Abstract
Alzheimer’s disease (AD) is an ascending, neurodegenerative disorder that attacks the brain’s nerve cells, i.e., neurons, resulting in loss of memory, language skills, and thinking and behavioural changes. It is one of the most common causes of dementia, a group of disorders that is marked by the decline of cognitive functioning. Probiotics are living microorganisms that are beneficial for human well-being. They help in balancing the extent of bacteria in the gut and support the defensive immune system of the body. Studies have found that probiotics can help with a variety of conditions, including mental health. Probiotics are beneficial bacteria that can help to maintain and strengthen a healthy gut microbiome. The gut microbiome is important for healthy brain function, as it is linked to the production of neurotransmitters and hormones that regulate mood and behaviour. This review article includes detailed review on the origination of probiotics and its significance in the treatment of AD.
1. Introduction
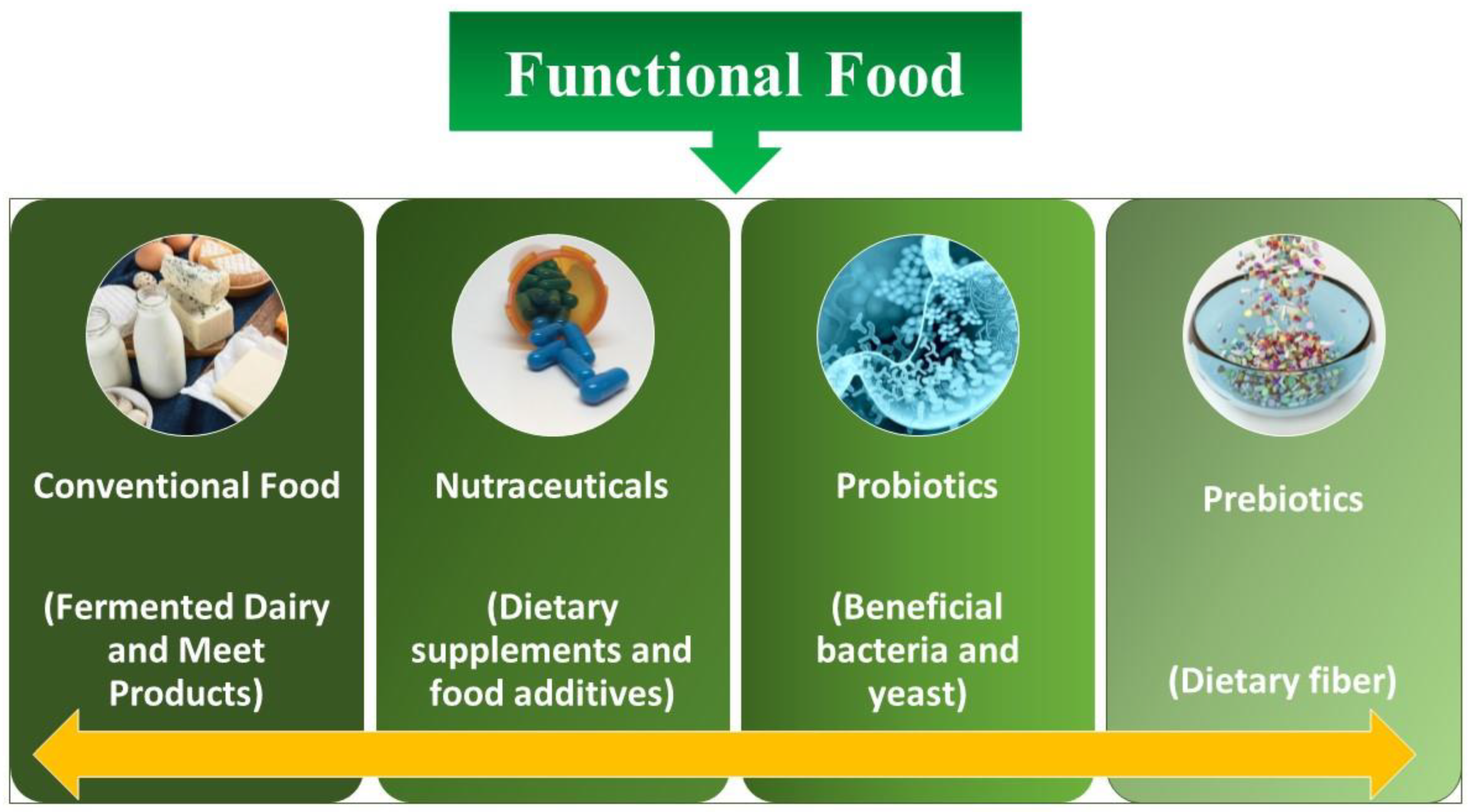
The concept of functional food is discussed thousands of times on different platforms [1]. Although the accurate definition is still a mystery, experts normally concur that in general, additives are involved in functional foods (containing microbes such as probiotics, mentioned underneath), which hold the benefit of health along with the basic nutrient content of the food as well (Figure 1). Functional foods have the potential to improve physiological mechanisms and enhance neuronal functioning at the level of the gastrointestinal tract (GIT) via altering biochemical parameters. Probiotics are considered one of the primary pathways for health benefits and are included in functional foods. It has been demonstrated that the host’s energy supply is significantly affected by the activities of microbiota present in the gut. The GI microbiota is the total population attributed to live microorganisms that inhabit a host organism’s GI tract [2].
Figure 1.
Different kinds of functional food.
Jäger et al. [3] stated that probiotics comprises of living microbes that, once ingested in sufficient quantities, are good for human health. The Greek words “pro” and “biotic”, which both imply “life”, are the origin of the phrase “probiotic” [3]. These microscopic organisms, which are primarily bacteria but can also incorporate certain yeasts, exist naturally in our own bodies as well as in certain foods and dietary supplements. The microbial community or microbiome, which consists of billions of bacteria, inhabits in the human body. It also exists in other parts, including the mouth, genital tract, and skin, but microbes are mostly found in our digestive system. Our general health is maintained by this intricate ecology [4]. The cornerstone of our health is made up of these live nutrients. Dental disease, yeast infections, eczema, and food allergies are prevented and treated by maintaining a healthy microbiome. Probiotics are a broad category of microbes. All of them have unique advantages, although the majority belong to just two groups. Lactobacillaceae is the most widely used probiotic. It is found in yoghurt and other fermented foods. Countless varieties may aid those who struggle to digest the milk sugar lactose as well as those who experience diarrhoea [5]. Bifidobacterium is frequently discovered in various dairy products and could lessen the symptoms and signs of irritable bowel syndrome (IBS) and a few other disorders [6,7]. Probiotics encompass the yeast Saccharomyces boulardii. It seems to aid in the prevention of diarrhoea and other intestinal problems. Slightly over 4 million (1.6%) American adults reported using probiotics or prebiotics in the previous 30 days, according to the 2012 National Health Interview Survey (NHIS). Probiotics and prebiotics were the third most popular dietary supplement among adults beside vitamins and minerals. From 2007 to 2012, consumption of probiotics in adults increased by four times. The 2012 NHIS also revealed that 300,000 children between the age of 4 and 17 (or 0.5% of the population) reported using probiotics or prebiotics in the 30 days prior to a questionnaire [8].
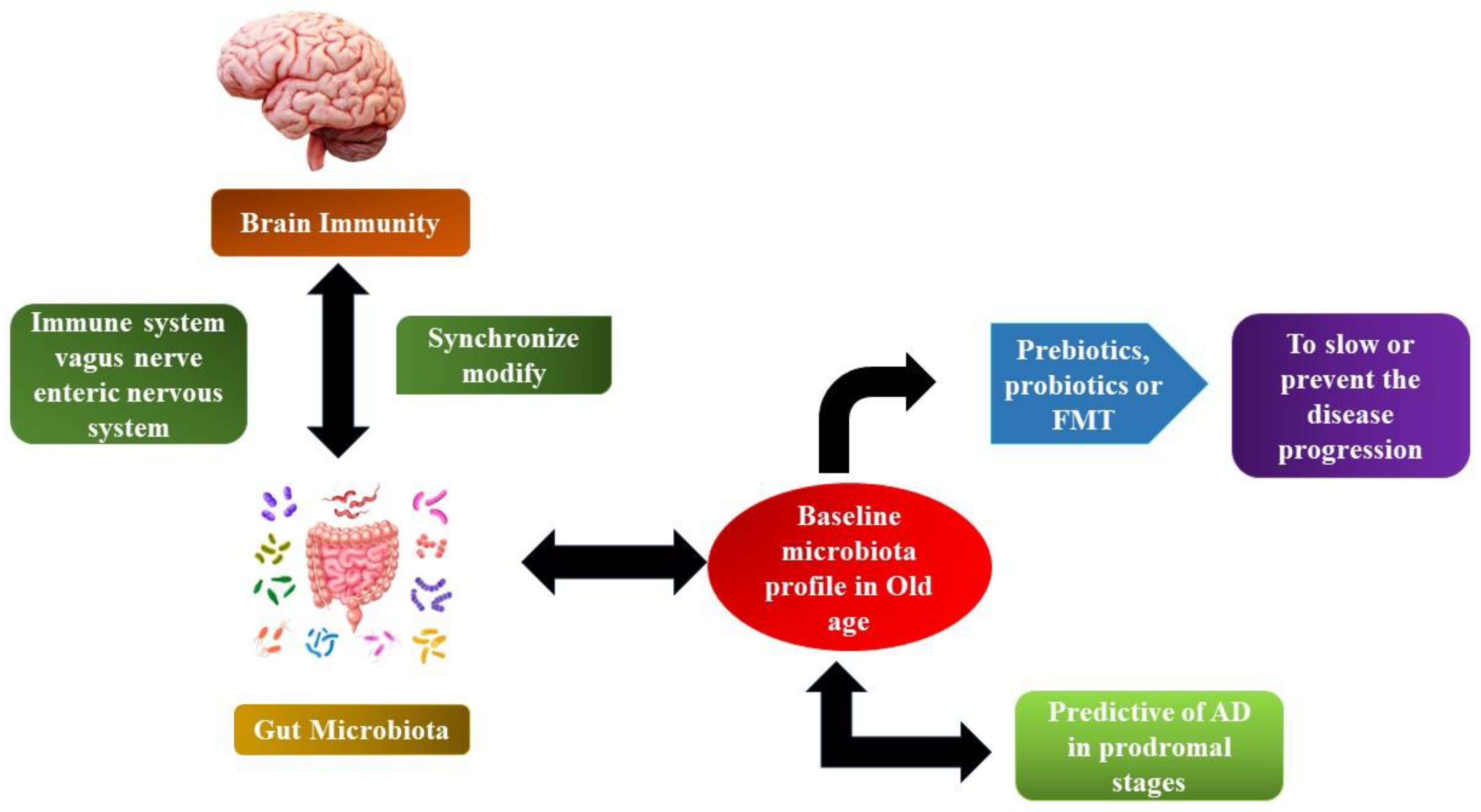
Probiotics have demonstrated potential for an assortment of medical conditions, such as the management of infant colic, the prevention of necrotizing enterocolitis and sepsis in premature infants, the treatment of periodontal disease, and the induction or maintenance of remission in ulcerative colitis [9]. An unbalanced microbiota in the gut that causes the alteration of the microbiome (dysbiosis) has been strongly associated with psychological disorders. The gut produces several neurotransmitters that are linked to anxiety and depression symptoms, including the neurotransmitters glutamate, serotonin, and GABA (gamma-aminobutyric acid). Numerous mental diseases are believed to be associated with gut dysbiosis, and there is strong evidence linking gut health with psychological wellness. One of the main characteristics and pathological traits linked to a wide range of neurodegenerative illnesses, including Alzheimer’s, Parkinson’s, and Huntington’s diseases, is neuroinflammation such as microgliosis and astrogliosis. Furthermore, owing to the constant presence of inflammation in the brain, classic neuroinflammatory illnesses like multiple sclerosis clearly highlight the significance of neuroinflammation in these neurodegenerative diseases. The most prevalent dementia amongst them is AD, which may be identified by an increase in neurofibrillary plaque build-up in the brain. There is strong evidence that GM (gastrointestinal microbiota) plays a role in the pathogenesis of AD, which is largely mediated by altered microglial activity in the brain. AD is just one neurodegenerative condition where microglial dysfunction has been identified [10].
The “gut–microbiota–brain axis” is a term coined for representing such relationship: a link uniting the gut microbiome and AD and also representing the role played by inflammation in the progression as well as onset of AD. The considerably discussed topic of the microbiota–gut-brain axis holds that the gut microbiota has an impact on the activities of central nervous system (CNS) [11,12,13]. By encouraging the development of advantageous microbes, a diet high in prebiotics supports the health of the digestive system. Throughout the fermentation of prebiotics, short-chain fatty acids (SCFAs) including butyrate, acetate, and propionate are created. They are crucial for maintaining metabolic and intestinal health. Likewise, according to the study, probiotics affect brain function and behaviour, and knowledge of these biological causes may help to improve psychological well-being in both healthy people and, possibly, those with mental illnesses [11]. Probiotics have been shown to enhance stress response, improve memory function, and even lessen anxiety and sadness in preclinical experiments with rodents [14,15].
Many theoretical concepts have been proposed regarding the contribution of the gut microbiota to AD chronic neuroinflammation. The highly significant concept includes the following ideas: (1) A neuroinflammatory condition in the brains of patients with AD that can be caused by direct microorganism infection; (2) an age-related dysbacteriosis that hypothesizes the aging immune system to be a cause of AD development; (3) a microbicidal safeguard theory indicating the immune reaction against the build-up of harmful bacteria as the cause of build-up of AD in brain; and A notable, promising result in the decrement of the progression of AD is observed when probiotics are taken as a sole supplement. The key driving force behind the exploration of overall therapeutic efficacy is now their clinically tested anti-inflammatory effects and antioxidant capabilities and their capacity to improve a patient’s cognitive function [16]. Fermented milk has the potential to be used in the treatment of mental and functional CNS disorders as well as modulating stress adaptivity and mood in disease and well-being [17]. Various foods with probiotic activity are being created from the dairy products that are fermented, including yogurt, dairy serums, and kefir containing Lactobacillaceae and Bifidobacterium [18,19]
Role of Psychobiotics in the Improving Mental Health
Probiotics, in particular psychobiotics, have demonstrated promise for treating AD and other mental health problems. These substances have a significant impact on cognitive and emotional functions as well as brain function. The gut microbiota has been linked to mental diseases including AD, and dysbiosis in the gut microbiota has been linked to brain maturation and neurogenesis. Psychobiotics can assist in re-establishing a healthy balance of gut flora, which may have a good impact on AD symptoms and cognitive performance [20]. Psychobiotics have been shown to have anti-inflammatory properties, and by controlling the immune system and blocking the production of inflammatory substances and chemicals, they can reduce neurological inflammation. By lowering neuroinflammation, psychobiotics may save brain cells and slow the onset of AD. It has been shown that neuroprotective substances like brain-derived neurotrophic factor (BDNF), which promotes the development and longevity of neurons, are produced by some psychobiotics. Since inadequate amounts of BDNF have been associated with AD, boosting BDNF synthesis by using psychobiotics may aid in preserving the health and functionality of neurons. The capacity of the stomach to create and employ neurotransmitters like serotonin and gamma-aminobutyric acid (GABA), which are essential for regulating emotions, cognition, and recollection, may be impacted by psychobiotics [21]. By altering neurotransmitter levels, psychobiotics may improve cognitive performance and emotional well-being in persons with AD. Increased intestinal permeability, a side effect of AD, facilitates the bloodstream entry of harmful substances and can lead to cognitive decline. Psychobiotics have been shown to boost the intestinal barrier’s capacity to prevent the entrance of contaminants by promoting the production of mucins and tight junction proteins. By enhancing gut barrier function, psychobiotics may aid in the reduction in systemic inflammation and may improve memory retention in AD [22]. Oxidative stress is one of the key elements in the development and progression of AD. Psychobiotics are antioxidants that can scavenge free radicals to decrease oxidative damage to brain cells. By reducing oxidative stress, psychobiotics may help to preserve neurons and decrease the neurodegenerative processes linked to AD [23]. Psychobiotics therapies may improve emotional behaviour and cognitive performance in children and adolescents, according to a comprehensive review by [24]. Another study by Cheng et al. [25] discovered that certain psychobiotic strains reduced cortisol levels and suppressed inflammation, which improved the signs and symptoms associated with anxiety and depression.
2. Production of Probiotics from Dairy and Non-Dairy Products
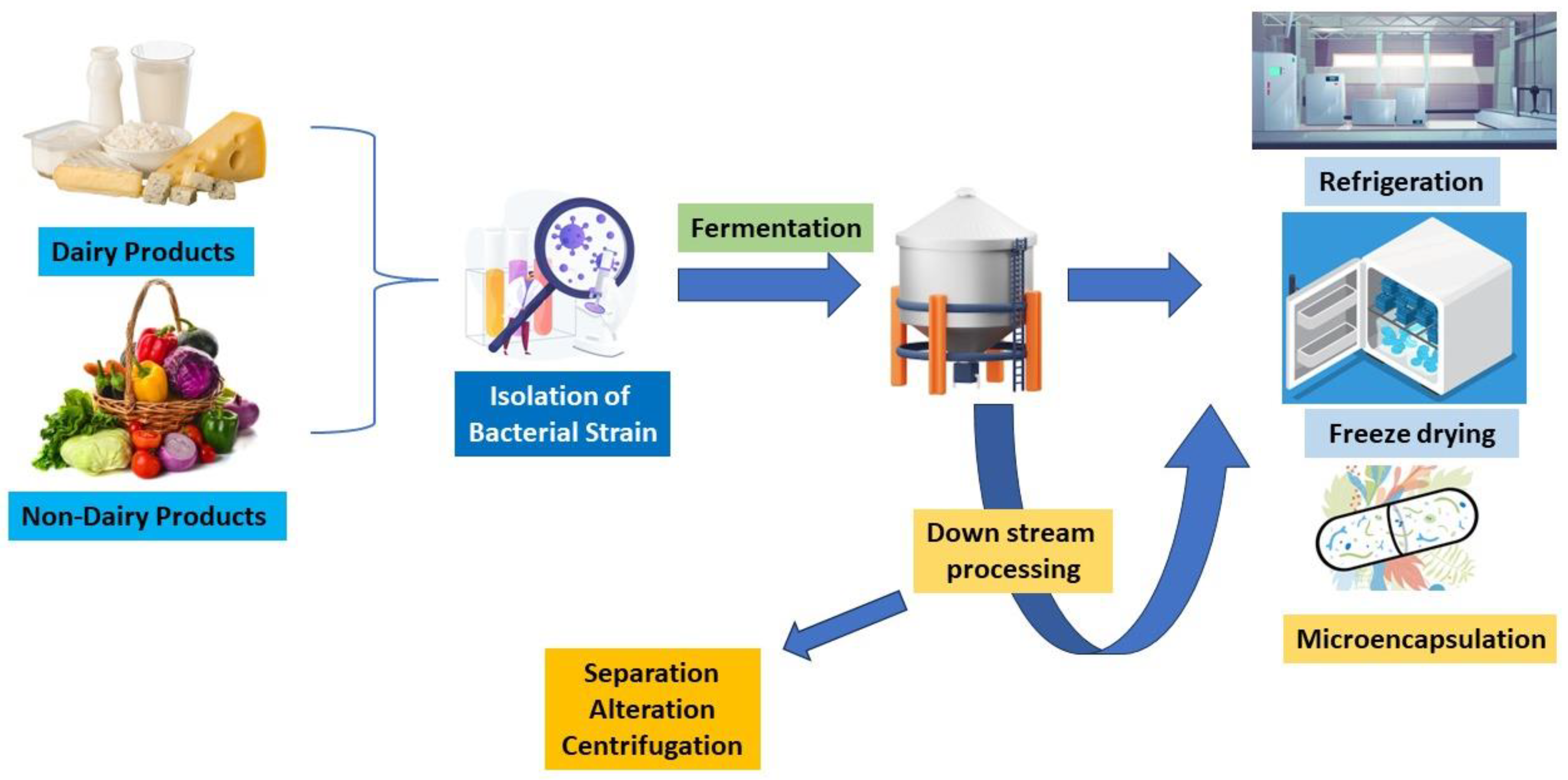
Generally, probiotics play a crucial role in the development of the human body, including digestive systems, mental disorders, immunological disorders, and so on. They can be found in many dairy and non-dairy products and are considered to have several health advantages. Kefir and yoghurt are two dairy products that are widely recognized for their probiotic properties [26,27]. Probiotics are formulated by fermenting milk with certain bacterial strains such as Lactobacillaceae and Bifidobacterium. Lactose, a natural milk sugar, undergoes fermentation and is transformed into lactic acid. This trims down the product’s pH and creates the acidic environment required for the enhancement of valuable microorganisms. Dairy probiotics are made by several processes (Figure 2) [28]. To get rid of dangerous microorganisms, milk is first pasteurized. The milk is then infused with the specific strains of microbes, and then, the milk is kept at a particular temperature to encourage bacterial development. The bacteria break down the lactose in the milk during fermentation to generate lactic acid, which gives the final product its distinctively sour flavour [29]. Non-dairy probiotic supplements are a great alternative for people who are intolerant to lactose or consume a plant-based diet. Probiotics that are non-dairy can be made from coconut, soy, and almond milk, among other sources. Dairy probiotics are produced using identical fundamental principles, but certain alterations are needed to account for the distinctive properties of non-dairy components. One common method of producing non-dairy probiotics is using starter cultures. Using starter cultures is one popular technique for creating non-dairy probiotics. Starter cultures are combinations of certain bacterial strains that are introduced to non-dairy products. These cultures have been chosen because of their propensity to successfully ferment milk and generate favourable microbial strains. The gratification and nutritional accessibility of non-dairy products replacements might vary, as the fermentation process for non-dairy products may take longer than for dairy products [30,31].
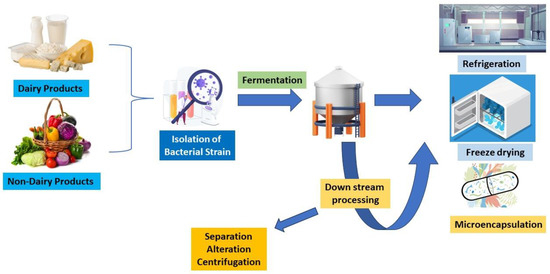
Figure 2.
Diagrammatic representation of process of generating probiotics from dairy and non-dairy products.
According to da Silva et al. [32], fermentations of cereal and fruit have a lot of potential for developing new probiotic drinks. The scientific community has performed in-depth investigations on several substrates, including mango, blueberry, orange, cherry, apple, and pineapple, either separately or in combination. It is important to recognize the various nutritional and health-enhancing advantages that grains and fruits offer. For the manufacturing of non-dairy probiotics, other methods such as freeze-drying, starter cultures, and encapsulation are also active. Probiotic cultures can be freeze-dried to extend their ability, to remove moisture, and extend shelf life. Encapsulation entails covering the probiotic bacteria in a barrier to protect them from the hostile environment of the gastrointestinal tract. They can improve a variety of biological processes when used in conjunction with probiotics, as shown in Table 1 below, which depicts the various dairy and non-dairy probiotic strains and their effect on human health, including mental health. The development of probiotic-infused non-dairy fermented drinks shows an increasing trend in the functional food industry. However, compared to its dairy-based predecessors, this field’s research is still in its infancy. Therefore, substantial research examining probiotic strain adaptability to plant environments is necessary and must be carried out [32].

Table 1.
Various bacterial strains present in dairy and non-dairy products, their mechanisms of action, and their effects on mental health.
Probiotics emitted from dairy products all contain essential nutrients and minerals, like protein, vitamin D, and calcium. For healthy teeth and bones, calcium plays a vital role, and its absorption requires vitamin D, both of which are present in milk. To build body and mend muscles, we need protein [54]. Dairy products also include minerals such as phosphorus and potassium as well as the vitamins B12, B2, and A. Together, these nutrients assist to maintain healthy skin, a strong immune system, and good bone health. Conversely, non-dairy products made from plant-based milks (such as soy, almond, and coconut) and cheese and yoghurt substitutes are accessible for people who are unable or prefer not to ingest dairy owing to lactose intolerance, allergies, or dietary restrictions. Alternatives to dairy milk are frequently enriched with calcium and vitamin D, making them equally as nutrient-dense as dairy milk. They provide diversity and accommodate customers with certain dietary needs or preferences, such as lactose-free or vegan customers. In comparison to dairy products, non-dairy foods frequently have lower levels of cholesterol and saturated fat. People who are concerned about their heart health or who want to consume less saturated fat may find this helpful [55]. Plant-based replacements could provide extra nutrients like antioxidants and dietary fibre that are good for digestion and general health. The decision between dairy and alternatives to it ultimately comes down to personal tastes, dietary requirements, and health objectives [56]. Both dairy and non-dairy ingredients may be used to make probiotics, providing alternatives for those with various dietary preferences. Specific bacterial strains, fermentation, and occasionally extra processes like freeze-drying and encapsulation are all used during the manufacturing process. Consuming probiotic foods, whether they come from dairy or non-dairy sources, might enhance digestion, and boost the immune system, among other positive health effects [57].
3. Comparison of Probiotics Originated from Lactobacilli and Other Strains
The probiotics fabricated from Lactobacilli, Enterococcus, Bifidobacterium, and Clostridium butyricum all have beneficial repercussions on health. The tough acidic environment of the stomach is naturally suitable for lactobacillus strains to thrive and colonise. They contribute to the preservation of a balanced gut microbiota by generating lactic acid, which makes it difficult for dangerous bacteria to grow [58]. Lactobacillus strains aid in microbial balance restoration and the development of advantageous bacteria, which improves digestion and nutrient absorption. Immunological responses are significantly influenced by the health of the gut, and Lactobacillus strains have been proven to boost immune system responses. They increase immune cell activity, promote antibody synthesis, and control inflammation. Lactobacillus strains can decrease the risk of infections and enhance general immunological health by enhancing the immune system’s performance [59]. In parallel to the other probiotics, lactobacilli offer certain advantages and distinctions. It is generally known that lactobacilli can endure the stomach’s acidic environment as well as the bile salts found in the small intestine. Due to this ability for resistance, lactobacilli can enter the intestines alive and start working with strong efficiency [60,61]. Some probiotics, including Enterococcus, might not be as resistant to bile and acid. The gut lining can be colonised and adhered to by lactobacilli, creating an obstacle that keeps infections out. This colonisation assists lactobacilli in growing more firmly in the gut, which could boost their proficiency [62,63]. In terms of colonisation competencies, Bifidobacterium and Enterococcus are comparable, although Clostridium butyricum can have different modes of action. The propensity of lactobacilli to create lactic acid by fermenting carbohydrates is quite strong. A healthy microbial equilibrium is encouraged by the acidity of the gut surroundings, thereby rendering it unsuitable for pathogenic bacteria [64,65]. Bifidobacterium can also create lactic acid, whereas Enterococcus and Clostridium butyricum have different metabolic assets. A healthy immune response has been demonstrated to be promoted by the lactobacilli’s modulation of the immune system. They may enhance the functioning of our immune response and encourage generation of special immune cells, both of which contribute to the maintenance of a healthy immune system [66]. Even though Bifidobacterium and Enterococcus are additional probiotics that may potentially have immunomodulatory properties, still lactobacilli still boost the immune system in more efficient manner. In Table 2 below, the difference between Lactobacillus-originated probiotics and probiotics originated from other bacterial strains is detailed for better understanding.

Table 2.
Comparison between the probiotics originated from Lactobacillus and other bacterial strains.
A wide variety of lactobacilli strains are available, providing flexibility in probiotic formulations and uses. Diverse lactobacilli strains may have unique characteristics and functions that enable targeted therapies for a range of medical problems [77]. Due to the variety of strains, it is possible to choose the best lactobacilli-based probiotic depending on the situation. The success of a probiotic is dependent on several variables, including the strain, dose, and particular medical circumstances [78]. There are many advantages to taking different Lactobacillus strains, but it is vital to keep in mind that an appropriate composition and strain must be utilised to achieve the intended results. The characteristics and functions of various Lactobacillaceae species and strains differ. A probiotic’s effectiveness is also influenced by the bacteria’s dosage, stability, and survivability [79].
4. The Effects of Fermentation on Probiotic-Generating Microbiota
Fermentation plays a significant role in the synthesis of probiotics by fostering an environment that promotes the emergence and development of advantageous microorganisms. Fermentation influences probiotic-forming microbes by providing a favourable environment for their growth, initiating competition with other microorganisms, promoting metabolic interactions, and enhancing the survival and stability of probiotic strains. The composition and interactions within probiotic communities established during fermentation can influence the health benefits, as they are helpful in gut colonisation potential and synergistic effects on the host [80]. Fermented foods and beverages often require the addition of specific starter cultures, which are composed of selected strains of beneficial microorganisms, including probiotics. These starter cultures initiate the fermentation process and establish a community of microorganisms that contribute to the development of desirable flavours, textures, and health benefits. During fermentation, the selected probiotic strains in the starter cultures compete with other microorganisms present in the food substrate [44,81]. The conditions of fermentation, such as the pH, nutrient availability, and oxygen levels, are often optimized for the growth of probiotics, giving them a competitive advantage over harmful or undesirable microorganisms. This competition helps to establish and maintain a dominant population of probiotics in fermented products. Different strains of probiotics within a community can interact metabolically during fermentation. For example, some probiotic strains may produce metabolites or enzymes that provide benefits to other strains, promoting their growth and activity. These metabolic interactions can enhance the overall probiotic effect of the microbes and contribute to the development of unique characteristics in the fermented product [82]. Probiotic-producing microbes in fermented foods often consist of multiple strains that complement each other’s functionalities. These strains may have different metabolic capabilities, including the breakdown of specific nutrients or the production of bioactive compounds. By working together, the probiotic community can have a broader range of health benefits and significantly impact the host in a positive way. Fermentation can improve the survival and stability of probiotic-producing microorganisms. The controlled conditions of fermentation, such as low pH and the absence of oxygen, can help in the protection of probiotic strains from environmental stress and extend their shelf life. This is particularly important for probiotic viability during storage and transit, ensuring that an adequate number of viable microorganisms reached the consumer [83]. Probiotic microbiota established during fermentation can have implications for gut colonisation. When consumed, these microbes can reach the digestive system and potentially colonise the gut, contributing to the restoration or maintenance of healthy gut microbiota. The ability of probiotics to survive the harsh conditions of the gastrointestinal tract and adhere to intestinal surfaces is crucial for their colonisation potential and long-term health benefits [84].
An anaerobic metabolic process called fermentation enables the synthesis of energy when oxygen is scarce. During the fermentation process, carbohydrates are oxidised to produce a variety of by-products, such as carbon dioxide organic acids, and alcohol. The phosphotransferase system (PTS) helps in the translocation of glucose through the membrane, which is utilised in the glycolysis pathway to create pyruvate. This route metabolizes NAD+ and produces energy. Pyruvate is then transformed into L- and D-lactate by the stereospecific NAD-dependent lactate dehydrogenases (LDHs), low-density lipoprotein (LdhL) and lactate dehydrogenase D (LdhD), respectively, which replenish NAD+ and maintain the redox equilibrium. A specific kind of fermentation called lactic acid fermentation is carried out by bacteria called lactobacilli, which produce lactic acid as a by-product [85]. A significant amount of investigation has been performed on the possible health benefits of lactobacilli. When lactobacilli are found in fermented foods, they can function as probiotics and offer several advantages [86].
These are a few ways in which fermentation influences the production of lactobacilli, as discussed below.
4.1. Increased Viability
Lactobacilli can grow and survive better in environments produced by fermentation. The development of these bacteria is supported by the controlled factors of fermentation, such as temperature, pH, and the availability of nutrients, ensuring their survival [87,88].
4.2. Metabolic Activity
During fermentation, lactobacilli break down the sugars in the food substrate to produce lactic acid. The characteristic tart flavour and acidic pH of foods that have undergone fermentation are a result of this metabolic process. Food safety is enhanced by the acidic environment that lactobacilli provide by helping to stop the growth of dangerous germs and diseases [89].
4.3. Increased Availability of Nutrients
Proteins and complex carbohydrates in the food substrate are broken down during fermentation into simpler, more easily absorbed forms. Enzymes produced by lactobacilli assist in the digestion of certain nutrients, making them easier for the digestive system to absorb [90].
4.4. Bioactive Molecule Synthesis
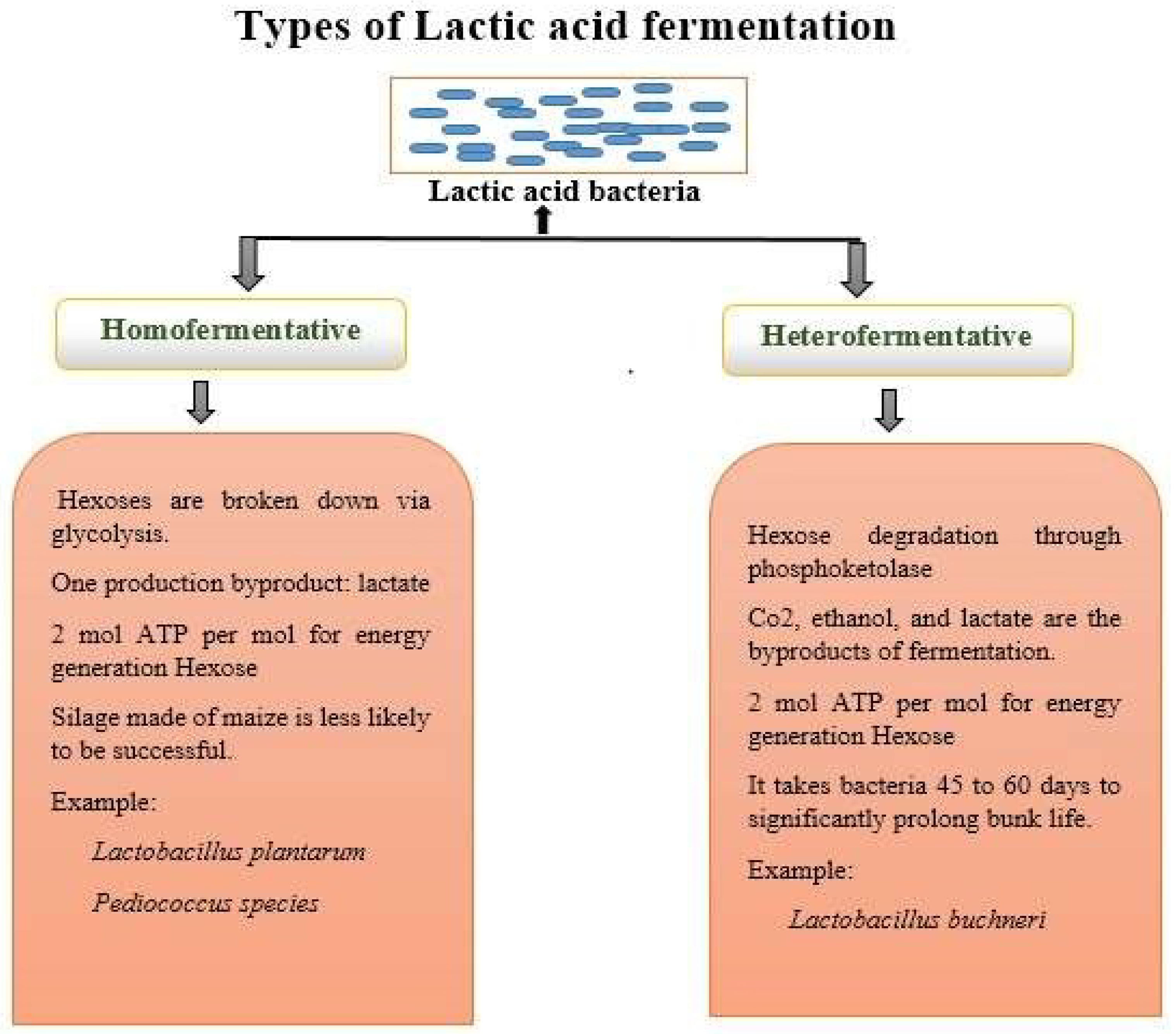
Lactobacilli can produce a variety of bioactive chemicals via fermentation. For instance, they can generate enzymes, antibacterial compounds, and vitamins (such as vitamin K and specific B vitamins), which enhance the nutritive value of fermented foods [91,92]. There are two lactic acid bacteria (LAB) routes for fermentation, as explained in Figure 3.
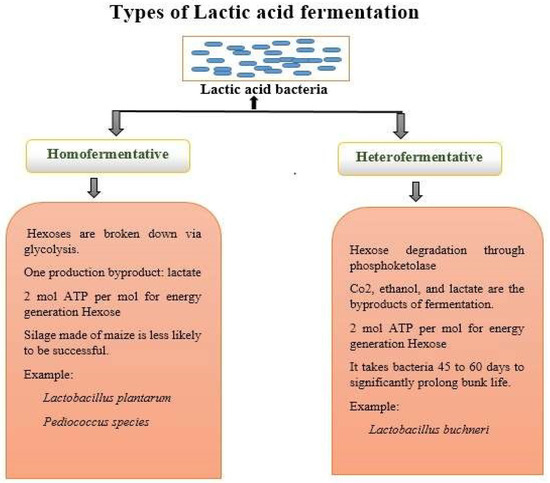
Figure 3.
Schematic representation of types of lactic acid fermentation.
By modifying environmental factors like pH, glucose content, and resource constraint, one can modify the homofermentative state of Lactococcus [89]. To make cheese, thermophilic strains of Lactobacillaceae helveticus are also employed. Streptococcus salivarius, Lactobacillaceae acidophilus, and Lactobacillaceae delbruckii are some of the homofermentative bacteria utilised in the yoghurt business. Other homofermentative bacteria employed in the milk industry include Streptococcus spp., Enterococcus, Pediococcus, and Aerococcus, albeit they are rarely utilized as starter cultures. The detection of CO2 gas is a requirement for heterofermentative bacterial testing. They are infrequently employed as starter cultures in the dairy business despite being uncommon in the milk and dairy industries [93]. Heterofermentative bacteria can occasionally result in flaws like slits in hard cheeses and bulging packaging in other dairy products if they enable development in considerable quantities. Leuconostoc spp., Lactobacillaceae brevis, Lactobacillaceae fermentum, Lactobacillaceae reuteri, Lactobacillaceae plantarium, Lactobacillaceae casei, and Lactobacillaceae curvatus are a few examples of heterofermentative bacteria. LAB that creates ethanol/acetic acid and CO2 instead of lactic acid as by-products of the fermentation of glucose are known as heterofermentative bacteria. The main distinction between homofermentative and heterofermentative bacteria is already mentioned in Figure 3 [94]. The dairy industry frequently uses starter cultures for homofermentative bacteria. On the contrary, the dairy sector employs starter cultures of heterofermentative bacteria occasionally. As the by-product of hexose metabolism during glycolysis, homofermentation almost exclusively yields lactic acid. In contrast, the phosphoketolase route is used in heterofermentative process to produce equimolar levels of lactic acid, carbon dioxide, ethanol, and acetate from hexose. Facultative heterofermenters adopt this route. In facultative heterofermentative Lactobacillaceae, hexoses are essentially converted to lactic acid by the Embden–Meyerhof–Parnas (EMP) route. While Lactobacillaceae acidophilus is categorized as an obligatory homofermentative member of the Lactobacillaceae, where over 85% of hexoses are fermented to lactic acid by the glycolysis pathway, Lactobacillus casei, Lactobacillaceae rhamnosus, Lactobacillaceae paracasei, and Lactobacillaceae plantarium are defined as facultative heterofermenters [95].
A pH between slightly acidic and neutral is required for homofermentative LAB to ferment glucose. Low pH, however, inhibits cellular metabolism and, as a result, lactic acid generation. At lower than pH 4, a huge number of LAB cannot grow [96]. Two methods are employed to maintain cell survival.
To maintain a pH of 7, lime is frequently added to the fermenters, which results in the production of calcium lactate (>90% of the lactic acid). The calcium-lactate-containing broth undergoes fermentation before being acidified with sulfuric acid to produce lactic acid. High sulphuric acid consumption results in higher levels of insoluble calcium sulphate in the form of gypsum than is produced by lactic acid as well as issues with waste disposal, additional corrosion issues, and a substantial cost in the process of recovering the product in commercial operations. In a perfect world, microbial fermentation would occur in a medium whose pH is at or below the pKa of lactic acid (3.78), allowing for the straightforward filtration of the acid form. Metabolic engineering has been used to create Lactobacillus sp. variations that are able to tolerate the acidified medium produced during fermentation. Following UV and nitrosoguanidine treatment, improved strains have been obtained, and they are now able to make lactic acid at rates and yields comparable to those of the conventional, neutral-pH lactic acid methods [97].
5. Importance of Probiotics for Mental Health
Probiotics are living microorganisms that are thought to gain health benefits when ingested. They are commonly found in certain foods such as yoghurt, kimchi, sauerkraut, and kefir. An emerging interest in the role of probiotics on mental health has been a burning topic recently. Studies have proposed the positive effect of probiotics on mental health via reducing inflammation, regulating the gut microbiota, and boosting stress response (Table 3). There is evidence to suggest that probiotics may help reduce symptoms of depression and anxiety, minimize stress-associated eating conduct, and improve cognitive outcomes [98]. Probiotics may help improve sleep quality, reduce fatigue, and boost mood. Furthermore, while more research work is necessary to better acknowledge the mechanisms and effects of probiotics on mental health, current evidence suggests that probiotics may be beneficial for overall mental health. It has been observed that the microbiota residing in the gastrointestinal tract (GIT) show an effect on the synaptic or neural signals produced by the CNS [99]. The term gut-brain axis depicts the association between the gut microbiota and the CNS. This association describes a relationship of the gut whose basis of communication is performed through the endocrine system, inflammatory responses, neural system, etc. Several researchers performed clinical trials to clarify this mutual association and have shown that a nerve, namely the vagus nerve, is responsible for the maintenance of the microbiota–gut-brain axis and performs a significant function in neurotransmitter signalling. The microbiota–gut-brain axis is a two-way mechanism. The microbiota present in the gut potentially influence the cognitive functions performed by the brain, such as remembrance power, memory, creativity, and controlling the centre of emotions, feelings, and sensations [100]. A clinical trial of probiotics containing Lactobacillus spp. was performed on mice, which resulted in lowering their anxiety levels. Many types of research are ongoing to specify the role of probiotics in the body, especially in the neural system. Probiotics show a positive effect on the mental health of an organism, and they help in stress management, act as anti-depressants, and cure various diseases like Parkinson’s disease, AD, autism, etc. In current studies, it has been found that the diet crucially contributes to the cognitive processes controlled by brain. The CNS involves the hypothalamic region that regulates stress and responds to stimuli. Dysfunction of the hypothalamic region or CNS can lead to neurodegenerative disorders [101].

Table 3.
Different probiotic strains with their effect on neurological disorder.
6. Aetiology of AD
Around the world, the chronic neurological condition AD (AD) accounts for about 80% of all cases of dementia, mainly in persons over 60 years of age. In accordance with a thorough investigation for the Global Burden of Disease study, in the year 2016, about 43.8 million people worldwide were affected by AD. It is expected that by the year 2050, around 131 million people worldwide will be suffering from AD, constituting it as one of the biggest worldwide health and hygiene challenges of the upcoming future generation. Significant memory, cognitive, and motor deficits are hallmarks of AD [110]. This is primarily caused by the deposition of beta-amyloid plaque protein outside the neuron or the existence of tau tangles inside the neurons. This may change the calcium balance and cause neuroinflammation, vascular degeneration, and ultimately neuronal death. AD is tightly linked to neuron loss, synaptic dysfunction, and neuropil threads. Amyloid precursor protein (APP), a crucial component involved in the system for digesting proteins, is strongly associated with the aetiology of AD [111]. The noteworthy factors contributing to the prior development of AD include transmutations in the presenilin 1 (PSEN1) and presenilin (PSEN 2) proteins and APP. A delayed start in AD is caused due to a variety of factors involving lifestyle, ageing, food, environment, and overexpression of the apolipoprotein (Apo) E4 gene. In AD, beta-amyloid accumulation is prevalent. Beta-amyloid is a peptide produced when APP is cleaved by a proteolytic enzyme. By clathrin-mediated endocytosis, amyloid precursor protein derived from trans-Golgi networks is delivered into the endosomal compartment. A portion of the APP is recycled back to the surface of the cell during this process in the endosome [112]. A non-amyloidogenic route controls APP on the cell surface and -secretase functions at N-terminal terminus in the A domain. These produce 83 membrane-tethered amino acids with the carboxy-terminal ends and APP as a result (CTF). As a result, -secretase will continue to cleave CTF-83 to produce the living intracellular domain of P3 fragment and APP [113].
In the endosomal compartment, the APP will take the amyloidogenic pathway. A non-amyloidogenic route controls APP on the cell surface and secretase functions on the N-terminal terminus in the A domain. It produces 83 membrane-tethered amino acids with carboxy terminal ends and APP as a result (CTF). As a result, -secretase will continue to cleave CTF-83 to produce the functional intracellular domain of P3 fragment and APP [113]. The APP will take the amyloidogenic pathway in the endosomal compartment. In this route, the -secretase binds with the APP’s extracellular domain, resulting in 99 membrane-bound amino acids made up of APP and CTF-(C99). To create soluble A fragment and the APP intracellular domain, -secretase will further cleave C99 [114]. In the presence of transition metal ions like Fe2+ and Cu2+, which create H2O2, the A peptide oligomerizes. By promoting lipid peroxidation, this will eventually lead to the formation of 4-hydroxynonenal (4HNE). In contrast, impaired glucose and glutamate transport effects in an expansion in inositol 1,4,5-triphosphate (IP3) production and Ca2+ influx led to the efflux of Ca2+ from the storage of the endoplasmic reticulum. When calpain, which is calcium-dependent, becomes activated, CDK5 is induced, which leads to tau hyperphosphorylation, neurofibrillary tangle development, impairment of axonal transport, and microtubule disassembly. In the end, it results in neuronal death and synaptic dysfunction [115]. The reactive oxygen species (ROS) and the increased inflow for Ca2+ ions into the mitochondria drive the production of mitochondrial cyclophilin D. As a result, proapoptotic substances such as apoptosis-inducing factor (AIF) and cytochrome C are released. An activation of the caspase cascade eventually causes the death of neuronal cells. Moreover, plaques cause a release of certain cytokines like IL-1, TNF, and IL-6 as well as a few chemokines like macrophage inflammatory protein-1 and IL8 from microglial cells. All of these in turn trigger the astrocytes’ production of acute-phase proteins, cytokines, and chemokines, which activates the microglial cells. The pathophysiology of AD is neuroinflammation, which is produced in the brain by activated astrocytes and microglia [116]. According to recent research, AD patients have an elevated concentration of immune cells that are peripheral, and these cells actively contribute to regional irritation. The pro-inflammatory cytokinin cells can penetrate through the blood–brain barrier (BBB) and furthermore incite some inflammatory responses like heat, soreness, and redness, which are particular to the brain but are nonetheless sources for peripheral inflammation, whether through obesity or systemic inflammation. As a result, the BBB becomes more permeable and porous, allowing peripheral immune cells to enter. Microglial cells will then become overactive. This will at first manifest in AD patients as reduced hippocampal-dependent learning [117].
7. Significance of Probiotics in Curing AD
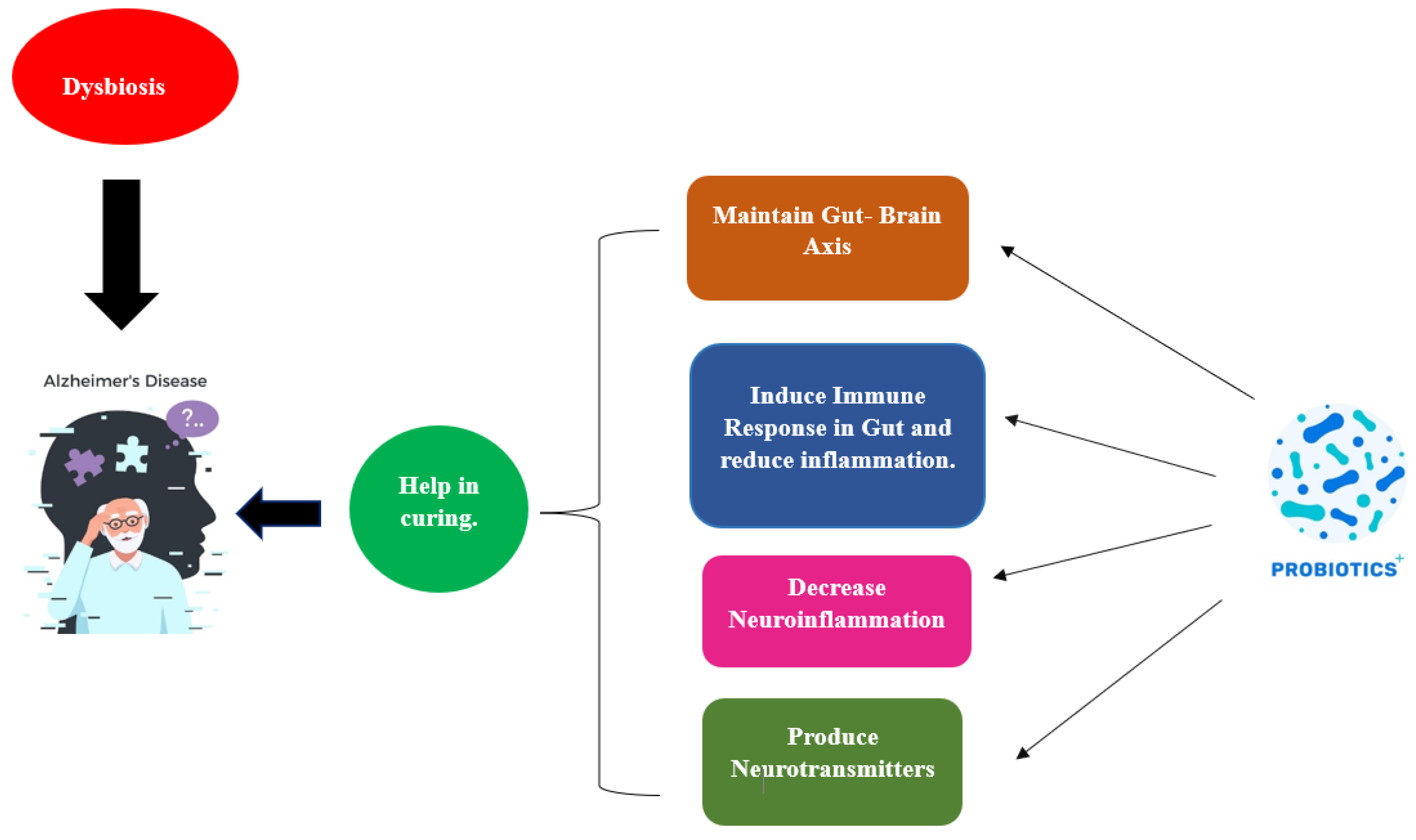
Probiotics work through a wide range of processes depicted in Figure 4, albeit the precise way they do so is still not fully understood.
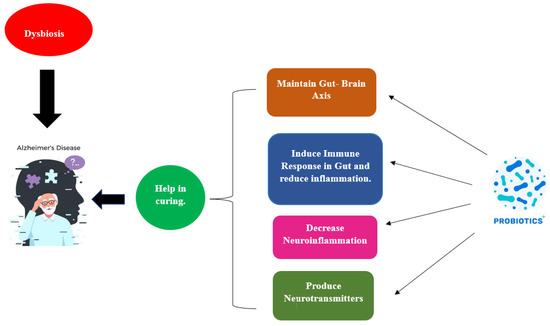
Figure 4.
Influence of probiotics in curing AD.
Various probiotic strains that are helpful in curing AD are mentioned in Table 4 below, which gives information regarding different probiotic strain and their effects in AD along with the mechanism.

Table 4.
Different probiotic strains and their effects on AD with mechanisms of actions.
The creation of short-chain fatty acids, bacteriocins, modulation, the struggle for nutrition, and the stimulation of the gut-brain axis’s function are all examples of how this happens. Figure 5 illustrates a probiotic’s potential mode of action in AD in relation to this. Based on the amount of fibre in the diet, The formation of short-chain fatty acids (SCFAs), which are generally saturated fatty acids, takes place in the gut. The fermentation process, which is mediated by the microbial species Lactobacillaceae, Clostridium, Bacteroides, Eubacterium, and Bifidobacterium, produces the metabolites acetate, butyrate, and propionate [126]. Three key mechanisms—neuronal routes, immune modulation, and endocrine factors—may all play a role in how SCFAs affect brain function. By enhancing barrier integrity and maintaining mucus production through immunological regulation, SFCAs can affect intestinal mucosa susceptibility and barrier function. SCFAs influence the release of cytokines, which influences the immune cells’ ability to proliferate and differentiate [127]. Because of this association, pro-inflammatory cytokines (such as IL-1, TNF, and IL-6) are suppressed, whereas an anti-inflammatory reaction is produced. Moreover, the expression of tight junction proteins is increased, and short SFCAs could impact the integrity of the BBB and traverse BBB using monocarboxylate transporters. By altering the function and form of neural microglia cells in the CNS, SCFAs have an impact on neuroinflammation, avoiding the death of neuronal cells. The ejection of gut hormones is modulated by SCFAs, which function as endocrine signalling molecules. SFCAs improve boundary strength and preserve mucus through immunological regulation. Propionate and acetate greatly increase the production of PYY and glucagon-like peptide-1 (GLP-1) in mouse colonic cells by activating the G-protein-coupled receptor. Neurons and intestinal enteroendocrine L-cells in the nucleus of the brainstem of the tractus solitarius generate and secrete GLP-1 [128]. Glucagon-like peptide-1 functions in preventing cell death and neuronal apoptosis and as a neuroprotective agent in the brain. PYY serves as a gut hormone that reduces appetite. Studies on animals have shown that the neuropeptide Y is highly raised in the hippocampus, which is part of the temporal lobe, of mice with AD and has neuroprotective effects by activating the PI3K-XBP1-induced Gip78/BiP path as well as hindering caspase-4 and caspase-3 actions. It also reduces oxidative stress by preventing the Aβ-induced lipid peroxidation that causes modifications at the level of the neurotransmitter glutamate and oxidative stress [129].
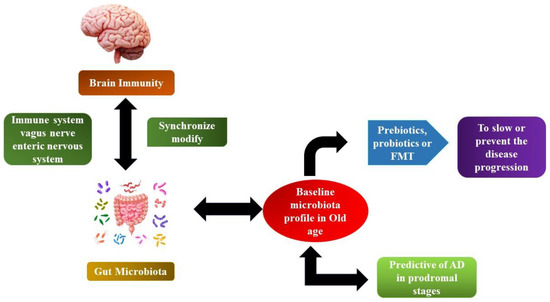
Figure 5.
The microbiome–gut-brain axis in AD.
Furthermore, neurotransmitters and neurotrophic factors may be modulated by short-chain fatty acids SCFAs. Investigations have shown that the gut microbiota can either create antecedents for neurotransmitters or catalyse their production and distribution through food consumption or even both [129]. Via secretory enterochromaffin (EC) cells, neurotransmitter precursors encourage the release of some neurotransmitters like GABA and 5-HT. Butyrate as well as propionate influences the production of 5-HT host from the serum and colonic endoplasmic reticulum, according to the findings of [130]. Cells of the EC generate several neuroactive by-products such as tryptophan, peptide tyrosine tyrosine (PYY), and histamine. Certain neuroactive metabolites and precursors of neurotransmitters can penetrate through the blood–brain barrier (BBB) and participate in signalling in the CNS and in the brain, where neurotransmitter production takes place. Evident types of gut bacteria directly affect the signalling of the vagus nerve by stimulating the dorsal motor nucleus of the vagus nerve (DMV). An expanding body of evidence points to lengthy stress as a high-risk factor in the development of AD, which may quicken the progression of the disease. Stress and anxiety have a strong link to dementia risk [131,132]. Stress originating from outside or from the environment could cause psychological distress, which can be made worse by oxidative damage and inflammation. In response to psychological stress, the hypothalamic–pituitary–adrenal axis (HPA) is stimulated, which results in the discharge of glucocorticoids into the blood system and their passage through the blood–brain barrier into the brain for the activation of nutrient corticosteroid receptors in the mouse model and glucocorticoid receptors in humans, including both [133]. By reducing the hypothalamic–pituitary–adrenal axis’s hyperactivity that results from inflammatory processes and gut microbiota dysbiosis, probiotics have a pragmatic outcome on the microbiota–gut-brain axis. According to research by Mindus et al. [134] on the various Lactobacillus strains, Lactobacillaceae rhamnoses, a probiotic, reduced anxiety-like behaviour and decreased corticosterone levels in non-stressed mice. It is currently thought that stress and HPA dysregulation are related, but the precise mechanism is still unknown. In the case of a mouse model with ongoing anxiety and stress brought on via maternal separation, a probiotic, namely Bifidobacterium pseudocatenulatum, enhanced glucocorticoid responsiveness and reduced inflammation induced by stress [131]. By restoring the brain expression levels of important glucose transporters (GLUT3 and GLUT1) and insulin-like growth factor receptor along with the diminished phosphorylation of adenosine-monophosphate-activated protein kinase and protein-kinase B (Akt), the study showed that oral administration of probiotics improves glucose uptake in 3xTg-AD mice. Parallel to this, mice treated by Bonfili et al. [135] showed a reduction in phosphorylated tau clumps. In line with memory enhancement, probiotics prevent the time-dependent rise in glycated haemoglobin and the build-up of advanced glycation end products in AD mice.
There were 35 total research studies carried out by Ji and Shen [136] including 26 studies using animal models and 9 studies using humans. In the 26 animal model studies, mice were employed in 24 of them, whereas AD models from Caenorhabditis elegans and Drosophila melanogaster were used in two of them, respectively. Thirteen studies used single-strain probiotics, while the remaining studies used multi-strain probiotics (ranging from two to nine probiotic strains); four used probiotic-fermented milk or probiotic-fermented soybean; two studies used engineered probiotic strains; and four studies concentrated on the synergistic effect of probiotics with the AD drug memantine, selenium, or exercise. The most often used probiotics in the trials that were included were Bifidobacterium and Lactobacillaceae species. These investigations demonstrated that probiotic treatment had neuroprotective effects, could lessen cognitive impairments, and could control gut microbiota dysbiosis, which may be connected to oxidative and inflammatory pathways. Probiotics appear to be an appealing strategy to treat AD, which opens the door for further study through carefully planned, large-scale clinical research. The effectiveness and safety of Lactobacillaceae plantarium C29-fermented soybean (DW2009) as a dietary supplement for improving cognition were examined by Hwang et al. [137]. For 12 weeks, 100 people with mild cognitive impairment (MCI) were randomly assigned to receive either DW2009 (800 mg/day, n = 50) or a placebo (800 mg/day, n = 50). The change in the composite score of memory- and attention-related cognitive abilities as determined by computerised neurocognitive function assessments served as the major outcome measure. For every single therapy group, correlations between changes in serum brain-derived neurotrophic factor (BDNF) levels and cognitive function were assessed. The DW2009 group had larger gains in the total cognitive functions as opposed to the placebo group (z = 2.36, p for interaction = 0.02), particularly in the attention domain (z = 2.34, p for interaction = 0.02). Following ingestion of DW2009, elevated blood BDNF levels were linked to improved cognition (t = 2.83, p = 0.007). The outcomes of this clinical research indicate that DW2009 can be provided without risk to MCI patients to improve their cognitive abilities. Increased serum BDNF levels after DW2009 administration may offer a glimpse into the mechanisms driving cognitive recovery, which supports the role of the gut-brain axis in treating MCI’s cognitive abnormalities [137].
8. Probiotics Safety Considerations
In accordance with the American Food and Drug Administration (FDA), probiotics are considered safe. The efficacy of Lactobacillaceae, Bifidobacterium, Clostridium, and Streptococcus species in AD has not yet been subject to any recorded data. Probiotics should not always be given to patients with ongoing AD, especially the ones who are accepting drugs that act as immunosuppressors, such as chemotherapy [138]. Many incidences of fungemia, bacteraemia, and sepsis demonstrated in people who received S. boulardii have been documented. Probiotic bacteria might occasionally include genes for antibiotic resistivity, which can be passed on to additional another bacterium, to potentially pathogenic strains that can lead to infection. Probiotic safety should consider the characteristics of the probiotic, the patient/consumer, and the manufacturing process. (Contaminated probiotics products represent a safety concern). While some can be determined right away, others require more research before any meaningful recommendations can be made [139].
9. Conclusions
Disorders of the twenty-first century, including obesity, anxiety, depression, Parkinson’s disease, diabetes, and AD, may seem to have completely different aetiologies, yet they have a few similarities, as they are all non-communicable, non-transmissible illnesses or disorders for which there is now no effective treatment to completely cure their symptoms. A wealth of literature connects these illnesses to changes in the microbiota. It has been demonstrated that several probiotics improve microbiome stability, which has positive effects on brain health and is especially helpful in preventing neurodegenerative diseases like AD. In summary, this review offers a wealth of research that is supported by evidence regarding the contribution of probiotics to slowing the progression of AD. Clinical trials and in vivo research have demonstrated the value of probiotics in the therapeutic arena for the treatment of AD. According to this claim, there have been no negative effects concerned with the consumption of probiotics for AD. For the sake of identifying changes in the gut microbiome that are specific to AD and that might further reveal new information about probiotics as an excellent curative source in the future, more clinical trials must be carried out. Furthermore, extensive research linking cognitive function with microbial diversity and the development of disease in AD patients might yield useful, predictive information.
Author Contributions
Conceptualization, A.T.; methodology, V.T. and R.M.; validation, V.K.P. and K.K.D.; formal analysis and investigation, A.M.S.; writing—original draft preparation, A.T. and V.K.P.; writing—review and editing, A.M.S.; supervision, E.H. and B.K.; administration, B.K.; funding acquisition, E.H. All authors have read and agreed to the published version of the manuscript.
Funding
Project No. TKP2021-NKTA-32 has been implemented with support from the National Research, Development, and Innovation Fund of Hungary, financed under the TKP2021-NKTA funding scheme.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Monteiro, S.S.; Almeida, R.L.; Santos, N.C.; Pereira, E.M.; Silva, A.P.; Oliveira, H.M.L.; Pasquali, M.A.B. New Functional Foods with Cactus Components: Sustainable Perspectives and Future Trends. Foods 2023, 12, 2494. [Google Scholar] [CrossRef] [PubMed]
- Wieërs, G.; Belkhir, L.; Enaud, R.; Leclercq, S.; Philippart de Foy, J.M.; Dequenne, I.; de Timary, P.; Cani, P.D. How Probiotics Affect the Microbiota. Front. Cell. Infect. Microbiol. 2019, 9, 454. [Google Scholar] [CrossRef]
- Jäger, R.; Mohr, A.E.; Carpenter, K.C.; Kerksick, C.M.; Purpura, M.; Moussa, A.; Townsend, J.R.; Lamprecht, M.; West, N.P.; Black, K.; et al. International Society of Sports Nutrition Position Stand: Probiotics. J. Int. Soc. Sports Nutr. 2019, 16, 62. [Google Scholar] [CrossRef]
- Berg, G.; Rybakova, D.; Fischer, D.; Cernava, T.; Vergès, M.-C.C.; Charles, T.; Chen, X.; Cocolin, L.; Eversole, K.; Corral, G.H.; et al. Microbiome Definition Re-visited: Old Concepts and New Challenges. Microbiome 2020, 8, 103. [Google Scholar] [CrossRef] [PubMed]
- Widyastuti, Y.; Febrisiantosa, A.; Tidona, F. Health-Promoting Properties of Lactobacilli in Fermented Dairy Products. Front. Microbiol. 2021, 12, 673890. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Santamarina, A.; Gonzalez, E.G.; Lamas, A.; Mondragon, A.D.C.; Regal, P.; Miranda, J.M. Probiotics as a Possible Strategy for the Prevention and Treatment of Allergies. A Narrative Review. Foods 2021, 10, 701. [Google Scholar] [CrossRef]
- Shaikh, S.D.; Sun, N.; Canakis, A.; Park, W.Y.; Weber, H.C. Irritable Bowel Syndrome and the Gut Microbiome: A Comprehensive Review. J. Clin. Med. 2023, 12, 2558. [Google Scholar] [CrossRef]
- Lenoir-Wijnkoop, I.; Merenstein, D.; Korchagina, D.; Broholm, C.; Sanders, M.E.; Tancredi, D. Probiotics Reduce Health Care Cost and Societal Impact of Flu-Like Respiratory Tract Infections in the USA: An Economic Modeling Study. Front. Pharmacol. 2019, 10, 980. [Google Scholar] [CrossRef]
- Barta, D.G.; Cornea-Cipcigan, M.; Margaoan, R.; Vodnar, D.C. Biotechnological Processes Simulating the Natural Fermentation Process of Bee Bread and Therapeutic Properties—An Overview. Front. Nutr. 2022, 9, 871896. [Google Scholar] [CrossRef]
- Madabushi, J.S.; Khurana, P.; Gupta, N.; Gupta, M. Gut Biome and Mental Health: Do Probiotics Work? Cureus 2023, 15, e40293. [Google Scholar] [CrossRef]
- Chudzik, A.; Orzyłowska, A.; Rola, R.; Stanisz, G.J. Probiotics, Prebiotics and Postbiotics on Mitigation of Depression Symptoms: Modulation of the Brain–Gut–Microbiome Axis. Biomolecules 2021, 11, 1000. [Google Scholar] [CrossRef] [PubMed]
- Reis, D.J.; Ilardi, S.S.; Punt, S.E.W. The Anxiolytic Effect of Probiotics: A Systematic Review and Meta-analysis of the Clinical and Preclinical Literature. PLoS ONE 2018, 13, e0199041. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lee, I.S.; Braun, C.; Enck, P. Effect of Probiotics on Central Nervous System Functions in Animals and Humans: A Systematic Review. J. Neurogastroenterol. Motil. 2016, 22, 589–605. [Google Scholar] [CrossRef] [PubMed]
- Mayer, E.A.; Knight, R.; Mazmanian, S.K.; Cryan, J.F.; Tillisch, K. Gut Microbes and the Brain: Paradigm Shift in Neuroscience. J. Neurosci. 2014, 34, 15490–15496. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Li, X.; Zhang, G.; Sadiq, F.A.; Simal-Gandara, J.; Xiao, J.; Sang, Y. Probiotics in the Dairy Industry—Advances and Opportunities. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3937–3982. [Google Scholar] [CrossRef]
- Arora, K.; Green, M.; Prakash, S. The Microbiome and Alzheimer’s Disease: Potential and Limitations of Prebiotic, Synbiotic, and Probiotic Formulations. Front. Bioeng. Biotechnol. 2020, 8, 537847. [Google Scholar] [CrossRef] [PubMed]
- Nagaoka, S. Yogurt Production. In Methods in Molecular Biology; Humana Press: New York, NY, USA, 2019; pp. 45–54. [Google Scholar] [CrossRef]
- Chen, W.; Narbad, A. Lactic Acid Bacteria in Foodborne Hazards Reduction; Springer: Singapore, 2018. [Google Scholar]
- Du, Y.; Xu, W.; Wu, T.; Li, H.; Hu, X.; Chen, J. Enhancement of Growth, Survival, Immunity and Disease Resistance in Litopenaeus Vannamei, by the Probiotic, Lactobacillus plantarum Ep-M17. Fish Shellfish Immunol. 2022, 129, 36–51. [Google Scholar] [CrossRef]
- Basso, M.; Johnstone, N.; Knytl, P.; Nauta, A.; Groeneveld, A.; Cohen Kadosh, K. A Systematic Review of Psychobiotic Interventions in Children and Adolescents to Enhance Cognitive Functioning and Emotional Behavior. Nutrients 2022, 14, 614. [Google Scholar] [CrossRef]
- Vasiliu, O. The Current State of Research for Psychobiotics Use in the Management of Psychiatric Disorders–A Systematic Literature Review. Front. Psychiatry 2023, 14, 1074736. [Google Scholar] [CrossRef]
- Zhu, R.; Fang, Y.; Li, H.; Liu, Y.; Wei, J.; Zhang, S.; Wang, L.; Fan, R.; Wang, L.; Li, S.; et al. Psychobiotic Lactobacillus plantarum JYLP-326 Relieves Anxiety, Depression, and Insomnia Symptoms in Test Anxious College via Modulating the Gut Microbiota and Its Metabolism. Front. Immunol. 2023, 14, 1158137. [Google Scholar] [CrossRef]
- Roy, S.; Banerjee, S.; Bhowmick, P.; Choudhury, L. Psychobiotics: Deciphering Its Role in Neuropsychiatry. World J. Bio. Pharm. Health Sci. 2023, 13, 457–464. [Google Scholar] [CrossRef]
- Sharma, R.; Gupta, D.; Mehrotra, R.; Mago, P. Psychobiotics: The Next-Generation Probiotics for the Brain. Curr. Microbiol. 2021, 78, 449–463. [Google Scholar] [CrossRef]
- Cheng, L.H.; Liu, Y.W.; Wu, C.C.; Wang, S.; Tsai, Y.C. Psychobiotics in Mental Health, Neurodegenerative and Neurodevelopmental Disorders. J. Food Drug Anal. 2019, 27, 632–648. [Google Scholar] [CrossRef]
- Kelesidis, T.; Pothoulakis, C. Efficacy and Safety of the Probiotic Saccharomyces boulardii for the Prevention and Therapy of Gastrointestinal Disorders. Ther. Adv. Gastroenterol. 2012, 5, 111–125. [Google Scholar] [CrossRef] [PubMed]
- da Silva Vale, A.; Venturim, B.C.; da Silva Rocha, A.R.F.; Martin, J.G.P.; Maske, B.L.; Balla, G.; De Dea Lindner, J.; Soccol, C.R.; de Melo Pereira, G.V. Exploring Microbial Diversity of Non-Dairy Fermented Beverages with a Focus on Functional Probiotic Microorganisms. Fermentation 2023, 9, 496. [Google Scholar] [CrossRef]
- Stavropoulou, E.; Bezirtzoglou, E. Probiotics in Medicine: A Long Debate. Front. Immunol. 2020, 11, 2192. [Google Scholar] [CrossRef]
- Farahmand, N.; Ouoba, L.I.I.; Naghizadeh Raeisi, S.; Sutherland, J.; Ghoddusi, H.B. Probiotic Lactobacilli in Fermented Dairy Products: Selective Detection, Enumeration and Identification Scheme. Microorganisms 2021, 9, 1600. [Google Scholar] [CrossRef]
- Owusu-Kwarteng, J.; Akabanda, F.; Agyei, D.; Jespersen, L. Microbial Safety of Milk Production and Fermented Dairy Products in Africa. Microorganisms 2020, 8, 752. [Google Scholar] [CrossRef]
- Dahiya, D.; Nigam, P.S. Nutrition and Health Through the Use of Probiotic Strains in Fermentation to Produce Non-dairy Functional Beverage Products Supporting Gut Microbiota. Foods 2022, 11, 2760. [Google Scholar] [CrossRef]
- Küçükgöz, K.; Trząskowska, M. Nondairy Probiotic Products: Functional Foods That Require More Attention. Nutrients 2022, 14, 753. [Google Scholar] [CrossRef]
- Aprea, G.; Del Matto, I.; Tucci, P.; Marino, L.; Scattolini, S.; Rossi, F. In Vivo Functional Properties of Dairy Bacteria. Microorganisms 2023, 11, 1787. [Google Scholar] [CrossRef] [PubMed]
- Karami, S.; Roayaei, M.; Hamzavi, H.; Bahmani, M.; Hassanzad-Azar, H.; Leila, M.; Rafieian-Kopaei, M. Isolation and Identification of Probiotic Lactobacillus from Local Dairy and Evaluating Their Antagonistic Effect on Pathogens. Int. J. Pharm. Investig. 2017, 7, 137–141. [Google Scholar] [CrossRef]
- Aspri, M.; Papademas, P.; Tsaltas, D. Review on Non-dairy Probiotics and Their Use in Non-dairy Based Products. Fermentation 2020, 6, 30. [Google Scholar] [CrossRef]
- Rasika, D.M.D.; Vidanarachchi, J.K.; Luiz, S.F.; Azeredo, D.R.P.; Cruz, A.G.; Ranadheera, C.S. Probiotic Delivery Through Non-dairy Plant-Based Food Matrices. Agriculture 2021, 11, 599. [Google Scholar] [CrossRef]
- Ballini, A.; Charitos, I.A.; Cantore, S.; Topi, S.; Bottalico, L.; Santacroce, L. About Functional Foods: The Probiotics and Prebiotics State of Art. Antibiotics 2023, 12, 635. [Google Scholar] [CrossRef] [PubMed]
- Jeffrey, M.P.; Jones Taggart, H.J.; Strap, J.L.; Edun, G.; Green-Johnson, J.M. Milk Fermented with Lactobacillus rhamnosus R0011 Induces a Regulatory Cytokine Profile in LPS-Challenged U937 and THP-1 Macrophages. Curr. Res. Food Sci. 2020, 3, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Chen, H.; Qiao, Y.; Liu, G.; Leng, C.; Zhang, Y.; Lv, X.; Feng, Z. The Nutrient Requirements of Lactobacillus rhamnosus GG and Their Application to Fermented Milk. J. Dairy Sci. 2019, 102, 5971–5978. [Google Scholar] [CrossRef]
- Syiemlieh, I.; Morya, S. Dairy and Non-dairy Based Probiotics: A Review. J. Pharm. Innov. J. 2022, 11, 2956–2964. [Google Scholar] [CrossRef]
- Klindt-Toldam, S.; Larsen, S.K.; Saaby, L.; Olsen, L.R.; Svenstrup, G.; Müllertz, A.; Knøchel, S.; Heimdal, H.; Nielsen, D.S.; Zielińska, D. Survival of Lactobacillus acidophilus NCFM® and Bifidobacterium lactis HN019 Encapsulated in Chocolate During In Vitro Simulated Passage of the Upper Gastrointestinal Tract. LWT 2016, 74, 404–410. [Google Scholar] [CrossRef]
- Ambrogi, V.; Bottacini, F.; Cao, L.; Kuipers, B.; Schoterman, M.; van Sinderen, D. Galacto-Oligosaccharides as Infant Prebiotics: Production, Application, Bioactive Activities and Future Perspectives, Application. Crit. Rev. Food Sci. Nutr. 2023, 63, 753–766. [Google Scholar] [CrossRef]
- Alcántara, C.; Perez, M.; Huedo, P.; Altadill, T.; Espadaler-Mazo, J.; Arqués, J.L.; Zúñiga, M.; Monedero, V. Study of the Biosynthesis and Functionality of Polyphosphate in Bifidobacterium longum KABP042. Sci. Rep. 2023, 13, 11076. [Google Scholar] [CrossRef] [PubMed]
- Ansari, F.; Alian Samakkhah, S.; Bahadori, A.; Jafari, S.M.; Ziaee, M.; Khodayari, M.T.; Pourjafar, H. Health-Promoting Properties of Saccharomyces cerevisiae var. boulardii as a Probiotic; Characteristics, Isolation, and Applications in Dairy Products. Crit. Rev. Food Sci. Nutr. 2023, 63, 457–485. [Google Scholar] [CrossRef]
- dos Santos, D.C.; da Oliveira Filho, J.G.; Andretta, J.R.; Silva, F.G.; Egea, M.B. Challenges in Maintaining the Probiotic Potential in Alcoholic Beverage Development. Food Biosci. 2023, 52, 102485. [Google Scholar] [CrossRef]
- A, A.J.; Suresh, A. Oral Microbial Shift Induced by Probiotic Bacillus Coagualans along with Its Clinical Perspectives. J. Oral Biol. Craniofac. Res. 2023, 13, 398–402. [Google Scholar] [CrossRef]
- Zhao, N.; Yu, T.; Yan, F. Probiotic Role and Application of Thermophilic Bacillus as Novel Food Materials. Trends Food Sci. Technol. 2023, 138, 1–15. [Google Scholar] [CrossRef]
- Mileriene, J.; Aksomaitiene, J.; Kondrotiene, K.; Asledottir, T.; Vegarud, G.E.; Serniene, L.; Malakauskas, M. Whole-Genome Sequence of Lactococcus lactis Subsp. lactis LL16 Confirms Safety, Probiotic Potential, and Reveals Functional Traits. Microorganisms 2023, 11, 1034. [Google Scholar] [CrossRef]
- Saeed, A.; Yasmin, A.; Baig, M.; Khan, K.; Heyat, M.B.B.; Akhtar, F.; Batool, Z.; Kazmi, A.; Wahab, A.; Shahid, M.; et al. Isolation and Characterization of Lactobacillus crispatus, Lactococcus lactis, and Carnobacterium divergens as Potential Probiotic Bacteria from Fermented Black and Green Olives (Olea europaea): An Exploratory Study. BioMed Res. Int. 2023, 2023, 8726320. [Google Scholar] [CrossRef]
- Martinović, A.; Chittaro, M.; Mora, D.; Arioli, S. The Ability of Streptococcus thermophilus BT01 to Modulate Urease Activity in Healthy Subjects’ Fecal Samples Depends on the Biomass Production Process. Mol. Nutr. Food Res. 2023, 67, e2200529. [Google Scholar] [CrossRef]
- Lavelle, K.; McDonnell, B.; Fitzgerald, G.; van Sinderen, D.; Mahony, J. Bacteriophage-Host Interactions in Streptococcus thermophilus and Their Impact on Co-evolutionary Processes. FEMS Microbiol. Rev. 2023, 47, fuad032. [Google Scholar] [CrossRef]
- Çam, G.; Akın, N.; Konak Göktepe, Ç.; Demirci, T. Pea (Pisum sativum L.) Pod Powder as a Potential Enhancer of Probiotic Enterococcus faecium M74 in Ice Cream and Its Physicochemical, Structural, and Sensory Effects. J. Sci. Food Agric. 2023, 103, 3184–3193. [Google Scholar] [CrossRef]
- Aljohani, A.B.; Al-Hejin, A.M.; Shori, A.B. Bacteriocins as Promising Antimicrobial Peptides, Definition, Classification, and Their Potential Applications in Cheeses. Food Sci. Technol. 2023, 43, e118021. [Google Scholar] [CrossRef]
- Ratajczak, A.E.; Zawada, A.; Rychter, A.M.; Dobrowolska, A.; Krela-Kaźmierczak, I. Milk and Dairy Products: Good or Bad for Human Bone? Practical Dietary Recommendations for the Prevention and Management of Osteoporosis. Nutrients 2021, 13, 1329. [Google Scholar] [CrossRef]
- Vanga, S.K.; Raghavan, V. How Well Do Plant Based Alternatives Fare Nutritionally Compared to Cow’s Milk? J. Food Sci. Technol. 2018, 55, 10–20. [Google Scholar] [CrossRef]
- Hever, J.; Cronise, R.J. Plant-Based Nutrition for Healthcare Professionals: Implementing Diet as a Primary Modality in the Prevention and Treatment of Chronic Disease. J. Geriatr. Cardiol. JGC 2017, 14, 355–368. [Google Scholar] [CrossRef]
- Şanlıbaba, P. Fermented Nondairy Functional Foods Based on Probiotics. Ital. J. Food Sci. 2023, 35, 91–105. [Google Scholar] [CrossRef]
- Rizzoli, R.; Biver, E. Are Probiotics the New Calcium and Vitamin D for Bone Health? Curr. Osteoporos. Rep. 2020, 18, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Chelladhurai, K.; Ayyash, M.; Turner, M.S.; Kamal-Eldin, A. Lactobacillus helveticus: Health Effects, Current Applications, and Future Trends in Dairy Fermentation. Trends Food Sci. Technol. 2023, 136, 159–168. [Google Scholar] [CrossRef]
- Kulkarni, R.R.; Gaghan, C.; Gorrell, K.; Sharif, S.; Taha-Abdelaziz, K. Probiotics as Alternatives to Antibiotics for the Prevention and Control of Necrotic Enteritis in Chickens. Pathogens 2022, 11, 692. [Google Scholar] [CrossRef]
- Bernardeau, M.; Vernoux, J.P. Overview of Differences Between Microbial Feed Additives and Probiotics for Food Regarding Regulation, Growth Promotion Effects and Health Properties and Consequences for Extrapolation of Farm Animal Results to Humans. Clin. Microbiol. Infect. 2013, 19, 321–330. [Google Scholar] [CrossRef]
- Cai, J.; Rimal, B.; Jiang, C.; Chiang, J.Y.L.; Patterson, A.D. Bile Acid Metabolism and Signaling, the Microbiota, and Metabolic Disease. Pharmacol. Ther. 2022, 237, 108238. [Google Scholar] [CrossRef]
- Collins, S.L.; Stine, J.G.; Bisanz, J.E.; Okafor, C.D.; Patterson, A.D. Bile Acids and the Gut Microbiota: Metabolic Interactions and Impacts on Disease. Nat. Rev. Microbiol. 2023, 21, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Kavitake, D.; Tiwari, S.; Shah, I.A.; Devi, P.B.; Delattre, C.; Reddy, G.B.; Shetty, P.H. Antipathogenic Potentials of Exopolysaccharides Produced by Lactic Acid Bacteria and Their Food and Health Applications. Food Control 2023, 152, 109850. [Google Scholar] [CrossRef]
- Langa, S.; Peirotén, Á.; Curiel, J.A.; de la Bastida, A.R.; Landete, J.M. Isoflavone Metabolism by Lactic Acid Bacteria and Its Application in the Development of Fermented Soy Food with Beneficial Effects on Human Health. Foods 2023, 12, 1293. [Google Scholar] [CrossRef]
- Vlasova, A.N.; Kandasamy, S.; Chattha, K.S.; Rajashekara, G.; Saif, L.J. Comparison of Probiotic Lactobacilli and Bifidobacteria Effects, Immune Responses and Rotavirus Vaccines and Infection in Different Host Species. Vet. Immunol. Immunopathol. 2016, 172, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Pahumunto, N.; Dahlen, G.; Teanpaisan, R. Evaluation of Potential Probiotic Properties of Lactobacillus and Bacillus Strains Derived from Various Sources for Their Potential Use in Swine Feeding. Probiotics Antimicrob. Proteins 2023, 15, 479–490. [Google Scholar] [CrossRef]
- Coimbra-Gomes, J.; Reis, P.J.M.; Tavares, T.G.; Faria, M.A.; Malcata, F.X.; Macedo, A.C. Evaluating the Probiotic Potential of Lactic Acid Bacteria Implicated in Natural Fermentation of Table Olives, cv. Cobrançosa. Molecules 2023, 28, 3285. [Google Scholar] [CrossRef]
- Foongsawat, N.; Sunthornthummas, S.; Nantavisai, K.; Surachat, K.; Rangsiruji, A.; Sarawaneeyaruk, S.; Insian, K.; Sukontasing, S.; Suwannasai, N.; Pringsulaka, O. Isolation, Characterization, and Comparative Genomics of the Novel Potential Probiotics from Canine Feces. Food Sci. Anim. Resour. 2023, 43, 685–702. [Google Scholar] [CrossRef]
- Mirzabekyan, S.; Harutyunyan, N.; Manvelyan, A.; Malkhasyan, L.; Balayan, M.; Miralimova, S.; Chikindas, M.L.; Chistyakov, V.; Pepoyan, A. Fish Probiotics: Cell Surface Properties of Fish Intestinal Lactobacilli and Escherichia coli. Microorganisms 2023, 11, 595. [Google Scholar] [CrossRef]
- Haryani, Y.; Halid, N.A.; Guat, G.S.; Nor-Khaizura, M.A.R.; Hatta, M.A.M.; Sabri, S.; Radu, S.; Hasan, H. High Prevalence of Multiple Antibiotic Resistance in Fermented Food-Associated Lactic Acid Bacteria in Malaysia. Food Control 2023, 147, 109558. [Google Scholar] [CrossRef]
- Asan-Ozusaglam, M.; Gunyakti, A. Lactobacillus fermentum Strains from Human Breast Milk with Probiotic Properties and Cholesterol-Lowering Effects. Food Sci. Biotechnol. 2019, 28, 501–509. [Google Scholar] [CrossRef]
- Keresztény, T.; Libisch, B.; Orbe, S.C.; Nagy, T.; Kerényi, Z.; Kocsis, R.; Posta, K.; Papp, P.P.; Olasz, F. Isolation and Characterization of Lactic Acid Bacteria with Probiotic Attributes from Different Parts of the Gastrointestinal Tract of Free-Living Wild Boars in Hungary. Probiotics Antimicrob. Proteins 2023, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Moiseenko, K.V.; Begunova, A.V.; Savinova, O.S.; Glazunova, O.A.; Rozhkova, I.V.; Fedorova, T.V. Biochemical and Genomic Characterization of Two New Strains of Lacticaseibacillus paracasei Isolated from the Traditional Corn-Based Beverage of South Africa, Mahewu, and Their Comparison with Strains Isolated from Kefir Grains. Foods 2023, 12, 223. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.H.; Yang, R.S.; Lin, Y.C.; Xin, W.G.; Zhou, H.Y.; Wang, F.; Zhang, Q.L.; Lin, L.B. Assessment of the Safety and Probiotic Characteristics of Lactobacillus salivarius CGMCC20700 Based on Whole-Genome Sequencing and Phenotypic Analysis. Front. Microbiol. 2023, 14, 1120263. [Google Scholar] [CrossRef] [PubMed]
- Yalçin, S.K.; Ozbas, Z.Y. Yeasts from Traditional Cheeses for Potential Applications. Glob. Food Secur. Wellness 2017, 3, 277–293. [Google Scholar]
- Mazziotta, C.; Tognon, M.; Martini, F.; Torreggiani, E.; Rotondo, J.C. Probiotics Mechanism of Action on Immune Cells and Beneficial Effects on Human Health. Cells 2023, 12, 184. [Google Scholar] [CrossRef] [PubMed]
- National Institutes of Health. Probiotics: Fact Sheet for Health Professionals; National Institutes of Health: Bethesda, MD, USA, 2020.
- Dempsey, E.; Corr, S.C. Lactobacillus spp. for Gastrointestinal Health: Current and Future Perspectives. Front. Immunol. 2022, 13, 840245. [Google Scholar] [CrossRef]
- Sharma, R.; Diwan, B.; Singh, B.P.; Kulshrestha, S. Probiotic Fermentation of Polyphenols: Potential Sources of Novel Functional Foods. Food Prod. Process. Nutr. 2022, 4, 21. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Rodrigues, C.F.; Stojanović-Radić, Z.; Dimitrijević, M.; Aleksić, A.; Neffe-Skocińska, K.; Zielińska, D.; Kołożyn-Krajewska, D.; Salehi, B.; Milton Prabu, S.; et al. Probiotics: Versatile Bioactive Components in Promoting Human Health. Medicina 2020, 56, 433. [Google Scholar] [CrossRef]
- Mohammadi, M.; Shadnoush, M.; Sohrabvandi, S.; Yousefi, M.; Khorshidian, N.; Mortazavian, A.M. Probiotics as Potential Detoxification Tools for Mitigation of Pesticides: A Mini Review. Int. J. Food Sci. Technol. 2021, 56, 2078–2087. [Google Scholar] [CrossRef]
- Maske, B.L.; de Melo Pereira, G.V.; Vale, A.D.S.; De Carvalho Neto, D.P.; Karp, S.G.; Viesser, J.A.; De Dea Lindner, J.; Pagnoncelli, M.G.; Soccol, V.T.; Soccol, C.R. A Review on Enzyme-Producing Lactobacilli Associated with the Human Digestive Process: From Metabolism to Application. Enzyme Microb. Technol. 2021, 149, 109836. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, P.; Zhang, X. Probiotics Regulate Gut Microbiota: An Effective Method to Improve Immunity. Molecules 2021, 26, 6076. [Google Scholar] [CrossRef]
- Xie, N.; Zhang, L.; Gao, W.; Huang, C.; Huber, P.E.; Zhou, X.; Li, C.; Shen, G.; Zou, B. NAD+ Metabolism: Pathophysiologic Mechanisms and Therapeutic Potential. Signal Transduct. Target. Ther. 2020, 5, 227. [Google Scholar] [CrossRef]
- Grujović, M.Ž.; Mladenović, K.G.; Semedo-Lemsaddek, T.; Laranjo, M.; Stefanović, O.D.; Kocić-Tanackov, S.D. Advantages and Disadvantages of Non-starter Lactic Acid Bacteria from Traditional Fermented Foods: Potential Use as Starters or Probiotics. Compr. Rev. Food Sci. Food Saf. 2022, 21, 1537–1567. [Google Scholar] [CrossRef] [PubMed]
- Rolfe, C.; Daryaei, H. Intrinsic and Extrinsic Factors Affecting Microbial Growth in Food Systems. In Food Safety Engineering; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Śliżewska, K.; Chlebicz-Wójcik, A. Growth Kinetics of Probiotic Lactobacillus Strains in the Alternative, Cost-Efficient Semi-solid Fermentation Medium. Biology 2020, 9, 423. [Google Scholar] [CrossRef] [PubMed]
- Abedi, E.; Hashemi, S.M.B. Lactic Acid Production–Producing Microorganisms and Substrates Sources-State of Art. Heliyon 2020, 6, e04974. [Google Scholar] [CrossRef] [PubMed]
- Nkhata, S.G.; Ayua, E.; Kamau, E.H.; Shingiro, J.B. Fermentation and Germination Improve Nutritional Value of Cereals and Legumes Through Activation of Endogenous Enzymes. Food Sci. Nutr. 2018, 6, 2446–2458. [Google Scholar] [CrossRef]
- Abdul Hakim, B.N.; Xuan, N.J.; Oslan, S.N.H. A Comprehensive Review of Bioactive Compounds from Lactic Acid Bacteria: Potential Functions as Functional Food in Dietetics and the Food Industry. Foods 2023, 12, 2850. [Google Scholar] [CrossRef]
- Szutowska, J. Functional Properties of Lactic Acid Bacteria in Fermented Fruit and Vegetable Juices: A Systematic Literature Review. Eur. Food Res. Technol. 2020, 246, 357–372. [Google Scholar] [CrossRef]
- Plaza-Vinuesa, L.; Sánchez-Arroyo, A.; López De Felipe, F.; de Las Rivas, B.; Muñoz, R. Non-redundant Functionality of Lactiplantibacillus plantarum Phospho-β-Glucosidases Revealed by Carbohydrate Utilization Signatures Associated to pbg2 and pbg4 Gene Mutants. J. Appl. Microbiol. 2023, 134, lxad077. [Google Scholar] [CrossRef]
- Li, J.; Jia, S.; Ma, D.; Deng, X.; Tian, J.; Wang, R.; Li, J.; Shan, A. Effects of Citric Acid and Heterofermentative Inoculants on Anaerobic Co-fermentation of Chinese Cabbage Waste and Wheat Bran. Bioresour. Technol. 2023, 377, 128942. [Google Scholar] [CrossRef]
- Lacroux, J.; Llamas, M.; Dauptain, K.; Avila, R.; Steyer, J.P.; van Lis, R.; Trably, E. Dark Fermentation and Microalgae Cultivation Coupled Systems: Outlook and Challenges. Sci. Total Environ. 2023, 865, 161136. [Google Scholar] [CrossRef] [PubMed]
- Jabłońska-Ryś, E.; Sławińska, A.; Skrzypczak, K.; Goral, K. Dynamics of Changes in pH and the Contents of Free Sugars, Organic Acids and LAB in Button Mushrooms During Controlled Lactic Fermentation. Foods 2022, 11, 1553. [Google Scholar] [CrossRef] [PubMed]
- Upadhyaya, B.P.; DeVeaux, L.C.; Christopher, L.P. Metabolic Engineering as a Tool for Enhanced Lactic Acid Production. Trends Biotechnol. 2014, 32, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Sivamaruthi, B.S.; Prasanth, M.I.; Kesika, P.; Chaiyasut, C. Probiotics in Human Mental Health and Diseases-A Minireview. Trop. J. Pharm. Res. 2019, 18, 889–895. [Google Scholar] [CrossRef]
- Eastwood, J.; Walton, G.; Van Hemert, S.; Williams, C.; Lamport, D. The Effect of Probiotics on Cognitive Function Across the Human Lifespan: A Systematic Review. Neurosci. Biobehav. Rev. 2021, 128, 311–327. [Google Scholar] [CrossRef]
- Socała, K.; Doboszewska, U.; Szopa, A.; Serefko, A.; Włodarczyk, M.; Zielińska, A.; Poleszak, E.; Fichna, J.; Wlaź, P. The Role of Microbiota-Gut-Brain Axis in Neuropsychiatric and Neurological Disorders. Pharmacol. Res. 2021, 172, 105840. [Google Scholar] [CrossRef]
- Papalini, S.; Michels, F.; Kohn, N.; Wegman, J.; van Hemert, S.; Roelofs, K.; Arias-Vasquez, A.; Aarts, E. Stress Matters: Randomized Controlled Trial on the Effect of Probiotics on Neurocognition. Neurobiol. Stress 2019, 10, 100141. [Google Scholar] [CrossRef]
- Roussin, L.; Prince, N.; Perez-Pardo, P.; Kraneveld, A.D.; Rabot, S.; Naudon, L. Role of the Gut Microbiota in the Pathophysiology of Autism Spectrum Disorder: Clinical and Preclinical Evidence. Microorganisms 2020, 8, 1369. [Google Scholar] [CrossRef]
- Li, Q.; Han, Y.; Dy, A.B.C.; Hagerman, R.J. The Gut Microbiota and Autism Spectrum Disorders. Front. Cell. Neurosci. 2017, 11, 120. [Google Scholar] [CrossRef]
- Vellingiri, B.; Aishwarya, S.Y.; Benita Jancy, S.; Sriram Abhishek, G.; Winster Suresh Babu, H.; Vijayakumar, P.; Narayanasamy, A.; Mariappan, S.; Sangeetha, R.; Valsala Gopalakrishnan, A.; et al. An Anxious Relationship Between Autism Spectrum Disorder and Gut Microbiota: A Tangled Chemistry? J. Clin. Neurosci. 2022, 99, 169–189. [Google Scholar] [CrossRef]
- Savignac, H.M.; Kiely, B.; Dinan, T.G.; Cryan, J.F. Bifidobacteriaexert Exert Strain-Specific Effects on Stress-Related Behavior and Physiology in BALB/C Mice. Neurogastroenterol. Motil. 2014, 26, 1615–1627. [Google Scholar] [CrossRef]
- Srivastav, S.; Neupane, S.; Bhurtel, S.; Katila, N.; Maharjan, S.; Choi, H.; Hong, J.T.; Choi, D.Y. Probiotics Mixture Increases Butyrate, and Subsequently Rescues the Nigral Dopaminergic Neurons from MPTP and Rotenone-Induced Neurotoxicity. J. Nutr. Biochem. 2019, 69, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.H.; Hor, J.W.; Chong, C.W.; Lim, S.Y. Probiotics for Parkinson’s Disease: Current Evidence and Future Directions. JGH Open 2021, 5, 414–419. [Google Scholar] [CrossRef]
- Allen, A.P.; Hutch, W.; Borre, Y.E.; Kennedy, P.J.; Temko, A.; Boylan, G.; Murphy, E.; Cryan, J.F.; Dinan, T.G.; Clarke, G. Bifidobacterium longum 1714 as a Translational Psychobiotic: Modulation of Stress, Electrophysiology and Neurocognition in Healthy Volunteers. Transl. Psychiatry 2016, 6, e939. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Braun, C.; Murphy, E.F.; Enck, P. Bifidobacterium longum 1714™ Strain Modulates Brain Activity of Healthy Volunteers During Social Stress. Am. J. Gastroenterol. 2019, 114, 1152–1162. [Google Scholar] [CrossRef]
- Kumar, A.; Sidhu, J.; Goyal, A.; Tsao, J.W.; Doerr, C. Alzheimer Disease (Nursing). In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- DeTure, M.A.; Dickson, D.W. The Neuropathological Diagnosis of Alzheimer’s Disease. Mol. Neurodegener. 2019, 14, 32. [Google Scholar] [CrossRef] [PubMed]
- Simoes, S.; Guo, J.; Buitrago, L.; Qureshi, Y.H.; Feng, X.; Kothiya, M.; Cortes, E.; Patel, V.; Kannan, S.; Kim, Y.H.; et al. Alzheimer’s Vulnerable Brain Region Relies on a Distinct Retromer Core Dedicated to Endosomal Recycling. Cell Rep. 2021, 37, 110182. [Google Scholar] [CrossRef]
- Tan, J.Z.A.; Gleeson, P.A. The Role of Membrane Trafficking in the Processing of Amyloid Precursor Protein and Production of Amyloid Peptides in Alzheimer’s Disease. Biochim. Biophys. Acta Biomembr. 2019, 1861, 697–712. [Google Scholar] [CrossRef]
- Evrard, C.; Gilet, A.L.; Colombel, F.; Dufermont, E.; Corson, Y. Now You Make False Memories; Now You Do Not: The Order of Presentation of Words in DRM Lists Influences the Production of the Critical Lure in Alzheimer’s Disease. Psychol. Res. 2018, 82, 429–438. [Google Scholar] [CrossRef]
- Naomi, R.; Embong, H.; Othman, F.; Ghazi, H.F.; Maruthey, N.; Bahari, H. Probiotics for Alzheimer’s Disease: A Systematic Review. Nutrients 2021, 14, 20. [Google Scholar] [CrossRef]
- Steiner, H.; Fukumori, A.; Tagami, S.; Okochi, M. Making the Final Cut: Pathogenic Amyloid-β Peptide Generation by γ-Secretase. Cell Stress 2018, 2, 292–310. [Google Scholar] [CrossRef]
- Kinney, J.W.; Bemiller, S.M.; Murtishaw, A.S.; Leisgang, A.M.; Salazar, A.M.; Lamb, B.T. Inflammation as a Central Mechanism in Alzheimer’s Disease. Alzheimers. Dement. 2018, 4, 575–590. [Google Scholar] [CrossRef] [PubMed]
- Chandra, S.; Sisodia, S.S.; Vassar, R.J. The Gut Microbiome in Alzheimer’s Disease: What We Know and What Remains to Be Explored. Mol. Neurodegener. 2023, 18, 9. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Yang, D.; Sun, J.; Li, Y. Probiotic Supplements Are Effective in People with Cognitive Impairment: A Meta-analysis of Randomized Controlled Trials. Nutr. Rev. 2023, 81, 1091–1104. [Google Scholar] [CrossRef]
- Drljača, J.; Milošević, N.; Milanović, M.; Abenavoli, L.; Milić, N. When the Microbiome Helps the Brain-Current Evidence. CNS Neurosci. Ther. 2023, 29 (Suppl. S1), 43–58. [Google Scholar] [CrossRef]
- Zhu, G.; Zhao, J.; Wang, G.; Chen, W. Bifidobacterium breve HNXY26M4 Attenuates Cognitive Deficits and Neuroinflammation by Regulating the gut-brain Axis in APP/PS1 Mice. J. Agric. Food Chem. 2023, 71, 4646–4655. [Google Scholar] [CrossRef]
- Song, X.; Zhao, Z.; Zhao, Y.; Wang, Z.; Wang, C.; Yang, G.; Li, S. Lactobacillus plantarum DP189 Prevents Cognitive Dysfunction in D-Galactose/AlCl3 Induced Mouse Model of Alzheimer’s Disease via Modulating Gut Microbiota and PI3K/Akt/GSK-3β Signaling Pathway. Nutr. Neurosci. 2022, 25, 2588–2600. [Google Scholar] [CrossRef]
- Ohsawa, K.; Uchida, N.; Ohki, K.; Nakamura, Y.; Yokogoshi, H. Lactobacillus helveticus–Fermented Milk Improves Learning and Memory in Mice. Nutr. Neurosci. 2015, 18, 232–240. [Google Scholar] [CrossRef]
- Bairamian, D.; Sha, S.; Rolhion, N.; Sokol, H.; Dorothée, G.; Lemere, C.A.; Krantic, S. Microbiota in Neuroinflammation and Synaptic Dysfunction: A Focus on Alzheimer’s Disease. Mol. Neurodegener. 2022, 17, 19. [Google Scholar] [CrossRef]
- Bernier, F.; Kuhara, T.; Xiao, J. Probiotic Bifidobacterium breve MCC1274 Protects Against Oxidative Stress and Neuronal Lipid Droplet Formation via PLIN4 Gene Regulation. Microorganisms 2023, 11, 791. [Google Scholar] [CrossRef]
- Verbeke, K.A.; Boobis, A.R.; Chiodini, A.; Edwards, C.A.; Franck, A.; Kleerebezem, M.; Nauta, A.; Raes, J.; van Tol, E.A.; Tuohy, K.M. Towards Microbial Fermentation Metabolites as Markers for Health Benefits of Prebiotics. Nutr. Res. Rev. 2015, 28, 42–66. [Google Scholar] [CrossRef]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids from Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Psichas, A.; Sleeth, M.L.; Murphy, K.G.; Brooks, L.; Bewick, G.A.; Hanyaloglu, A.C.; Ghatei, M.A.; Bloom, S.R.; Frost, G. The Short Chain Fatty Acid Propionate Stimulates GLP-1 and PYY Secretion via Free Fatty Acid Receptor 2 in Rodents. In Int. J. Obes. 2015, 39, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Y.; Du, Y.F.; Chen, L. Neuropeptides Exert Neuroprotective Effects in Alzheimer’s Disease. Front. Mol. Neurosci. 2018, 11, 493. [Google Scholar] [CrossRef]
- Nimgampalle, M.; Kuna, Y. Anti-Alzheimer Properties of Probiotic, Lactobacillus plantarum MTCC 1325 in Alzheimer’s Disease Induced Albino Rats. J. Clin. Diagn. Res. 2017, 11, KC01–KC05. [Google Scholar] [CrossRef] [PubMed]
- Moya-Pérez, A.; Perez-Villalba, A.; Benítez-Páez, A.; Campillo, I.; Sanz, Y. Bifidobacterium CECT 7765 Modulates Early Stress-Induced Immune, Neuroendocrine, and Behavioral Alterations in Mice. Brain Behav. Immun. 2017, 65, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.S.; Begeny, C.T.; Boyle, P.A.; Schneider, J.A.; Bennett, D.A. Vulnerability to Stress, Anxiety, and Development of Dementia in Old Age. Am. J. Geriatr. Psychiatry 2011, 19, 327–334. [Google Scholar] [CrossRef]
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Nagler, C.R.; Ismagilov, R.F.; Mazmanian, S.K.; Hsiao, E.Y. Indigenous Bacteria from the Gut Microbiota Regulate Host Serotonin Biosynthesis. Cell 2015, 161, 264–276. [Google Scholar] [CrossRef]
- Mindus, C.; Ellis, J.; Van Staaveren, N.; Harlander-Matauschek, A. Lactobacillus-Based Probiotics Reduce the Adverse Effects of Stress in Rodents: A Meta-analysis. Front. Behav. Neurosci. 2021, 15, 642757. [Google Scholar] [CrossRef]
- Bonfili, L.; Cecarini, V.; Gogoi, O.; Berardi, S.; Scarpona, S.; Angeletti, M.; Rossi, G.; Eleuteri, A.M. Gut Microbiota Manipulation Through Probiotics Oral Administration Restores Glucose Homeostasis in a Mouse Model of Alzheimer’s Disease. Neurobiol. Aging 2020, 87, 35–43. [Google Scholar] [CrossRef]
- Ji, H.F.; Shen, L. Probiotics as Potential Therapeutic Options for Alzheimer’s Disease. Appl. Microbiol. Biotechnol. 2021, 105, 7721–7730. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.H.; Park, S.; Paik, J.W.; Chae, S.W.; Kim, D.H.; Jeong, D.G.; Ha, E.; Kim, M.; Hong, G.; Park, S.H.; et al. Efficacy and Safety of Lactobacillus plantarum C29-Fermented Soybean (DW2009) in Individuals with Mild Cognitive Impairment: A 12-Week, Multi-center, Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients 2019, 11, 305. [Google Scholar] [CrossRef] [PubMed]
- Dudek-Wicher, R.; Junka, A.; Paleczny, J.; Bartoszewicz, M. Clinical Trials of Probiotic Strains in Selected Disease Entities. Int. J. Microbiol. 2020, 2020, 8854119. [Google Scholar] [CrossRef] [PubMed]
- Merenstein, D.; Pot, B.; Leyer, G.; Ouwehand, A.C.; Preidis, G.A.; Elkins, C.A.; Hill, C.; Lewis, Z.T.; Shane, A.L.; Zmora, N.; et al. Emerging Issues in Probiotic Safety: 2023 Perspectives. Gut Microbes 2023, 15, 2185034. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).