Research Progress of Lytic Chitin Monooxygenase and Its Utilization in Chitin Resource Fermentation Transformation
Abstract
1. Introduction
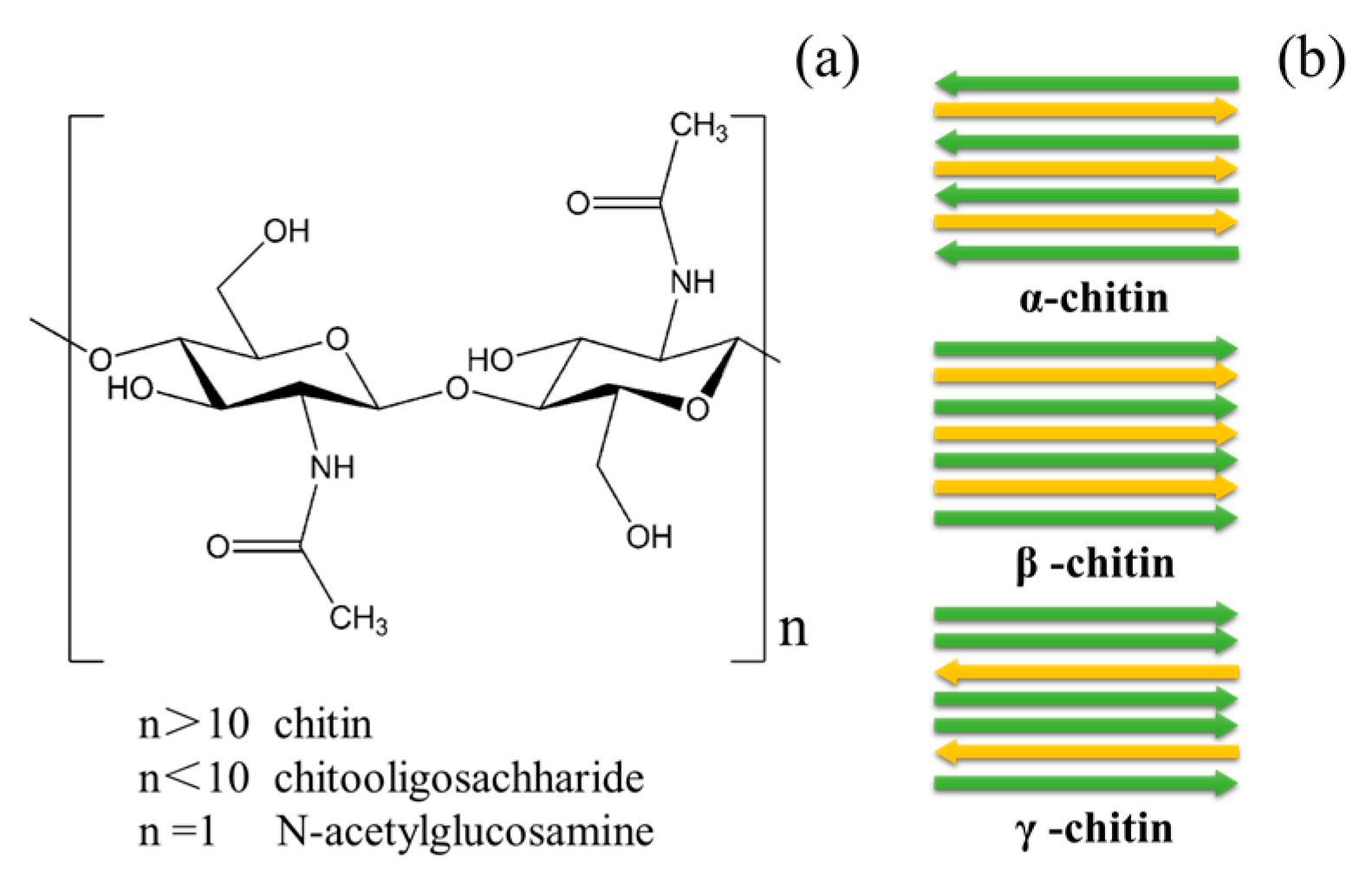
2. Present Situation of Biomass Resources Development in Chitin
3. Research History and Classification of LPMO
4. LCMs with Chitin Activity
4.1. Phylogenetic Analysis of LCMs
4.2. Structural Characteristics of LCMs
4.3. LCMs Active Sites and Their Catalytic Mechanism for Chitin
4.4. Types and Functions of CBMs in LCMs
4.5. Roles of FnIII and Linker in LPMOs
4.6. Multifunctional LCMs
5. Activity Determination and Screening of LCMs
5.1. Detection Methods for LCMs
5.2. LCMs Screening Obtained
6. Preparation of LPMOs
7. Engineering of LCMs
8. Study on the Synergistic Effect of LCMs on Chitin Enzyme and Its Application
9. Current Challenges and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cho, E.J.; Trinh, L.T.P.; Song, Y.; Lee, Y.G.; Bae, H.-J. Bioconversion of Biomass Waste into High Value Chemicals. Bioresour. Technol. 2020, 298, 122386. [Google Scholar] [CrossRef]
- Guo, X.; An, Y.; Liu, F.; Lu, F.; Wang, B. Lytic Polysaccharide Monooxygenase—A New Driving Force for Lignocellulosic Biomass Degradation. Bioresour. Technol. 2022, 362, 127803. [Google Scholar] [CrossRef]
- Mohan, K.; Ganesan, A.R.; Muralisankar, T.; Jayakumar, R.; Sathishkumar, P.; Uthayakumar, V.; Chandirasekar, R.; Revathi, N. Recent Insights into the Extraction, Characterization, and Bioactivities of Chitin and Chitosan from Insects. Trends Food Sci. Technol. 2020, 105, 17–42. [Google Scholar] [CrossRef]
- Shi, X.; Ye, X.; Zhong, H.; Wang, T.; Jin, F. Sustainable Nitrogen-Containing Chemicals and Materials from Natural Marine Resources Chitin and Microalgae. Mol. Catal. 2021, 505, 111517. [Google Scholar] [CrossRef]
- Zhou, D.; Shen, D.; Lu, W.; Song, T.; Wang, M.; Feng, H.; Shentu, J.; Long, Y. Production of 5-Hydroxymethylfurfural from Chitin Biomass: A Review. Molecules 2020, 25, 541. [Google Scholar] [CrossRef]
- Kamal, M.; Adly, E.; Alharbi, S.A.; Khaled, A.S.; Rady, M.H.; Ibrahim, N.A. Exploring Simplified Methods for Insect Chitin Extraction and Application as a Potential Alternative Bioethanol Resource. Insects 2020, 11, 788. [Google Scholar] [CrossRef]
- Liaqat, F.; Eltem, R. Chitooligosaccharides and Their Biological Activities: A Comprehensive Review. Carbohydr. Polym. 2018, 184, 243–259. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Jhariya, U.; Purohit, H.J.; Dafale, N.A. Synergistic Action of Lytic Polysaccharide Monooxygenase with Gly-coside Hydrolase for Lignocellulosic Waste Valorization: A Review. Biomass Conv. Bioref. 2021, 13, 8272–8745. [Google Scholar] [CrossRef]
- Yu, X.; Zhao, Y.; Yu, J.; Wang, L. Recent Advances in the Efficient Degradation of Lignocellulosic Metabolic Networks by Lytic Polysaccharide Monooxygenase. Acta Biochim. Biophys. Sin. 2023, 55, 529. [Google Scholar] [CrossRef] [PubMed]
- Yan, N.; Chen, X. Don’t Waste Seafood Waste. Nature 2015, 524, 155–157. [Google Scholar] [CrossRef] [PubMed]
- Rovaletti, A.; De Gioia, L.; Fantucci, P.; Greco, C.; Vertemara, J.; Zampella, G.; Arrigoni, F.; Bertini, L. Recent Theoretical Insights into the Oxidative Degradation of Biopolymers and Plastics by Metalloenzymes. Int. J. Mol. Sci. 2023, 24, 6368. [Google Scholar] [CrossRef] [PubMed]
- Merzendorfer, H. Insect Chitin Synthases: A Review. J. Comp. Physiol. B 2005, 176, 1–15. [Google Scholar] [CrossRef]
- Santos, V.P.; Marques, N.S.S.; Maia, P.C.S.V.; de Lima, M.A.B.; de Oliveira Franco, L.; de Campos-Takaki, G.M. Seafood Waste as Attractive Source of Chitin and Chitosan Production and Their Applications. Int. J. Mol. Sci. 2020, 21, 4290. [Google Scholar] [CrossRef] [PubMed]
- Arnold, N.D.; Brück, W.M.; Garbe, D.; Brück, T.B. Enzymatic Modification of Native Chitin and Conversion to Specialty Chemical Products. Mar. Drug 2020, 18, 93. [Google Scholar] [CrossRef]
- Liu, Y.; Qin, Z.; Wang, C.; Jiang, Z. N-Acetyl-d-Glucosamine-Based Oligosaccharides from Chitin: Enzymatic Production, Characterization and Biological Activities. Carbohydr. Polym. 2023, 315, 121019. [Google Scholar] [CrossRef]
- Wani, A.K.; Akhtar, N.; Mir, T.u.G.; Rahayu, F.; Suhara, C.; Anjli, A.; Chopra, C.; Singh, R.; Prakash, A.; El Messaoudi, N.; et al. Eco-Friendly and Safe Alternatives for the Valorization of Shrimp Farming Waste. Environ. Sci. Pollut. Res. 2023, 30, 1–30. [Google Scholar] [CrossRef]
- Cao, S.; Liu, Y.; Shi, L.; Zhu, W.; Wang, H. N-Acetylglucosamine as a Platform Chemical Produced from Renewable Resources: Opportunity, Challenge, and Future Prospects. Green Chem. 2022, 24, 493–509. [Google Scholar] [CrossRef]
- Anil, S. Potential Medical Applications of Chitooligosaccharides. Polymers 2022, 14, 3558. [Google Scholar] [CrossRef]
- Kumar, M.; Brar, A.; Vivekanand, V.; Pareek, N. Bioconversion of Chitin to Bioactive Chitooligosaccharides: Amelioration and Coastal Pollution Reduction by Microbial Resources. Mar. Biotechnol. 2018, 20, 269–281. [Google Scholar] [CrossRef]
- Gonçalves, C.; Ferreira, N.; Lourenço, L. Production of Low Molecular Weight Chitosan and Chitooligosaccharides (COS): A Review. Polymers 2021, 13, 2466. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Mo, X.; Zhou, N.; Wang, Y.; Wei, G.; Hao, Z.; Chen, K. Identification of Chitinolytic Enzymes in Chitinolyticbacter Meiyuanensis and Mechanism of Efficiently Hydrolyzing Chitin to N-Acetyl Glucosamine. Front. Microbiol. 2020, 11, 572053. [Google Scholar] [CrossRef]
- Ling, M.; Li, J.; Du, G.; Liu, L. Metabolic Engineering for the Production of Chitooligosaccharides: Advances and Perspectives. Emerg. Top. Life Sci. 2018, 2, 377–388. [Google Scholar] [CrossRef]
- Sharma, A.; Arya, S.K.; Singh, J.; Kapoor, B.; Bhatti, J.S.; Suttee, A.; Singh, G. Prospects of Chitinase in Sustainable Farming and Modern Biotechnology: An Update on Recent Progress and Challenges. Biotechnol. Genet. Eng. Rev. 2023, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Chakdar, H.; Pandiyan, K.; Thapa, S.; Shahid, M.; Singh, A.; Srivastava, A.K.; Saxena, A.K. Bacterial Chitinases: Genetics, Engineering and Applications. World J. Microbiol. Biotechnol. 2022, 38, 252. [Google Scholar] [CrossRef]
- Aktuganov, G.E.; Melent’ev, A.I. Specific Features of Chitosan Depolymerization by Chitinases, Chitosanases, and Nonspe-cific Enzymes in the Production of Bioactive Chitooligosaccharides (Review). Appl. Biochem. Microbiol. 2017, 53, 611–627. [Google Scholar] [CrossRef]
- Ahuja, V.; Bhatt, A.; Sharma, V.; Rathour, R.; Rana, N.; Bhatia, R.; Varjani, S.; Kumar, M.; Magdouli, S.; Yang, Y.-H.; et al. Advances in Glucosamine Production from Waste Biomass and Microbial Fermentation Technology and Its Applications. Biomass Convers. Biorefinery 2021, 1–23. [Google Scholar] [CrossRef]
- Hemsworth, G.R.; Johnston, E.M.; Davies, G.J.; Walton, P.H. Lytic Polysaccharide Monooxygenases in Biomass Conversion. Trends Biotechnol. 2015, 33, 747–761. [Google Scholar] [CrossRef] [PubMed]
- Courtade, G.; Aachmann, F.L. Chitin-active lytic polysaccharide monooxygenases. In Targeting Chitin-Containing Organisms; Springer: Singapore, 2019; pp. 115–129. [Google Scholar]
- Vandhana, T.M.; Reyre, J.; Sushmaa, D.; Berrin, J.; Bissaro, B.; Madhuprakash, J. On the Expansion of Biological Functions of Lytic Polysaccharide Monooxygenases. New Phytol. 2022, 233, 2380–2396. [Google Scholar] [CrossRef]
- Reese, E.T.; Gilligan, W. The Swelling Factor in Cellulose Hydrolysis. Text. Res. J. 1954, 24, 663–669. [Google Scholar] [CrossRef]
- Eriksson, K.-E.; Pettersson, B.; Westermark, U. Oxidation: An Important Enzyme Reaction in Fungal Degradation of Cellulose. FEBS Lett. 1974, 49, 282–285. [Google Scholar] [CrossRef]
- Sunna, A.; Gibbs, M.D.; Bergquist, P.L. A Novel Thermostable Multidomain 1,4-β-Xylanase from ‘Caldibacillus Cellulovorans’ and Effect of Its Xylan-Binding Domain on Enzyme Activity. Microbiology 2000, 146, 2947–2955. [Google Scholar] [CrossRef] [PubMed]
- Saloheimo, M.; Nakari-SetaLa, T.; Tenkanen, M.; Penttila, M. CDNA Cloning of a Trichoderma Reesei Cellulase and Demonstration of Endoglucanase Activity by Expression in Yeast. Eur. J. Biochem. 1997, 249, 584–591. [Google Scholar] [CrossRef]
- Harris, P.V.; Welner, D.; McFarland, K.C.; Re, E.; Navarro Poulsen, J.-C.; Brown, K.; Salbo, R.; Ding, H.; Vlasenko, E.; Merino, S.; et al. Stimulation of Lignocellulosic Biomass Hydrolysis by Proteins of Glycoside Hydrolase Family 61: Structure and Function of a Large, Enigmatic Family. Biochemistry 2010, 49, 3305–3316. [Google Scholar] [CrossRef]
- Vaaje-Kolstad, G.; Westereng, B.; Horn, S.J.; Liu, Z.; Zhai, H.; Sørlie, M.; Eijsink, V.G.H. An Oxidative Enzyme Boosting the Enzymatic Conversion of Recalcitrant Polysaccharides. Science 2010, 330, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Horn, S.; Vaaje-Kolstad, G.; Westereng, B.; Eijsink, V.G. Novel Enzymes for the Degradation of Cellulose. Biotechnol. Biofuels 2012, 5, 45. [Google Scholar] [CrossRef]
- Levasseur, A.; Drula, E.; Lombard, V.; Coutinho, P.M.; Henrissat, B. Expansion of the Enzymatic Repertoire of the CAZy Database to Integrate Auxiliary Redox Enzymes. Biotechnol. Biofuels 2013, 6, 41. [Google Scholar] [CrossRef]
- Long, L.; Hu, Y.; Sun, F.; Gao, W.; Hao, Z.; Yin, H. Advances in Lytic Polysaccharide Monooxygenases with the Cellu-lose-Degrading Auxiliary Activity Family 9 to Facilitate Cellulose Degradation for Biorefinery. Int. J. Biol. Macromol. 2022, 219, 68–83. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, T.L.R.; Júnior, A.T.; Wolf, L.D.; Buckeridge, M.S.; dos Santos, L.V.; Murakami, M.T. An Actinobacteria Lytic Poly-saccharide Monooxygenase Acts on Both Cellulose and Xylan to Boost Biomass Saccharification. Biotechnol. Biofuels 2019, 12, 117. [Google Scholar] [CrossRef]
- Hemsworth, G.R.; Henrissat, B.; Davies, G.J.; Walton, P.H. Discovery and Characterization of a New Family of Lytic Poly-saccharide Monooxygenases. Nat. Chem. Biol. 2013, 10, 122–126. [Google Scholar] [CrossRef]
- Wang, D.; Li, J.; Wong, A.C.Y.; Aachmann, F.L.; Hsieh, Y.S.Y. A Colorimetric Assay to Rapidly Determine the Activities of Lytic Polysaccharide Monooxygenases. Biotechnol. Biofuels 2018, 11, 215. [Google Scholar] [CrossRef] [PubMed]
- Vu, V.V.; Beeson, W.T.; Span, E.A.; Farquhar, E.R.; Marletta, M.A. A Family of Starch-Active Polysaccharide Monooxygen-ases. Proc. Natl. Acad. Sci. USA 2014, 111, 13822–13827. [Google Scholar] [CrossRef] [PubMed]
- Haddad Momeni, M.; Leth, M.L.; Sternberg, C.; Schoof, E.; Nielsen, M.W.; Holck, J.; Workman, C.T.; Hoof, J.B.; Abou Hachem, M. Loss of AA13 LPMOs Impairs Degradation of Resistant Starch and Reduces the Growth of Aspergillus Nidulans. Biotechnol. Biofuels 2020, 13, 135. [Google Scholar] [CrossRef] [PubMed]
- Couturier, M.; Ladevèze, S.; Sulzenbacher, G.; Ciano, L.; Fanuel, M.; Moreau, C.; Villares, A.; Cathala, B.; Chaspoul, F.; Frandsen, K.E.; et al. Lytic Xylan Oxidases from Wood-Decay Fungi Unlock Biomass Degradation. Nat. Chem. Biol. 2018, 14, 306–310. [Google Scholar] [CrossRef]
- Sabbadin, F.; Hemsworth, G.R.; Ciano, L.; Henrissat, B.; Dupree, P.; Tryfona, T.; Marques, R.D.S.; Sweeney, S.T.; Besser, K.; Elias, L.; et al. An Ancient Family of Lytic Polysaccharide Monooxygenases with Roles in Arthropod Development and Bio-mass Digestion. Nat. Commun. 2018, 9, 756. [Google Scholar] [CrossRef]
- Filiatrault-Chastel, C.; Navarro, D.; Haon, M.; Grisel, S.; Herpoël-Gimbert, I.; Chevret, D.; Fanuel, M.; Henrissat, B.; Heiss-Blanquet, S.; Margeot, A.; et al. AA16, a New Lytic Polysaccharide Monooxygenase Family Identified in Fungal Se-cretomes. Biotechnol. Biofuels 2019, 12, 55. [Google Scholar] [CrossRef]
- Sabbadin, F.; Urresti, S.; Henrissat, B.; Avrova, A.O.; Welsh, L.R.J.; Lindley, P.J.; Csukai, M.; Squires, J.N.; Walton, P.H.; Davies, G.J.; et al. Secreted Pectin Monooxygenases Drive Plant Infection by Pathogenic Oomycetes. Science 2021, 373, 774–779. [Google Scholar] [CrossRef]
- Nakagawa, Y.S.; Kudo, M.; Loose, J.S.M.; Ishikawa, T.; Totani, K.; Eijsink, V.G.H.; Vaaje-Kolstad, G. A Small Lytic Polysac-charide Monooxygenase FromStreptomyces Griseustargeting α- and β-Chitin. FEBS J. 2015, 282, 1065–1079. [Google Scholar] [CrossRef]
- Book, A.J.; Yennamalli, R.M.; Takasuka, T.E.; Currie, C.R.; Phillips, G.N.; Fox, B.G. Evolution of Substrate Specificity in Bac-terial AA10 Lytic Polysaccharide Monooxygenases. Biotechnol. Biofuels 2014, 7, 109. [Google Scholar] [CrossRef]
- Zhou, X.; Qi, X.; Huang, H.; Zhu, H. Sequence and Structural Analysis of AA9 and AA10 LPMOs: An Insight into the Basis of Substrate Specificity and Regioselectivity. Int. J. Mol. Sci. 2019, 20, 4594. [Google Scholar] [CrossRef]
- Frandsen, K.E.H.; Lo Leggio, L. Lytic Polysaccharide Monooxygenases: A Crystallographer’s View on a New Class of Bio-mass-Degrading Enzymes. Int. Union Crystallogr. J. 2016, 3, 448–467. [Google Scholar] [CrossRef] [PubMed]
- Tandrup, T.; Frandsen, K.E.H.; Johansen, K.S.; Berrin, J.-G.; Lo Leggio, L. Recent Insights into Lytic Polysaccharide Monooxygenases (LPMOs). Biochem. Soc. Trans. 2018, 46, 1431–1447. [Google Scholar] [CrossRef] [PubMed]
- Vaaje-Kolstad, G.; Forsberg, Z.; Loose, J.S.; Bissaro, B.; Eijsink, V.G. Structural Diversity of Lytic Polysaccharide Monooxy-genases. Curr. Opin. Struct. Biol. 2017, 44, 67–76. [Google Scholar] [CrossRef]
- Phillips, C.M.; Beeson, W.T.; Cate, J.H.; Marletta, M.A. Cellobiose Dehydrogenase and a Copper-Dependent Polysaccharide Monooxygenase Potentiate Cellulose Degradation by Neurospora Crassa. ACS Chem. Biol. 2011, 6, 1399–1406. [Google Scholar] [CrossRef]
- Vaaje-Kolstad, G.; Bøhle, L.A.; Gåseidnes, S.; Dalhus, B.; Bjørås, M.; Mathiesen, G.; Eijsink, V.G.H. Characterization of the Chitinolytic Machinery of Enterococcus Faecalis V583 and High-Resolution Structure of Its Oxidative CBM33 Enzyme. J. Mol. Biol. 2012, 416, 239–254. [Google Scholar] [CrossRef] [PubMed]
- Gudmundsson, M.; Kim, S.; Wu, M.; Ishida, T.; Momeni, M.H.; Vaaje-Kolstad, G.; Lundberg, D.; Royant, A.; Ståhlberg, J.; Eijsink, V.G.H.; et al. Structural and Electronic Snapshots during the Transition from a Cu(II) to Cu(I) Metal Center of a Lytic Polysaccharide Monooxygenase by X-Ray Photoreduction. J. Biol. Chem. 2014, 289, 18782–18792. [Google Scholar] [CrossRef] [PubMed]
- Walton, P.H.; Davies, G.J. On the Catalytic Mechanisms of Lytic Polysaccharide Monooxygenases. Curr. Opin. Chem. Biol. 2016, 31, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Forsberg, Z.; Røhr, Å.K.; Mekasha, S.; Andersson, K.K.; Eijsink, V.G.H.; Vaaje-Kolstad, G.; Sørlie, M. Comparative Study of Two Chitin-Active and Two Cellulose-Active AA10-Type Lytic Polysaccharide Monooxygenases. Biochemistry 2014, 53, 1647–1656. [Google Scholar] [CrossRef] [PubMed]
- Forsberg, Z.; Courtade, G. On the Impact of Carbohydrate-Binding Modules (CBMs) in Lytic Polysaccharide Monooxygenases (LPMOs). Essays Biochem. 2023, 67, 561–574. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, H.J.; Knox, J.P.; Boraston, A.B. Advances in Understanding the Molecular Basis of Plant Cell Wall Polysaccharide Recognition by Carbohydrate-Binding Modules. Curr. Opin. Struct. Biol. 2013, 23, 669–677. [Google Scholar] [CrossRef]
- Crasson, O.; Courtade, G.; Léonard, R.R.; Aachmann, F.L.; Legrand, F.; Parente, R.; Baurain, D.; Galleni, M.; Sørlie, M.; Vandevenne, M. Human Chitotriosidase: Catalytic Domain or Carbohydrate Binding Module, Who’s Leading HCHT’s Bio-logical Function. Sci. Rep. 2017, 7, 2768. [Google Scholar] [CrossRef]
- Boraston, A.B.; Bolam, D.N.; Gilbert, H.J.; Davies, G.J. Carbohydrate-Binding Modules: Fine-Tuning Polysaccharide Recognition. Biochem. J. 2004, 382, 769–781. [Google Scholar] [CrossRef]
- Forsberg, Z.; Nelson, C.E.; Dalhus, B.; Mekasha, S.; Loose, J.S.M.; Crouch, L.I.; Røhr, Å.K.; Gardner, J.G.; Eijsink, V.G.H.; Vaaje-Kolstad, G. Structural and Functional Analysis of a Lytic Polysaccharide Monooxygenase Important for Efficient Uti-lization of Chitin in Cellvibrio japonicus. J. Biol. Chem. 2016, 291, 7300–7312. [Google Scholar] [CrossRef]
- Madland, E.; Forsberg, Z.; Wang, Y.; Lindorff-Larsen, K.; Niebisch, A.; Modregger, J.; Eijsink, V.G.H.; Aachmann, F.L.; Courtade, G. Structural and Functional Variation of Chitin-Binding Domains of a Lytic Polysaccharide Monooxygenase from Cellvibrio japonicus. J. Biol. Chem. 2021, 297, 101084. [Google Scholar] [CrossRef]
- Mutahir, Z.; Mekasha, S.; Loose, J.S.M.; Abbas, F.; Vaaje-Kolstad, G.; Eijsink, V.G.H.; Forsberg, Z. Characterization and Synergistic Action of a Tetra-modular Lytic Polysaccharide Monooxygenase from Bacillus cereus. FEBS Lett. 2018, 592, 2562–2571. [Google Scholar] [CrossRef] [PubMed]
- Manjeet, K.; Madhuprakash, J.; Mormann, M.; Moerschbacher, B.M.; Podile, A.R. A Carbohydrate Binding Module-5 Is Es-sential for Oxidative Cleavage of Chitin by a Multi-Modular Lytic Polysaccharide Monooxygenase from Bacillus thuringiensis Serovar Kurstaki. Int. J. Biol. Macromol. 2019, 127, 649–656. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, H.; Zhao, Y.; Li, T.; Yin, H. Comparative Studies of Two AA10 Family Lytic Polysaccharide Monooxygenases from Bacillus thuringiensis. PeerJ 2023, 11, e14670. [Google Scholar] [CrossRef]
- Sato, K.; Chiba, D.; Yoshida, S.; Takahashi, M.; Totani, K.; Shida, Y.; Ogasawara, W.; Nakagawa, Y.S. Functional Analysis of a Novel Lytic Polysaccharide Monooxygenase from Streptomyces Griseus on Cellulose and Chitin. Int. J. Biol. Macromol. 2020, 164, 2085–2091. [Google Scholar] [CrossRef] [PubMed]
- Bissaro, B.; Røhr, Å.K.; Müller, G.; Chylenski, P.; Skaugen, M.; Forsberg, Z.; Horn, S.J.; Vaaje-Kolstad, G.; Eijsink, V.G.H. Oxidative Cleavage of Polysaccharides by Monocopper Enzymes Depends on H2O2. Nat. Chem. Biol. 2017, 13, 1123–1128. [Google Scholar] [CrossRef]
- Forsberg, Z.; Bissaro, B.; Gullesen, J.; Dalhus, B.; Vaaje-Kolstad, G.; Eijsink, V.G.H. Structural Determinants of Bacterial Lytic Polysaccharide Monooxygenase Functionality. J. Biol. Chem. 2018, 293, 1397–1412. [Google Scholar] [CrossRef] [PubMed]
- Meier, K.K.; Jones, S.M.; Kaper, T.; Hansson, H.; Koetsier, M.J.; Karkehabadi, S.; Solomon, E.I.; Sandgren, M.; Kelemen, B. Oxygen Activation by Cu LPMOs in Recalcitrant Carbohydrate Polysaccharide Conversion to Monomer Sugars. Chem. Rev. 2017, 118, 2593–2635. [Google Scholar] [CrossRef]
- Courtade, G.; Forsberg, Z.; Heggset, E.B.; Eijsink, V.G.H.; Aachmann, F.L. The Carbohydrate-Binding Module and Linker of a Modular Lytic Polysaccharide Monooxygenase Promote Localized Cellulose Oxidation. J. Biol. Chem. 2018, 293, 13006–13015. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Nagar, P.; Rathore, S.; Adlakha, N. The Linker Region Promotes Activity and Binding Efficiency of Modular LPMO towards Polymeric Substrate. Microbiol. Spectr. 2022, 10, 1. [Google Scholar] [CrossRef]
- Guo, X.; An, Y.; Jiang, L.; Zhang, J.; Lu, F.; Liu, F. The Discovery and Enzymatic Characterization of a Novel AA10 LPMO from Bacillus amyloliquefaciens with Dual Substrate Specificity. Int. J. Biol. Macromol. 2022, 203, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Mekasha, S.; Forsberg, Z.; Dalhus, B.; Bacik, J.-P.; Choudhary, S.; Schmidt-Dannert, C.; Vaaje-Kolstad, G.; Eijsink, V.G.H. Structural and Functional Characterization of a Small Chitin-Active Lytic Polysaccharide Monooxygenase Domain of a Multi-Modular Chitinase FromJonesia Denitrificans. FEBS Lett. 2015, 590, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Bacik, J.-P.; Mekasha, S.; Forsberg, Z.; Kovalevsky, A.Y.; Vaaje-Kolstad, G.; Eijsink, V.G.H.; Nix, J.C.; Coates, L.; Cuneo, M.J.; Unkefer, C.J.; et al. Neutron and Atomic Resolution X-Ray Structures of a Lytic Polysaccharide Monooxygenase Reveal Copper-Mediated Dioxygen Binding and Evidence for N-Terminal Deprotonation. Biochemistry 2017, 56, 2529–2532. [Google Scholar] [CrossRef]
- Mekasha, S.; Tuveng, T.R.; Askarian, F.; Choudhary, S.; Schmidt-Dannert, C.; Niebisch, A.; Modregger, J.; Vaaje-Kolstad, G.; Eijsink, V.G.H. A Trimodular Bacterial Enzyme Combining Hydrolytic Activity with Oxidative Glycosidic Bond Cleavage Efficiently Degrades Chitin. J. Biol. Chem. 2020, 295, 9134–9146. [Google Scholar] [CrossRef]
- Wang, D.; Li, Y.; Zheng, Y.; Hsieh, Y.S.Y. Recent Advances in Screening Methods for the Functional Investigation of Lytic Polysaccharide Monooxygenases. Front. Chem. 2021, 9, 653754. [Google Scholar] [CrossRef]
- Westereng, B.; Arntzen, M.Ø.; Østby, H.; Agger, J.W.; Vaaje-Kolstad, G.; Eijsink, V.G.H. Analyzing activities of lytic poly-saccharide monooxygenases by liquid chromatography and mass spectrometry. In Protein-Carbohydrate Interactions: Methods and Protocols; Springer: New York, NY, USA, 2023; pp. 27–51. [Google Scholar]
- Vaaje-Kolstad, G.; Horn, S.J.; van Aalten, D.M.F.; Synstad, B.; Eijsink, V.G.H. The Non-Catalytic Chitin-Binding Protein CBP21 from Serratia marcescens Is Essential for Chitin Degradation. J. Biol. Chem. 2005, 280, 28492–28497. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Liu, Y.; Liu, Y.; Li, Y.; Yu, H. Heterologous Expression and Characterization of a Novel Lytic Polysaccharide Monooxygenase from Natrialbaceae Archaeon and Its Application for Chitin Biodegradation. Bioresour. Technol. 2022, 354, 127174. [Google Scholar] [CrossRef]
- Munzone, A.; El Kerdi, B.; Fanuel, M.; Rogniaux, H.; Ropartz, D.; Réglier, M.; Royant, A.; Simaan, A.J.; Decroos, C. Char-acterization of a Bacterial Copper-dependent Lytic Polysaccharide Monooxygenase with an Unusual Second Coordination Sphere. FEBS J. 2020, 287, 3298–3314. [Google Scholar] [CrossRef] [PubMed]
- Franco Cairo, J.P.L.; Cannella, D.; Oliveira, L.C.; Gonçalves, T.A.; Rubio, M.V.; Terrasan, C.R.F.; Tramontina, R.; Mofatto, L.S.; Carazzolle, M.F.; Garcia, W.; et al. On the Roles of AA15 Lytic Polysaccharide Monooxygenases Derived from the Termite Coptotermes gestroi. J. Inorg. Biochem. 2021, 216, 111316. [Google Scholar] [CrossRef]
- Sethupathy, S.; Morales, G.M.; Li, Y.; Wang, Y.; Jiang, J.; Sun, J.; Zhu, D. Harnessing Microbial Wealth for Lignocellulose Biomass Valorization through Secretomics: A Review. Biotechnol. Biofuels 2021, 14, 154. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.-X.; Li, P.-Y.; Chen, X.-L.; Zhang, Y.-S.; Wang, J.-P.; Wang, Y.-J.; Sheng, Q.; Sun, Z.-Z.; Qin, Q.-L.; Ren, X.-B.; et al. A Pathway for Chitin Oxidation in Marine Bacteria. Nat. Commun. 2022, 13, 5889. [Google Scholar] [CrossRef] [PubMed]
- Song, X.-F.; Feng, C. Lytic polysaccharide monooxygenase and its application. Acta Microbiol. Sin. 2023, 63, 2534–2551. [Google Scholar] [CrossRef]
- Gregory, R.C.; Hemsworth, G.R.; Turkenburg, J.P.; Hart, S.J.; Walton, P.H.; Davies, G.J. Activity, Stability and 3-D Structure of the Cu(<scp>ii</Scp>) Form of a Chitin-Active Lytic Polysaccharide Monooxygenase from Bacillus amyloliquefaciens. Dalton Trans. 2016, 45, 16904–16912. [Google Scholar] [CrossRef]
- Ghatge, S.S.; Telke, A.A.; Waghmode, T.R.; Lee, Y.; Lee, K.-W.; Oh, D.-B.; Shin, H.-D.; Kim, S.-W. Multifunctional Cellulolytic Auxiliary Activity Protein HcAA10-2 from Hahella Chejuensis Enhances Enzymatic Hydrolysis of Crystalline Cellulose. Appl. Microbiol. Biotechnol. 2014, 99, 3041–3055. [Google Scholar] [CrossRef]
- Courtade, G.; Le, S.B.; Sætrom, G.I.; Brautaset, T.; Aachmann, F.L. A Novel Expression System for Lytic Polysaccharide Monooxygenases. Carbohydr. Res. 2017, 448, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, J.; Liu, X.; Pan, X.; Hou, J.; Ran, C.; Zhou, Z. Improving Extracellular Production of Serratia marcescens Lytic Polysaccharide Monooxygenase CBP21 and Aeromonas Veronii B565 Chitinase Chi92 in Escherichia coli and Their Syner-gism. AMB Expr. 2017, 7, 170. [Google Scholar] [CrossRef] [PubMed]
- Chaplin, A.K.; Wilson, M.T.; Hough, M.A.; Svistunenko, D.A.; Hemsworth, G.R.; Walton, P.H.; Vijgenboom, E.; Worrall, J.A.R. Heterogeneity in the Histidine-Brace Copper Coordination Sphere in Auxiliary Activity Family 10 (AA10) Lytic Poly-saccharide Monooxygenases. J. Biol. Chem. 2016, 291, 12838–12850. [Google Scholar] [CrossRef]
- Fowler, C.A.; Sabbadin, F.; Ciano, L.; Hemsworth, G.R.; Elias, L.; Bruce, N.; McQueen-Mason, S.; Davies, G.J.; Walton, P.H. Discovery, Activity and Characterisation of an AA10 Lytic Polysaccharide Oxygenase from the Shipworm Symbiont Teredinibacter Turnerae. Biotechnol. Biofuels 2019, 12, 232. [Google Scholar] [CrossRef] [PubMed]
- Gaber, Y.; Rashad, B.; Hussein, R.; Abdelgawad, M.; Ali, N.S.; Dishisha, T.; Várnai, A. Heterologous Expression of Lytic Polysaccharide Monooxygenases (LPMOs). Biotechnol. Adv. 2020, 43, 107583. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.-J.; Yoon, S.-H.; Kim, Y.-W. Overproduction and Characterization of a Lytic Polysaccharide Monooxygenase in Bacillus Subtilis Using an Assay Based on Ascorbate Consumption. Enzym. Microb. Technol. 2016, 93–94, 150–156. [Google Scholar] [CrossRef]
- Schallmey, M.; Singh, A.; Ward, O.P. Developments in the Use of Bacillus Species for Industrial Production. Can. J. Microbiol. 2004, 50, 1–17. [Google Scholar] [CrossRef]
- Guo, X.; Chai, C.; An, Y.; Peng, C.; Shi, N.; Wang, W.; Lu, F.; Dai, Y.; Liu, F. Rational Design of Signal Peptides for Improved MtC1LPMO Production in Bacillus amyloliquefaciens. Int. J. Biol. Macromol. 2021, 175, 262–269. [Google Scholar] [CrossRef]
- Russo, D.A.; Zedler, J.A.Z.; Wittmann, D.N.; Möllers, B.; Singh, R.K.; Batth, T.S.; van Oort, B.; Olsen, J.V.; Bjerrum, M.J.; Jensen, P.E. Expression and Secretion of a Lytic Polysaccharide Monooxygenase by a Fast-Growing Cyanobacterium. Biotechnol. Biofuels 2019, 12, 74. [Google Scholar] [CrossRef]
- Votvik, A.K.; Røhr, Å.K.; Bissaro, B.; Stepnov, A.A.; Sørlie, M.; Eijsink, V.G.H.; Forsberg, Z. Structural and Functional Characterization of the Catalytic Domain of a Cell-Wall Anchored Bacterial Lytic Polysaccharide Monooxygenase from Streptomyces coelicolor. Sci. Rep. 2023, 13, 5145. [Google Scholar] [CrossRef]
- Li, F.; Zhao, H.; Liu, Y.; Zhang, J.; Yu, H. Chitin Biodegradation by Lytic Polysaccharide Monooxygenases from Streptomyces coelicolor In Vitro and In Vivo. Int. J. Mol. Sci. 2022, 24, 275. [Google Scholar] [CrossRef] [PubMed]
- Støpamo, F.G.; Røhr, Å.K.; Mekasha, S.; Petrović, D.M.; Várnai, A.; Eijsink, V.G.H. Characterization of a Lytic Polysaccharide Monooxygenase from Aspergillus fumigatus Shows Functional Variation among Family AA11 Fungal LPMOs. J. Biol. Chem. 2021, 297, 101421. [Google Scholar] [CrossRef]
- Sabbadin, F.; Henrissat, B.; Bruce, N.C.; McQueen-Mason, S.J. Lytic Polysaccharide Monooxygenases as Chitin-Specific Vir-ulence Factors in Crayfish Plague. Biomolecules 2021, 11, 1180. [Google Scholar] [CrossRef]
- Qu, M.; Guo, X.; Tian, S.; Yang, Q.; Kim, M.; Mun, S.; Noh, M.Y.; Kramer, K.J.; Muthukrishnan, S.; Arakane, Y. AA15 Lytic Polysaccharide Monooxygenase Is Required for Efficient Chitinous Cuticle Turnover during Insect Molting. Commun. Biol. 2022, 5, 518. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Rollán, C.; Falkenberg, K.B.; Rennig, M.; Bertelsen, A.B.; Ipsen, J.Ø.; Brander, S.; Daley, D.O.; Johansen, K.S.; Nørholm, M.H.H. LyGo: A Platform for Rapid Screening of Lytic Polysaccharide Monooxygenase Production. ACS Synth. Biol. 2021, 10, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Loose, J.S.M.; Arntzen, M.Ø.; Bissaro, B.; Ludwig, R.; Eijsink, V.G.H.; Vaaje-Kolstad, G. Multipoint Precision Binding of Substrate Protects Lytic Polysaccharide Monooxygenases from Self-Destructive Off-Pathway Processes. Biochemistry 2018, 57, 4114–4124. [Google Scholar] [CrossRef]
- Jensen, M.S.; Klinkenberg, G.; Bissaro, B.; Chylenski, P.; Vaaje-Kolstad, G.; Kvitvang, H.F.; Nærdal, G.K.; Sletta, H.; Forsberg, Z.; Eijsink, V.G.H. Engineering Chitinolytic Activity into a Cellulose-Active Lytic Polysaccharide Monooxygenase Provides Insights into Substrate Specificity. J. Biol. Chem. 2019, 294, 19349–19364. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Xu, Z.; Li, Y.; He, J.; Zhu, H. Improvement of the Stability and Activity of an LPMO Through Rational Disulfide Bonds Design. Front. Bioeng. Biotechnol. 2022, 9, 815990. [Google Scholar] [CrossRef] [PubMed]
- Forsberg, Z.; Stepnov, A.A.; Nærdal, G.K.; Klinkenberg, G.; Eijsink, V.G.H. Engineering Lytic Polysaccharide Monooxygenases (LPMOs). In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–34. [Google Scholar]
- Lo Leggio, L.; Weihe, C.D.; Poulsen, J.-C.N.; Sweeney, M.; Rasmussen, F.; Lin, J.; De Maria, L.; Wogulis, M. Structure of a Lytic Polysaccharide Monooxygenase from Aspergillus fumigatus and an Engineered Thermostable Variant. Carbohydr. Res. 2018, 469, 55–59. [Google Scholar] [CrossRef]
- Tanghe, M.; Danneels, B.; Last, M.; Beerens, K.; Stals, I.; Desmet, T. Disulfide Bridges as Essential Elements for the Thermo-stability of Lytic Polysaccharide Monooxygenase LPMO10C from Streptomyces coelicolor. Protein Eng. Des. Sel. 2017, 30, 401–408. [Google Scholar] [CrossRef]
- Sørlie, M.; Keller, M.B.; Westh, P. The Interplay between Lytic Polysaccharide Monooxygenases and Glycoside Hydrolases. Essays Biochem. 2023, 67, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Suzuki, M.; Taiyoji, M.; Nikaidou, N.; Watanabe, T. Chitin Binding Protein (CBP21) in the Culture Supernatant of Serratia marcescens 2170. Biosci. Biotechnol. Biochem. 1998, 62, 128–135. [Google Scholar] [CrossRef]
- Hamre, A.G.; Eide, K.B.; Wold, H.H.; Sørlie, M. Activation of Enzymatic Chitin Degradation by a Lytic Polysaccharide Monooxygenase. Carbohydr. Res. 2015, 407, 166–169. [Google Scholar] [CrossRef]
- Nakagawa, Y.S.; Kudo, M.; Onodera, R.; Ang, L.Z.P.; Watanabe, T.; Totani, K.; Eijsink, V.G.; Vaaje-Kolstad, G. Analysis of Four Chitin-Active Lytic Polysaccharide Monooxygenases from Streptomyces Griseus Reveals Functional Varia-tion. J. Agric. Food Chem. 2020, 68, 13641–13650. [Google Scholar] [CrossRef]
- Pentekhina, I.; Hattori, T.; Tran, D.M.; Shima, M.; Watanabe, T.; Sugimoto, H.; Suzuki, K. Chitinase System of Aeromonas Salmonicida, and Characterization of Enzymes Involved in Chitin Degradation. Biosci. Biotechnol. Biochem. 2020, 84, 1936–1947. [Google Scholar] [CrossRef]
- Sun, X.-B.; Gao, D.-Y.; Cao, J.-W.; Liu, Y.; Rong, Z.-T.; Wang, J.-K.; Wang, Q. BsLPMO10A from Bacillus Subtilis Boosts the Depolymerization of Diverse Polysaccharides Linked via β-1,4-Glycosidic Bonds. Int. J. Biological Macromol. 2023, 230, 123133. [Google Scholar] [CrossRef]
- Ma, L.; Liu, Z.; Kong, Z.; Wang, M.; Li, T.; Zhu, H.; Wan, Q.; Liu, D.; Shen, Q. Functional Characterization of a Novel Copper-Dependent Lytic Polysaccharide Monooxygenase TgAA11 from Trichoderma Guizhouense NJAU 4742 in the Oxi-dative Degradation of Chitin. Carbohydr. Polym. 2021, 258, 117708. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Pan, D.; Xiao, P.; Xu, Q.; Geng, F.; Zhang, X.; Zhou, X.; Xu, H. A Novel Lytic Polysaccharide Monooxygenase from Enrichment Microbiota and Its Application for Shrimp Shell Powder Biodegradation. Front. Microbiol. 2023, 14, 1097492. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Haider, J.; Liu, P.; Yang, J.; Tan, Z.; Huang, T.; Lin, J.; Jiang, M.; Liu, H.; Zhu, L. Engineered LPMO Significantly Boosting Cellulase-Catalyzed Depolymerization of Cellulose. J. Agric. Food Chem. 2020, 68, 15257–15266. [Google Scholar] [CrossRef] [PubMed]
- Jagadeeswaran, G.; Veale, L.; Mort, A.J. Do Lytic Polysaccharide Monooxygenases Aid in Plant Pathogenesis and Herbivory? Trends Plant Sci. 2020, 26, 142–155. [Google Scholar] [CrossRef] [PubMed]
- Loose, J.S.M.; Boudes, M.; Bergoin, M.; Coulibaly, F.; Vaaje-Kolstad, G. The Melolontha Melolontha Entomopoxvirus Fusolin Protein Is a Chitin-active Lytic Polysaccharide Monooxygenase That Displays Extreme Stability. FEBS Lett. 2023, 597, 1375–1383. [Google Scholar] [CrossRef] [PubMed]





| LCMs | Family | PDB Code | Organism | References |
|---|---|---|---|---|
| CjLPMO10A | AA10 | 5FJQ | Cellvibrio japonicus | [63] |
| JdLPMO10A | AA10 | 5AA7 | Jonesia denitrificans | [75] |
| CBP21 | AA10 | 2BEM | Serratia marcescens | [80] |
| NaLPMO10A | AA10 | Natrialbaceae archaeon | [81] | |
| PlAA10 | AA10 | Photorhabdus luminescen | [82] | |
| AoLpmo11 | AA11 | 4MAH | Aspergillus oryzae | [40] |
| TdAA15A | AA15 | 5MSZ | Thermobia domestica | [45] |
| TdAA15B | AA15 | Thermobia domestica | [45] |
| LCMs | Chitinase | Substrate | Product | Increase | Reference |
|---|---|---|---|---|---|
| CBP21 (50 nM) | ChitABC (50 nM) | β-Chitin (0.1 mg/mL) | Chitobiose (μM) | 127 to 162 μM * | [111] |
| CBP21 (1 μM) | ChitA (170 nM) | β-Chitin (20 mg/mL) | (GlcNAc)2 (μM) | 275 to 1680 μM * | [112] |
| CBP21 (1 μM) | ChitA (170 nM) | β-Chitin (20 mg/mL) | (GlcNAc)2 (μM) | 262 to 2188 μM * | [112] |
| SgLPMO10B (1.5 μM) | SmChiC (0.2μM) | β-Chitin (4 mg/mL) | (GlcNAc)2 (μM) | 0.383 to 1.044 mM * | [113] |
| SgLPMO10C (0.3 μM) | SmChiC (0.2μM) | β-Chitin (4 mg/mL) | (GlcNAc)2 (μM) | 0.199 to 0.844 mM * | [113] |
| SgLPMO10D (0.3 μM) | SmChiC (0.2μM) | β-Chitin (4 mg/mL) | (GlcNAc)2 (μM) | 0.212 to 0.691 mM * | [113] |
| SgLPMO10F (1.5 μM) | SmChiC (0.2μM) | β-Chitin (4 mg/mL) | (GlcNAc)2 (μM) | 0.169 to 0.712 mM * | [113] |
| AsLPMO10A (5 μg/1.5 mL) | AsChiABCD (5 μg/1.5 mL) | Chitin powder (4 mg/1.4 mL) | Reducing sugar (μM) | 0.213 to 0.425 μM * | [114] |
| NaLPMO10A (2 μM) | Commercial chitinase (15 μg/mL) | Chitin (10 mg/mL) | Reducing sugar (μM) | 0.29 to 0.44 (mg/mL) | [81] |
| BsLPMO10A (0.75 μM) | BtCHI18-1 (7.5 μL) | Chitin (5 mg/mL) | Reducing sugar (μM) | 2063 to 2596 μM * | [115] |
| TgAA11 (5 μM) | Sg-chi 10 μg | α-Chitin 1% w/v | Reducing sugar | Increase by 39.9% (Relative value) | [116] |
| TgAA11 (5 μM) | Sg-chi 10 μg | β-Chitin 1% w/v | Reducing sugar | Increase by 288.2% (Relative value) | [116] |
| M2822 | Commercial chitinase (1 mg/mL) | α-Chitin (10 mg/mL) | GlcNAc (μM) | 2.32 to 4.26 μM | [117] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, D.; Liu, J.; Xiao, P.; Xie, Y.; Zhou, X.; Zhang, Y. Research Progress of Lytic Chitin Monooxygenase and Its Utilization in Chitin Resource Fermentation Transformation. Fermentation 2023, 9, 754. https://doi.org/10.3390/fermentation9080754
Pan D, Liu J, Xiao P, Xie Y, Zhou X, Zhang Y. Research Progress of Lytic Chitin Monooxygenase and Its Utilization in Chitin Resource Fermentation Transformation. Fermentation. 2023; 9(8):754. https://doi.org/10.3390/fermentation9080754
Chicago/Turabian StylePan, Delong, Jinze Liu, Peiyao Xiao, Yukun Xie, Xiuling Zhou, and Yang Zhang. 2023. "Research Progress of Lytic Chitin Monooxygenase and Its Utilization in Chitin Resource Fermentation Transformation" Fermentation 9, no. 8: 754. https://doi.org/10.3390/fermentation9080754
APA StylePan, D., Liu, J., Xiao, P., Xie, Y., Zhou, X., & Zhang, Y. (2023). Research Progress of Lytic Chitin Monooxygenase and Its Utilization in Chitin Resource Fermentation Transformation. Fermentation, 9(8), 754. https://doi.org/10.3390/fermentation9080754






