Abstract
Cistus creticus L. (rockrose), a species of ecological and medicinal significance, constitutes a valuable component of the Mediterranean ecosystem. The present study investigated the effect of the inorganic salt concentration of Murashige and Skoog medium (MS), woody plant medium (WPM), and Driver and Kuniyaki Walnut medium (DKW) at several strengths (1/8×, 1/4×, 1/2×, 1×, and 2×) on the in vitro growth and organogenesis of rockrose. Significant interactions were observed throughout the experiments between pairs of plant origins, medium types, and strengths, and we also examined the extent to which they affected the studied traits was examined. The types of nutrient medium affected all studied traits except shoot and root percentages. The maximum growth percentage (143.49%) was gained using full-strength WPM. The best performance in shoot percentage was obtained using MS (100%) at several strengths along with 1× WPM (100%). The topmost rooting percentage values (98.61%) were obtained using 1× WPM and 1/2× DKW. The highest number of shoots and roots were observed using full-strength MS (9.39) and half-strength WPM (6.49), respectively. The maximum values for shoot and root length were achieved using 1/2× MS (0.78 cm) and 1/8× WPM (1.55 cm), respectively. The origin of the plant material did not influence any studied trait. Moreover, the genetic relations among the populations used in the in vitro culture were assessed using simple sequence repeats (SSR) markers. Twenty-eight alleles were identified across all five STR loci. The different and effective alleles per locus were 5.60 and 4.72, respectively. The average observed and expected heterozygosity was estimated at 0.52 and 0.72, respectively. Shannon’s information index and the inbreeding coefficient (F) were assessed at 1.48 and 0.30, respectively, revealing a narrow genetic base and high genetic similarity among origins, suggesting that they belong to the same population.
Keywords:
rockrose; MS; WPM; DKW; in vitro micropropagation; shoot induction; root induction; microsatellites; population genetics 1. Introduction
Cistus creticus L. (rockrose) and other species of Cistaceae, a medium-sized taxon of shrubs and commonly herbs, are prominent characteristics of the Mediterranean flora, covering broad dry and sun-exposed areas [1]. Evolutionarily, they developed survival adaptations to overcome the disturbances of the frequent fires that Mediterranean ecosystems experience [2]. The Cistus species’ ecological value has been acknowledged since they are considered typical pyrophytes that spread through seeds and creat pure stands after fire [3,4]. Cistaceae distribution is extensive; i.e., several species are distributed over most of Europe [5], North Africa, and even North and South America [6], although they are present mainly in the Mediterranean region, with some species distributed almost exclusively in the Mediterranean rim [7,8].
Cistus species are major components of Greek phryganic ecosystems [9]. In Greece, the species have been known since antiquity [10,11] because of their resin properties, called ladanon [12], which are used in Mediterranean folk medicine for the healing of several diseases [10]. The resin is a source of pharmaceutical and aromatic properties, is secreted from the glandular hairs of leaves and stems, and contains labdane diterpenes, which have antimicrobial [13], anti-inflammatory [14], and cytotoxic activity [15,16,17].
The economic interest of several Cistus spp. is due to their use as ornamental and melliferous flora [18]. Also, ladanon resin is traditionally used in medicine and perfumery [19,20,21,22]. A quite impressive use is to inoculate them with the mycorrhizae of Tuber nigrum Bull (a black truffle) and then plant them in the primary stage of truffle forest repopulation [23].
In a previous study [1], an efficient in vitro propagation protocol was developed for the large-scale production of C. creticus L. Prior to our propagation protocol, there were many successful efforts in in vitro propagation for other Cistus species [18,24] and only one in rockrose [25]. To complement our initial research as well as that of other authors [20,26,27], in the present work, we investigated the effects of three different agar-solidified nutrient mediums, i.e., MS, WPM, and DKW, at several strengths, i.e., 1/8×, 1/4×, 1/2×, 1×, and 2×, on in vitro growth and organogenesis. The studied traits concerned the shoot proliferation and root induction of three populations of C. creticus plantlets for the large-scale clonal propagation of selected rockrose clones. Moreover, the current research aimed to assess the genetic population structure of the three populations of C. creticus used in an in vitro propagation study. To the best of our knowledge, this research is the first attempt at a population genetic analysis of C. creticus L. based on SSR markers. Our genetic analysis of the three populations used in the in vitro culture experiments was based on prior studies performed by Astuti et al. [28] for Cistus laurifolius L. and by Bertolasi et al. [29] for Cistus albidus L.
2. Materials and Methods
2.1. In Vitro Culture
2.1.1. Plant Material
Three large, healthy mature C. creticus L. individuals with attractive purple flowers and green leaves, growing in three natural environments of Attica (Greece), i.e., Mt. Parnitha, Mt. Pateras, and Mt Pendeli, were selected as explant sources (Supplementary Materials, Figure S1). The lateral shoots of mother plants (explant donors) were collected in April. These were derived from actively growing stems during the most recent vegetative growth season, i.e., they were 1–2 months old. They were stored in a moist cotton cloth at 4 °C until subsequent handling. The following day, explants that were 1.0–1.5 cm long, i.e., nodal segments and apical shoot tips, were excised from the explant donors.
2.1.2. Explant Surface Sterilization
Explants were effectively disinfected through consecutive immersions in two distinct aqueous solutions. Initially, the explants were immersed in a solution of 70% (v/v) ethanol with continuous stirring for 1 min. Subsequently, they were treated with a second aqueous solution of sodium hypochlorite (10% w/v NaOCl, Merck KGaA, Darmstadt, Germany) at a concentration of 1.5% (v/v), supplemented with 0.05% (v/v) Tween 20 (Fisher Bioreagents, Pittsburgh, PA, USA), under continuous stirring for 15 min. After each immersion, a sequence of three rinses with sterile deionized water, each lasting three minutes, was conducted.
2.1.3. Initial Culture Establishment
Single node explants were placed in culture vessels containing basal medium. Three media were employed to initiate the in vitro culture: the Murashige and Skoog (MS) [30] (Duchefa Biochemie, Haarlem, The Netherlands), the Lloyd and McCown wood plant medium (WPM) [31] (Duchefa Biochemie, Haarlem, The Netherlands), and the Driver and Kuniyaki Walnut (DKW) medium [32] (Duchefa Biochemie, Haarlem, The Netherlands). Each medium contained 3% (w/v) sucrose (Duchefa Biochemie, Haarlem, The Netherlands) and was solidified with 2.4 g/L agar (Phytagel, Sigma, Burlington, MA, USA). Before the addition of agar, each medium was adjusted to pH 5.8. All media were autoclaved at 121 °C and 122 kPa for 20 min. The cultures were grown in a growth chamber at 22 ± 1 °C under a 16 h light/8 h dark photoperiod. The photosynthetic photon flux density at culture level was maintained at 100 µmol m−2 s−1, which was supplied by cool-white fluorescent lamps.
2.1.4. Initial Culture Shoot Multiplication
Contaminated-free explants derived from those three C. creticus clones were subcultured in each WPM, MS and DKW nutrient media. The media had no growth regulators, and multiple shoot induction was performed to achieve an adequate number of shoots for subsequent experiments, i.e., shoot regeneration and root induction. The cultures were maintained under the same conditions as previously described.
2.1.5. Cultures for the Evaluation of Growth, Shooting and Rooting Performance
Nodal segments, from the previous initial shoot multiplication cultures, were placed onto five different strengths (1/8×, 1/4×, 1/2×, 1× and 2×) in three different nutrient media (WPM, MS and DKW), containing no growth regulators. To avoid the effect of different salt concentration deriving from different media, explants were subcultured in the same medium. The media were prepared as described above. The cultures were maintained for 4 weeks in the same conditions as mentioned. The effect of the three solid nutrient media of different strengths had on growth percentage (%, (initial height-terminal height)/initial height), shoot and root formation percentage (%), number and length of shoots, and roots was assessed after a 4-week period of culture. The height was determined as the distance from the cut to the top of the explant. Three replications of eight explants per replication were used for each medium and its strength. Each experiment was arranged in a growth chamber in a completely randomized design.
2.1.6. Statistical Analysis
Analysis was based on the individual values of average percentage of growth, shoot and root formation, the average number of shoots and roots per explant, and the average length of shoots and roots per explant per treatment. The following linear model was used in the analysis, at a significance level of a = 0.05, to determine the influence of the clone, the nutrient medium, the strength of the nutrient medium and the interactions among clone–nutrient medium, clone–medium strength, and finally clone–nutrient medium–medium strength:
where yijkl is the phenotypic measurement for a trait of the lth explant, the jth nutrient medium, the kth strength of jth nutrient medium and the ith explant clone, as dependent variables; μ is the fixed population mean of all explants; ci is the fixed effect of the ith clone; mj is the random effect of the jth nutrient medium, sk is the random effect of the kth strength of the nutrient medium; ci*mj is the interaction of the ith clone with the jth nutrient medium; ci*sk is the interaction of the ith clone with the kth strength of jth nutrient medium; ci*mj*sk is the interaction of the ith clone with the jth nutrient medium being at the kth strength; and eijkl is the random residual error of the lth explant, the kth strength, the jth nutrient medium and the ith clone. The restricted maximum likelihood (REML) method was performed to assess the variance components. Analysis of variance (ANOVA) and Duncan’s multiple range test (MRT) at a = 0.05 were performed on the mean shoot and root number, the mean shoot and root length and the shooting and rooting percentage per treatment. The data, initially expressed as percentages, underwent appropriate log or arcsine transformation for statistical analysis. Subsequently, the transformed data were reverted to percentages presented in the relevant tables and graphs. All statistical analyses were performed using SPSS v.20 software for Windows (IBM SPSS Statistics 2011, IBM Corp., Armonk, NY, USA).
yijkl = μ + ci + mj + sk + ci*mj + ci*sk + ci*mj*sk + eijkl
2.2. Genetic Population Analysis
2.2.1. Plant Material—DNA Isolation and Quantification
Twelve plants from the three regions (populations) were used in the genetic population analysis. Genomic DNA extraction was performed on 100 mg fresh leaf samples, using the Higher Purity Plant DNA Purification Kit (Canvax, Córdoba, Spain) following the manufacturer’s instructions. Before storing at −20 °C, the purified total DNA of the samples underwent quantification, and its quality was documented using the UV-Vis spectrophotometer Q5000 UV-Vis (Quawell, San Jose, CA, USA).
2.2.2. Microsatellite Loci
Five microsatellite loci developed by Astuti et al. [28] for Cistus laurifolius L. and had been successfully tested for their ability to amplify C. albidus DNA samples according to Bertolasi et al. [29] were used to analyze the genetic population structure of the three C. creticus L. populations. The sequences and traits of the SSR markers are presented in Table 1.

Table 1.
Documentation on the 5 microsatellite markers used in the genetic analysis of C. creticus L.
2.2.3. PCR Reaction Mix and Amplification
The PCR reaction was performed in a total volume of 20 μL comprising 30 ng genomic DNA. The final concentrations of reaction mix components were 1×PCR buffer (10×) (Nippon Genetics, Tokyo, Japan), 2 mM of MgCl2 (50 mM) (Nippon Genetics, Tokyo, Japan), including the amount of MgCl2 contained in PCR buffer, 1 U Fast Gene Taq DNA polymerase (5 U μL−1, Nippon Genetics, Tokyo, Japan), 100 μM each of dNTPs (10 mM) (Nippon Genetics, Tokyo, Japan) and 0.8 μM of each forward and reverse primer (Eurofins Genomics, Ebersberg, Germany).
The amplifications reactions were performed twice in the 96-well thermal cycler Bio Rad C1000 Touch (Bio-Rad, Hercules, CA, USA) as follows: 95 °C for 3 min, 30 cycles at 95 °C for 45 s, 60 °C for 90 s and 72 °C for 1 min and a final extension of 8 min at 72 °C. The ultimate holding temperature was set at 4 °C. Each reaction comprised both a negative and a positive control.
2.2.4. Capillary Electrophoresis, Genotyping and Statistical Data
DNA fragment capillary electrophoresis was performed in a 3730 Genetic Analyzer (Applied Biosystems, Thermo Fisher Scientific Co., Waltham, MA, USA) using LIZ500 (Applied Biosystems, Thermo Fisher Scientific Co., Waltham, MA, USA) as the molecular weight standard. Geneious Prime v. 2022.1.1 software (Dotmatics, Boston, MA, USA) was employed for genotyping. The analytical threshold was set at 150 relative fluorescence units (RFUs).
Genetic diversity, i.e., the number of observed alleles (Na), effective number of alleles (Ne), Shannon’s information index (I), percentage of polymorphic loci (PPL), Nei genetic distance (Nei D) and Nei Genetic Identity (Nei I), was estimated using the GenAlEx package [33]. The PIC (Polymorphism Information Content) of each SSR marker was calculated using Cervus 3.0.7 [34,35]. Hardy–Weinberg tests were not conducted due to small within-population sample sizes.
3. Results
3.1. In Vitro Culture
Table 2 presents the source of variation retrieved from the ANOVA table of the in vitro culture of rockrose explants for the studied traits, according to GLM: yijkl = μ + ci + mj + sk + ci*mj + ci*sk + ci*mj*sk + eijkl.

Table 2.
Source of variation retrieved from ANOVA table of in vitro culture of C. creticus explants for the studied traits, according to GLM: yijkl = μ + ci + mj + sk + ci*mj + ci*sk + ci*mj*sk + eijkl.
Shoot formation was achieved after two weeks of culture and root initiation of in vitro-propagated explants commenced during the third week, both depending on the applied treatment (Figure 1). Blastogenesis was not affected by the origin of C. creticus explants (Table 2) but from the nutrient medium and its strength. The origin of plant material did not influence any studied traits. The medium type had a significant effect on the growth percentage (p ≤ 0.001), number (p ≤ 0.001) and length (p ≤ 0.01) of shoots per explant. Conversely, the medium type had no effect on shooting and rooting percentage. Moreover, statistically significant differences in the mean number (p ≤ 0.001) and length (p ≤ 0.05) of roots per explant among different nutrient mediums were observed. The strength of the medium had an impact on all the studied traits. The medium strength had a greater effect on the growth and rooting percentage (p ≤ 0.001) compared to the shooting percentage (p ≤ 0.05) as well as on the number and length of shoots and roots (p ≤ 0.001) (Figure 2, Table 2).

Figure 1.
Media and strength selection have affected the number and length of C. creticus L. explants’ shoots after 4 weeks of culture: (a) explants in full strength MS; (b) explants in half-strength DKW. Bar = 25 mm.

Figure 2.
Media and strength selection have affected the number and length of C. creticus L. explant roots after 4 weeks of culture: (a) explants in half-strength MS; (b) explants in half-strength WPM. Bar = 25 mm.
Significant interactions were observed among clone–nutrient medium, clone–medium strength, and nutrient medium–medium strength as well as among clone–nutrient medium–medium strength in relation to studied traits (Table 2). Significant interactions between explant origins and medium types were noticed only for explant growth (p ≤ 0.01) and root number (p ≤ 0.05). The interaction between explant origin and medium strength was significant only for growth (p ≤ 0.05) and rooting (p ≤ 0.05) percentage. The interaction among plant origin, medium type and its strength had a greater effect on growth (p ≤ 0.001) and rooting percentage (p ≤ 0.001) compared to shooting percentage (p ≤ 0.05), as well as on the number and length of shoots and roots (p ≤ 0.001).
The influence of various media on the overall mean growth, blastogenesis and root induction percentage (%), mean number of shoots and roots per explant as well as on the mean length of shoots and roots of C. creticus L. explants are presented in Figure 3 and Figure 4 and in the Supplementary Material Table S1.
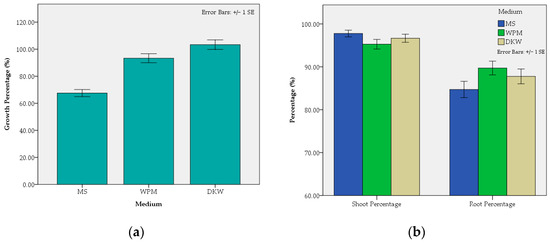
Figure 3.
Effect of the medium on the mean growth percentage of shoots (%) (a) and on the mean shoot and root percentage (%) (b) of C. creticus explants.
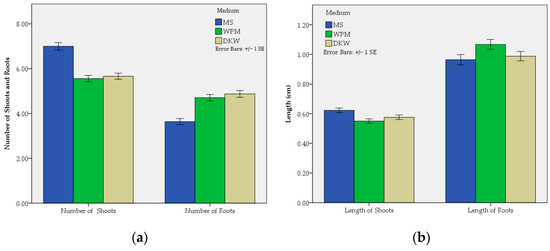
Figure 4.
Effect of the medium on the mean shoot and root number (a) and on the mean shoot and root length (b) per C. creticus explants.
The applied treatment, i.e., the type of nutrient medium, significantly affected the average growth percentage of explant, blastogenesis and root induction (Figure 3, Supplementary Material Table S1). Explants cultured in DKW exhibited superior growth (103.32%) compared to those cultured in WPM and MS. Conversely, MS showed the lowest value (67.52%), which exhibited a statistically significant difference compared to DKW and WPM (93.30%). No differences in shoot percentage were observed among the treatments using full-strength mediums, i.e., MS (97.78%), DKW (96.67%) and WPM (95.28%). Likewise, no differences in rooting percentage were observed among the treatments using full-strength mediums, i.e., WPM (89.72%), DKW (87.78%) and MS (84.72%), too.
The MS medium presented the highest mean number of shoots per explant (7.00), which was statistically significant different (p ≤ 0.001) compared to WPM (5.56) and DKW (5.66). The last two treatments were not statistically different from each other. A similar trend was presented concerning the average length of shoots per explant, in which the best results were obtained using MS (0.62 cm), being statistically significant different (p ≤ 0.01) compared to WPM (0.55 cm) and DKW (0.58 cm). The last two treatments presented non-significant differences.
The DKW and WPM did not exhibit statistical differences in the number of roots per explant with the first medium showing the highest value (4.87) and the second showing a similar value (4.71). The number of roots using MS was the lowest (3.64) and statistically different compared to the previous treatments (p ≤ 0.001). Statistically significant differences (p ≤ 0.05) were observed between WPM and MS in the mean length of roots per explant with WPM presenting the best performance (1.07 cm) followed by the DKW (0.99 cm) and MS (0.96 cm). The last two treatments showed non-significant differences.
Additionally, the effect of different media on the examined parameters in relation to the rockrose origins used in in vitro culture are presented in Supplementary Material Figures S2–S4.
The impact of the combination of different nutrient media and their strengths on the overall mean growth, blastogenesis and root induction percentage (%), mean number of shoots and roots per explant as well as on the mean length of shoots and roots of C. creticus L. explants are presented in Figure 5, Figure 6 and Figure 7 and in Supplementary Material Tables S2–S4.
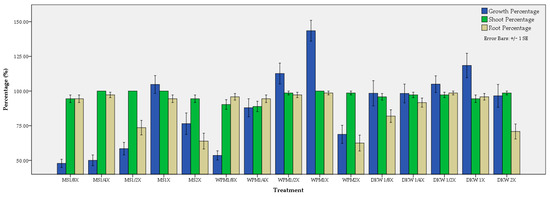
Figure 5.
Effect of medium and its strength on the average growth, shoot and root percentage (%) of C. creticus explants.
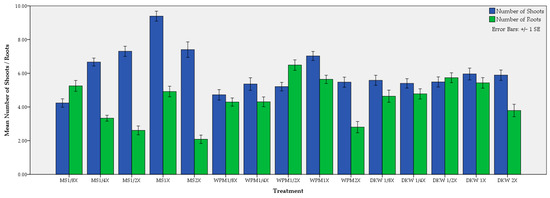
Figure 6.
Effect of medium and its strength on the average shoot and root number of C. creticus explants.
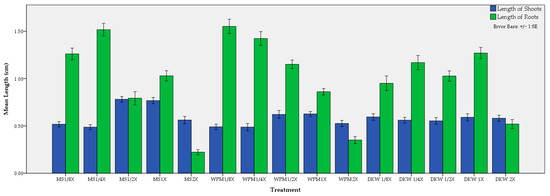
Figure 7.
Effect of medium and its strength on the average shoot and root length of C. creticus explants.
Regarding the growth percentage, the maximum (143.49%) was obtained in the full-strength WPM medium followed by the full-strength DKW (118.45%) and half-strength WPM (112.66%) (Figure 5). Full-strength MS also showed good results (104.75%). In general, when the media were diluted, the growth decreased. This was obvious for MS and WPM media but not for DKW. The best performance in shooting percentage was obtained in MS (100%) at several strengths (1/4×, 1/2× and 1×) as well as in full-strength WPM (100%). Overall, the shooting percentage was adequate in all treatments presenting non-significant differences. To some extent, the same pattern was observed for rooting percentage with the highest values (98.61%) obtained in full-strength WPM and in half-strength DKW. Dilution of media lead to sufficient rooting percentage (97.22%) in some treatments, e.g., 1/4× MS and 1/2× WPM (Figure 5).
Concerning the number of shoots, highest values were observed in all media when these were at full strength, i.e., MS (9.39), WPM (7.03) and DKW (5.96) (Figure 6). The best medium proved to be MS except when diluted to 1/8 of its strength. Normally, a shift from full strength resulted in a decrease in shoot number values that were often statistically significant different. To a limited degree, the above motif was applied for number of roots with reference to WPM and DKW media. Both half-strength WPM and DKW presented the highest root number values, 6.49 and 5.74, respectively, while the best MS score (5.25) was exhibited in the 1/8 dilution. On the contrary, the lowest values were obtained in 2× MS (2.08), 1/2× MS (2.61), and 2× WPM (2.81) (Figure 6).
No differences were observed in shoot length between 1/2× MS (0.78 cm) and 1× MS (0.77 cm). These two values were significantly different from those of the other treatments that also involved the same nutrient medium (Figure 7). The same pattern was noticed concerning WPM, where no statistical differences were observed between 1× WPM (0.63 cm) and 1/2× WPM (0.62 cm). Whether diluting or doubling the DKW, the mean shoot length per explant, which was close to 0.57 cm, was not actually affected. In general, when those media were diluted, the mean length of roots was increased. This was obvious for MS and WPM media but did not apply to DKW. The best performance in root length was achieved in 1/8× WPM (1.55 cm) and in 1/4× MS (1.52 cm) followed by the 1/4× WPM (1.42). Regarding the DKW medium, the maximum mean root length (1.27 cm) was attained in full strength (Figure 7).
3.2. Genetic Population Analysis
All SSR primers generated amplicons in all three populations. The number of alleles and their range for each locus and C. creticus population are presented in Table 3.

Table 3.
Characteristics of PCR amplicons for studied STR loci and plant material of C. creticus populations.
In total, 28 alleles were identified across all five STR loci for the 36 C. creticus L. samples derived from the three populations of Attica. The average allele per locus was 5.60 ± 1.23 (mean ± standard error). The mean of different alleles per locus (Na) was estimated at 5.60 ± 1.50, while the effective alleles (Ne) were estimated at 4.72 ± 1.14 (Table 4) respectively. The mean observed (Ho) and expected (He) heterozygosity were evaluated at 0.52 ± 0.08 and 0.72 ± 0.07, respectively. The inbreeding coefficient (fixation index F) and Shannon’s information index (I) were calculated at 0.30 ± 0.06 and 1.48 ± 0.28, respectively. The percentage of polymorphic loci (PPL) in all populations was estimated at 100% (Table 4). The FST indices between the pairs of populations, i.e., Mt. Pateras and Mt. Parnitha, Mt. Pateras and Mt. Pendeli and Mt. Parnitha and Mt. Pendeli were 0.009, 0.004 and 0.007, respectively. The overall mean FST value was estimated at 0.009 ± 0.002. Nei genetic distances (Nei D) for the same pairs of populations were calculated at 0.046, 0.021 and 0.040, respectively. The genetic identities (Nei I) were evaluated at 0.955, 0.979 and 0.961, respectively (Table 5). The minimum polymorphism information content (PIC) values were observed in two loci, i.e., cislau14-1 (0.374) and cislau11-1 (0.535) (Table 6). The most informative marker was cislau1-1 (PIC = 0.853) followed by cislau12-1 (PIC = 0.845) (Table 6).

Table 4.
Genetic informative parameters of C. creticus populations.

Table 5.
Pairwise population matrices of genetic distance, genetic identity, and inbreeding coefficient of C. creticus populations.

Table 6.
Characteristics of studied STR locus of C. creticus L. populations.
4. Discussion
4.1. In Vitro Culture
Successful shoot organogenesis was observed across all types of nutrient media. All cultures exhibited satisfactory shooting percentages, and the newly formed shoots exhibited significant elongation. Our most favorable results in terms of growth were obtained using the DKW medium, which has an intermediate nitrogen concentration compared to the other two media. In contrast to MS, WPM also demonstrated superior results, as it was the medium with the lowest nitrogen availability. The success of in vitro blastogenesis relies on the composition and concentration of basal salts, growth regulators, and organic components [36]. Specifically, the nitrogen content in the nutrient medium appears to affect the formation of shoots in explants [37].
Maximum growth percentage was achieved in full- or half-strength WPM and DKW. The same results were those of Hatzilazarou et al. [38] in Nerium oleander L. They reported higher shoot elongation on WPM or MS, regardless of their strengths, and stressed that the cultures on DKW or B5 nutrient media produced shorter shoots. Conversely, Rezali et al. [39] reported that an increase in MS strength resulted in a decrease in shoot height concerning Typhonium flagelliforme.
The shoot percentage was higher on MS, but there was no significant difference in the other two media. Our results were contrary to the findings of Bell et al. [40], where DKW presented superiority to WPM and MS for shoot proliferation and shoot number per explant of pear cultivars. The dilution of MS has a significant negative effect on shoot formation but does not impact the mean shoot length. In line with the findings of this study were those of Hatzilazarou et al. [38] in Nerium oleander L., where higher shoot formation was achieved on full-strength WPM or MS media, with no statistically significant differences between them, as opposed to DKW or B5. The shoot number obtained in full-strength medium was higher compared to the diluted media as reported by Wan Nurul Hidayah et al. [41] in Pogostemon cablin (patchouli). They reported that the number of shoots obtained in full-strength medium was greater compared to the diluted media. In addition, varieties of Cannabis sativa L. displayed better response in the full-strength MS compared to the 1/2× MS medium [42]. Kumar et al. [43] also reported similar findings in Litchi chinensis. Full-strength MS exhibited higher shoot regeneration rates in Harpagophytum procumbens with no significant differences observed when half-strength MS medium was used [44].
The results of this study are consistent with the previously mentioned findings, especially concerning goji berry (Lycium barbarum L.). The highest shoot multiplication was recorded on MS and DKW media compared to WPM [45]. Similar results were found by Parris et al. [46] in a Magnolia cultivar. The DKW medium exhibited the highest shoot multiplication rate in Betula pendula L [47]. In contrast to our results, Fadel et al. [48], in Mentha spicata L., observed the maximum number of shoots on half-strength MS medium. Tetsumura et al. [49] in Vaccinium corymbosum and V. virgatum observed that a reduction in the strength of MS medium resulted in the increase in in vitro shoot formation. In similar experiments, Villamor [50] in Zingiber officinale, Rezali et al. [39] in Typhonium flagelliforme as well as Taheri et al. [51] in Ziziphora persica observed a decrease in both shoot number and length with the reduction in MS strength. Grigoriadou et al. [52] reported conflicting results regarding the dilution of nutrient media for two pear cultivars. Moreover, Bertsouklis et al. [53] reported that using DKW increased Juniperus phoenicea L. (Phoenicean juniper) shoot formation, which were findings that differed from ours. The type of nutrient medium had a significant impact on the average number of shoots formed in Juniperus oxycedrus L. [54,55]. Jain et al. [44] found that MS medium produced more shoots compared to WPM, which exhibited a significantly lower number of shoots in Harpagophytum procumbens cultures.
Our results, regarding shoot length, were similar to those of Hatzilazarou et al. [38] in Nerium oleander L. The maximum shoot length was achieved on full-strength WPM or MS, showing a significant difference compared to DKW or B5 media. Similar findings were those of Sokolov et al. [56], where MS was found to be superior to DKW in terms of shoot length in Magnolia sp. However, DKW performed better in shoot number. In contrast, Halstead et al. [57] reported a better shoot height performance in DKW compared to MS. DKW was proved to be the best medium for shoot length in a Magnolia cultivar [46] and in Juglans regia L. [58]. DKW was also identified as the most effective medium for shoot length in Brazilian ginseng (Pfaffia glomerata (Spreng.) Pedersen) [59]. The results of Villamor [50] indicated that MS dilution decreased shoot length in Zingiber officinale Rosc., too. According to Jain et al. [44], reducing the salt concentration in the media resulted in a decrease in shoot elongation capacity. On half- and quarter-strength WPM, shoot elongation was significantly reduced compared to the MS medium in Harpagophytum procumbens. Conversely, Fadel et al. [48] observed the maximum shoot length on half-strength MS medium in Mentha spicata L.. Our results did not coincide with those of Loureiro et al. [60], who observed that Juniperus phoenicea L. explants growing in DKW exhibited significantly better results compared to those growing in WPM or MS. Several studies in Juniperus species [61,62,63,64,65,66,67] have showed that optimal results in blastogenesis were achieved in media with a lower nitrogen content, i.e., diluted media. However, Bertsouklis et al. [53] encountered challenges using the MS medium with low nitrogen content in an in vitro propagation of Juniperus phoenicea.
Nodal explants exhibited satisfactory rooting in all types and strengths of nutrient media. All cultures demonstrated a satisfactory rooting percentage with notable root elongation. The optimal rooting percentages and the highest root number per explant were observed in half- and full-strength DKW and WPM. High rooting percentage was also observed in quarter-strength MS. Diluting WPM and MS to 1/8 and 1/4 resulted in achieving the maximum root length. Our findings aligned with those of Halstead et al. [57] who reported the best root percentage in full-strength DKW compared to MS. Many researchers have also documented the advantageous impact of reducing the medium strength on root initiation [68,69]. The strength dilution of MS basal medium increased root induction in Rosa spp. [68], Cannabis sativa L. [42,65], Typhonium flagelliforme (G. Lodd.) Blume [39], Camellia sinensis L. [70] Mentha spicata L. [48], Zingiber officinale Roscoe [50] and Syzygium alternifolium (Wight) Walp. [71]. Reducing the strength of MS medium by half resulted in an increase in the rooting of Mentha arvensis L. explants, too [72]. Tetsumura et al. also observed that lowering the strength of the MS medium resulted in the increase in in vitro root formation in Vaccinium corymbosum and V. virgatum. Decreasing the strength of the medium was advantageous for the in vitro root induction of Cynara scolymus L. [73,74,75]. Moreover, Li and Eaton [76], in grapevine, reported that rooting in half-strength MS salts was superior compared to full strength. The beneficial effect of reducing the concentration of the MS basal medium on in vitro rooting ability has also been demonstrated in trees such as Quercus sobur L. [77] and Wrightia tomentosa Roem. & Schult. [78].
In the in vitro culture of Magnolia cultivar, WPM outperformed DKW and MS in terms of root number [46]. Villamor [50] in Zingiber officinale reported that the root number was significantly increased in half-strength MS basal medium, which are results that align to ours. Patel and Shah [69] mentioned that the root number and root length of Stevia rebaudiana explants were influenced by the strength of the MS medium, although these results were not statistically significant, with the best results achieved in quarter-strength MS medium.
DKW proved to be the most successful medium for Brazilian ginseng (Pfaffia glomerata (Spreng.) Pedersen) in terms of root length [59]. Controversial results were reported by Fadel et al. [48], in Mentha spicata L., where the highest root length was observed on full-strength MS, while no roots were induced on 1/4 MS. In Prunus sp., MS failed to induce roots, too [79]. Rezali et al. [39] recorded an increment in the number of roots in Typhonium flagelliforme by decreasing the MS strength. Bidarigh and Azarpour [80] also reported that the highest root length and root number in tea explants (Camellia sinensis L.) were obtained by reducing the medium strength. Reducing the strength of the MS medium by half resulted in enhanced rooting characteristics of Mentha spicata L. [48] and Mentha arvensis L. [72]. The root number in Zingiber officinale Roscoe [50] was increased with the dilution of the MS basal medium. The highest rooting percentage, root number and root length in Syzygium alternifolium were obtained in quarter-strength and half-strength MS medium [71].
The variations in the effects of medium strength are likely associated with the specific components of the culture medium [48] and may vary among origins, depending on the type and physiological condition of the explants [38]. Even minor alterations in the concentration of trace elements can dramatically affect in vitro plant organogenesis [48]. In the in vitro culture of Populus alba L., an increase in zinc concentration in the medium resulted in a significant reduction in the number and length of induced roots [81]. In addition, the maximum in vitro growth of the epiphyte bromeliads Vriesea friburguensis Mez, V. hieroglyphica E. Morren and V. unilateralis Mez were obtained on the medium which was full of Ca [82]. The omission of having omitted KNO3 in both full and half-strength MS media was proven to be significantly deleterious to root formation [50], thus having a detrimental effect on the growth of all the genotypes tested according to Jean and Cappadocia [83]. In other cases, the observed deleterious effect could not be solely attributed to potassium deficiency. Instead, this result might be attributed to a high ratio of NO3:NH4 in the media, as observed in Dioscorea opposita Thunb [84]. Bell et al. [40] also highlighted the differential responses of explants in in vitro cultures due to various salt contents.
4.2. Genetic Population Analysis
Allele peaks were detected within the size range reported in the literature [28,29]. The most diverse loci were cislau1-1 followed by cislau12-1, presenting 10 and 8 alleles, respectively, which were findings that were comparable to the results of Astuti et al. [28] and Bertolasi et al. [29]. This specific SSR combination may detect the variation and the structure among different Cistus populations. In general, the system could allow the discrimination among populations, sub-populations and possibly identifying divergent individuals within different geographical regions [28,29].
Our results provided, to a certain extent, allele frequency estimations for the three C. creticus L. populations. However, they did not deliver a substantial genetic population survey due to the limited geographic area included in the analysis. The genetic base was relatively narrow, which can be explained by the fact that only twenty-eight alleles were identified. The genetic diversity, though, between the three origins was very low due to minimal, close to zero, FST values [85,86]. This conclusion is confirmed by both values—the very low value of Nei D (<0.05) and the especially high value of Nei I (>0.95) [87,88,89]—thus suggesting the genetic similarity of the three populations and indicating that they belong to the same population. Furthermore, as the F value was not close to zero, it could be assumed that the plants coming from all three origins are not undergoing random mating [90]. The results of Paolini et al. (REF), who studied the differences between two subspecies of C. creticus using ISSR markers, revealed significant divergence between the two groups alongside low genetic diversity within them. In addition, the results of Astuti et al. [28] suggested low genetic diversity within the population of Cistus laurifolius L. Another study on Cistus ladanifer L. also reported low levels of variability [91]. In contrast, Bertolasi et al. [29] found a good reservoir of genetic variability in a population of Cistus albidus L. using SSR markers.
5. Conclusions
In vitro culture of Cistus creticus L. occurred effortlessly. An absence of complication was observed in both blastogenesis and root induction. The results showed that in addition to the type of nutrient medium and its strength, their combination had a significant effect on in vitro growth, shoot and root formation of rockrose explants in vitro culture. DKW demonstrated the highest values for explant growth and rooting percentage along with a parallel increase in root number. On the other hand, MS medium presented the best results in shooting percentage and mean number and length of shoots per explant, while WPM mainly affected root length. The origin of plant material did not influence any of the studied in vitro culture traits. Correspondingly, specific types of nutrient media combined with certain strengths could be employed at particular stages of in vitro culture, i.e., culture establishment, shoot multiplication or root formation. Moreover, the SSR markers used to assess the genetic relations of the three populations of C. creticus L. plants, which were involved in in vitro culture, indicated that the genetic base was relatively narrow. The very low estimated genetic diversity was attributed to the limited geographic sampling area included in the analysis. Low genetic diversity, along with the high evaluated genetic similarity, suggested that the three origins belonged to the same population.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/horticulturae10010104/s1, Figure S1. The sampling sites of the C. creticus L. populations in Attica (Greece). Figure S2. Effect of the medium on the mean shoot growth percentage (%) (a), on the mean shoot percentage (%) (b) and on the mean root percentage (%) (c) in relation to clone origin of C. creticus L. explants. Figure S3. Effect of the medium on the mean shoot number (a) and mean shoot length (b) in relation to clone origin of C. creticus L. explants. Figure S4. Effect of the medium on the mean root number (a) and mean root length (b) in relation to clone origin of C. creticus L. explants. Table S1: Effect of the medium type on the studied traits of C. creticus L. explants. Means followed by the same letter do not differ statistically at p ≤ 0.05 according to the Duncan test; Table S2: Effect of medium and its strength on the average growth, shoot and root percentage (%) of C. creticus L. explants. (Means followed by the same letter do not differ statistically at p ≤ 0.05 according to the Duncan test.) MS: Murashige and Skoog medium, WPM: wood plant medium, DKW: Driver and Kuniyaki Walnut medium; Table S3: Effect of medium and its strength on the average number of shoots and length per C. creticus L. explants. (Means followed by the same letter do not differ statistically at p ≤ 0.05 according to the Duncan test.) MS: Murashige and Skoog medium, WPM: wood plant medium, DKW: Driver and Kuniyaki Walnut medium; Table S4: Effect of medium and its strength on the average number of roots and length per C. creticus L. > explants. (Means followed by the same letter do not differ statistically at p ≤ 0.05 according to the Duncan test.) MS: Murashige and Skoog medium, WPM: wood plant medium, DKW: Driver and Kuniyaki Walnut medium.
Author Contributions
Conceptualization, K.I.; methodology, K.I. and P.K.; validation, K.I. and P.K.; formal analysis, K.I. and P.K.; investigation, K.I. and P.K.; resources, K.I. and P.K.; data curation, K.I. and P.K.; writing—original draft preparation, K.I.; writing—review and editing, K.I. and P.K.; visualization, K.I.; supervision, K.I.; project administration K.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no funding.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Acknowledgments
The authors want to express their sincere thanks to the librarian of the Forest Research Institute of Athens, D. Panayiotopoulou (MSc Library and Information Science), for information seeking and retrieving processes as well as her additional proofreading services.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zygomala, A.M.; Ioannidis, C.; Koropouli, X. In Vitro Propagation of Cistus creticus L. Acta Hortic. 2003, 616, 391–396. [Google Scholar] [CrossRef]
- Trabaud, L. Fire and Survival Traits of Plants. In The Role of Fire in Ecological Systems; Trabaud, L., Ed.; SPBAcademic Publishing: The Hague, The Netherlands, 1987; pp. 65–89. [Google Scholar]
- Le Houérou, H.N. Fire and Vegetation in the Mediterranean Basin. In Proceedings of the 13th Annual Tall Timbers Fire Ecology Conference, Talahassee, FL, USA, 22–23 March 1973. [Google Scholar]
- Proctor, M. Cistaceae. In Flowering Plants of the World; Heywood, V., Ed.; Oxford University Press: Oxford, UK, 1978; pp. 100–108. [Google Scholar]
- Tutin, T.G.; Heywood, V.H.; Burges, N.A.; Valentine, D.H.; Walters, S.M.; Webb, D.A. Flora Europaea: Cistaceae; Cambridge University Press: Cambridge, UK, 1968; Volume 2. [Google Scholar]
- Meusel, H.-J.; Rauschert, E.; Weinert, S.E. Vergleichende Chorologie Der Zentraleuropäischen Flora. Band II; VEB Gustav Fischer Verlag: Jena, Germany, 1978.
- Dansereau, P.M. Monographie du Genre Cistus L.; Boissiera 4; Conservatoire de Botanique: Nord, France, 1939; pp. 1–90. [Google Scholar]
- Greuter, W.; Burdet, H.; Long, G. Cistaceae. In Med-Checklist; Med-Checklist Trust of OPTIMA; Greuter, W., Burdet, H.M., Long, G., Eds.; Conservatoire et Jardin Botaniques: Geneve, Switzerland, 1984; Volume 1, pp. 315–330. [Google Scholar]
- Margaris, N.S. Structure and Dynamics in a Phryganic (East Mediterranean) Ecosystem. J. Biogeogr. 1976, 3, 249–259. [Google Scholar] [CrossRef]
- Pedanius, D.; Osbaldeston, T.A.; Wood, R.P. De Materia Medica: Being an Herbal with Many Other Medicinal Materials: Written in Greek in the First Century of the Common Era: A New Indexed Version in Modern English; IBIDIS: Johannesburg, South Africa, 2000; ISBN 978-0-620-23435-1. [Google Scholar]
- Hort, F. Theophrastus: De Signis Vol. II in the Enquire into PlantsHeinemann: London, UK; Harvard University Press: Cambridge, UK, 1926. [Google Scholar]
- Rosén, H.B. Herodoti Historiae. Vol. I: Libros I–IV; Springer: Berlin, Germany, 1987; ISBN 3-322-00359-0. [Google Scholar]
- Moujir, L.; Gutiérrez-Navarro, A.M.; San Andrés, L.; Luis, J.G. Structure—Antimicrobial Activity Relationships of Abietane Diterpenes from Salvia Species. Phytochemistry 1993, 34, 1493–1495. [Google Scholar] [CrossRef]
- Demetzos, C.; Dimas, K.; Hatziantoniou, S.; Anastasaki, T.; Angelopoulou, D. Cytotoxic and Anti-Inflammatory Activity of Labdane and Cis-Clerodane Type Diterpenes. Planta Med. 2001, 67, 614–618. [Google Scholar] [CrossRef] [PubMed]
- Chinou, I.; Demetzos, C.; Harvala, C.; Roussakis, C.; Verbist, J. Cytotoxic and Antibacterial Labdane-Type Diterpenes from the Aerial Parts of Cistus incanus subsp. Creticus. Planta Med 1994, 60, 34–36. [Google Scholar] [CrossRef]
- Demetzos, C.; Mitaku, S.; Couladis, M.; Harvala, C.; Kokkinopoulos, D. Natural Metabolites of Ent-13-Epi-Manoyl Oxide and Other Cytotoxic Diterpenes from the Resin “LADANO” of Cistus creticus. Planta Med. 1994, 60, 590–591. [Google Scholar] [CrossRef]
- Dimas, K.; Demetzos, C.; Marsellos, M.; Sotiriadou, R.; Malamas, M.; Kokkinopoulos, D. Cytotoxic Activity of Labdane Type Diterpenes against Human Leukemic Cell Lines in Vitro. Planta Med. 1998, 64, 208–211. [Google Scholar] [CrossRef]
- Iriondo, J.M.; Moreno, C.; Pérez, C. Micropropagation of Six Rockrose (Cistus) Species. HortScience 1995, 30, 1080–1081. [Google Scholar] [CrossRef]
- Polunin, O.; Huxley, A. Flowers of the Mediterranean; Chatto and Windus: London, UK, 1972. [Google Scholar]
- López González, G. La Guía de Incafo de Los Árboles y Arbustos de La Península Ibérica; Guias verdes de INFACO, 4; Infaco: Madrid, Spain, 1982; ISBN 9788485389346. [Google Scholar]
- Brickell, C. Royal Horticultural Society Gardeners’ Encyclopedia of Plants and Flowers; Dorling Kindersley: London, UK, 1989; ISBN 0-86318-386-7. [Google Scholar]
- Heywood, V.H. Flowering Plants of the World; Dorling Kindersley Publishers Ltd.: London, UK, 1993; ISBN 0-7134-7422-X. [Google Scholar]
- Pacioni, G. El Cultivo Moderno y Rentable de La Trufa; De Vecchi: Milan, Italy, 1992; ISBN 84-315-0516-8. [Google Scholar]
- M’Kada, J.; Dorion, N.; Bigot, C. In Vitro Propagation of Cistus × purpureus Lam. Sci. Hortic. 1991, 46, 155–160. [Google Scholar] [CrossRef]
- Pela, Z.; Pentcheva, M.; Gerasopoulos, D.; Maloupa, E. In Vitro Induction of Adventitious Roots and Proliferation of Cistus creticus creticus L. Plants. Acta Hortic. 2000, 317–322. [Google Scholar] [CrossRef]
- Ruta, C.; Morone-Fortunato, I. In Vitro Propagation of Cistus clusii Dunal, an Endangered Plant in Italy. Vitr. Cell. Dev. Biol. Plant 2010, 46, 172–179. [Google Scholar] [CrossRef]
- Airò, M.; Farruggia, G.; Giardina, G.; Giovino, A. Micropropagation protocol of a threatened species: Cistus crispus L. In Proceedings of the Acta Horticulturae; International Society for Horticultural Science (ISHS), Leuven, Belgium, 26 May 2015; pp. 549–552. [Google Scholar]
- Astuti, G.; Roma-Marzio, F.; D’Antraccoli, M.; Bedini, G.; Carta, A.; Sebastiani, F.; Bruschi, P.; Peruzzi, L. Conservation Biology of the Last Italian Population of Cistus laurifolius (Cistaceae): Demographic Structure, Reproductive Success and Population Genetics. Nat. Conserv. 2017, 22, 169–190. [Google Scholar] [CrossRef]
- Bertolasi, B.; Zago, L.; Gui, L.; Sitzia, T.; Vanetti, I.; Binelli, G.; Puppi, G.; Buldrini, F.; Pezzi, G. Phenological and Genetic Characterization of Mediterranean Plants at the Peripheral Range: The Case of Cistus albidus near Lake Garda. Flora 2019, 252, 26–35. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Lloyd, G.; McCown, B. Woody Plant Medium: A Mineral Nutrient Formulation for Microculture of Woody Plant Species. HortScience 1981, 16, 453. [Google Scholar]
- Driver, J.A.; Kuniyuki, A.H. In Vitro Propagation of Paradox Walnut Rootstock. HortScience 1984, 19, 507–509. [Google Scholar] [CrossRef]
- Peakall, R.; Smouse, P.E. GENALEX 6: Genetic Analysis in Excel. Population Genetic Software for Teaching and Research. Mol. Ecol. Notes 2006, 6, 288–295. [Google Scholar] [CrossRef]
- Botstein, D.; White, R.L.; Skolnick, M.; Davis, R.W. Construction of a Genetic Linkage Map in Man Using Restriction Fragment Length Polymorphisms. Am. J. Hum. Genet. 1980, 32, 314. [Google Scholar]
- Kalinowski, S.T.; Taper, M.L.; Marshall, T.C. Revising How the Computer Program Cervus Accommodates Genotyping Error Increases Success in Paternity Assignment. Mol. Ecol. 2007, 16, 1099–1106. [Google Scholar] [CrossRef]
- Ge, X.; Chu, Z.; Lin, Y.; Wang, S. A Tissue Culture System for Different Germplasms of Indica Rice. Plant Cell Rep. 2006, 25, 392–402. [Google Scholar] [CrossRef]
- Momeni, M.; Ganji-Moghadam, E.; Kazemzadeh-Beneh, H.; Asgharzadeh, A. Direct Organogenesis from Shoot Tip Explants of Juniperus polycarpos L.: Optimizing Basal Media and Plant Growth Regulators on Proliferation and Root Formation. Plant Cell Biotechnol. Mol. Biol. 2018, 19, 40–50. [Google Scholar]
- Hatzilazarou, S.; Kostas, S.; Economou, A.; Scaltsoyiannes, A. Efficient propagation of Nerium oleander L. through tissue culture. Propag. Ornam. Plants 2017, 17, 12. [Google Scholar]
- Rezali, N.I.; Jaafar Sidik, N.; Saleh, A.; Osman, N.I.; Mohd Adam, N.A. The Effects of Different Strength of MS Media in Solid and Liquid Media on in Vitro Growth of Typhonium flagelliforme. Asian Pac. J. Trop. Biomed. 2017, 7, 151–156. [Google Scholar] [CrossRef]
- Bell, R.L.; Srinivasan, C.; Lomberk, D. Effect of Nutrient Media on Axillary Shoot Proliferation and Preconditioning for Adventitious Shoot Regeneration of Pears. Vitr. Cell. Dev. Biol. Plant 2009, 45, 708–714. [Google Scholar] [CrossRef]
- Wan Nurul Hidayah, W.A.; Norrizah, J.S.; Sharifah Aminah, S.M.; Sharipah Ruzaina, S.A.; Faezah, P. Effect of Medium Strength and Hormones Concentration on Regeneration of Pogostemon Cablin Using Nodes Explant. Asian J. Biotechnol. 2012, 4, 46–52. [Google Scholar] [CrossRef][Green Version]
- Ioannidis, K.; Dadiotis, E.; Mitsis, V.; Melliou, E.; Magiatis, P. Biotechnological Approaches on Two High CBD and CBG Cannabis sativa L. (Cannabaceae) Varieties: In Vitro Regeneration and Phytochemical Consistency Evaluation of Micropropagated Plants Using Quantitative 1H-NMR. Molecules 2020, 25, 5928. [Google Scholar] [CrossRef]
- Kumar, M.; Shiva Prakash, N.; Prasad, U.S.; Bhalla-Sarin, N. A Novel Approach of Regeneration from Nodal Explants of Field-Grown Litchi (Litchi chinensis Sonn.) Fruit Trees. J. Plant Sci. 2006, 1, 240–246. [Google Scholar] [CrossRef]
- Jain, N.; Bairu, M.W.; Stirk, W.A.; Van Staden, J. The Effect of Medium, Carbon Source and Explant on Regeneration and Control of Shoot-Tip Necrosis in Harpagophytum procumbens. S. Afr. J. Bot. 2009, 75, 117–121. [Google Scholar] [CrossRef][Green Version]
- Silvestri, C.; Sabbatini, G.; Marangelli, F.; Rugini, E.; Cristofori, V. Micropropagation and Ex Vitro Rooting of Wolfberry. HortScience 2018, 53, 1494–1499. [Google Scholar] [CrossRef]
- Parris, J.K.; Touchell, D.H.; Ranney, T.G.; Adelberg, J. Basal Salt Composition, Cytokinins, and Phenolic Binding Agents Influence In Vitro Growth and Ex Vitro Establishment of Magnolia ‘Ann’. HortScience 2012, 47, 1625–1629. [Google Scholar] [CrossRef]
- Rathwell, R.; Shukla, M.R.; Jones, A.M.P.; Saxena, P.K. In Vitro Propagation of Cherry Birch (Betula lenta L.). Can. J. Plant Sci. 2016, 96, 571–578. [Google Scholar] [CrossRef]
- Fadel, D.; Kintzios, S.; Economou, A.S.; Moschopoulou, G.; Constantinidou, H.-I.A. Effect of Different Strength of Medium on Organogenesis, Phenolic Accumulation and Antioxidant Activity of Spearmint (Mentha spicata L.). Open Hortic. J. 2010, 3, 31–35. [Google Scholar] [CrossRef]
- Tetsumura, T.; Matsumoto, Y.; Sato, M.; Honsho, C.; Yamashita, K.; Komatsu, H.; Sugimoto, Y.; Kunitake, H. Evaluation of Basal Media for Micropropagation of Four Highbush Blueberry Cultivars. Sci. Hortic. 2008, 119, 72–74. [Google Scholar] [CrossRef]
- Villamor, C.C. Influence of Media Strength and Sources of Nitrogen on Micropropagation of Ginger, Zingiber officinale Rosc. Int. Sci. Res. J. 2010, 2, 6. [Google Scholar]
- Taheri, A.; Kosari-Nasab, M.; Movafeghi, A. Effects of Different Strengths of Medium on Production of Phenolic and Flavonoid Compounds in Regenerated Shoots of Ziziphora persica. Russ. Agric. Sci. 2015, 41, 225–229. [Google Scholar] [CrossRef]
- Grigoriadou, K.; Leventakis, N.; Vasilakakis, M. Effect of Various Culture Conditions on Proliferation and Shoot Tip Necrosis in the Pear Cultivars ‘William’s’ and ‘Highland’ Grown In Vitro. Acta Hortic. 2000, 520, 103–108. [Google Scholar] [CrossRef]
- Bertsouklis, K.; Paraskevopoulou, A.; Zarkadoula, N. In Vitro Propagation of Juniperus phoenicea L. In Proceedings of the International Symposium on Botanical Gardens and Landscapes, Bangkok, Thailand, 2–4 December 2019; pp. 331–334. [Google Scholar]
- Gómez, M.P.; Segura, J. Axillary Shoot Proliferation in Cultures of Explants from Mature Juniperus oxycedrus Trees. Tree Physiol. 1995, 15, 625–628. [Google Scholar] [CrossRef]
- Gómez, M.P.; Segura, J. Factors Controlling Adventitious Bud Induction and Plant Regeneration in mature Juniperus oxycedrus Leaves Cultured in Vitro. Vitr. Plant 1994, 30, 210–218. [Google Scholar] [CrossRef]
- Sokolov, R.S.; Atanassova, B.Y.; Iakimova, E.T. Physiological Response of Cultured. to Nutrient Medium Composition. J. Hortic. Res. 2014, 22, 49–61. [Google Scholar] [CrossRef][Green Version]
- Halstead, M.A.; Garfinkel, A.R.; Marcus, T.C.; Hayes, P.M.; Carrijo, D.R. Hemp Growth in Vitro and in Vivo: A Comparison of Growing Media and Growing Environments across 10 Accessions. HortScience 2022, 57, 1041–1047. [Google Scholar] [CrossRef]
- Saadat, Y.A.; Hennerty, M.J. Factors Affecting the Shoot Multiplication of Persian Walnut (Juglans regia L.). Sci. Hortic. 2002, 95, 251–260. [Google Scholar] [CrossRef]
- Silva, T.D.; Chagas, K.; Batista, D.S.; Felipe, S.H.S.; Louback, E.; Machado, L.T.; Fernandes, A.M.; Buttrós, V.H.T.; Koehler, A.D.; Farias, L.M.; et al. Morphophysiological in Vitro Performance of Brazilian Ginseng (Pfaffia Glomerata (Spreng.) Pedersen) Based on Culture Medium Formulations. Vitr. Cell. Dev. Biol. Plant 2019, 55, 454–467. [Google Scholar] [CrossRef]
- Loureiro, J.; Capelo, A.; Brito, G.; Rodriguez, E.; Silva, S.; Pinto, G.; Santos, C. Micropropagation of Juniperus phoenicea from Adult Plant Explants and Analysis of Ploidy Stability Using Flow Cytometry. Biol. Plant. 2007, 51, 7–14. [Google Scholar] [CrossRef]
- Khater, N.; Benbouza, H. Preservation of Juniperus thurifera L.: A Rare Endangered Species in Algeria through In Vitro Regeneration. J. For. Res. 2019, 30, 77–86. [Google Scholar] [CrossRef]
- Salih, A.M.; Al-Qurainy, F.; Khan, S.; Tarroum, M.; Nadeem, M.; Shaikhaldein, H.O.; Alabdallah, N.M.; Alansi, S.; Alshameri, A. Mass Propagation of Juniperus procera Hoechst. Ex Endl. From Seedling and Screening of Bioactive Compounds in Shoot and Callus Extract. BMC Plant Biol. 2021, 21, 192. [Google Scholar] [CrossRef]
- Thorpe, T.A.; Harry, I.S.; Kumar, P.P. Application of Micropropagation to Forestry. In Micropropagation: Technology and Application; Debergh, P.C., Zimmerman, R.H., Eds.; Springer: Dordrecht, The Netherlands, 1991; pp. 311–336. ISBN 978-94-009-2075-0. [Google Scholar]
- Al-Ramamneh, E.A.; Dura, S.; Daradkeh, N. Propagation Physiology of Juniperus phoenicea L. from Jordan Using Seeds and in Vitro Culture Techniques: Baseline Information for a Conservation Perspective. Afr. J. Biotechnol. 2012, 11, 7684–7692. [Google Scholar] [CrossRef]
- Ślusarkiewicz-Jarzina, A.S.; Ponitka, A.; Kaczmarek, Y. Influence of Cultivar, Explant Source and Plant Growth Regulator on Callus Induction and Plant Regeneration of Cannabis sativa L. Acta Biol. Crac. Ser. Bot 2005, 47, 145–151. [Google Scholar]
- Ioannidis, K.; Tomprou, I.; Panayiotopoulou, D.; Boutsios, S.; Daskalakou, E.N. Potential and Constraints on In Vitro Micropropagation of Juniperus drupacea Labill. Forests 2023, 14, 142. [Google Scholar] [CrossRef]
- Al-Ramamneh, E.A.-D.; Daradkeh, N.; Rababah, T.; Pacurar, D.; Al-Qudah, M. Effects of Explant, Media and Growth Regulators on in Vitro regeneration and Antioxidant Activity of Juniperus phoenicea. Aust. J. Crop Sci. 2017, 11, 828–837. [Google Scholar] [CrossRef]
- Sauer, A.; Walther, F.; Preil, W. Different Suitability for in Vitro Propagation of Rose Cultivars. Gartenbauwissenschaft 1985, 3, 133–138. [Google Scholar]
- Patel, R.M.; Shah, R.R. Regeneration of Stevia Plant through Callus Culture. Indian J. Pharm. Sci. 2009, 71, 46–50. [Google Scholar] [CrossRef]
- Bidarigh, S.; Hatamzadeh, A.; Azarpour, E. The Study Effect of IBA Hormone Levels on Rooting in Micro Cuttings of Tea (Camellia sinensis L.). World Appl. Sci. J. 2012, 20, 1051–1054. [Google Scholar]
- Sha Valli Khan, P.S.; Hausman, J.F.; Rao, K.R. Effect of Agar, MS Medium Strength, Sucrose and Polyamines on in Vitro Rooting of Syzygium alternifolium. Biol. Plant. 1999, 42, 333–340. [Google Scholar] [CrossRef]
- Phatak, S.V.; Heble, M.R. Organogenesis and Terpenoid Synthesis in Mentha arvensis. Fitoterapia 2002, 73, 32–39. [Google Scholar] [CrossRef]
- Ancora, G. Globe Artichoke (Cynara scolymus L.). In Crops I; Bajaj, Y.P.S., Ed.; Springer: Berlin/Heidelberg, Germany, 1986; pp. 471–519. ISBN 978-3-642-61625-9. [Google Scholar]
- Iapichino, G. Micropropagation of Globe Artichoke (Cynara scolymus L.) from Underground Dormant Buds (“Ovoli”). Vitr. Cell. Dev. Biol. Plant 1996, 32, 249–252. [Google Scholar] [CrossRef]
- Lauzer, D.; Vieth, J. Micropropagation of Seed-Derived Plants of Cynara scolymus L., Cv. ‘Green Globe’. Plant Cell Tissue Organ Cult. 1990, 21, 237–244. [Google Scholar] [CrossRef]
- Li, J.-R.; Eaton, G. Growth and Rooting of Grape Shoot Apices In Vitro. HortScience 1984, 19, 64–66. [Google Scholar] [CrossRef]
- Manzanera, J.A.; Pardos, J.A. Micropropagation of Juvenile and Adult Quercus suber L. Plant Cell Tissue Organ Cult. 1990, 21, 1–8. [Google Scholar] [CrossRef]
- Purohit, S.D.; Kukda, G.; Sharma, P.; Tak, K. In Vitro Propagation of an Adult Tree Wrightia Tomentosa through Enhanced Axillary Branching. Plant Sci. 1994, 103, 67–72. [Google Scholar] [CrossRef]
- Fallahpour, M.; Miri, S.M.; Bouzari, N. Effects of Media Cultures and Plant Growth Regulators on Micropropagation of CAB-6P Cherry Semi-Dwarf Rootstock. Iran. J. Hortic. Sci. 2019, 50, 187–196. [Google Scholar]
- Bidarigh, S.; Azarpour, E. Evaluation of the Effect of MS Medium Levels on Rooting in Micro Cuttings of Tea (Camellia sinensis L.) under In-Vitro Culture Condition. ARPN J. Agric. Biol. Sci. 2013, 8, 5. [Google Scholar]
- Castiglione, S.; Franchin, C.; Fossati, T.; Lingua, G.; Torrigiani, P.; Biondi, S. High Zinc Concentrations Reduce Rooting Capacity and Alter Metallothionein Gene Expression in White Poplar (Populus alba L. Cv. Villafranca). Chemosphere 2007, 67, 1117–1126. [Google Scholar] [CrossRef]
- Aranda-Peres, A.N.; Peres, L.E.P.; Higashi, E.N.; Martinelli, A.P. Adjustment of Mineral Elements in the Culture Medium for the Micropropagation of Three Vriesea Bromeliads from the Brazilian Atlantic Forest: The Importance of Calcium. HortScience 2009, 44, 106–112. [Google Scholar] [CrossRef]
- Jean, M.; Cappadocia, M. In Vitro Tuberization in Dioscorea alata L. ‘Brazo Fuerte’ and ‘Florido’ and D. abyssinica Hoch. Plant Cell Tissue Organ Cult. 1991, 26, 147–152. [Google Scholar] [CrossRef]
- Asahira, T.; Yazawa, S. Bulbil Formation of Dioscorea opposita Cultured In Vitro. Mem. Coll. Agric. Kyoto Univ. 1979, 113, 39–51. [Google Scholar]
- Nei, M. Genetic Distance between Populations. Am. Nat. 1972, 106, 283–292. [Google Scholar] [CrossRef]
- Nei, M. Estimation of Average Heterozygosity and Genetic Distance from a Small Number of Individuals. Genetics 1978, 89, 583–590. [Google Scholar] [CrossRef]
- Wright, S. The Interpretation of Population Structure by F-Statistics with Special Regard to Systems of Mating. Evolution 1965, 19, 395–420. [Google Scholar] [CrossRef]
- Wright, S. Evolution and the Genetics of Populations. Volume 4. Variability within and among Natural Populations; University of Chicago Press: Chicago, IL, USA, 1978. [Google Scholar]
- Bairu, M.W.; Aremu, A.O.; Van Staden, J. Somaclonal Variation in Plants: Causes and Detection Methods. Plant Growth Regul. 2011, 63, 147–173. [Google Scholar] [CrossRef]
- Larkin, P.J.; Scowcroft, W.R. Somaclonal Variation—A Novel Source of Variability from Cell Cultures for Plant Improvement. Theor. Appl. Genet. 1981, 60, 197–214. [Google Scholar] [CrossRef] [PubMed]
- Quintela-Sabarís, C.; Ribeiro, M.M.; Poncet, B.; Costa, R.; Castro-Fernández, D.; Fraga, M.I. AFLP analysis of the pseudometallophyte Cistus ladanifer: Comparison with cpSSRs and exploratory genome scan to investigate loci associated to soil variables. Plant Soil 2012, 359, 397–413. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).