Integrative Transcriptomic and Metabolomic Analyses of the Mechanism of Anthocyanin Accumulation and Fruit Coloring in Three Blueberry Varieties of Different Colors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Identification of Pigments
2.3. Relative Quantification of Anthocyanin by Ultraviolet–Visible (UV/Vis) Spectroscopy
2.4. Anthocyanins Identification by Metabolite Profiling
2.5. Total RNA Extraction and Quality Assessment
2.6. Illumina Transcriptome (RNA-Seq) Library Preparation, Sequencing, and Expression Level Estimation
2.7. Phylogenetic Analysis and Sequence Alignment
2.8. Co-Expression Network Construction
2.9. Quantitative Real-Time PCR Analysis
3. Results
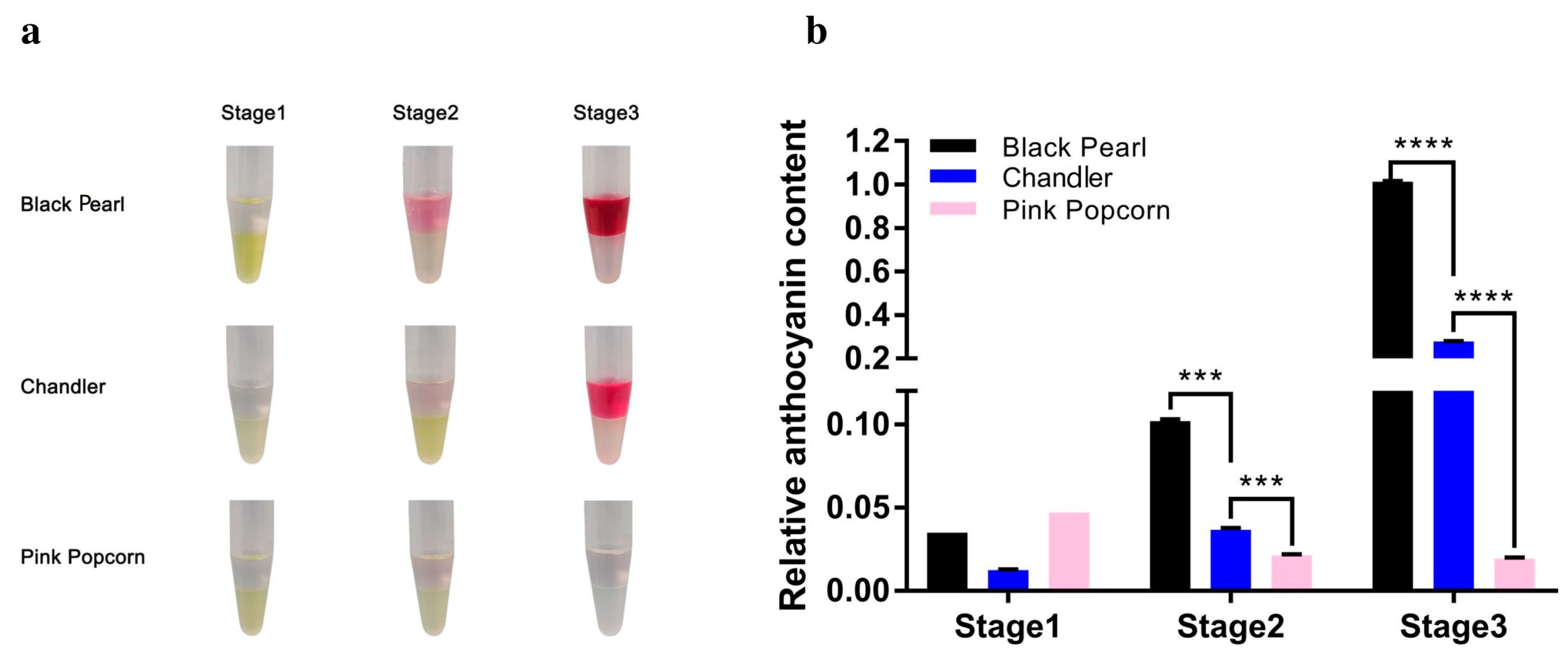
3.1. Color and Pigment Contents Are Significantly Different during Fruit Ripening of the Three Blueberry Varieties
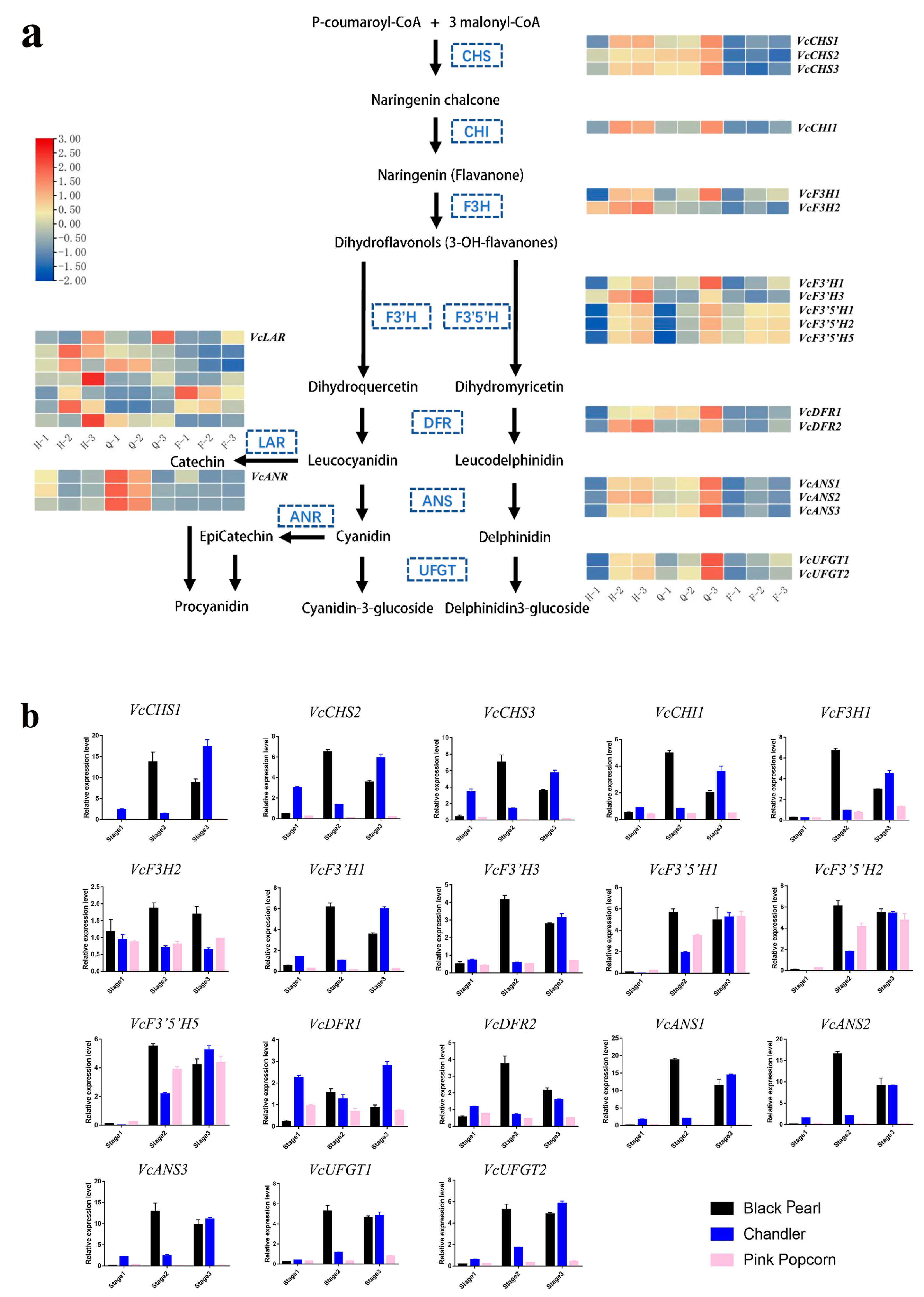
3.2. Key Structural Genes Identified in Anthocyanin Metabolic Pathway through Gene Expression
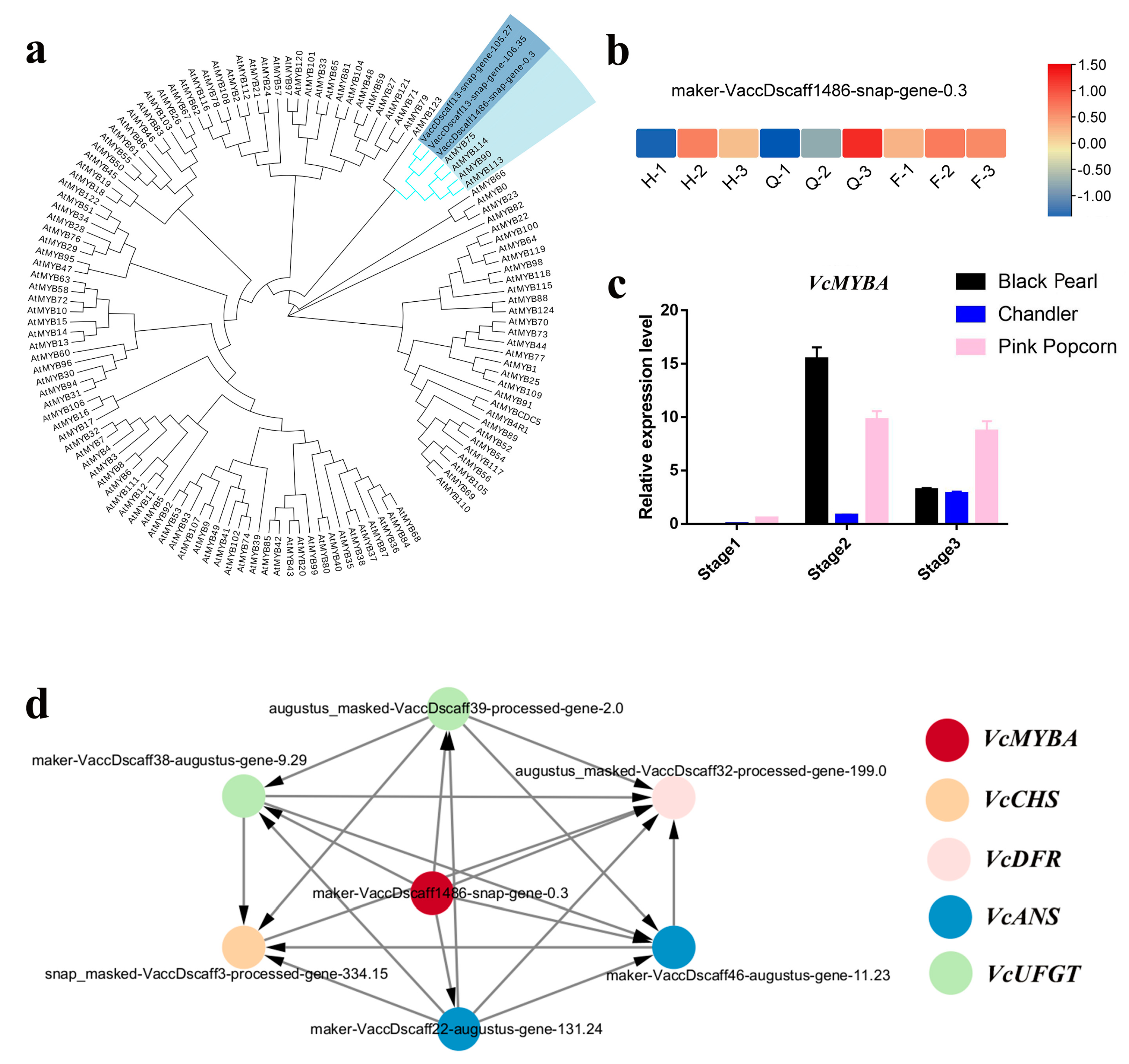
3.3. Anthocyanin Biosynthesis-Related Transcription Factors (TFs) by WGCNA and Correlation Analysis
4. Discussion
4.1. Delphinidin Is the Main Anthocyanin Component for Mature Blueberry Fruit Color Difference between Black, Blue, and Pink
4.2. VcANS Is the Key Candidate Genes Responsible for Mature Blueberry Fruit Color
4.3. VcMYBA Is a Key Candidate TFs Involved in Anthocyanin Synthesis in Vaccinium spp.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Eck, P. Blueberry Science; Rutgers University Press: New Brunswick, NJ, USA, 1988. [Google Scholar]
- Kim, B.; Lee, S.G.; Park, Y.K.; Ku, C.S.; Pham, T.X.; Wegner, C.J.; Lee, J.Y. Blueberry, blackberry, and blackcurrant differentially affect plasma lipids and pro-inflammatory markers in diet-induced obesity mice. Nutr. Res. Pract. 2016, 10, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Routray, W.; Orsat, V. Blueberries and their anthocyanins: Factors affecting biosynthesis and properties. Compr. Rev. Food Sci. Food Saf. 2011, 10, 303–320. [Google Scholar] [CrossRef]
- Duan, Y.; Tarafdar, A.; Chaurasia, D.; Singh, A.; Bhargava, P.C.; Yang, J.; Awasthi, M.K. Blueberry fruit valorization and valuable constituents: A review. Int. J. Food Microbiol. 2022, 381, 109890. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Liu, G.; Yan, D.; Zhang, M.; Cui, Q.; Zhao, Q.; Xu, G. Manipulation of ploidy for blueberry breeding: In vitro chromosome doubling of diploid Vaccinium duclouxii (Lévl.) Hand-Mazz by trifluralin. Sci. Hortic. 2023, 317, 112056. [Google Scholar] [CrossRef]
- Wu, J.H.; Miller, S.A.; Hall, H.K.; Mooney, P.A. Factors affecting the efficiency of micropropagation from lateral buds and shoot tips of Rubus. Plant Cell Tissue Organ Cult. 2009, 99, 17–25. [Google Scholar] [CrossRef]
- Kalt, W.; Cassidy, A.; Howard, L.R.; Krikorian, R.; Stull, A.J.; Tremblay, F.; Zamora-Ros, R. Recent research on the health benefits of blueberries and their anthocyanins. Adv. Nutr. 2009, 11, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Beecher, G.R.; Holden, J.M.; Haytowitz, D.B.; Gebhardt, S.E.; Prior, R.L. Concentrations of anthocyanins in common foods in the United States and estimation of normal consumption. J. Agric. Food Chem. 2006, 54, 4069–4075. [Google Scholar] [CrossRef]
- Ma, L.; Sun, Z.; Zeng, Y.; Luo, M.; Yang, J. Molecular mechanism and health role of functional ingredients in blueberry for chronic disease in human beings. Int. J. Mol. Sci. 2018, 19, 2785. [Google Scholar] [CrossRef]
- Yamuangmorn, S.; Prom-u-Thai, C. The potential of high-anthocyanin purple rice as a functional ingredient in human health. Antioxidants 2021, 10, 833. [Google Scholar] [CrossRef]
- Suresh, S.; Begum, R.F.; Singh, A.; Chitra, V. Anthocyanin as a therapeutic in Alzheimer’s disease: A systematic review of preclinical evidences. Ageing Res. Rev. 2022, 76, 101595. [Google Scholar] [CrossRef]
- Wood, E.; Hein, S.; Heiss, C.; Williams, C.; Rodriguez-Mateos, A. Blueberries and cardiovascular disease prevention. Food Funct. 2019, 10, 7621–7633. [Google Scholar] [CrossRef] [PubMed]
- Felgus-Lavefve, L.; Howard, L.; Adams, S.H.; Baum, J.I. The effects of blueberry phytochemicals on cell models of inflammation and oxidative stress. Adv. Nutr. 2022, 13, 1279–1309. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Li, X.; Pan, Z.; Zhu, Y.; Tuo, J.; Meng, Q.; Pan, Y. Anthocyanin ameliorates hypoxia and ischemia induced inflammation and apoptosis by increasing autophagic flux in SH-SY5Y cells. Eur. J. Pharmacol. 2020, 883, 173360. [Google Scholar] [CrossRef]
- Karlova, R.; Chapman, N.; David, K.; Angenent, G.C.; Seymour, G.B.; De Maagd, R.A. Transcriptional control of fleshy fruit development and ripening. J. Exp. Bot. 2014, 65, 4527–4541. [Google Scholar] [CrossRef] [PubMed]
- Saigo, T.; Wang, T.; Watanabe, M.; Tohge, T. Diversity of anthocyanin and proanthocyanin biosynthesis in land plants. Curr. Opin. Plant Biol. 2020, 55, 93–99. [Google Scholar] [CrossRef]
- Günther, C.S.; Dare, A.P.; McGhie, T.K.; Deng, C.; Lafferty, D.J.; Plunkett, B.J.; Espley, R.V. Spatiotemporal modulation of flavonoid metabolism in blueberries. Front. Plant Sci. 2020, 11, 545. [Google Scholar] [CrossRef]
- Lepiniec, L.; Debeaujon, I.; Routaboul, J.M.; Baudry, A.; Pourcel, L.; Nesi, N.; Caboche, M. Genetics and biochemistry of seed flavonoids. Annu. Rev. Plant Biol. 2006, 57, 405–430. [Google Scholar] [CrossRef]
- Cassidy, A.; Mukamal, K.J.; Liu, L.; Franz, M.; Eliassen, A.H.; Rimm, E.B. High anthocyanin intake is associated with a reduced risk of myocardial infarction in young and middle-aged women. Circulation 2013, 127, 188–196. [Google Scholar] [CrossRef]
- Liu, H.; Liu, Z.; Wu, Y.; Zheng, L.; Zhang, G. Regulatory mechanisms of anthocyanin biosynthesis in apple and pear. Int. J. Mol. Sci. 2021, 22, 8441. [Google Scholar] [CrossRef]
- Zhang, A.; Yang, H.; Ji, S.; Tian, C.; Chen, N.; Gong, H.; Li, J. Metabolome and transcriptome analyses of anthocyanin accumulation mechanisms reveal metabolite variations and key candidate genes involved in the pigmentation of Prunus tomentosa Thunb. cherry fruit. Front. Plant Sci. 2022, 13, 938908. [Google Scholar] [CrossRef]
- Zhao, J.; Huhman, D.; Shadle, G.; He, X.Z.; Sumner, L.W.; Tang, Y.; Dixon, R.A. MATE2 mediates vacuolar sequestration of flavonoid glycosides and glycoside malonates in Medicago truncatula. Plant Cell 2011, 23, 1536–1555. [Google Scholar] [CrossRef] [PubMed]
- Goodman, C.D.; Casati, P.; Walbot, V. A multidrug resistance–associated protein involved in anthocyanin transport in Zea mays. Plant Cell 2004, 16, 1812–1826. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zhang, X.; Zhang, Y.; Lin, X.; Li, B.; Chen, Z. Integrated metabolomic and transcriptomic strategies to understand the effects of dark stress on tea callus flavonoid biosynthesis. Plant Physiol. Biochem. 2020, 155, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Jaakola, L. New insights into the regulation of anthocyanin biosynthesis in fruits. Trends Plant Sci. 2013, 18, 477–483. [Google Scholar] [CrossRef]
- Xu, W.; Dubos, C.; Lepiniec, L. Transcriptional control of flavonoid biosynthesis by MYB–bHLH–WDR complexes. Trends in Plant Science 2015, 20, 176–185. [Google Scholar] [CrossRef]
- Zhang, B.; Chopra, D.; Schrader, A.; Hülskamp, M. Evolutionary comparison of competitive protein-complex formation of MYB, bHLH, and WDR proteins in plants. J. Exp. Bot. 2019, 70, 3197–3209. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, F.; Wang, B.; Wu, H.; Wu, J.; Liu, J.; Sun, Y.; Cheng, C.; Qiu, D. Identification, characterization and expression analysis of anthocyanin biosynthesis-related bHLH genes in bluberry (Vaccinium corymbosum L.). Int. J. Mol. Sci. 2021, 22, 13274. [Google Scholar] [CrossRef]
- Cao, Y.; Li, K.; Li, Y.; Zhao, X.; Wang, L. MYB transcription factors as regulators of secondary metabolism in plants. Biology 2020, 9, 61. [Google Scholar] [CrossRef]
- Yan, H.; Pei, X.; Zhang, H.; Li, X.; Zhang, X.; Zhao, M.; Zhao, X. MYB-mediated regulation of anthocyanin biosynthesis. Int. J. Mol. Sci. 2021, 22, 3103. [Google Scholar] [CrossRef]
- Allan, A.C.; Espley, R.V. MYBs drive novel consumer traits in fruits and vegetables. Trends Plant Sci. 2018, 23, 693–705. [Google Scholar] [CrossRef]
- Broun, P. Transcriptional control of flavonoid biosynthesis: A complex network of conserved regulators involved in multiple aspects of differentiation in Arabidopsis. Curr. Opin. Plant Biol. 2005, 8, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Borevitz, J.O.; Xia, Y.; Blount, J.; Dixon, R.A.; Lamb, C. Activation tagging identifies a conserved MYB regulator of phenylpropanoid biosynthesis. Plant Cell 2000, 12, 2383–2393. [Google Scholar] [CrossRef] [PubMed]
- Vanderauwera, S.; Zimmermann, P.; Rombauts, S.; Vandenabeele, S.; Langebartels, C.; Gruissem, W.; Van Breusegem, F. Genome-wide analysis of hydrogen peroxide-regulated gene expression in Arabidopsis reveals a high light-induced transcriptional cluster involved in anthocyanin biosynthesis. Plant Physiol. 2005, 139, 806–821. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Goto-Yamamoto, N.; Hirochika, H. Retrotransposon-induced mutations in grape skin color. Science 2004, 304, 982. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.R.; Lee, E.; Bogs, J.; McDavid, D.A.; Thomas, M.R.; Robinson, S.P. White grapes arose through the mutation of two similar and adjacent regulatory genes. Plant J. 2007, 49, 772–785. [Google Scholar] [CrossRef] [PubMed]
- Takos, A.M.; Jaffé, F.W.; Jacob, S.R.; Bogs, J.; Robinson, S.P.; Walker, A.R. Light-induced expression of a MYB gene regulates anthocyanin biosynthesis in red apples. Plant Physiol. 2006, 142, 1216–1232. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.; Zhao, M.; Leavitt, J.M.; Lloyd, A.M. Regulation of the anthocyanin biosynthetic pathway by the TTG1/bHLH/Myb transcriptional complex in Arabidopsis seedlings. Plant J. 2008, 53, 814–827. [Google Scholar] [CrossRef] [PubMed]
- LaFountain, A.M.; Yuan, Y.W. Repressors of anthocyanin biosynthesis. New Phytol. 2021, 231, 933–949. [Google Scholar] [CrossRef]
- Karppinen, K.; Zoratti, L.; Nguyenquynh, N.; Häggman, H.; Jaakola, L. On the developmental and environmental regulation of secondary metabolism in Vaccinium spp. berries. Front. Plant Sci. 2016, 7, 655. [Google Scholar] [CrossRef]
- Srivastava, A.; Akoh, C.C.; Fischer, J.; Krewer, G. Effect of anthocyanin fractions from selected cultivars of Georgia-grown blueberries on apoptosis and phase II enzymes. J. Agric. Food Chem. 2007, 55, 3180–3185. [Google Scholar] [CrossRef]
- Alkharouf, N.W.; Dhanaraj, A.L.; Naik, D.; Overall, C.; Matthews, B.F.; Rowland, L.J. BBGD: An online database for blueberry genomic data. BMC Plant Biol. 2007, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Colle, M.; Leisner, C.P.; Wai, C.M.; Ou, S.; Bird, K.A.; Wang, J.; Edger, P.P. Haplotype-phased genome and evolution of phytonutrient pathways of tetraploid blueberry. GigaScience 2019, 8, giz012. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.; Ye, X.; Li, X.; Yang, Y.; Hu, Z.; Overmyer, K.; Salojärvi, J. Chromosome-level genome assembly of the diploid blueberry Vaccinium darrowii provides insights into its subtropical adaptation and cuticle synthesis. Plant Commun. 2022, 3, 100307. [Google Scholar] [CrossRef] [PubMed]
- Zeng, T.; He, Z.; He, J.; Lv, W.; Huang, S.; Li, J.; Wang, L. The telomere-to-telomere gap-free reference genome of wild blueberry (Vaccinium duclouxii) provides its high soluble sugar and anthocyanin accumulation. Hortic. Res. 2023, 10, uhad209. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, M.; Shen, M.; Bu, S.; Zhu, B.; He, F.; Xiao, J. Chromosome-level genome assembly and annotation of the native Chinese wild blueberry Vaccinium bracteatum. Fruit Res. 2022, 2, 8. [Google Scholar] [CrossRef]
- Zifkin, M.; Jin, A.; Ozga, J.A.; Zaharia, L.I.; Schernthaner, J.P.; Gesell, A.; Constabel, C.P. Gene expression and metabolite profiling of developing highbush blueberry fruit indicates transcriptional regulation of flavonoid metabolism and activation of abscisic acid metabolism. Plant Physiol. 2012, 158, 200–224. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Estrada, A.D.; Blakley, I.; Reid, R.; Patel, K.; Meyer, M.D.; Loraine, A.E. RNA-Seq analysis and annotation of a draft blueberry genome assembly identifies candidate genes involved in fruit ripening, biosynthesis of bioactive compounds, and stage-specific alternative splicing. Gigascience 2015, 4, s13742-015. [Google Scholar] [CrossRef]
- Jaakola, L.; Määttä, K.; Pirttilä, A.M.; Törrönen, R.; Kärenlampi, S.; Hohtola, A. Expression of genes involved in anthocyanin biosynthesis in relation to anthocyanin, proanthocyanidin, and flavonol levels during bilberry fruit development. Plant Physiol. 2002, 130, 729–739. [Google Scholar] [CrossRef]
- Li, L.; Zhang, H.; Liu, Z.; Cui, X.; Zhang, T.; Li, Y.; Zhang, L. Comparative transcriptome sequencing and de novo analysis of Vaccinium corymbosum during fruit and color development. BMC Plant Biol. 2016, 16, 223. [Google Scholar] [CrossRef]
- Mengist, M.F.; Grace, M.H.; Mackey, T.; Munoz, B.; Pucker, B.; Bassil, N.; Iorizzo, M. Dissecting the genetic basis of bioactive metabolites and fruit quality traits in blueberries (Vaccinium corymbosum L.). Front. Plant Sci. 2022, 13, 964656. [Google Scholar] [CrossRef]
- Montanari, S.; Thomson, S.; Cordiner, S.; Günther, C.S.; Miller, P.; Deng, C.H.; Espley, R. High-density linkage map construction in an autotetraploid blueberry population and detection of quantitative trait loci for anthocyanin content. Frontiers in Plant Science 2022, 13, 965397. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Lu, Y.; Zhou, Y.; Hao, X.; Chen, W. Functional analysis of a methyltransferase involved in anthocyanin biosynthesis from blueberries (Vaccinium corymbosum). J. Agric. Food Chem. 2022, 70, 16253–16262. [Google Scholar] [CrossRef] [PubMed]
- Plunkett, B.J.; Espley, R.V.; Dare, A.P.; Warren, B.A.; Grierson, E.R.; Cordiner, S.; Schwinn, K.E. MYBA from blueberry (Vaccinium section Cyanococcus) is a subgroup 6 type R2R3MYB transcription factor that activates anthocyanin production. Front. Plant Sci. 2018, 9, 1300. [Google Scholar] [CrossRef] [PubMed]
- Karppinen, K.; Lafferty, D.J.; Albert, N.W.; Mikkola, N.; McGhie, T.; Allan, A.C.; Jaakola, L. MYBA and MYBPA transcription factors co-regulate anthocyanin biosynthesis in blue-coloured berries. New Phytol. 2021, 232, 1350–1367. [Google Scholar] [CrossRef] [PubMed]
- Lafferty, D.J.; Espley, R.V.; Deng, C.H.; Dare, A.P.; Günther, C.S.; Jaakola, L.; Albert, N.W. The coordinated action of MYB activators and repressors controls proanthocyanidin and anthocyanin biosynthesis in Vaccinium. Front. Plant Sci. 2022, 13, 910155. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Li, J.; Zhu, L.; Chang, P.; Li, L.; Zhang, L. Identification and characterization of MYB-bHLH-WD40 regulatory complex members controlling anthocyanidin biosynthesis in blueberry fruits development. Genes 2019, 10, 496. [Google Scholar] [CrossRef] [PubMed]
- Li, B.J.; Zheng, B.Q.; Wang, J.Y.; Tsai, W.C.; Lu, H.C.; Zou, L.H.; Wang, Y. New insight into the molecular mechanism of colour differentiation among floral segments in orchids. Commun. Biol. 2020, 3, 89. [Google Scholar] [CrossRef]
- Chu, L.; Zhao, P.; Wang, K.; Zhao, B.; Li, Y.; Yang, K.; Wan, P. VaSDC1 is involved in modulation of flavonoid metabolic pathways in black and red seed coats in Adzuki bean (Vigna angularis L.). Front. Plant Sci. 2021, 12, 679892. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef]
- Petroni, K.; Tonelli, C. Recent advances on the regulation of anthocyanin synthesis in reproductive organs. Plant Sci. 2011, 181, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Koes, R.; Verweij, W.; Quattrocchio, F. Flavonoids: A colorful model for the regulation and evolution of biochemical pathways. Trends Plant Sci. 2005, 10, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, J.; Li, P.; Sun, C.; Dong, W. Integrative metabolome and transcriptome analyses reveals the black fruit coloring mechanism of Crataegus maximowiczii CK Schneid. Plant Physiol. Biochem. 2023, 194, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Shakeel, M.; Wang, D.; Qu, C.; Yang, S.; Ahmad, S.; Song, Z. Metabolome and transcriptomes of light-induced anthocyanin synthesis in rabbiteye blueberry (Vaccinium ashei: Reade). BMC Plant Biol. 2022, 22, 223. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Q.; Fu, N.; Song, M.; Han, X.; Yang, Q.; Zhang, J. Cyanidin-3-O-glucoside Contributes to Leaf Color Change by Regulating Two bHLH Transcription Factors in Phoebe bournei. Int. J. Mol. Sci. 2023, 24, 3829. [Google Scholar] [CrossRef]
- Smith, S.D.; Wang, S.; Rausher, M.D. Functional evolution of an anthocyanin pathway enzyme during a flower color transition. Mol. Biol. Evol. 2013, 30, 602–612. [Google Scholar] [CrossRef]
- Zhang, J.; Han, Z.Y.; Tian, J.; Zhang, X.; Song, T.T.; Yao, Y.C. The expression level of anthocyanidin synthase determines the anthocyanin content of crabapple (Malus sp.) petals. Acta Physiol. Plant. 2015, 37, 1–10. [Google Scholar] [CrossRef]
- Saito, K.; Kobayashi, M.; Gong, Z.; Tanaka, Y.; Yamazaki, M. Direct evidence for anthocyanidin synthase as a 2-oxoglutarate-dependent oxygenase: Molecular cloning and functional expression of cDNA from a red forma of Perilla frutescens. Plant J. 1999, 17, 181–189. [Google Scholar] [CrossRef]
- Gong, Z.; Yamazaki, M.; Sugiyama, M.; Tanaka, Y.; Saito, K. Cloning and molecular analysis of structural genes involved in anthocyanin biosynthesis and expressed in a forma-specific manner in Perilla frutescens. Plant Mol. Biol. 1997, 35, 915–927. [Google Scholar] [CrossRef]
- Shimada, S.; Inoue, Y.T.; Sakuta, M. Anthocyanidin synthase in non-anthocyanin-producing Caryophyllales species. Plant J. 2005, 44, 950–959. [Google Scholar] [CrossRef] [PubMed]
- Rosati, C.; Cadic, A.; Duron, M.; Ingouff, M.; Simoneau, P. Molecular characterization of the anthocyanidin synthase gene in Forsythia × intermedia reveals organ-specific expression during flower development. Plant Sci. 1999, 149, 73–79. [Google Scholar] [CrossRef]
- Nakatsuka, T.; Nishihara, M.; Mishiba, K.; Yamamura, S. Temporal expression of flavonoid biosynthesis-related genes regulates flower pigmentation in gentian plants. Plant Sci. 2005, 168, 1309–1318. [Google Scholar] [CrossRef]
- Nakatsuka, T.; Nishihara, M.; Mishiba, K.; Yamamura, S. Two different mutations are involved in the formation of white-flowered gentian plants. Plant Sci. 2005, 169, 949–958. [Google Scholar] [CrossRef]
- Szankowski, I.; Flachowsky, H.; Li, H.; Halbwirth, H.; Treutter, D.; Regos, I.; Fischer, T.C. Shift in polyphenol profile and sublethal phenotype caused by silencing of anthocyanidin synthase in apple (Malus sp.). Planta 2009, 229, 681–692. [Google Scholar] [CrossRef]
- Lo Piero, A.R. The state of the art in biosynthesis of anthocyanins and its regulation in pigmented sweet oranges [(Citrus sinensis) L. Osbeck]. J. Agric. Food Chem. 2015, 63, 4031–4041. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, Z.; Yu, X.; Zhao, L.; Zhao, M.; Han, X.; Qi, S. Identification of two novel R2R3-MYB transcription factors, PsMYB114L and PsMYB12L, related to anthocyanin biosynthesis in Paeonia suffruticosa. Int. J. Mol. Sci. 2019, 20, 1055. [Google Scholar] [CrossRef]
- Sakai, M.; Yamagishi, M.; Matsuyama, K. Repression of anthocyanin biosynthesis by R3-MYB transcription factors in lily (Lilium spp.). Plant Cell Rep. 2019, 38, 609–622. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, L.; Du, Q.; Li, A.; Liu, G.; Wang, H.; Cui, Q.; Liu, Z.; Liu, H.; Lu, Y.; Deng, Y.; et al. Integrative Transcriptomic and Metabolomic Analyses of the Mechanism of Anthocyanin Accumulation and Fruit Coloring in Three Blueberry Varieties of Different Colors. Horticulturae 2024, 10, 105. https://doi.org/10.3390/horticulturae10010105
Chu L, Du Q, Li A, Liu G, Wang H, Cui Q, Liu Z, Liu H, Lu Y, Deng Y, et al. Integrative Transcriptomic and Metabolomic Analyses of the Mechanism of Anthocyanin Accumulation and Fruit Coloring in Three Blueberry Varieties of Different Colors. Horticulturae. 2024; 10(1):105. https://doi.org/10.3390/horticulturae10010105
Chicago/Turabian StyleChu, Liwei, Qianhui Du, Aizhen Li, Guiting Liu, Hexin Wang, Qingqing Cui, Zhichao Liu, Haixia Liu, Yani Lu, Yanqiong Deng, and et al. 2024. "Integrative Transcriptomic and Metabolomic Analyses of the Mechanism of Anthocyanin Accumulation and Fruit Coloring in Three Blueberry Varieties of Different Colors" Horticulturae 10, no. 1: 105. https://doi.org/10.3390/horticulturae10010105
APA StyleChu, L., Du, Q., Li, A., Liu, G., Wang, H., Cui, Q., Liu, Z., Liu, H., Lu, Y., Deng, Y., & Xu, G. (2024). Integrative Transcriptomic and Metabolomic Analyses of the Mechanism of Anthocyanin Accumulation and Fruit Coloring in Three Blueberry Varieties of Different Colors. Horticulturae, 10(1), 105. https://doi.org/10.3390/horticulturae10010105




