Process Sustainability Analysis of Biorefineries to Produce Biofertilizers and Bioenergy from Biodegradable Residues
Abstract
:1. Introduction
1.1. Overview
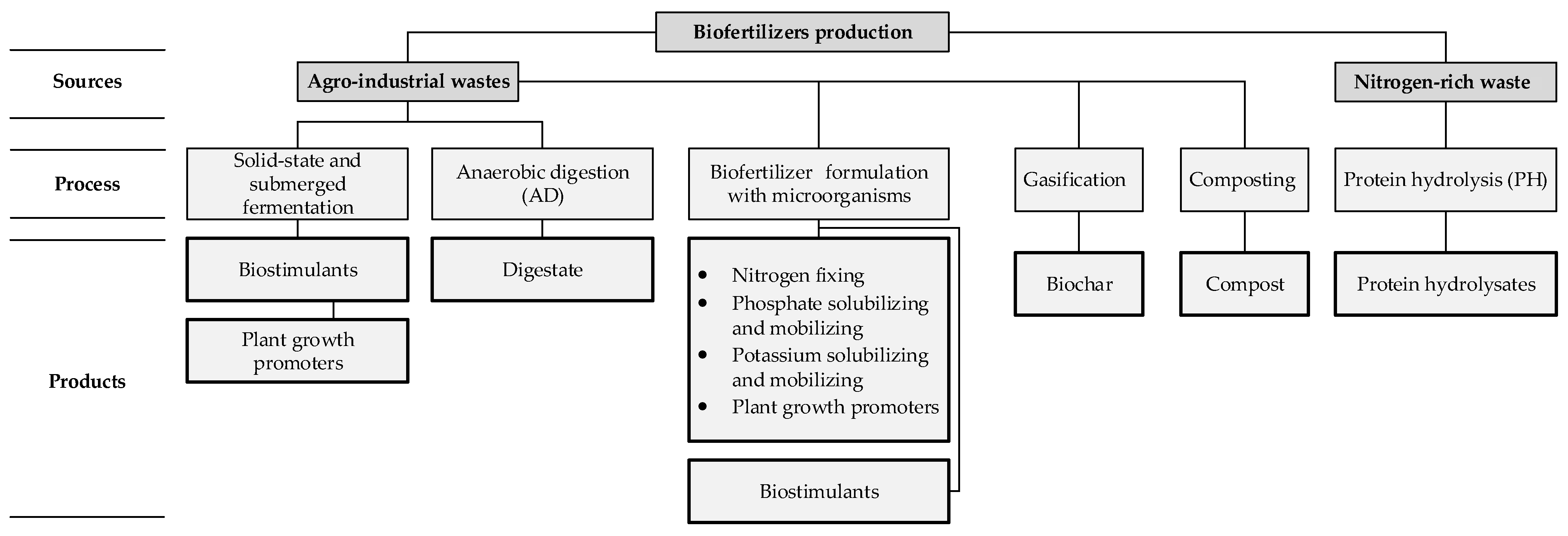
1.2. Substrates and Processes in Biofertilizer Production
1.2.1. Agro-Industrial Wastes
1.2.2. Nitrogen-Rich Wastes
1.2.3. Biofertilizers Formulation with Microorganisms
1.2.4. Biofertilizers Associated with Soil Organic Carbon
1.3. Process Design and Sustainability
2. Methodology
2.1. Description of Biofertilizer Production Processes
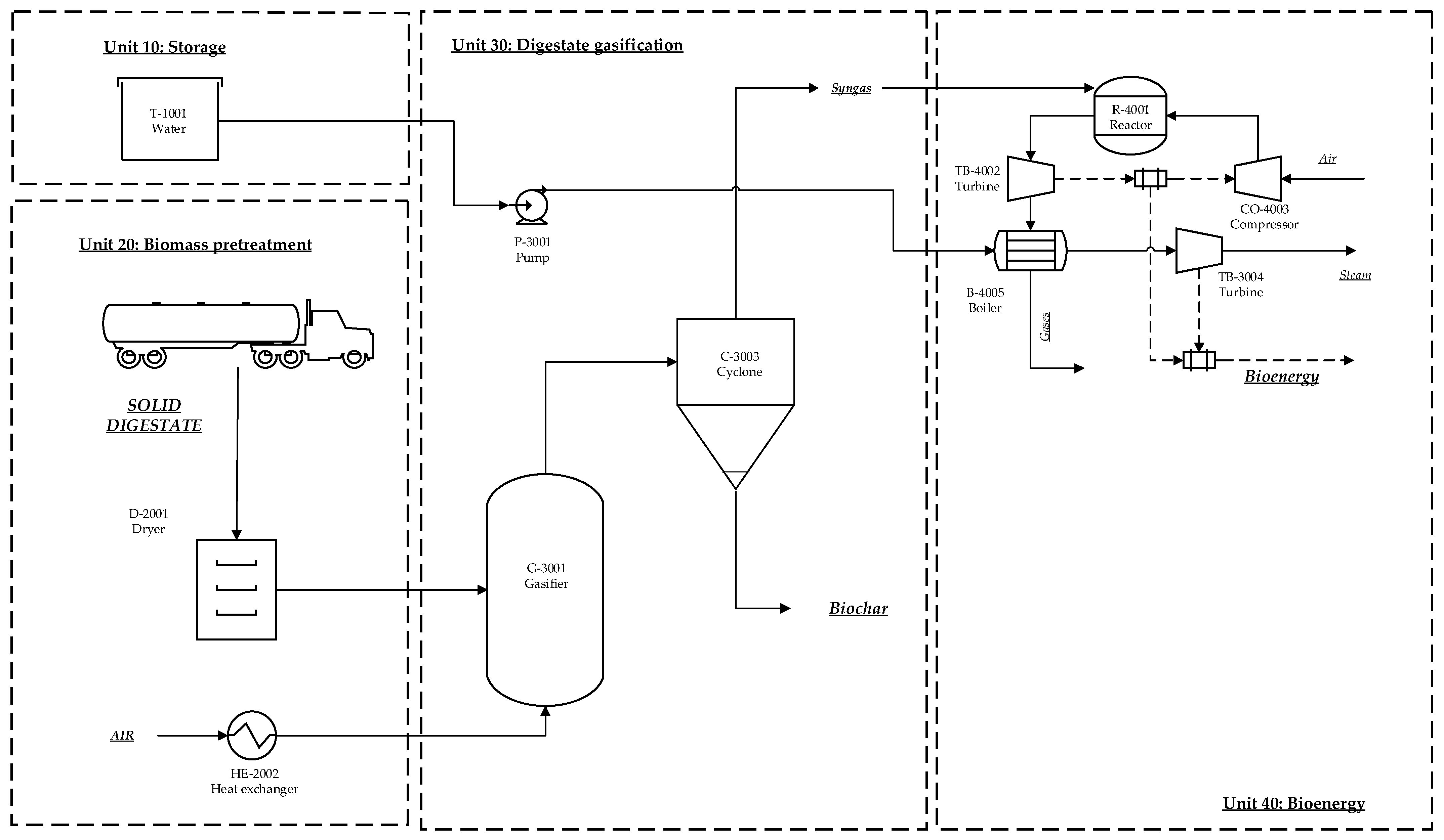
2.1.1. Biofertilizer Production from AD Digestate
2.1.2. Biofertilizer Production from Biochar
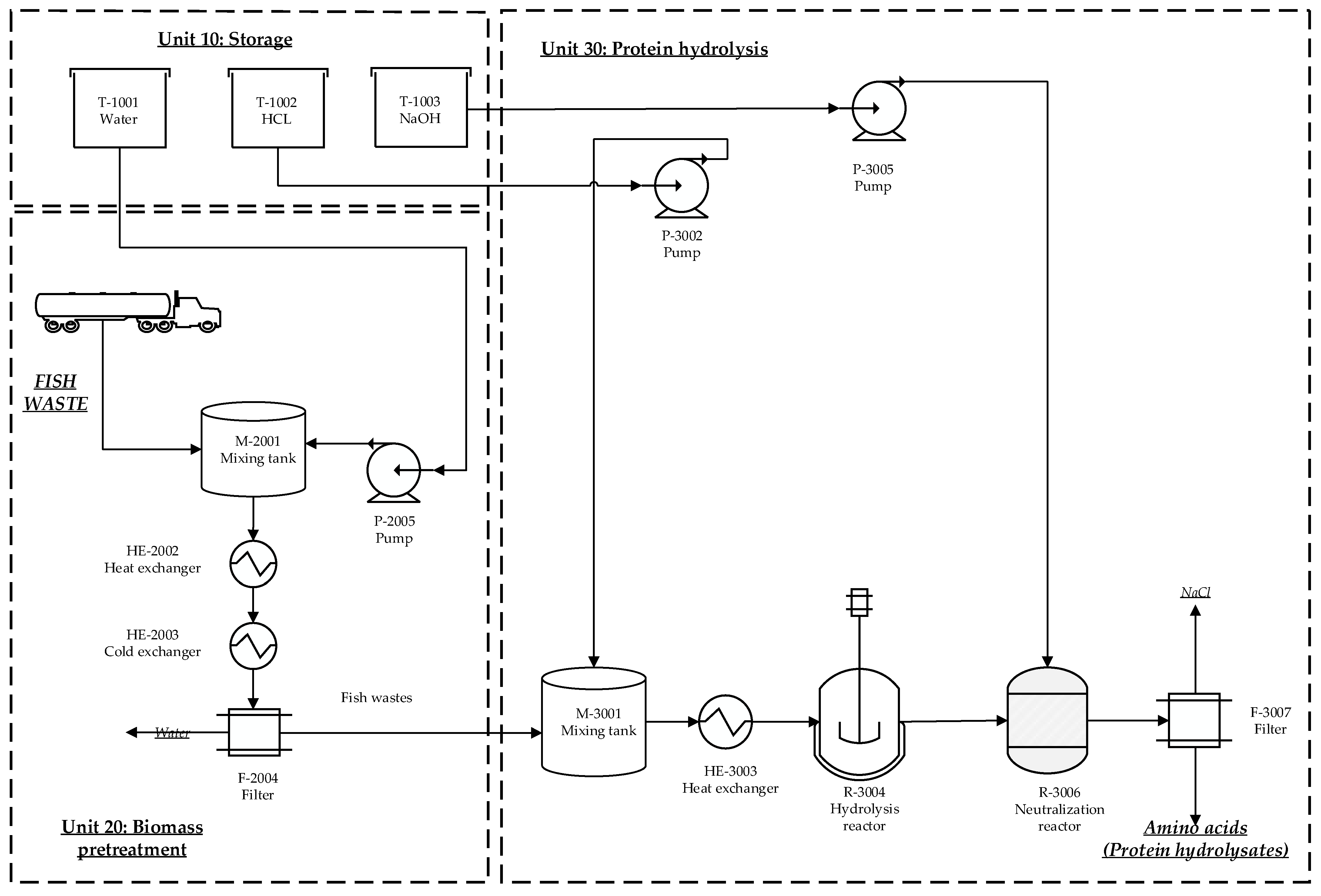
2.1.3. Biofertilizer Production from Protein Hydrolysates
2.2. Simulation Procedure
2.3. Technical and Energy Assessment
2.4. Economic Assessment
Sensitivity Analysis
2.5. Environmental Assessment
2.6. Social Assessment
2.7. Sustainability Assessment
3. Results and Discussion
3.1. Biofertilizer Production from AD Digestate
3.2. Biofertilizer Production from Biochar
3.3. Biofertilizer Production from Protein Hydrolysates
3.4. Technical and Energetic Assessment
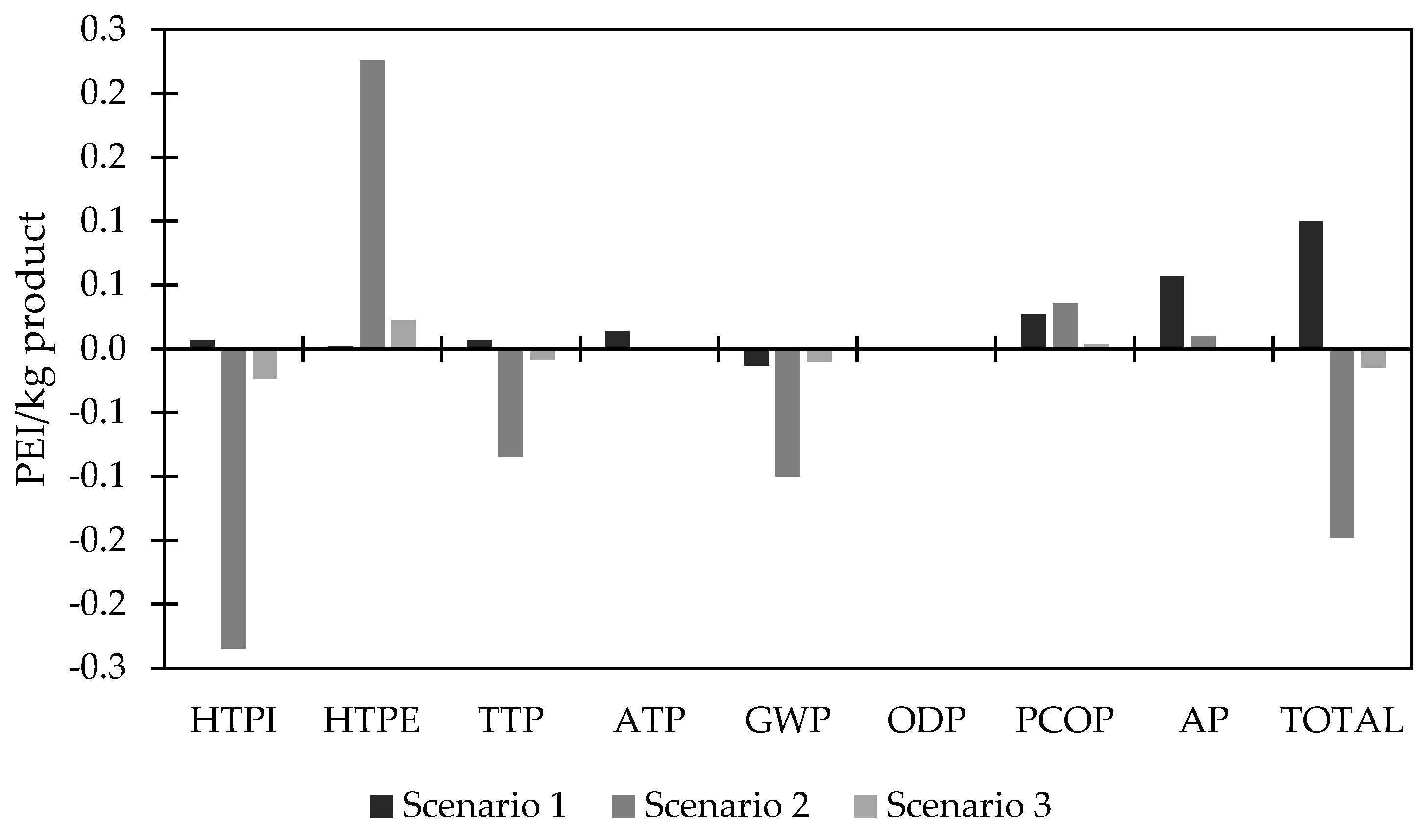
3.5. Environmental Assessment
3.6. Economic Assessment
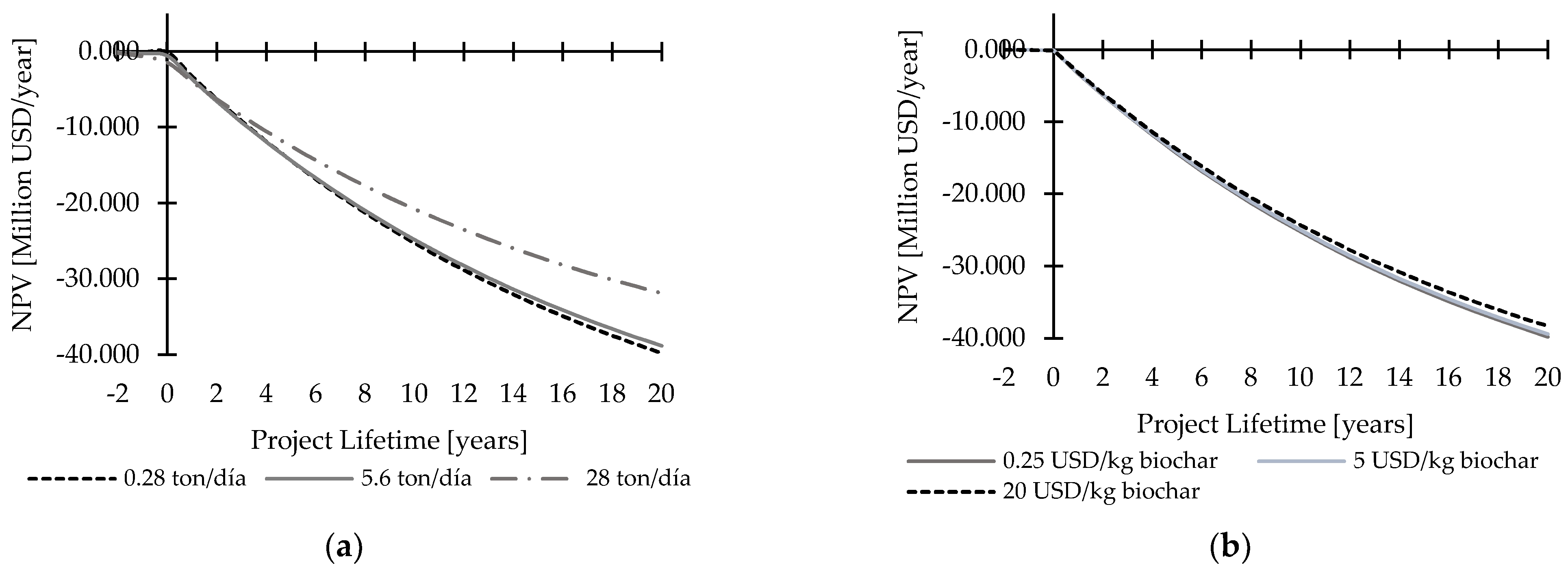
3.6.1. Biofertilizer Production from AD Digestate
3.6.2. Biofertilizer Production from Biochar
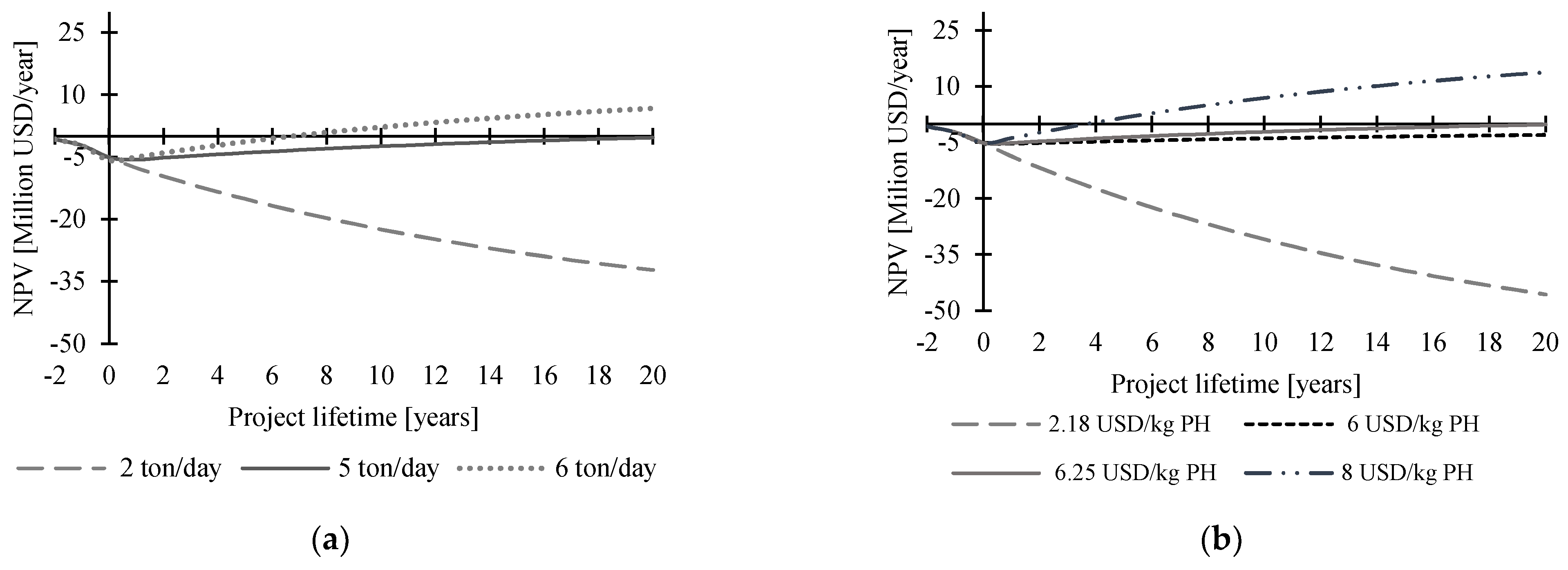
3.6.3. Biofertilizer Production from Protein Hydrolysates
3.6.4. Social Assessment
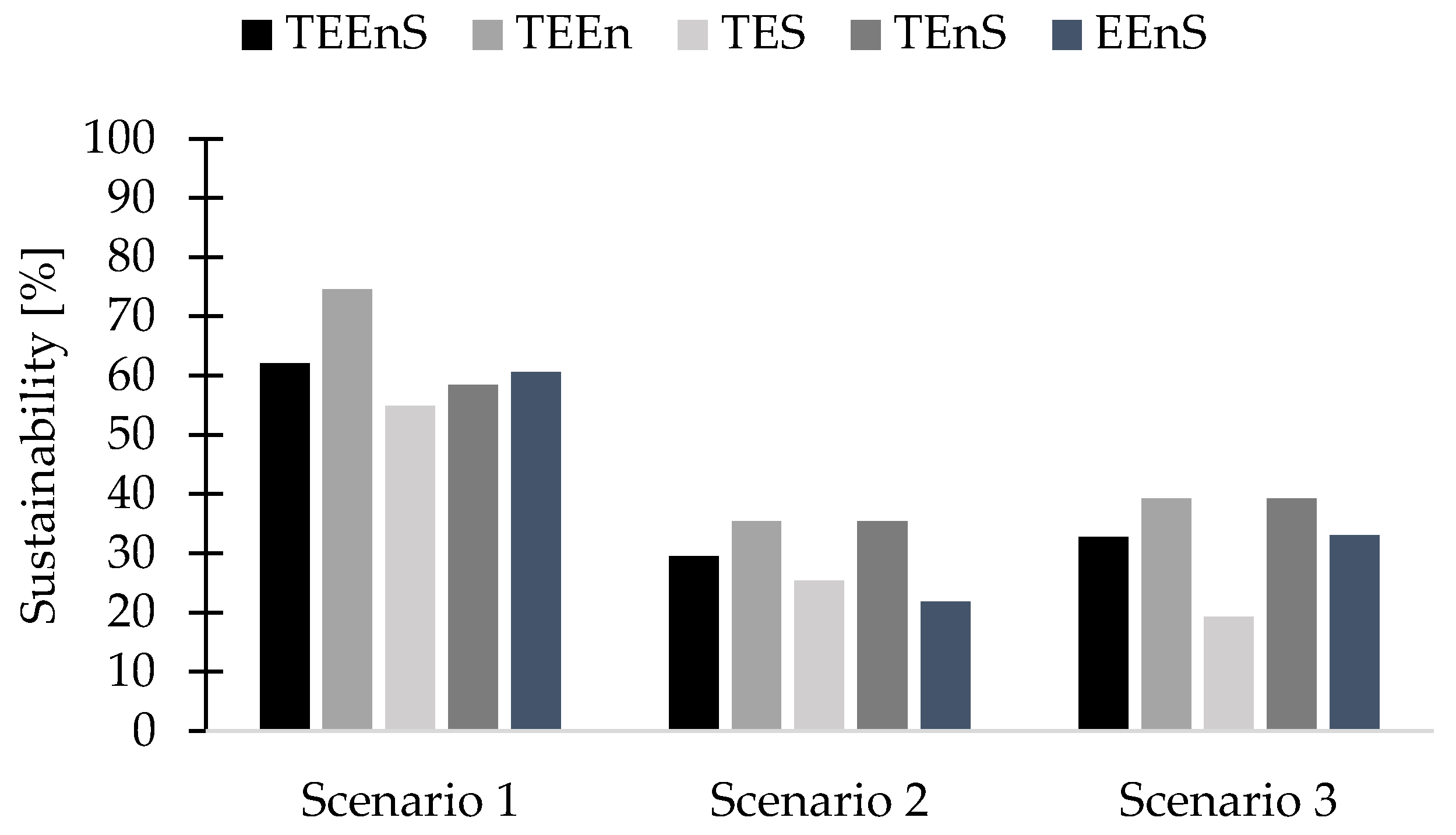
3.6.5. Sustainability Assessment
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| Product mass flow [kg/h] | Mass inflow of renewable raw material [kg/h] | ||
| Mass inflow of raw material or reactants [kg/h] | Enthalpy of product current [kJ/kg] | ||
| Thermal energy consumption [MJ/h] | System Working Consumption [MJ/h] | ||
| Investment capital (CAPEX) | Value calculated with process data | ||
| Product price [USD/year] | Value associated with the worst case | ||
| Raw material mass flow [kg/h] | Value associated with the best case | ||
| Process products | Process raw materials |
References
- Food and Agriculture Organization of the United Nations. World Fertilizers Trends and Outlook to 2022; FAO: Rome, Italy, 2019. [Google Scholar]
- Pahalvi, H.N.; Rafiya, L.; Rashid, S.; Nisar, B.; Kamili, A.N. Chemical Fertilizers and Their Impact on Soil Health. In Microbiota and Biofertilizers; Volume 2: Ecofriendly Tools for Reclamation of Degraded Soil Environs; Springer: Berlin/Heidelberg, Germany, 2021; pp. 1–20. [Google Scholar] [CrossRef]
- Chen, Y.; Lyu, Y.; Yang, X.; Zhang, X.; Pan, H.; Wu, J.; Lei, Y.; Zhang, Y.; Wang, G.; Xu, M.; et al. Performance Comparison of Urea Production Using One Set of Integrated Indicators Considering Energy Use, Economic Cost and Emissions’ Impacts: A Case from China. Energy 2022, 254, 124489. [Google Scholar] [CrossRef]
- Meessen, J. Urea Synthesis. Chem. Ing. Tech. 2014, 86, 2180–2189. [Google Scholar] [CrossRef]
- Humphreys, J.; Lan, R.; Tao, S. Development and Recent Progress on Ammonia Synthesis Catalysts for Haber–Bosch Process. Adv. Energy Sustain. Res. 2021, 2, 2000043. [Google Scholar] [CrossRef]
- Boer, M.A.; Wolzak, L.; Slootweg, J.C. Phosphorus: Reserves, Production, and Applications. In Phosphorus Recovery and Recycling; Springer: Singapore, 2018; pp. 75–100. [Google Scholar] [CrossRef]
- Ji, G.; Wang, W.; Chen, H.; Yang, S.; Sun, J.; Fu, W.; Yang, B.; Huang, Z. Sustainable Potassium Chloride Production from Concentrated KCl Brine via a Membrane-Promoted Crystallization Process. Desalination 2022, 521, 115389. [Google Scholar] [CrossRef]
- Nosheen, S.; Ajmal, I.; Song, Y. Microbes as Biofertilizers, a Potential Approach for Sustainable Crop Production. Sustainability 2021, 13, 1868. [Google Scholar] [CrossRef]
- Chakraborty, T.; Akhtar, N. Biofertilizers: Prospects and Challenges for Future. In Biofertilizers; Wiley: Hoboken, NJ, USA, 2021; pp. 575–590. [Google Scholar] [CrossRef]
- Mahapatra, D.M.; Chanakya, H.N.; Joshi, N.V.; Ramachandra, T.V.; Murthy, G.S. Algae-Based Biofertilizers: A Biorefinery Approach. In Microorganisms for Green Revolution. Microorganisms for Sustainability; Springer: Singapore, 2018; pp. 177–196. [Google Scholar] [CrossRef]
- Research and Markets Biofertilizers Global Market Report. 2023. Available online: https://www.researchandmarkets.com/reports/5766642/biofertilizers-global-market-report (accessed on 5 June 2023).
- Suthar, H.; Hingurao, K.; Vaghashiya, J.; Parmar, J. Fermentation: A Process for Biofertilizer Production. In Microorganisms for Green Revolution. Microorganisms for Sustainability; Springer: Singapore, 2017; pp. 229–252. [Google Scholar] [CrossRef]
- Massi, E. Anaerobic Digestion. Green Energy Technol. 2012, 45, 47–63. [Google Scholar] [CrossRef]
- Samoraj, M.; Mironiuk, M.; Izydorczyk, G.; Witek-Krowiak, A.; Szopa, D.; Moustakas, K.; Chojnacka, K. The Challenges and Perspectives for Anaerobic Digestion of Animal Waste and Fertilizer Application of the Digestate. Chemosphere 2022, 295, 133799. [Google Scholar] [CrossRef]
- Doyeni, M.O.; Stulpinaite, U.; Baksinskaite, A.; Suproniene, S.; Tilvikiene, V. The Effectiveness of Digestate Use for Fertilization in an Agricultural Cropping System. Plants 2021, 10, 1734. [Google Scholar] [CrossRef]
- Lee, M.E.; Steiman, M.W.; St. Angelo, S.K. Biogas Digestate as a Renewable Fertilizer: Effects of Digestate Application on Crop Growth and Nutrient Composition. Renew. Agric. Food Syst. 2021, 36, 173–181. [Google Scholar] [CrossRef]
- Errico, M.; Fjerbaek Sotoft, L.; Kjærhuus Nielsen, A.; Norddahl, B. Treatment Costs of Ammonia Recovery from Biogas Digestate by Air Stripping Analyzed by Process Simulation. Clean Technol. Env. Policy 2018, 20, 1479–1489. [Google Scholar] [CrossRef]
- Callegaro, K.; Brandelli, A.; Daroit, D.J. Beyond Plucking: Feathers Bioprocessing into Valuable Protein Hydrolysates. Waste Manag. 2019, 95, 399–415. [Google Scholar] [CrossRef] [PubMed]
- Bhari, R.; Kaur, M.; Sarup Singh, R. Chicken Feather Waste Hydrolysate as a Superior Biofertilizer in Agroindustry. Curr. Microbiol. 2021, 78, 2212–2230. [Google Scholar] [CrossRef] [PubMed]
- Kalayu, G. Phosphate Solubilizing Microorganisms: Promising Approach as Biofertilizers. Int. J. Agron. 2019, 2019, 4917256. [Google Scholar] [CrossRef]
- Olaniyan, F.T.; Alori, E.T.; Adekiya, A.O.; Ayorinde, B.B.; Daramola, F.Y.; Osemwegie, O.O.; Babalola, O.O. The Use of Soil Microbial Potassium Solubilizers in Potassium Nutrient Availability in Soil and Its Dynamics. Ann. Microbiol. 2022, 72, 45. [Google Scholar] [CrossRef]
- Michalak, I.; Górka, B.; Wieczorek, P.P.; Rój, E.; Lipok, J.; Łęska, B.; Messyasz, B.; Wilk, R.; Schroeder, G.; Dobrzyńska-Inger, A.; et al. Supercritical Fluid Extraction of Algae Enhances Levels of Biologically Active Compounds Promoting Plant Growth. Eur. J. Phycol. 2016, 51, 243–252. [Google Scholar] [CrossRef]
- Messyasz, B.; Michalak, I.; Łęska, B.; Schroeder, G.; Górka, B.; Korzeniowska, K.; Lipok, J.; Wieczorek, P.; Rój, E.; Wilk, R.; et al. Valuable Natural Products from Marine and Freshwater Macroalgae Obtained from Supercritical Fluid Extracts. J. Appl. Phycol. 2018, 30, 591–603. [Google Scholar] [CrossRef]
- Stockmann, U.; Padarian, J.; McBratney, A.; Minasny, B.; de Brogniez, D.; Montanarella, L.; Hong, S.Y.; Rawlins, B.G.; Field, D.J. Global Soil Organic Carbon Assessment. Glob. Food Sec. 2015, 6, 9–16. [Google Scholar] [CrossRef]
- Ermolaev, D.V.; Karaeva, J.V.; Timofeeva, S.S.; Kovalev, A.A.; Kovalev, D.A.; Litti, Y.V. Modeling of Air Gasification of Dark Fermentation Digestate in a Downdraft Gasifier. Int. J. Hydrogen Energy 2023, 48, 24255–24263. [Google Scholar] [CrossRef]
- Gwenzi, W.; Chaukura, N.; Wenga, T.; Mtisi, M. Biochars as Media for Air Pollution Control Systems: Contaminant Removal, Applications and Future Research Directions. Sci. Total Environ. 2021, 753, 142249. [Google Scholar] [CrossRef]
- Fedeli, R.; Alexandrov, D.; Celletti, S.; Nafikova, E.; Loppi, S. Biochar Improves the Performance of Avena sativa L. Grown in Gasoline-Polluted Soils. Environ. Sci. Pollut. Res. 2023, 30, 28791–28802. [Google Scholar] [CrossRef]
- Song, B.; Almatrafi, E.; Tan, X.; Luo, S.; Xiong, W.; Zhou, C.; Qin, M.; Liu, Y.; Cheng, M.; Zeng, G.; et al. Biochar-Based Agricultural Soil Management: An Application-Dependent Strategy for Contributing to Carbon Neutrality. Renew. Sustain. Energy Rev. 2022, 164, 112529. [Google Scholar] [CrossRef]
- Fechter, M.; Petrova, I.P.; Kraume, M. Balance of Total Mass and Nitrogen Fluxes through Consecutive Digestate Processing Steps: Two Application Cases. J. Environ. Manag. 2023, 326, 116791. [Google Scholar] [CrossRef]
- Ayilara, M.S.; Olanrewaju, O.S.; Babalola, O.O.; Odeyemi, O. Waste Management through Composting: Challenges and Potentials. Sustainability 2020, 12, 4456. [Google Scholar] [CrossRef]
- Wei, Z.; Ahmed Mohamed, T.; Zhao, L.; Zhu, Z.; Zhao, Y.; Wu, J. Microhabitat Drive Microbial Anabolism to Promote Carbon Sequestration during Composting. Bioresour. Technol. 2022, 346, 126577. [Google Scholar] [CrossRef] [PubMed]
- Smith, R. Chemical Process Design and Integration; John Wiley & Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- Costamagna, P.; Delucchi, M.; Busca, G.; Giordano, A. System for Ammonia Removal from Anaerobic Digestion and Associated Ammonium Sulfate Production: Simulation and Design Considerations. Process Saf. Environ. Prot. 2020, 144, 133–142. [Google Scholar] [CrossRef]
- Zhu, K.; Gu, S.; Liu, J.; Luo, T.; Khan, Z.; Zhang, K.; Hu, L. Wood Vinegar as a Complex Growth Regulator Promotes the Growth, Yield, and Quality of Rapeseed. Agronomy 2021, 11, 510. [Google Scholar] [CrossRef]
- Petrova, I.; Tolstorebrov, I.; Eikevik, T.M. Production of Fish Protein Hydrolysates Step by Step: Technological Aspects, Equipment Used, Major Energy Costs and Methods of Their Minimizing. Int. Aquat. Res. 2018, 10, 223–241. [Google Scholar] [CrossRef]
- Jajic, I.; Krstovic, S.; Glamocic, D.; Jakšic, S.; Abramovic, B. Validation of an HPLC Method for the Determination of Amino Acids in Feed. J. Serbian Chem. Soc. 2013, 78, 839–850. [Google Scholar] [CrossRef]
- Szopa, D.; Skrzypczak, D.; Izydorczyk, G.; Chojnacka, K.; Korczyński, M.; Witek-Krowiak, A. Evaluation of Tenebrio Molitor Protein Hydrolysates as Biostimulants Improving Plants Growth and Root Architecture. J. Clean Prod. 2023, 401, 136812. [Google Scholar] [CrossRef]
- Solarte-Toro, J.C.; Ortiz-Sanchez, M.; Cardona Alzate, C.A. Sustainability Analysis of Biorefineries Based on Country Socio-Economic and Environmental Context: A Step-by-Step Way for the Integral Analysis of Biomass Upgrading Processes. Renew. Energy 2023, 206, 1147–1157. [Google Scholar] [CrossRef]
- Ministerio de Desarrollo y Agricultura Rural Agronet-Estadísticas. Available online: https://www.agronet.gov.co/estadistica/Paginas/home.aspx?cod=1 (accessed on 21 July 2023).
- Vanegas Salazar, C.M. Manejo Del Bagazo En La Agroindustria de Caña Panelraen El Nordeste Antioqueño a Partir de La Gestion Integral de Residuos: Estudio de Caso Municipio de Yolombo; Universidad De Manizales: Manizales, Colombia, 2016. [Google Scholar]
- Restrepo-Serna, D.L.; Martínez-Ruano, J.A.; Cardona-Alzate, C.A. Energy Efficiency of Biorefinery Schemes Using Sugarcane Bagasse as Raw Material. Energies 2018, 11, 3474. [Google Scholar] [CrossRef]
- Rajendran, K.; Kankanala, H.R.; Lundin, M.; Taherzadeh, M.J. A Novel Process Simulation Model (PSM) for Anaerobic Digestion Using Aspen Plus. Bioresour. Technol. 2014, 168, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Ruano, J.A.; Restrepo-Serna, D.L.; Carmona-Garcia, E.; Giraldo, J.A.P.; Aroca, G.; Cardona, C.A. Effect of Co-Digestion of Milk-Whey and Potato Stem on Heat and Power Generation Using Biogas as an Energy Vector: Techno-Economic Assessment. Appl. Energy. 2019, 241, 504–518. [Google Scholar] [CrossRef]
- Karaeva, J.V.; Timofeeva, S.S.; Islamova, S.I.; Gerasimov, A.V. Pyrolysis Kinetics of New Bioenergy Feedstock from Anaerobic Digestate of Agro-Waste by Thermogravimetric Analysis. J. Environ. Chem. Eng. 2022, 10, 107850. [Google Scholar] [CrossRef]
- Solarte-Toro, J.C.; Chacón-Pérez, Y.; Cardona-Alzate, C.A. Evaluation of Biogas and Syngas as Energy Vectors for Heat and Power Generation Using Lignocellulosic Biomass as Raw Material. Electron. J. Biotechnol. 2018, 33, 52–62. [Google Scholar] [CrossRef]
- Torres Barrera, N.H.; Grandas Rincón, I.A. Estimación de Los Desperdicios Generados Por La Producción de Trucha Arcoíris En El Lago de Tota, Colombia. Cienc. Tecnol. Agropecu. 2017, 18, 247–255. [Google Scholar] [CrossRef]
- Chalamaiah, M.; Dinesh Kumar, B.; Hemalatha, R.; Jyothirmayi, T. Fish Protein Hydrolysates: Proximate Composition, Amino Acid Composition, Antioxidant Activities and Applications: A Review. Food Chem. 2012, 135, 3020–3038. [Google Scholar] [CrossRef]
- Popa, C.; Oprescu, E.E.; Popescu, M. Study of the Influence of Process Parameters on Biomass Gasification Using UniSim Design Environment. In Proceedings of the 2022 14th International Conference on Electronics, Computers and Artificial Intelligence, Ploiesti, Romania, 30 June–1 July 2022. [Google Scholar] [CrossRef]
- Wooley, R.J.; Putsche, V. Development of an ASPEN PLUS Physical Property Database for Biofuels Components; National Renewable Energy Lab. (NREL): Golden, CO, USA, 1996. [Google Scholar]
- Ruiz-Mercado, G.J.; Smith, R.L.; Gonzalez, M.A. Sustainability Indicators for Chemical Processes: II. Data Needs. Ind. Eng. Chem. Res. 2012, 51, 2329–2353. [Google Scholar] [CrossRef]
- Smith, R.L.; Gonzales, M.; Ruíz Mercado, J.G. GREENSCOPE: A Method for Modeling Chemical Process Sustainability. In Proceedings of the AIChE National Meeting, Minneapolis, MN, USA, 16–21 October 2011. [Google Scholar]
- Peters, M.S.; Timmerhaus, K.D. Plants Design and Economics for Chemical Engineering; McGraw Hill: New York, NY, USA, 1991. [Google Scholar]
- Towler, G.; Sinnott, R. Principles, Practice and Economics of Plant and Process Design. In Chemical Engineering Design; Elsevier: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Rueda-Duran, C.A.; Ortiz-Sanchez, M.; Cardona-Alzate, C.A. Detailed Economic Assessment of Polylactic Acid Production by Using Glucose Platform: Sugarcane Bagasse, Coffee Cut Stems, and Plantain Peels as Possible Raw Materials. Biomass Convers. Biorefin. 2022, 12, 4419–4434. [Google Scholar] [CrossRef]
- Peters, M.S.; Timmerhaus, K.D. Plant Design and Costing for Chemical Engineers; McGraw Hill: New York, NY, USA, 1991. [Google Scholar]
- Towler, G.; Sinnott, R.A.Y. Chemical Engineering Design; Elsevier: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Lending Interest Rate (%)-Country Comparison. Available online: https://www.indexmundi.com/facts/indicators/FR.INR.LEND/compare#country=co (accessed on 21 July 2023).
- Corporate Income Tax (CIT) Rates. Available online: https://taxsummaries.pwc.com/quick-charts/corporate-income-tax-cit-rates#anchor-C (accessed on 21 July 2023).
- Ministerio del Trabajo Actas de Salarío Minímo. Available online: https://www.mintrabajo.gov.co/web/guest/relaciones-laborales/comision-permanente-de-concertacion?inheritRedirect=true (accessed on 21 July 2023).
- Aristizábal-Marulanda, V.; Solarte-Toro, J.C.; Cardona Alzate, C.A. Economic and Social Assessment of Biorefineries: The Case of Coffee Cut-Stems (CCS) in Colombia. Bioresour. Technol. Rep. 2020, 9, 100397. [Google Scholar] [CrossRef]
- Singh, N.; Singhania, R.R.; Nigam, P.S.; Dong, C.D.; Patel, A.K.; Puri, M. Global Status of Lignocellulosic Biorefinery: Challenges and Perspectives. Bioresour. Technol. 2022, 344, 126415. [Google Scholar] [CrossRef] [PubMed]
- Young, D.M.; Cabezas, H. Designing Sustainable Processes with Simulation: The Waste Reduction (WAR) Algorithm. Comput. Chem. Eng. 1999, 23, 1477–1491. [Google Scholar] [CrossRef]
- Fundación Natura Guía Para Los Inventarios Organizacionales de Emisiones de Gases de Efecto Invernadero. 2014. Available online: https://natura.org.co/publicaciones/guia-los-inventarios-organizacionales-gei-uso-combustibles-fosiles-actividades-comerciales-e-industriales/ (accessed on 21 July 2023).
- Unidad de Planeación Minero Energética (UPME) Factores de Emisión de Los Combustibles Colombianos (FECOC). Available online: http://www.upme.gov.co/calculadora_emisiones/aplicacion/calculadora.html (accessed on 21 July 2023).
- GreenDelta Product Social Impact Life Cycle Assessment Database v.3 (PSILCA). Available online: https://psilca.net/ (accessed on 21 July 2023).
- Global Living Wage Coalition Living Wage for Caribbean Coast of Colombia. Available online: https://www.globallivingwage.org/living-wage-benchmarks/living-wage-for-caribbean-coast-of-colombia/ (accessed on 21 July 2023).
- Al Seadi, T.; Drosg, B.; Fuchs, W.; Rutz, D.; Janssen, R. Biogas Digestate Quality and Utilization. In The Biogas Handbook: Science, Production and Applications; Woodhead Publishing Limited: Cambridge, UK, 2013; pp. 267–301. [Google Scholar] [CrossRef]
- Fechter, M.; Kraume, M. Digestate Treatment Techniques. 2016. Available online: https://www.ejournals.eu/Czasopismo-Techniczne/2016/Mechanika-Zeszyt-1-M-(1)-2016/art/7547 (accessed on 21 July 2023).
- Shafiq, S.; Akram, N.A.; Ashraf, M.; García-Caparrós, P.; Ali, O.M.; Abdel Latef, A.A.H. Influence of Glycine Betaine (Natural and Synthetic) on Growth, Metabolism and Yield Production of Drought-Stressed Maize (Zea Mays L.) Plants. Plants 2021, 10, 2540. [Google Scholar] [CrossRef] [PubMed]
- Ghodake, G.S.; Shinde, S.K.; Kadam, A.A.; Saratale, R.G.; Saratale, G.D.; Kumar, M.; Palem, R.R.; AL-Shwaiman, H.A.; Elgorban, A.M.; Syed, A.; et al. Review on Biomass Feedstocks, Pyrolysis Mechanism and Physicochemical Properties of Biochar: State-of-the-Art Framework to Speed up Vision of Circular Bioeconomy. J. Clean Prod. 2021, 297, 126645. [Google Scholar] [CrossRef]
- Colla, G.; Nardi, S.; Cardarelli, M.; Ertani, A.; Lucini, L.; Canaguier, R.; Rouphael, Y. Protein Hydrolysates as Biostimulants in Horticulture. Sci. Hortic. 2015, 196, 28–38. [Google Scholar] [CrossRef]
- Brentrup, F.; Hoxha, A.; Christensen, B. Carbon Footprint Analysis of Mineral Fertilizer Production in Europe and Other World Regions. In Proceedings of the 10th International Conference on Life Cycle Assessment of Food, Dublin, Ireland, 19–21 October 2016. [Google Scholar]
- Moncada, J.; Matallana, L.G.; Cardona, C.A. Selection of Process Pathways for Biorefinery Design Using Optimization Tools: A Colombian Case for Conversion of Sugarcane Bagasse to Ethanol, Poly-3-Hydroxybutyrate (PHB), and Energy. Ind. Eng. Chem. Res. 2013, 52, 4132–4145. [Google Scholar] [CrossRef]
- Indian Manufacturers Suppliers Exporters Directory. Available online: https://my.indiamart.com/ (accessed on 21 July 2023).
- Zhang, R. Presentation: Digestate Alone and with Compost—Designing for Specific End Uses. In Proceedings of the California Bioresources Alliance 2017 Symposium, Los Angeles, CA, USA, 2 November 2017. [Google Scholar]
- Sahoo, K.; Mani, S. Economic and Environmental Impacts of an Integrated-State Anaerobic Digestion System to Produce Compressed Natural Gas from Organic Wastes and Energy Crops. Renew. Sustain. Energy Rev. 2019, 115, 109354. [Google Scholar] [CrossRef]




| Moisture | Cellulose | Hemicellulose | Lignin | Protein | Ash |
|---|---|---|---|---|---|
| 49.33% | 23.38% | 11.89% | 11.54% | 2.37% | 1.48% |
| Proximal Snalysis | Moisture | Ash | Fixed Carbon | Volatile Material |
| 6.02% | 24.44% | 5.64% | 63.91% | |
| Ultimate analysis | C | H | O | N |
| 35.60% | 4.40% | 58.20% | 1.80% |
| Protein | Moisture | Ash | Fat |
|---|---|---|---|
| 80.57% | 4.31% | 11.46% | 3.67% |
| Global atmospheric impact | GWP: Global warming potential |
| ODP: Ozone-depleting potential | |
| AP: Acidification potential | |
| PCOP: Photochemical oxidation potential | |
| Global toxicological impact | HTPE: Human toxicity potential for exposure |
| HTPI: Human toxicity potential for ingestion | |
| ATP: Aquatic toxicity potential | |
| TTP: Terrestrial toxicity Potential |
| Parameter | Value |
|---|---|
| Pellets (solid digestate) [kg/h] | 2591.43 |
| Concentrated liquid digestate [kg/h] | 37640.83 |
| Ammonium sulphate [kg/h] | 38.19 |
| Ammonia recovery (% w/w) | 96 |
| H2SO4 flow (98% w/w) | 31.04 |
| Air stripping column stages | 12 |
| Absorption column stages | 5 |
| Indicator | Scenario | |||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| Technical | Product yield [%] | 60.90 | Total = 75.64 Biochar = 5.64 | 92.79 |
| Process mass intensity index [kg raw materials/kg product] | 1.64 | 1.32 | 16.13 | |
| Renewability material index [kg renewable raw material/kg raw materials—%] | 99.60 | 100.00 | 56.68 | |
| Energy | Self-generation index | 0.09 | 0.03 | 0.16 |
| Variable | Scenario 1 | Scenario 2 | Scenario 3 |
|---|---|---|---|
| Natural gas [m3/h] | 270.54 | - | 0.308 |
| Electricity [kWh] | 10.45 | - | - |
| kg CO2-eq/h | 14426.43 | - | 0.0767 |
| kg CO2-eq/kg product | 0.67 | 0.06 | 7.6 × 10−4 |
| Water use [kg/h] | 61797.85 | - | 88.72 |
| kg H2O/kg product | 1.47 | - | 0.88 |
| Raw material Price [USD/kg] | Scenario 1 | Ref | Scenario 2 | Value | Ref | Scenario 3 | Ref | |
|---|---|---|---|---|---|---|---|---|
| BSB | 0.035 | [73] | Fish wastes | 0.003 | [74] | |||
| H2SO4 | 0.730 | [74] | Digestate | 0.0027 | [75] | HCl | 0.12 | |
| Inoculum | 0.0027 | [75] | NaOH | 0.28 | ||||
| Product Price [USD/kg] | Scenario 1 | Ref | Scenario 2 | Value | Ref | Scenario 3 | Ref | |
| Pellets | 0.00176 | [75] | ||||||
| CLD | 0.00276 | [75] | Biochar | 0.218 | [74] | Amino | 2.18 | [74] |
| Ammonium sulfate | 0.25 | acids | ||||||
| Utilities | Price | Ref | ||||||
| Low pressure steam [USD/ton] | 7.89 | |||||||
| Process water [USD/m3] | 0.326 | [54] | ||||||
| Electricity [USD/kWh] | 0.055 | |||||||
| Cooling water [USD/m3] | 0.042 | |||||||
| M/L | Increase | #Operators | |||
|---|---|---|---|---|---|
| 9 | 12 | 15 | |||
| 0.69 | 0% | 5.16 | 4.46 | 3.76 | NPV (MUSD) |
| 0.76 | 10% | 4.95 | 4.18 | 3.41 | |
| 0.90 | 30% | 4.53 | 3.62 | 2.71 | |
| 0.97 | 40% | 4.32 | 3.34 | 2.36 | |
| 1.11 | 60% | 3.89 | 2.78 | 1.61 | |
| Dimension | Indicator | Scenario 1 | Scenario 2 | Scenario 3 | |||
|---|---|---|---|---|---|---|---|
| Best | Worst | Best | Worst | Best | Worst | ||
| Technical | PMI | 1.64 | 50.00 | 1.32 | 50.00 | 1.00 | 50.00 |
| RMI [%] | 100.00 | 0.00 | 100.00 | 0.00 | 100.00 | 0.00 | |
| SGI [%] | 1.00 | 0.00 | 1.00 | 0.00 | 1.00 | 0.00 | |
| Economic | PBP | 12.00 | 20.00 | 6.00 | 0.00 | 7.00 | 0.00 |
| TR | 0.54 | 0.36 | 85.80 | 0.08 | 33.43 | 5.76 | |
| Environmental | CF [kg CO2-eq] | 0.00 | 20.00 | 7.1 × 10−5 | 0.05 | 0.00 | 20.00 |
| WF [m3/kg] | 0.00 | 20.00 | 0.00 | 0.00 | 0.00 | 20.00 | |
| Social | M/Lmax | 0.69 | 1.00 | 0.69 | 1.00 | 0.69 | 1.00 |
| Dimension | Scale (ton/day) | Scenario 1 | Scenario 2 | Scenario 3 | Scenario 1 | Scenario 2 | Scenario 3 |
|---|---|---|---|---|---|---|---|
| 175 | 0.28 | 2 | 175 | 0.28 | 2 | ||
| Indicator | Actual | Normalization | |||||
| Technical | PMI | 1.64 | 1.32 | 16.13 | 1.00 | 1.01 | 0.70 |
| RMI [%] | 99.60 | 100.00 | 6.68 | 1.00 | 1.00 | 0.07 | |
| SGI [%] | 0.09 | 0.03 | 0.16 | 0.09 | 0.03 | 0.16 | |
| Economic | PBP | 12.00 | 0.00 | 0.00 | 1.00 | 0.00 | 0.00 |
| TR | 0.47 | 0.082 | 5.76 | 0.61 | 0.00 | 0.00 | |
| Environmental | CF [kg CO2-eq] | 0.67 | 10−5 | 7.6010−4 | 0.97 | 1.00 | 1.00 |
| WF [m3/kg] | 1.4710−3 | 0.00 | 8.8110−4 | 1.00 | 0.00 | 1.00 | |
| Social | M/Lmax | 1 | 0.79 | 0.99 | 1 | 0.65 | 0.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burbano-Cuasapud, J.M.; Solarte-Toro, J.C.; Restrepo-Serna, D.L.; Cardona Alzate, C.A. Process Sustainability Analysis of Biorefineries to Produce Biofertilizers and Bioenergy from Biodegradable Residues. Fermentation 2023, 9, 788. https://doi.org/10.3390/fermentation9090788
Burbano-Cuasapud JM, Solarte-Toro JC, Restrepo-Serna DL, Cardona Alzate CA. Process Sustainability Analysis of Biorefineries to Produce Biofertilizers and Bioenergy from Biodegradable Residues. Fermentation. 2023; 9(9):788. https://doi.org/10.3390/fermentation9090788
Chicago/Turabian StyleBurbano-Cuasapud, Johana Marisol, Juan Camilo Solarte-Toro, Daissy Lorena Restrepo-Serna, and Carlos Ariel Cardona Alzate. 2023. "Process Sustainability Analysis of Biorefineries to Produce Biofertilizers and Bioenergy from Biodegradable Residues" Fermentation 9, no. 9: 788. https://doi.org/10.3390/fermentation9090788
APA StyleBurbano-Cuasapud, J. M., Solarte-Toro, J. C., Restrepo-Serna, D. L., & Cardona Alzate, C. A. (2023). Process Sustainability Analysis of Biorefineries to Produce Biofertilizers and Bioenergy from Biodegradable Residues. Fermentation, 9(9), 788. https://doi.org/10.3390/fermentation9090788






