 7.40 ± 0.13 |
| Sensory Attribute * | Apparency | Odor | Taste | Texture | % Max. Overall Quality |
|---|---|---|---|---|---|
| Sample | |||||
| control cheese | 13.82 ± 0.45 | 18.43 ± 0.54 af | 33.00 ± 0.37 ai | 29.14 ± 0.28 ae | 94.39 |
| cheese + P. ostreatus | 13.93 ± 0.22 | 16.14 ± 0.49 abcd | 21.25 ± 0.74 abcde | 26.36 ± 0.54 abcd | 77.46 |
| cheese + A. bisporus | 14.04 ± 0.35 | 19.57 ± 0.20 bg | 34.25 ± 0.20 bgj | 29.36 ± 0.13 bf | 97.21 |
| cheese + L. reuteri | 14.13 ± 0.42 | 18.71 ± 0.38 ce | 32.50 ± 0.57 cfg | 28.71 ± 0.37 c | 94.05 |
| cheese + L. reuteri + P. ostreatus | 14.14 ± 0.40 | 17.00 ± 0.39 efgh | 26.75 ± 0.57 dfij | 26.79 ± 0.47 efg | 84.68 |
| cheese + L. reuteri + A. bisporus | 14.25 ± 0.40 | 19.00 ± 0.19 dh | 33.50 ± 0.27 e | 29.36 ± 0.20 dg | 96.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jovanović, M.; Vojvodić, P.; Tenji, D.; Tomić, N.; Nešić, J.; Mitić-Ćulafić, D.; Miočinović, J. Cheese Fermented with Human-Derived Limosilactobacillus reuteri DSM 17938 and Mushroom Powders: A Novel Psychobiotic Food with Enhanced Bioactivity and Sensory Acceptability. Fermentation 2023, 9, 745. https://doi.org/10.3390/fermentation9080745
Jovanović M, Vojvodić P, Tenji D, Tomić N, Nešić J, Mitić-Ćulafić D, Miočinović J. Cheese Fermented with Human-Derived Limosilactobacillus reuteri DSM 17938 and Mushroom Powders: A Novel Psychobiotic Food with Enhanced Bioactivity and Sensory Acceptability. Fermentation. 2023; 9(8):745. https://doi.org/10.3390/fermentation9080745
Chicago/Turabian StyleJovanović, Marina, Petar Vojvodić, Dina Tenji, Nina Tomić, Jovana Nešić, Dragana Mitić-Ćulafić, and Jelena Miočinović. 2023. "Cheese Fermented with Human-Derived Limosilactobacillus reuteri DSM 17938 and Mushroom Powders: A Novel Psychobiotic Food with Enhanced Bioactivity and Sensory Acceptability" Fermentation 9, no. 8: 745. https://doi.org/10.3390/fermentation9080745
APA StyleJovanović, M., Vojvodić, P., Tenji, D., Tomić, N., Nešić, J., Mitić-Ćulafić, D., & Miočinović, J. (2023). Cheese Fermented with Human-Derived Limosilactobacillus reuteri DSM 17938 and Mushroom Powders: A Novel Psychobiotic Food with Enhanced Bioactivity and Sensory Acceptability. Fermentation, 9(8), 745. https://doi.org/10.3390/fermentation9080745