The Phytochemical Profile and Antioxidant Activity of Thermally Processed Colorful Sweet Potatoes
Abstract
:1. Introduction
2. Materials and Methods
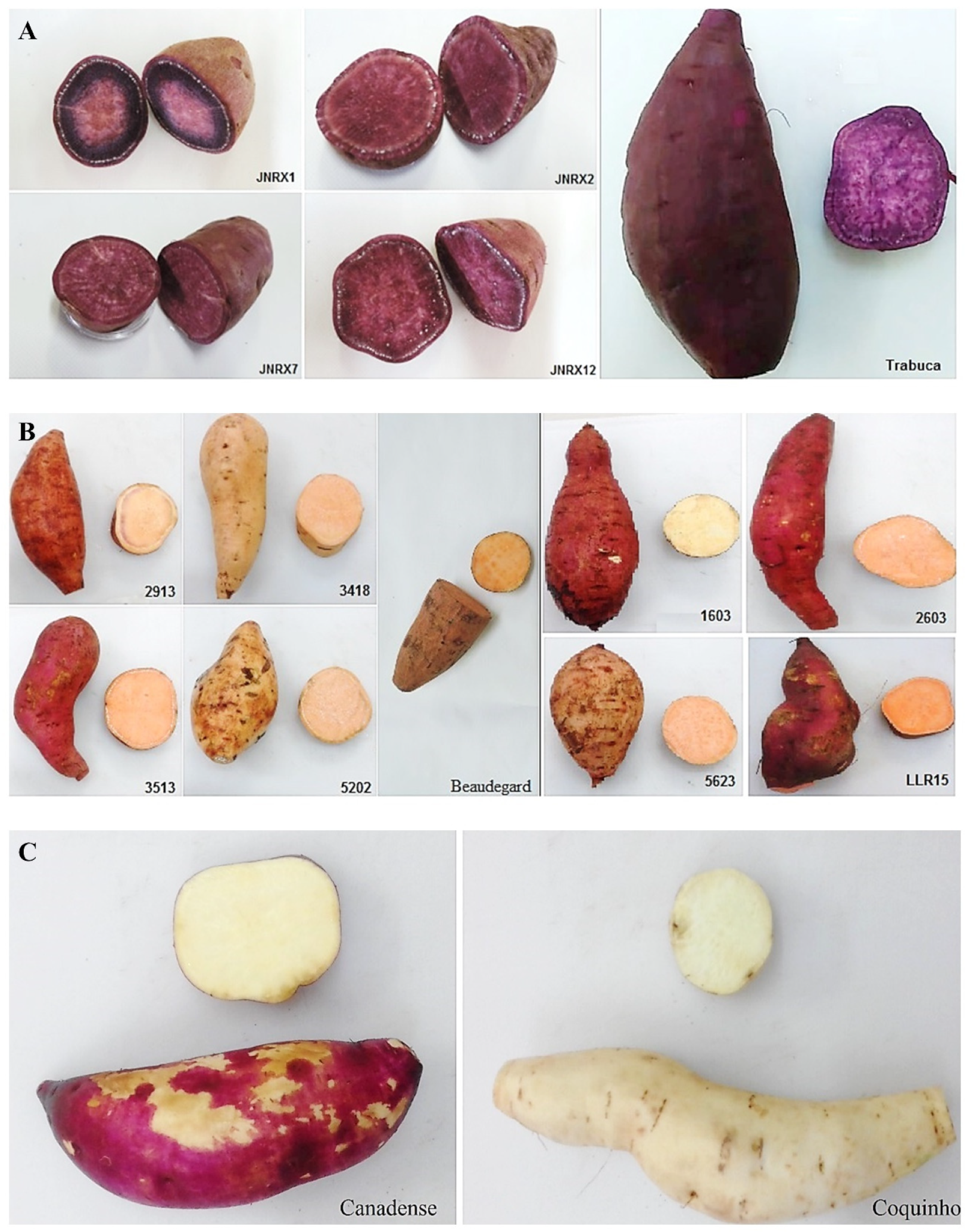
2.1. Plant Material
2.2. Cooking Analysis
2.3. Phytochemical Analysis and Antioxidant Activity
2.4. Profile of Phenolic Compounds
2.5. Profile of Biogenic Amines, Amino Acids, and the Amine Index (AI)
2.6. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hong, K.H.; Koh, E. Effects of Cooking Methods on Anthocyanins and Total Phenolics in Purple-Fleshed Sweet Potato: Cooking Effects on Anthocyanins. J. Food Process. Preserv. 2016, 40, 1054–1063. [Google Scholar] [CrossRef]
- Oloniyo, R.O.; Omoba, O.S.; Awolu, O.O.; Olagunju, A.I. Orange-fleshed Sweet Potatoes Composite Bread: A Good Carrier of Beta (β)-carotene and Antioxidant Properties. J. Food Biochem. 2021, 45, e13423. [Google Scholar] [CrossRef] [PubMed]
- Basílio, L.S.P.; Vanz Borges, C.; Minatel, I.O.; Vargas, P.F.; Tecchio, M.A.; Vianello, F.; Lima, G.P.P. New Beverage Based on Grapes and Purple-Fleshed Sweet Potatoes: Use of Non-Standard Tubers. Food Biosci. 2022, 47, 101626. [Google Scholar] [CrossRef]
- Guclu, G.; Dagli, M.M.; Aksay, O.; Keskin, M.; Kelebek, H.; Selli, S. Comparative Elucidation on the Phenolic Fingerprint, Sugars and Antioxidant Activity of White, Orange and Purple-Fleshed Sweet Potatoes (Ipomoea Batatas L.) as Affected by Different Cooking Methods. Heliyon 2023, 9, e18684. [Google Scholar] [CrossRef] [PubMed]
- Chintha, P.; Sarkar, D.; Pecota, K.; Dogramaci, M.; Hatterman-Valenti, H.; Shetty, K. Phenolic Bioactive-Linked Antioxidant, Anti-Hyperglycemic, and Antihypertensive Properties of Sweet Potato Cultivars with Different Flesh Color. Hortic. Environ. Biotechnol. 2023, 64, 877–893. [Google Scholar] [CrossRef]
- Gomez-Gomez, H.A.; Borges, C.V.; Minatel, I.O.; Luvizon, A.C.; Lima, G.P.P. Health benefits of dietary phenolic compounds and biogenic amines. In Bioactive Molecules in Food; Springer: Cham, Switzerland, 2018; pp. 1–25. [Google Scholar] [CrossRef]
- Lv, X.; Mu, J.; Wang, W.; Liu, Y.; Lu, X.; Sun, J.; Wang, J.; Ma, Q. Effects and mechanism of natural phenolic acids/fatty acids on copigmentation of purple sweet potato anthocyanins. Curr. Res. Food Sci. 2022, 5, 1243–1250. [Google Scholar] [CrossRef] [PubMed]
- Diamante, M.S.; Borges, C.V.; da Silva, M.B.; Minatel, I.O.; Corrêa, C.R.; Gomez Gomez, H.A.; Lima, G.P.P. Bioactive Amines Screening in Four Genotypes of Thermally Processed Cauliflower. Antioxidants 2019, 8, 311. [Google Scholar] [CrossRef] [PubMed]
- Borges, C.V.; Maraschin, M.; Coelho, D.S.; Leonel, M.; Gomez, H.A.G.; Belin, M.A.F.; Diamante, M.S.; Amorim, E.P.; Gianeti, T.; Castro, G.R.; et al. Nutritional value and antioxidant compounds during the ripening and after domestic cooking of bananas and plantains. Food Res. Int. 2020, 132, 109061. [Google Scholar] [CrossRef]
- Ruiz-Capillas, C.; Herrero, A. Impact of Biogenic Amines on Food Quality and Safety. Foods 2019, 8, 62. [Google Scholar] [CrossRef]
- Wójcik, W.; Łukasiewicz, M.; Puppel, K. Biogenic Amines: Formation, Action and Toxicity—A Review. J. Sci. Food Agric. 2021, 101, 2634–2640. [Google Scholar] [CrossRef]
- Til, H.P.; Falke, H.E.; Prinsen, M.K.; Willems, M.I. Acute and Subacute Toxicity of Tyramine, Spermidine, Spermine, Putrescine and Cadaverine in Rats. Food Chem. Toxicol. 1997, 35, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Sternberg, Z.; Podolsky, R.; Nir, A.; Yu, J.; Nir, R.; Halvorsen, S.W.; Quinn, J.F.; Kaye, J.; Kolb, C. Elevated Spermidine Serum Levels in Mild Cognitive Impairment, a Potential Biomarker of Progression to Alzheimer Dementia, a Pilot Study. J. Clin. Neurosci. 2022, 100, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Siener, R.; Seidler, A.; Hönow, R. Oxalate-Rich Foods. Food Sci. Technol. 2021, 41, 169–173. [Google Scholar] [CrossRef]
- Nicoletto, C.; Vianello, F.; Sambo, P. Effect of Different Home-cooking Methods on Textural and Nutritional Properties of Sweet Potato Genotypes Grown in Temperate Climate Conditions. J. Sci. Food Agric. 2018, 98, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A., Jr. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Pękal, A.; Pyrzynska, K. Evaluation of Aluminium Complexation Reaction for Flavonoid Content Assay. Food Anal. Methods 2014, 7, 1776–1782. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in Isolated Chloroplasts. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Giusti, M.M.; Wrolstad, R.E. Characterization and Measurement of Anthocyanins by UV-visible Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, 1–13. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and Carotenoids: Pigments of Photosynthetic Biomembranes. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1987; pp. 350–382. [Google Scholar] [CrossRef]
- Gahler, S.; Otto, K.; Böhm, V. Alterations of Vitamin C, Total Phenolics, and Antioxidant Capacity as Affected by Processing Tomatoes to Different Products. J. Agric. Food Chem. 2003, 51, 7962–7968. [Google Scholar] [CrossRef]
- Dini, I.; Tenore, G.C.; Dini, A. Effect of Industrial and Domestic Processing on Antioxidant Properties of Pumpkin Pulp. LWT Food. Sci. Technol. 2013, 53, 382–385. [Google Scholar] [CrossRef]
- Burgos, G.; Amoros, W.; Muñoa, L.; Sosa, P.; Cayhualla, E.; Sanchez, C.; Díaz, C.; Bonierbale, M. Total Phenolic, Total Anthocyanin and Phenolic Acid Concentrations and Antioxidant Activity of Purple-Fleshed Potatoes as Affected by Boiling. J. Food Compost. Anal. 2013, 30, 6–12. [Google Scholar] [CrossRef]
- Bernhardt, S.; Schlich, E. Impact of Different Cooking Methods on Food Quality: Retention of Lipophilic Vitamins in Fresh and Frozen Vegetables. J. Food Eng. 2006, 77, 327–333. [Google Scholar] [CrossRef]
- Gomez-Gomez, H.A.; Minatel, I.O.; Borges, C.V.; Marques, M.O.M.; da Silva, E.T.; Monteiro, G.C.; da Silva, M.J.R.; Tecchio, M.A.; Lima, G.P.P. Phenolic Compounds and Polyamines in Grape-Derived Beverages. J. Agric. Sci. 2018, 10, 65. [Google Scholar] [CrossRef]
- Humia, B.V.; Santos, K.S.; Schneider, J.K.; Leal, I.L.; de Abreu Barreto, G.; Batista, T.; Machado, B.A.S.; Druzian, J.I.; Krause, L.C.; da Costa Mendonça, M.; et al. Physicochemical and Sensory Profile of Beauregard Sweet Potato Beer. Food Chem. 2020, 312, 126087. [Google Scholar] [CrossRef] [PubMed]
- Murador, D.; Braga, A.R.; Da Cunha, D.; De Rosso, V. Alterations in Phenolic Compound Levels and Antioxidant Activity in Response to Cooking Technique Effects: A Meta-Analytic Investigation. Crit. Rev. Food Sci. Nutr. 2018, 58, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Zheng, R.L.; Huang, Z.Y. Reactive oxygen species. In Free Radical in Medical and Agricultural Science; Zheng, R.L., Huang, Z.Y., Eds.; China Higher Education Press: Beijing, China; Springer Press: Beijing, China, 2001; pp. 17–27. [Google Scholar]
- Oliveira Silva, E.; Batista, R. Ferulic Acid and Naturally Occurring Compounds Bearing a Feruloyl Moiety: A Review on Their Structures, Occurrence, and Potential Health Benefits. Compr. Rev. Food Sci. Food Saf. 2017, 16, 580–616. [Google Scholar] [CrossRef]
- Carrera, C.; Zelaya-Medina, C.F.; Chinchilla, N.; Ferreiro-González, M.; Barbero, G.F.; Palma, M. How Different Cooking Methods Affect the Phenolic Composition of Sweet Potato for Human Consumption (Ipomea batata (L.) Lam). Agronomy 2021, 11, 1636. [Google Scholar] [CrossRef]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomás-Barberán, F.A. The Effects of Polyphenols and Other Bioactives on Human Health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef]
- Carrillo, J.Á.; Zafrilla, M.P.; Marhuenda, J. Cognitive Function and Consumption of Fruit and Vegetable Polyphenols in a Young Population: Is There a Relationship? Foods 2019, 8, 507. [Google Scholar] [CrossRef]
- Conio, B.; Martino, M.; Magioncalda, P.; Escelsior, A.; Inglese, M.; Amore, M.; Northoff, G. Opposite Effects of Dopamine and Serotonin on Resting-State Networks: Review and Implications for Psychiatric Disorders. Mol. Psychiatry 2020, 25, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Henneberg, R.; Otuki, M.F.; Furman, A.E.F.; Hermann, P.; do Nascimento, A.J.; Leonart, M.S.S. Protective Effect of Flavonoids against Reactive Oxygen Species Production in Sickle Cell Anemia Patients Treated with Hydroxyurea. Rev. Bras. Hematol. Hemoter. 2013, 35, 52–55. [Google Scholar] [CrossRef]
- Borges, C.V.; Belin, M.A.F.; Amorim, E.P.; Minatel, I.O.; Monteiro, G.C.; Gomez Gomez, H.A.; Monar, G.R.S.; Lima, G.P.P. Bioactive Amines Changes during the Ripening and Thermal Processes of Bananas and Plantains. Food Chem. 2019, 298, 125020. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, M.; Lambropoulou, D.; Morrison, C.; Kłodzińska, E.; Namieśnik, J.; Płotka-Wasylka, J. Literature Update of Analytical Methods for Biogenic Amines Determination in Food and Beverages. Trends Analyt. Chem. 2018, 98, 128–142. [Google Scholar] [CrossRef]
- Liu, Q.; Gao, T.; Liu, W.; Liu, Y.; Zhao, Y.; Liu, Y.; Li, W.; Ding, K.; Ma, F.; Li, C. Functions of Dopamine in Plants: A Review. Plant Signal. Behav. 2020, 15, 1827782. [Google Scholar] [CrossRef]
- Kalač, P.; Krausová, P. A Review of Dietary Polyamines: Formation, Implications for Growth and Health and Occurrence in Foods. Food Chem. 2005, 90, 219–230. [Google Scholar] [CrossRef]
- Negri, S.; Commisso, M.; Avesani, L.; Guzzo, F. The Case of Tryptamine and Serotonin in Plants: A Mysterious Precursor for an Illustrious Metabolite. J. Exp. Bot. 2021, 72, 5336–5355. [Google Scholar] [CrossRef]
- Linares, D.M.; del Rio, B.; Redruello, B.; Ladero, V.; Martin, M.C.; Fernandez, M.; Ruas-Madiedo, P.; Alvarez, M.A. Comparative Analysis of the in Vitro Cytotoxicity of the Dietary Biogenic Amines Tyramine and Histamine. Food Chem. 2016, 197, 658–663. [Google Scholar] [CrossRef]
- Silber, B.Y.; Schmitt, J.A.J. Effects of Tryptophan Loading on Human Cognition, Mood, and Sleep. Neurosci. Biobehav. Rev. 2010, 34, 387–407. [Google Scholar] [CrossRef]
- Musilova, J.; Lidikova, J.; Vollmannova, A.; Frankova, H.; Urminska, D.; Bojnanska, T.; Toth, T. Influence of heat treatments on the content of bioactive substances and antioxidant properties of sweet potato (Ipomoea batatas L.) tubers. J. Food Qual. 2020, 2020, 8856260. [Google Scholar] [CrossRef]
- Del Rio, B.; Redruello, B.; Linares, D.M.; Ladero, V.; Ruas-Madiedo, P.; Fernandez, M.; Martin, M.C.; Alvarez, M.A. The Biogenic Amines Putrescine and Cadaverine Show in Vitro Cytotoxicity at Concentrations That Can Be Found in Foods. Sci. Rep. 2019, 9, 120. [Google Scholar] [CrossRef] [PubMed]






| Genotypes | Cooking | TPC (mg GEA 100 mg−1) | TF (mg GEA 100 mg−1) | TA (mg GEA 100 mg−1) | MDA (nmol TBARS g−1) | DPPH (mg Trolox 100 mg−1) |
|---|---|---|---|---|---|---|
| Purple pulp | ||||||
| JNRX1 | Crude | 147.2 ± 3.3 iC * | 135.0 ± 6.6 gD | 25.8 ± 3.3 gD | 27.2 ± 0.2 iE | 0.13 ± 0.0 eD |
| Mi | 351.5 ± 4.8 cA | 306.7 ± 8.8 cA | 159.5 ± 29.0 cA | 225.1 ± 13.8 bA | 29.4 ± 3.5 aA | |
| S | 299.5 ± 2.8 eB | 262.3 ± 5.3 eB | 81.9 ± 10.4 fC | 178.6 ± 32.6 cB | 18.0 ± 1.5 cC | |
| B | 238.0 ± 3.4 hB | 208.8 ± 12.8 fC | 38.5 ± 1.4 gD | 137.9 ± 2.4 eD | 21.4 ± 2.4 cB | |
| Op | 230.0 ± 0.8 hB | 214.8 ± 26.8 fC | 102.9 ± 19.7 eB | 169.5 ± 8.6 cC | 21.4 ± 1.7 cB | |
| Owp | 96.8 ± 8.5 lD | 74.3 ± 14.6 iE | 45.0 ± 4.8 gD | 174.1 ± 6.3 cB | 24.2 ± 0.7 bB | |
| JNRX2 | Crude | 136.6 ± 18.5 jC | 125.5 ± 22.0 gD | 94.8 ± 9.4 eD | 27.4 ± 1.4 iD | 0.20 ± 0.0 eC |
| Mi | 380.6 ± 12.8 bA | 328.9 ± 25.3 bA | 165.6 ± 12.4 cA | 43.9 ± 3.1 hD | 28.1 ± 2.4 aA | |
| S | 238.9 ± 10.1 hB | 212.4 ± 12.2 fB | 139.5 ± 19.6 dB | 180.9 ± 15.6 cC | 26.5 ± 1.5 bB | |
| B | 110.8 ± 4.6 kC | 89.4 ± 3.2 iC | 72.2 ± 3.3 fE | 187.7 ± 15.3 cB | 27.4 ± 0.5 aA | |
| Op | 289.4 ± 13.9 fB | 248.9 ± 11.5 eB | 182.3 ± 9.6 cA | 194.7 ± 6.4 cB | 26.3 ± 1.4 bB | |
| Owp | 77.9 ± 2.2 mD | 59.8 ± 3.0 jE | 67.8 ± 3.5 fE | 245.5 ± 6.5 aA | 27.5 ± 0.3 aA | |
| JNRX7 | Crude | 155.2 ± 20.3 iD | 129.5 ± 13.8 gC | 57.6 ± 15.6 gD | 39.0 ± 0.2 hE | 0.25 ± 0.0 eE |
| Mi | 389.1 ± 15.8 bA | 339.8 ± 7.8 bA | 250.3 ± 6.3 aA | 217.6 ± 1.3 bA | 28.5 ± 2.4 aA | |
| S | 261.9 ± 6.8 gC | 224.4 ± 6.3 fB | 181.5 ± 1.6 cB | 152.8 ± 14.6 dC | 19.8 ± 1.3 cB | |
| B | 129.9 ± 1.8 jE | 107.4 ± 4.1 hD | 94.3 ± 3.8 eD | 186.9 ± 6.3 cB | 19.9 ± 0.9 cB | |
| Op | 308.9 ± 15.6 eB | 276.6 ± 15.9 dB | 131.5 ± 18.5 dC | 84.2 ± 2.0 fD | 5.6 ± 0.4 dD | |
| Owp | 100.7 ± 1.0 lE | 82.2 ± 5.3 iE | 74.5 ± 7.2 fD | 158.0 ± 7.1 dC | 17.2 ± 1.6 cC | |
| JNRX12 | Crude | 167.4 ± 18.8 iD | 146.3 ± 13.4 gC | 73.5 ± 33.4 fC | 58.1 ± 4.9 gD | 0.25 ± 0.0 eB |
| Mi | 432.6 ± 8.4 aA | 366.5 ± 10.0 aA | 212.5 ± 8.4 bA | 172.7 ± 9.5 cC | 28.7 ± 2.6 aA | |
| S | 287.8 ± 7.3 fC | 238.6 ± 5.8 eB | 171.4 ± 14.3 cB | 182.7 ± 11.9 cB | 28.9 ± 1.4 aA | |
| B | 150.2 ± 3.3 iE | 121.0 ± 3.1 gC | 50.3 ± 8.0 gD | 190.6 ± 3.0 cA | 27.8 ± 1.2 aA | |
| Op | 330.9 ± 19.2 dB | 280.5 ± 16.4 dB | 187.7 ± 24.4 cB | 172.9 ± 14.3 cC | 28.0 ± 0.9 aA | |
| Owp | 116.7 ± 0.6 kE | 96.9 ± 3.5 iD | 45.1 ± 6.4 gD | 183.3 ± 8.9 cB | 28.0 ± 2.3 aA | |
| Trabuca | Crude | 125.8 ± 3.5 jD | 106.1 ± 5.4 hD | 47.6 ± 3.0 gC | 48.9 ± 6.6 hD | 0.77 ± 0.0 eC |
| Mi | 374.8 ± 16.5 bA | 318.5 ± 10.5 bA | 251.1 ± 16.4 aA | 131.7 ± 0.5 eC | 21.4 ± 0.7 cB | |
| S | 235.4 ± 6.8 hC | 198.8 ± 8.3 fC | 94.5 ± 30.8 eB | 213.2 ± 8.7 bA | 24.3 ± 1.6 bA | |
| B | 96.3 ± 2.5 lE | 76.1 ± 2.8 iE | 42.4 ± 11.6 gC | 176.8 ± 0.7 cB | 19.2 ± 0.2 cB | |
| Op | 275.9 ± 17.5 fB | 240.1 ± 12.8 eB | 120.9 ± 10.9 eB | 141.8 ± 1.0 eC | 25.3 ± 0.8 bA | |
| Owp | 63.0 ± 4.4 mF | 46.4 ± 3.3 jE | 37.5 ± 3.5 gD | 134.4 ± 0.8 eC | 18.8 ± 1.2 cB | |
| Orange pulp | ||||||
| LLR15 | Crude | 75.1 ± 5.0 dD | 62.1 ± 7.8 cC | 14.0 ± 2.6 iB | 15.5 ± 1.3 bA | 1.08 ± 0.3 iE |
| Mi | 109.7 ± 1.2 aA | 88.3 ± 3.8 aA | 28.9 ± 1.5 bA | 14.9 ± 2.1 bB | 9.55 ± 1.0 eA | |
| S | 92.7 ± 3.2 bB | 74.1 ± 4.1 bB | 29.6 ± 1.7 aA | 11.3 ± 0.4 cD | 3.87 ± 0.1 gC | |
| B | 94.2 ± 5.4 bB | 74.9 ± 9.9 bB | 28.6 ± 2.5 bA | 11.0 ± 0.8 cD | 2.72 ± 0.9 hD | |
| Op | 98.6 ± 3.2 bB | 85.1 ± 5.4 aA | 27.9 ± 1.6 bA | 12.6 ± 0.9 cC | 1.44 ± 0.2 iE | |
| Owp | 80.6 ± 5.1 cC | 58.7 ± 14.8 dD | 30.6 ± 0.7 aA | 9.89 ± 0.8 dE | 4.38 ± 0.8 gB | |
| 5623 | Crude | 48.6 ± 2.6 hD | 37.9 ± 3.0 fD | 11.4 ± 2.7 jC | 11.9 ± 0.1 cB | 1.33 ± 0.1 iD |
| Mi | 77.7 ± 0.9 dA | 67.3 ± 4.2 cA | 26.7 ± 1.8 cA | 11.3 ± 0.6 cB | 18.3 ± 1.7 aA | |
| S | 66.3 ± 1.0 eB | 51.8 ± 3.0 dB | 27.7 ± 2.7 bA | 10.5 ± 0.1 dC | 6.63 ± 1.0 fC | |
| B | 56.3 ± 2.3 fC | 43.3 ± 1.3 eC | 28.7 ± 0.6 bA | 8.81 ± 0.4 dD | 6.84 ± 0.6 fC | |
| Op | 59.0 ± 3.8 fC | 48.9 ± 3.2 eC | 23.5 ± 1.6 dB | 17.0 ± 2.0 aA | 17.7 ± 0.5 bB | |
| Owp | 53.0 ± 0.8 gC | 43.8 ± 2.6 eC | 28.3 ± 1.3 bA | 16.5 ± 1.5 aA | 17.6 ± 3.4 bB | |
| 2603 | Crude | 72.3 ± 0.6 dC | 54.6 ± 8.4 dD | 14.9 ± 1.8 hC | 13.5 ± 0.7 cC | 2.65 ± 0.0 hD |
| Mi | 104.0 ± 1.3 aA | 86.6 ± 2.2 aA | 28.0 ± 2.2 bA | 18.2 ± 0.4 aA | 17.5 ± 2.4 bB | |
| S | 88.2 ± 9.7 bB | 71.6 ± 2.9 cB | 29.7 ± 0.8 aA | 15.6 ± 1.0 bB | 17.6 ± 1.0 bB | |
| B | 82.1 ± 1.0 cB | 67.0 ± 3.6 cC | 29.3 ± 1.8 aA | 12.4 ± 0.2 cD | 17.7 ± 0.0 bB | |
| Op | 85.7 ± 3.8 cB | 63.2 ± 6.1 cC | 26.1 ± 1.2 cB | 12.6 ± 0.9 cD | 9.89 ± 0.6 dC | |
| Owp | 75.1 ± 0.3 dC | 60.8 ± 2.4 dC | 30.5 ± 1.2 aA | 18.7 ± 0.3 aA | 19.1 ± 2.1 aA | |
| Beauregard | Crude | 42.1 ± 4.6 iD | 33.6 ± 3.9 fD | 12.7 ± 1.0 jB | 8.72 ± 0.6 dA | 0.23 ± 0.0 iE |
| Mi | 109.4 ± 10.7 aA | 94.7 ± 9.9 aA | 27.4 ± 1.6 dA | 8.99 ± 0.1 dA | 11.0 ± 0.3 dB | |
| S | 73.9 ± 12.3 dC | 62.0 ± 12.7 cC | 27.9 ± 2.4 dA | 5.07 ± 1.3 eD | 6.63 ± 0.8 fD | |
| B | 91.3 ± 17.7 bB | 72.8 ± 10.3 cB | 28.2 ± 0.8 dA | 5.01 ± 0.7 eD | 6.84 ± 0.4 fD | |
| Op | 92.8 ± 1.7 bB | 71.1 ± 4.6 cB | 24.4 ± 1.1 fA | 7.58 ± 0.7 dC | 8.27 ± 0.5 eC | |
| Owp | 42.9 ± 11.5 iD | 32.7 ± 12.0 fD | 29.5 ± 1.6 cA | 6.23 ± 0.7 eB | 15.0 ± 0.8 cA | |
| 3513 | Crude | 68.6 ± 1.6 dE | 52.7 ± 7.0 dC | 16.4 ± 1.0 hB | 14.9 ± 0.1 bB | 2.62 ± 0.7 hD |
| Mi | 104.2 ± 2.4 aA | 90.8 ± 9.0 aA | 20.1 ± 1.7 fA | 13.4 ± 1.5 cC | 18.5 ± 3.1 aA | |
| S | 90.4 ± 8.7 bB | 66.1 ± 11.3 cB | 19.4 ± 2.0 fA | 14.4 ± 2.1 bB | 18.6 ± 2.7 aA | |
| B | 85.8 ± 3.6 cC | 65.5 ± 4.3 cB | 17.5 ± 3.4 gB | 12.2 ± 1.9 cC | 17.5 ± 1.6 bB | |
| Op | 84.4 ± 6.9 cC | 64.6 ± 11.2 cB | 11.9 ± 0.6 jC | 14.0 ± 0.4 bB | 10.7 ± 1.4 dC | |
| Owp | 74.1 ± 1.1 dD | 57.9 ± 1.9 dC | 21.4 ± 1.2 eA | 15.6 ± 2.2 bA | 18.9 ± 1.4 aA | |
| 3418 | Crude | 46.3 ± 0.6 hD | 35.6 ± 1.9 fD | 12.7 ± 2.1 iC | 17.9 ± 1.9 aA | 0.91 ± 0.0 iD |
| Mi | 75.7 ± 1.8 dA | 60.5 ± 5.8 dA | 14.1 ± 0.6 iA | 12.9 ± 1.1 cC | 7.23 ± 0.1 fB | |
| S | 65.5 ± 1.5 eB | 46.5 ± 6.6 eB | 15.9 ± 0.7 hA | 11.5 ± 0.2 cD | 6.96 ± 0.8 fB | |
| B | 54.6 ± 2.9 gC | 39.2 ± 3.7 fC | 13.6 ± 1.9 iB | 7.69 ± 2.9 dE | 4.54 ± 0.2 gC | |
| Op | 57.9 ± 1.3 fC | 41.0 ± 2.1 eC | 13.5 ± 0.8 iB | 14.7 ± 1.0 bC | 8.78 ± 1.5 eA | |
| Owp | 48.3 ± 0.4 hD | 37.2 ± 1.7 fC | 9.80 ± 1.6 kD | 16.9 ± 1.6 aB | 0.74 ± 0.0 iD | |
| 2913 | Crude | 53.0 ± 6.6 gD | 41.5 ± 3.8 eE | 6.16 ± 1.0 iE | 15.3 ± 0.4 bA | 0.54 ± 0.0 iB |
| Mi | 83.1 ± 0.7 cA | 66.8 ± 0.5 cA | 9.56 ± 1.5 kB | 15.6 ± 0.1 bA | 19.0 ± 2.1 aA | |
| S | 73.3 ± 0.9 dB | 57.9 ± 3.8 dB | 8.53 ± 2.6 kC | 9.46 ± 1.1 dC | 18.9 ± 2.0 aA | |
| B | 62.3 ± 1.3 eC | 50.1 ± 2.1 eC | 7.24 ± 1.3 lD | 8.88 ± 1.1 dC | 18.7 ± 1.1 aA | |
| Op | 65.8 ± 3.0 eC | 50.0 ± 7.0 eC | 8.26 ± 2.4 kC | 10.9 ± 3.4 cA | 18.4 ± 1.2 aA | |
| Owp | 56.8 ± 1.8 fD | 45.8 ± 3.4 eD | 12.9 ± 1.3 iA | 14.3 ± 1.3 bB | 18.6 ± 2.0 aA | |
| 5202 | Crude | 45.9 ± 0.6 hD | 31.9 ± 3.9 fD | 10.3 ± 2.2 jD | 9.42 ± 3.6 dC | 0.38 ± 0.0 iD |
| Mi | 75.9 ± 0.2 dA | 58.6 ± 7.9 dA | 13.2 ± 1.0 iC | 8.51 ± 0.2 dD | 17.8 ± 1.4 bA | |
| S | 64.1 ± 2.6 eB | 52.2 ± 1.5 dB | 15.9 ± 1.4 hB | 9.97 ± 0.9 dC | 18.7 ± 1.1 aA | |
| B | 55.3 ± 0.8 gC | 44.2 ± 3.7 eC | 10.1 ± 1.0 jD | 8.51 ± 0.1 dD | 14.5 ± 0.6 cC | |
| Op | 57.2 ± 0.6 fC | 45.9 ± 2.4 eC | 10.6 ± 0.3 jD | 10.9 ± 0.4 cB | 13.2 ± 0.4 cC | |
| Owp | 48.3 ± 0.7 hD | 34.1 ± 1.5 fD | 17.8 ± 1.1 gA | 15.2 ± 3.7 bA | 17.1 ± 1.6 bB | |
| Yellow pulp | ||||||
| 1603 | Crude | 52.9 ± 0.7 gD | 38.2 ± 0.5 fE | 9.04 ± 0.4 kC | 7.09 ± 1.2 dD | 0.05 ± 0.0 iD |
| Mi | 83.5 ± 2.1 cA | 65.0 ± 4.7 cA | 10.2 ± 0.6 jB | 8.66 ± 0.6 dC | 8.87 ± 1.2 eC | |
| S | 71.9 ± 2.0 dB | 56.6 ± 4.5 dB | 10.5 ± 0.5 jB | 9.21 ± 2.3 dB | 9.29 ± 0.9 eB | |
| B | 62.8 ± 0.6 eC | 45.3 ± 6.2 eD | 11.2 ± 0.3 jA | 6.40 ± 0.1 eE | 9.44 ± 0.4 eB | |
| Op | 64.9 ± 0.8 eC | 51.5 ± 1.9 dC | 10.6 ± 0.8 jB | 6.52 ± 0.4 eE | 9.71 ± 1.0 eB | |
| Owp | 55.6 ± 0.5 gD | 38.6 ± 4.0 fE | 11.2 ± 1.0 jA | 12.3 ± 3.1 cA | 10.4 ± 0.0 dA | |
| White pulp | ||||||
| Canadense | Crude | 26.3 ± 0.6 jD | 13.1 ± 3.2 hD | 2.08 ± 1.0 mC | 6.08 ± 1.8 eC | 0.16 ± 0.0 iC |
| Mi | 57.9 ± 0.6 fA | 40.9 ± 5.2 eA | 3.09 ± 1.1 mB | 3.00 ± 1.4 fD | 6.15 ± 0.5 fA | |
| S | 45.5 ± 1.8 hB | 31.1 ± 1.2 fB | 3.62 ± 0.6 mB | 10.4 ± 0.1 dA | 6.91 ± 0.3 fA | |
| B | 35.9 ± 0.9 iC | 23.5 ± 2.2 gC | 3.36 ± 1.0 mB | 8.67 ± 1.5 dB | 6.38 ± 0.3 fA | |
| Op | 38.9 ± 0.9 iC | 26.6 ± 3.8 gC | 3.10 ± 0.9 mB | 3.71 ± 0.1 fD | 0.11 ± 0.0 iC | |
| Owp | 28.5 ± 0.5 jD | 17.1 ± 2.5 hD | 5.05 ± 0.4 lA | 3.14 ± 0.6 fD | 0.68 ± 0.0 iB | |
| Coquinho | Crude | 18.7 ± 0.5 kD | 9.30 ± 1.0 hE | 1.37 ± 0.5 mC | 5.61 ± 0.3 eC | 0.14 ± 0.0 iE |
| Mi | 50.3 ± 0.5 hA | 33.9 ± 7.7 fA | 2.07 ± 0.7 mB | 7.77 ± 0.7 dB | 10.8 ± 1.0 dA | |
| S | 38.0 ± 2.1 iB | 25.4 ± 0.9 gB | 2.98 ± 0.2 mA | 7.74 ± 0.1 dB | 9.3 ± 0.8 eB | |
| B | 28.3 ± 0.7 jC | 16.5 ± 2.5 hC | 2.06 ± 0.6 mB | 1.21 ± 0.3 fD | 10.1 ± 0.1 dA | |
| Op | 30.3 ± 1.1 jB | 19.1 ± 0.5 hC | 2.38 ± 0.6 mB | 12.1 ± 1.2 cA | 5.23 ± 0.6 fD | |
| Owp | 20.7 ± 0.5 kD | 11.5 ± 0.7 hD | 2.41 ± 0.3 mA | 12.0 ± 0.4 cA | 7.74 ± 0.4 fC | |
| Gen. | Cook. | Gallic Acid | Catechin | t-Cinnamic Acid | Chlorogenic Acid | Caffeic Acid | p-Coumaric Acid | t-Ferulic Acid |
|---|---|---|---|---|---|---|---|---|
| Purple pulp | ||||||||
| JNRX1 | Crude | 53.4 ± 1.7 fD * | Nd | 0.1 ± 0.0 jC | 213.6 ± 5.3 fC | 17.4 ± 2.9 fE | 71.8 ± 8.8 eC | 33.1 ± 7.2 fE |
| Mi | 117.3 ± 3.8 dB | Nd | Nd | 1415.1 ± 11.6 dB | 114.5 ± 1.8 bC | 58.4 ± 6.5 eD | 528.0 ± 6.3 cC | |
| S | 28.7 ± 0.7 hE | Nd | 0.5 ± 0.1 iB | 1250.7 ± 7.1 dB | 66.2 ± 11.3 cD | 53.5 ± 8.4 eD | 690.1 ± 9.9 cD | |
| B | 19.6 ± 2.1 iF | Nd | 0.5 ± 0.2 iB | 2168.7 ± 8.9 cA | 143.7 ± 13.0 bB | 651.2 ± 6.9 bA | 713.4 ± 1.9 bB | |
| Op | 292.6 ± 25.4 aA | Nd | 0.8 ± 0.3 iA | 2101.2 ± 7.8 cA | 165.2 ± 12.0 aA | 568.8 ± 7.1 bB | 838.8 ± 8.9 bA | |
| Owp | 60.9 ± 8.7 eC | Nd | Nd | 1635.4 ± 8.1 dB | 133.2 ± 13.4 bB | 428.6 ± 8.2 bB | 488.8 ± 7.3 cC | |
| JNRX2 | Crude | 30.6 ± 2.4 gC | Nd | Nd | 387.2 ± 6.0 fC | 15.7 ± 12.3 fC | 39.6 ± 5.2 fC | 62.1 ± 6.8 eE |
| Mi | 35.4 ± 6.1 gB | Nd | 3.0 ± 0.4 gA | 2090.9 ± 5.9 cB | 77.5 ± 15.9 cB | 275.3 ± 4.9 dB | 871.5 ± 5.7 bB | |
| S | 26.0 ± 1.4 hD | Nd | 2.7 ± 0.9 gA | 3581.5 ± 7.1 aA | 167.2 ± 14.7 aA | 211.6 ± 3,9 dB | 936.7 ± 6.3 bA | |
| B | 32.9 ± 1.2 gB | Nd | 1.5 ± 0.6 hB | 2616.8 ± 8.3 cB | 84.6 ± 13.8 cB | 305.2 ± 4.7 cA | 587.4 ± 5.9 cD | |
| Op | 32.2 ± 1.1 gB | Nd | 1.4 ± 0.7 hB | 3809.2 ± 5.9 aA | 98.7 ± 15.3 cB | 258.5 ± 8.5 dB | 970.3 ± 6.0 bA | |
| Owp | 43.8 ± 1.5 fA | Nd | 1.4 ± 0.5 hB | 3253.7 ± 6.9 aA | 172.8 ± 16.7 aA | 356.9 ± 9.0 cA | 765.2 ± 8.4 bC | |
| JNRX7 | Crude | 75.9 ± 5.0 eC | 26.0 ± 0.7 hD | Nd | 227.6 ± 8.7 fD | 14.8 ± 1.9 fC | 82.2 ± 10.0 dD | 34.4 ± 7.5 fD |
| Mi | 23.4 ± 0.7 hE | 57.8 ± 5.4 eC | 0.9 ± 0.4 iD | 1274.5 ± 8.9 dC | 35.9 ± 3.7 eB | 554.8 ± 12.3 bC | 506.0 ± 6.3 cC | |
| S | 68.0 ± 1.9 eD | 56.7 ± 2.4 eC | 0.6 ± 0.1 iD | 1530.1 ± 9.3 dB | 47.5 ± 7.3 dA | 799.5 ± 15.7 aA | 785.5 ± 6.0 bA | |
| B | 76.6 ± 1.2 eC | 45.9 ± 0.7 fC | 64.2 ± 10.7 bB | 1984.5 ± 10.3 dA | 50.7 ± 9.1 dA | 684.6 ± 25.8 bB | 714.8 ± 5.7 bA | |
| Op | 178.2 ± 0.8 cA | 109.0 ± 2.4 bA | 116.5 ± 9.8 aA | 1240.1 ± 12.3 dC | 44.3 ± 7.0 dA | 452.9 ± 17.6 bC | 628.9 ± 5.9 cB | |
| Owp | 158.7 ± 1.4 dB | 60.4 ± 0.7 eB | 34.6 ± 8.7 dC | 1715.7 ± 19.7 dB | 47.7 ± 6.4 dA | 551.6 ± 18.3 bC | 516.3 ± 3.8 cC | |
| JNRX12 | Crude | 69.2 ± 2.1 eD | 13.6 ± 1.1 iE | 0.8 ± 0.1 iE | 1427.5 ± 0.7 d | 16.9 ± 0.5 fE | 125.0 ± 0.9 dC | 247.6 ± 0.9 dD |
| Mi | 32.7 ± 1.2 fE | 20.0 ± 0.7 bhD | 50.7 ± 0.5 cB | 2277.8 ± 0.7 cB | 38.2 ± 0.5 eD | 315.2 ± 0.8 cB | 549.0 ± 1.0 cC | |
| S | 123.9 ± 0.2 dC | 30.1 ± 0.9 gC | 17.0 ± 0.3 eC | 2944.4 ± 0.5 bA | 67.1 ± 0.3 dC | 525.6 ± 0.8 bA | 1147.0 ± 0.7 aB | |
| B | 107.9 ± 0,5 dC | 79.5 ± 0.0 dB | 49.4 ± 0.7 cB | 2802.3 ± 0.7 bA | 72.7 ± 0.7 cC | 343.4 ± 0.7 cB | 1257.8 ± 0.8 aA | |
| Op | 204.4 ± 5.4 aA | 111.5 ± 8.7 bA | 75.3 ± 0.8 bA | 2998.5 ± 0.9 bA | 135.1 ± 0.4 bA | 569.8 ± 1.2 bA | 1089.8 ± 0.8 aB | |
| Owp | 175.5 ± 2.6 cB | 81.9 ± 5.4 cB | 7.6 ± 0.6 fD | 2527.6 ± 0.3 cB | 94.4 ± 0.6 cB | 339.0 ± 0.8 cB | 1256.0 ± 0.6 aA | |
| Trabuca | Crude | 152.0 ± 9.1 dD | 31.9 ± 2.2 gD | 0.5 ± 0.1 iD | 49.9 ± 0.6 gE | 5.7 ± 0.2 gE | 13.0 ± 1.1 gE | 8.2 ± 0.8 gD |
| Mi | 149.4 ± 1.4 dD | 147.4 ± 2.3 bB | 1.2 ± 0.2 hC | 998.7 ± 0.9 eD | 154.3 ± 0.8 aA | 365.7 ± 0.7 cD | 600.1 ± 0.9 cC | |
| S | 188.9 ± 1.1 bB | 278.8 ± 48.9 aA | 28.3 ± 0.3 dB | 3257.0 ± 0.6 aA | 102.4 ± 0.9 bB | 927.0 ± 0.9 aA | 1286.8 ± 0.5 aA | |
| B | 174.6 ± 1.2 cC | 96.1 ± 8.4 cC | 25.9 ± 0.5 dB | 1452.2 ± 0.5 dC | 74.4 ± 0.1 cC | 546.3 ± 0.1 bC | 788.7 ± 0.9 bC | |
| Op | 213.8 ± 2.4 aA | 100.6 ± 5.1 bC | 29.9 ± 0.5 dB | 2082.2 ± 0.7 cB | 72.4 ± 0.9 cC | 663.2 ± 0.4 bB | 1260.7 ± 0.8 aA | |
| Owp | 256.3 ± 2.1 aA | 118.8 ± 5.1 bC | 36.8 ± 0.8 dA | 1664.4 ± 0.9 dC | 51.6 ± 0.7 dD | 658.8 ± 0.3 bB | 921.3 ± 0.7 bB | |
| Orange pulp | ||||||||
| LLR15 | Crude | 186.4 ± 0.8 cB | 3.7 ± 0.9 dD | 0.5 ± 0.1 eE | 63.67 ± 1.1 hD | 9.9 ± 1.0 fCA | 10.0 ± 1.5 hD | 25.2 ± 1.9 hE |
| Mi | 212.9 ± 0.7 bA | 22.5 ± 0.6 bA | 7.6 ± 0.8 dB | 1428.9 ± 1.2 dA | 40.5 ± 0.9 dB | 408.4 ± 1.7 dA | 1997.3 ± 1.7 bA | |
| S | 172.9 ± 0.7 dC | 13.7 ± 0.8 cB | 1.9 ± 0.2 eD | 989.2 ± 1.6 eB | 39.8 ± 0.8 dB | 110.7 ± 1.9 fC | 358.7 ± 1.5 eC | |
| B | 185.6 ± 0.8 cB | 13.1 ± 0.8 cB | 30.0 ± 0.9 bA | 1537.4 ± 1.7 dA | 55.2 ± 0.7 dA | 280.2 ± 2.0 eB | 768.6 ± 1.3 dB | |
| Op | 200.0 ± 0.6 bA | 5.5 ± 0.5 dC | 4.7 ± 0.7 dC | 840.7 ± 1.7 eB | 50.9 ± 0.6 dA | 132.9 ± 1.8 fC | 498.2 ± 1.1 eC | |
| Owp | 198.7 ± 0.7 bB | 6.1 ± 0.6 dC | 5.7 ± 0.8 dB | 472.1 ± 1.5 fC | 37.2 ± 0.5 dB | 57.3 ± 1.6 gD | 179.4 ± 0.9 fD | |
| 5623 | Crude | 132.2 ± 0.8 dB | 1.3 ± 0.9 eC | 0.2 ± 0.0 fE | 148.9 ± 0.8 gD | 22.3 ± 0.4 eD | 5.5 ± 1.4 iD | 55.9 ± 0.7 gE |
| Mi | 125.1 ± 0.9 dC | 22.6 ± 0.8 bA | 55.6 ± 0.6 aA | 2565.1 ± 1.0 cA | 143.4 ± 0.3 bB | 450.5 ± 1.2 dB | 1837.8 ± 0.5 bA | |
| S | 126.7 ± 1.0 dC | 14.7 ± 0.7 cB | 11.3 ± 0.7 cC | 2213.0 ± 1.9 cB | 102.0 ± 0.2 bC | 362.5 ± 1.0 eC | 840.4 ± 0.3 dC | |
| B | 108.2 ± 0.9 eD | 15.3 ± 0.9 cB | 2.4 ± 0.8 eD | 1523.9 ± 1.5 dC | 115.5 ± 0.1 cB | 300.8 ± 0.8 eC | 573.4 ± 0.1 eD | |
| Op | 197.2 ± 0.7 bA | 25.5 ± 0.8 bA | 36.1 ± 0.9 bB | 2924.6 ± 1.3 cA | 190.7 ± 0.2 bA | 727.3 ± 0.6 cA | 1375.2 ± 0.2 cB | |
| Owp | 139.8 ± 0.6 dB | 23.5 ± 0.6 bA | 45.8 ± 0.7 aA | 2593.6 ± 1.0 cA | 192.9 ± 0.3 bA | 391.7 ± 0.4 eB | 1855.4 ± 0.3 bA | |
| 2603 | Crude | 137.5 ± 1.2 dC | Nd | 0.1 ± 0.0 fB | 69.1 ± 1.3 hD | 7.7 ± 0.4 fD | 7.3 ± 0.2 iE | 18.4 ± 0.4 hE |
| Mi | 133.1 ± 1.3 dC | Nd | Nd | 2714.8 ± 1.1 cB | 111.7 ± 0.5 cB | 701.2 ± 0.3 cC | 2292.9 ± 0.5 bA | |
| S | 150.2 ± 0.8 dA | Nd | Nd | 2423.0 ± 1.4 cB | 105.5 ± 0.6 cB | 669.5 ± 0.4 cdC | 882.6 ± 0.6 dC | |
| B | 141.0 ± 0.9 dB | Nd | 6.5 ± 0.2 dA | 3451.4 ± 1.8 bA | 127.6 ± 0.7 cA | 1224.9 ± 0.5 bB | 1781.8 ± 0.7 cB | |
| Op | 110.6 ± 0.7 eD | Nd | Nd | 3119.8 ± 1.6 bA | 134.9 ± 0.8 cA | 1447.2 ± 0.6 bA | 1984.1 ± 0.8 bA | |
| Owp | 142.9 ± 1.0 dB | Nd | Nd | 998.9 ± 1,7 ec | 40.9 ± 0.9 dC | 240.3 ± 0.7 eD | 507.7 ± 0.9 eD | |
| Beauregard | Crude | 170.8 ± 1.1 cB | Nd | Nd | 769.8 ± 1.5 eD | Nd | 443.3 ± 0.8 dD | 711.9 ± 1.0 dD |
| Mi | 136.4 ± 1.3 dC | Nd | Nd | 2612.8 ± 1.9 cB | Nd | 1813.9 ± 0.9 bB | 2454.9 ± 1.1 bB | |
| S | 103.3 ± 0.9 eD | Nd | Nd | 1331.8 ± 1.7 dC | Nd | 1537.1 ± 1.0 bB | 1750.3 ± 1.2 cC | |
| B | 123.3 ± 0.8 dC | Nd | Nd | 1616.2 ± 1.8 dC | Nd | 1211.7 ± 1.1 bB | 919.8 ± 1.3 dD | |
| Op | 258.4 ± 0.9 aA | Nd | Nd | 3223.4 ± 1.9 bA | Nd | 5058.3 ± 1.2 aA | 4601.3 ± 1.4 aA | |
| Owp | 178.4 ± 1.0 cB | Nd | Nd | 1465.8 ± 0.8 dC | Nd | 922.6 ± 1.3 cC | 1256.1 ± 1.5 cC | |
| 3513 | Crude | 270.3 ± 1.1 aA | 10.7 ± 0.8 cD | 0.04 ± 0.0 gB | 114.4 ± 0.9 gD | 14.9 ± 0.8 eC | 7.8 ± 1.4 iD | 103.8 ± 1.6 fD |
| Mi | 205.1 ± 0.9 bB | 26.9 ± 0.6 bC | 12.1 ± 0.2 cA | 3195.9 ± 0.7 bB | 122.2 ± 0.7 cB | 549.1 ± 1.5 dB | 2080.4 ± 1.7 bB | |
| S | 243.3 ± 0.7 aA | 71.6 ± 0.8 aA | Nd | 5296.8 ± 0.8 aA | 235.3 ± 0.2 aA | 1255.9 ± 1.6 bA | 1621.3 ± 1.8 cC | |
| B | 187.1 ± 1.1 cC | 53.5 ± 0.9 aB | Nd | 3952.5 ± 0.9 bB | 235.3 ± 0.7 aA | 1164.1 ± 1.7 bA | 2334.0 ± 1.9 bB | |
| Op | 179.7 ± 0.9 cC | 29.0 ± 0.7 bC | Nd | 2011.4 ± 0.8 cC | 135.4 ± 0.9 cB | 244.4 ± 1.8 eC | 1013.1 ± 2.0 dC | |
| Owp | 201.1 ± 1.0 bB | 55.9 ± 0.9 aB | Nd | 5255.9 ± 0.5 aA | 269.3 ± 0.8 aA | 1060.1 ± 1.9 bA | 4508.1 ± 2.1 aA | |
| Gen. | Cook. | Rutin | Luteolin | Quercetin | 3-O-Methyl | Kaempferol | Cyan-3-O-glu | Cyan-3,5-di | Delphi-3-O-glu | Malv-3,5-di | Perlag-3-O-glu | Mal-3-O-glu | Peonid-3-O-glu |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Purple pulp | |||||||||||||
| JNRX1 | Crude | 16.2 ± 1.1 dC * | Nd | Nd | Nd | Nd | 203.3 ± 11.0 aA | 23.1 ± 1.1 cB | 231.1 ± 1.3 bA | 645.0 ± 1.6 aA | Nd | 321.5 ± 1.6 cA | 120.9 ± 1.9 cC |
| Mi | 126.6 ± 1.6 bA | Nd | Nd | Nd | Nd | 99.6 ± 1.9 dD | 18.5 ± 1.9 dC | 15.8 ± 1.0 fE | 192.6 ± 1.3 cD | Nd | 337.8 ± 1.5 cA | 119.6 ± 1.6 cC | |
| S | 134.0 ± 1.2 bA | Nd | Nd | Nd | Nd | 265.4 ± 1.8 aA | 17.0 ± 1.6 dD | 41.0 ± 0.9 eD | 215.9 ± 1.8 bC | Nd | 227.4 ± 1.8 cB | 82.3 ± 1.3 dD | |
| B | 7.5 ± 1.5 eD | Nd | Nd | Nd | Nd | 115.0 ± 1.7 dC | 57.3 ± 1.8 bA | Nd | 257.7 ± 1.9 bC | Nd | 244.4 ± 0.8 cB | 231.7 ± 1.7 aB | |
| Op | 100.2 ± 1.3 bB | Nd | Nd | Nd | Nd | 217.8 ± 1.9 aA | 19.8 ± 1.3 dC | 141.1 ± 1.7 cB | 323.6 ± 1.3 bB | Nd | 291.3 ± 1.9 cB | 189.8 ± 0.9 bA | |
| Owp | 92.8 ± 1.1 bB | Nd | Nd | Nd | Nd | 189.0 ± 1.8 bB | 17.6 ± 1.0 dD | 78.5 ± 1.3 dC | 222.7 ± 1.9 bC | Nd | 172.3 ± 1.8 dC | 62.7 ± 1.9 dd | |
| JNRX2 | Crude | 15.1 ± 0.9 dD | 4.5 ± 1.8 cB | 13.0 ± 1.3 dA | 0.2 ± 0.1 hC | 2.3 ± 0.2 dC | 279.9 ± 1.7 aA | 179.9 ± 1.9 aA | 831.4 ± 1.9 aA | 252.0 ± 1.4 bA | 673.3 ± 1.9 aA | 416.6 ± 1.7 bA | 205.1 ± 1.6 aA |
| Mi | 18.4 ± 0.9 dC | 5.8 ± 1.6 cB | 11.6 ± 1.2 dB | 0.9 ± 0.2 gA | 6.3 ± 2.1 cA | 134.4 ± 1.8 cC | 7.2 ± 0.9 eC | 133.7 ± 1.3 cD | 51.5 ± 1.7 dC | 246.6 ± 1.6 cC | 160.1 ± 1.3 dC | 103.8 ± 1.3 cB | |
| S | 42.3 ± 1.1 cA | 11.4 ± 1.4 bA | 9.4 ± 0.6 dC | 0.6 ± 0.3 gB | 4.3 ± 1.3 cB | 136.3 ± 1.9 cC | 6.2 ± 1.0 eC | 172.6 ± 1.6 cD | 59.2 ± 1.9 dC | 275.5 ± 1.3 cC | 273.1 ± 1.9 cB | 137.1 ± 1.7 cB | |
| B | 24.4 ± 1.3 dC | 5.1 ± 0.5 cB | 8.8 ± 1.0 eC | 0.3 ± 0.1 hC | 2.7 ± 0.9 dC | 116.4 ± 2.1 dD | 8.6 ± 1.1 eC | 149.8 ± 1.7 cD | 41.9 ± 1.5 eD | 250.1 ± 1.7 cC | 212.8 ± 1.7 cB | 123.9 ± 1.2 cB | |
| Op | 43.4 ± 1.8 cA | 2.1 ± 0.8 dC | 7.5 ± 0.9 eC | 0.2 ± 0.1 hC | 1.1 ± 0.3 eD | 152.0 ± 1.5 bB | 39.7 ± 1.7 cB | 310.6 ± 1.7 bB | 71.5 ± 1.0 dB | 345.8 ± 1.6 bB | 197.3 ± 1.3 dB | 133.8 ± 1.7 cB | |
| Owp | 35.9 ± 1.7 cB | 3.0 ± 0.3 dC | 4.8 ± 0.2 fF | Nd | 2.3 ± 0.3 dC | 20.1 ± 1.7 eE | 37.5 ± 1.1 cB | 252.5 ± 1.9 bC | 58.5 ± 1.3 dC | 317.7 ± 0.9 bB | 152.4 ± 1.0 dC | 122.9 ± 1.1 cB | |
| JNRX7 | Crude | 14.2 ± 1.8 dD | 1.0 ± 0.1 eC | 2.8 ± 0.8 gD | 0.1 ± 0.0 hD | 0.8 ± 0.2 fC | 27.0 ± 1.6 eA | 25.4 ± 1.6 cC | 236.1 ± 1.3 bA | 65.8 ± 1.0 dA | Nd | 493.4 ± 1.8 bA | 151.6 ± 1.6 cS |
| Mi | 24.6 ± 1.6 dC | 1.5 ± 0.3 eC | 8.5 ± 0.5 eB | 0.2 ± 0.1 hC | 1.5 ± 0.3 eA | 12.8 ± 1.9 fD | 22.7 ± 1.0 cC | 55.9 ± 1.8 eD | 23.0 ± 1.6 fC | Nd | 243.2 ± 1.9 cC | 88.7 ± 1.3 dB | |
| S | 34.3 ± 1.7 cB | 4.4 ± 0.2 cB | 4.7 ± 0.2 fC | 0.1 ± 0.0 hD | 1.0 ± 0.1 eC | 18.5 ± 1.7 fC | 29.2 ± 1.2 cC | 71.6 ± 1.4 dC | 31.8 ± 1,7 eB | Nd | 339.8 ± 1.8 cB | 92.6 ± 1.3 dB | |
| B | 37.2 ± 1.8 cB | 5.6 ± 0.8 cB | 7.4 ± 0.5 eB | 0.9 ± 0.3 gB | 1.2 ± 0.2 eB | 23.8 ± 1.5 eB | 46.2 ± 1.7 bB | 59.9 ± 1.7 eD | 37.4 ± 0.7 eB | Nd | 296.4 ± 0.9 cC | 99.4 ± 0.8 dB | |
| Op | 34.8 ± 2.0 cB | 8.2 ± 0.9 bA | 13.1 ± 0.3 dA | 4.2 ± 1.0 eA | 1.2 ± 0.4 eB | 23.9 ± 1.9 eB | 40.4 ± 1.5 bB | 78.3 ± 1.6 dB | 3.7 ± 1.0 gD | Nd | 221.0 ± 1.7 cC | 65.5 ± 1.8 dC | |
| Owp | 42.5 ± 1.3 cA | 8.8 ± 1.1 bA | 11.5 ± 0.9 dA | Nd | 1.2 ± 0.6 eB | 24.2 ± 1.3 eB | 54.0 ± 1.8 bA | 81.6 ± 1.1 dB | 34.7 ± 1.3 eB | Nd | 279.5 ± 1.9 cC | 74.0 ± 1.8 dC | |
| JNRX12 | Crude | 11.2 ± 1.0 dD | 37.4 ± 0.9 aA | 36.1 ± 0.6 cC | 14.8 ± 0.5 dE | 10.1 ± 0.7 bD | 28.7 ± 0.6 eB | Nd | 352.0 ± 0.9 bA | Nd | Nd | 1490.4 ± 0.7 aA | 367.5 ± 0.8 aA |
| Mi | 1.9 ± 1.1 fE | 34.5 ± 0.7 aB | 36.1 ± 0.9 cC | 35.2 ± 0.7 cD | 13.2 ± 0.4 bC | 19.1 ± 0.9 fB | Nd | 40.4 ± 0.8 eD | Nd | Nd | 274.9 ± 0.9 cD | 106.5 ± 0.9 cC | |
| S | 17.3 ± 0.9 dD | 31.8 ± 0.8 aC | 7.1 ± 0.8 eD | 81.2 ± 0.6 aA | 48.0 ± 0.8 aA | 23.1 ± 0.3 eB | Nd | 106.7 ± 1.7 cB | Nd | Nd | 450.5 ± 0.6 bB | 185.3 ± 0.7 bB | |
| B | 44.5 ± 0.6 cB | 32.9 ± 0.6 aB | 171.5 ± 0.7 bB | 48.2 ± 0.6 bC | 44.3 ± 0.3 aB | 13.0 ± 0.7 fC | Nd | 91.6 ± 1.9 dC | Nd | Nd | 166.0 ± 0.8 dE | 76.1 ± 0.8 dD | |
| Op | 57.7 ± 0.8 cA | 37.4 ± 1.1 aA | 369.9 ± 0.9 aA | 67.6 ± 0.7 bB | 44.8 ± 0.5 aB | 19.6 ± 0.5 eB | Nd | 88.2 ± 1.8 dC | Nd | Nd | 317.6 ± 0.8 cC | 186.5 ± 0.9 bB | |
| Owp | 33.7 ± 0.7 cC | 32.3 ± 1.2 aC | 6.8 ± 0.3 fD | 16.0 ± 0.9 dE | 12.6 ± 0.5 bD | 138.5 ± 0.4 cA | Nd | 95.2 ± 1.5 dC | Nd | Nd | 328.5 ± 0.6 cC | 165.3 ± 0.5 bB | |
| Trabuca | Crude | 30.0 ± 0.9 cD | 1.4 ± 0.1 eC | 3.6 ± 0.2 fC | 0.1 ± 0.0 hC | 1.1 ± 0.2 eD | 119.2 ± 1.1 dD | 107.7 ± 0.8 aA | 135.2 ± 1.3 bA | 51.5 ± 0.9 dB | 41.1 ± 0.9 fD | 37.6 ± 0.9 gD | 53.0 ± 0.8 dD |
| Mi | 111.1 ± 0.8 bC | 5.9 ± 0.3 cA | 17.0 ± 0.3 dA | 2.3 ± 0.3 fA | 12.6 ± 0.9 bA | 120.8 ± 1.0 cD | 43.4 ± 0.9 bD | 63.5 ± 1.1 eC | 61.5 ± 1.1 dB | 61.5 ± 0.6 eC | 79.1 ± 0.7 eB | 108.1 ± 0.9 cC | |
| S | 253.3 ± 1.0 aA | 5.6 ± 0.3 cA | 10.8 ± 0.6 dB | 1.7 ± 0.2 fB | 5.1 ± 0.6 cB | 126.5 ± 1.1 cC | 48.3 ± 0.7 bD | 72.8 ± 1.0 dB | 47.7 ± 1.0 eB | 74.4 ± 0.8 eB | 99.0 ± 0.9 eA | 356.8 ± 1.1 aA | |
| B | 133.4 ± 0.9 bC | 2.2 ± 0.1 dB | 2.7 ± 0.5 gD | 0.3 ± 0.1 hC | 2.0 ± 0.2 dC | 127.7 ± 0.9 cC | 41.7 ± 0.7 bD | 54.1 ± 0.9 eC | 8.2 ± 1.1 gC | 80.7 ± 1.1 eB | 51.3 ± 0.9 fC | 118.2 ± 0.9 cC | |
| Op | 160.5 ± 0.7 bB | 2.6 ± 0.3 dB | 2.1 ± 0.4 gD | 2.1 ± 0.3 fA | 2.5 ± 0.5 dC | 151.8 ± 1.1 bB | 56.7 ± 0.8 bC | 45.7 ± 0.7 eD | 135.0 ± 0.9 cA | 142.4 ± 1.2 dA | 55.2 ± 0.8 fC | 181.4 ± 0.7 bB | |
| Owp | 283.8 ± 0.5 aA | 1.8 ± 0.1 dC | 2.3 ± 0.3 gD | Nd | 1.3 ± 0.8 eD | 211.1 ± 0.8 aA | 61.4 ± 0.9 bB | 41.4 ± 1.0 eD | 100.5 ± 0.8 cA | 66.5 ± 0.9 eC | 73.8 ± 1.0 eB | 120.1 ± 0.8 cC | |
| Orange pulp | |||||||||||||
| LLR15 | Crude | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd |
| Mi | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | |
| S | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | |
| B | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | |
| Op | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | |
| Owp | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | |
| 5623 | Crude | 1.9 ± 0.4 bB | 3.9 ± 0.1 bB | 1.46 ± 0.2 bB | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd |
| Mi | 24.9 ± 0.4 aA | 10.5 ± 0.5 aA | 13.22 ± 0.6 aA | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | |
| S | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | |
| B | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | |
| Op | Nd | 4.7 ± 0.3 bB | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | |
| Owp | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | |
| 2603 | Crude | 0.4 ± 0.1 bA | Nd | 1.2 ± 0.2 bA | 0.5 ± 0.1 dA | 1.06 ± 0.2 dA | Nd | Nd | Nd | Nd | Nd | Nd | Nd |
| Mi | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | |
| S | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | |
| B | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | |
| Op | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | |
| Owp | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | |
| Beauregard | Crude | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd |
| Mi | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | |
| S | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | |
| B | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | |
| Op | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | |
| Owp | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | |
| 3513 | Crude | Nd | Nd | Nd | 1.9 ± 1.1 bB | 5.7 ± 0.5 bC | Nd | Nd | Nd | Nd | Nd | Nd | Nd |
| Mi | Nd | Nd | Nd | 0.6 ± 0.1 cC | 1.3 ± 0.7 dD | Nd | Nd | Nd | Nd | Nd | Nd | Nd | |
| S | Nd | Nd | Nd | 0.8 ± 0.2 cC | 3.9 ± 0.6 cC | Nd | Nd | Nd | Nd | Nd | Nd | Nd | |
| B | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | |
| Op | Nd | Nd | Nd | 3.2 ± 0.5 aA | 19.1 ± 0.4 aA | Nd | Nd | Nd | Nd | Nd | Nd | Nd | |
| Owp | Nd | Nd | Nd | Nd | 14.3 ± 0.5 aB | Nd | Nd | Nd | Nd | Nd | Nd | Nd | |
| Gen. | Cooking | Histamine | Putrescine | Cadaverine | Tyramine | Dopamine | Serotonin | Spermine | Spermidine | Tryptamine | AI |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Orange pulp | |||||||||||
| LLR15 * | Crude | 39.3 ± 1.2 fB * | 66.3 ± 1.6 cB | Nd | 1.4 ± 0.1 dC | 3.3 ± 0.4 eC | 11.4 ± 1.1 eC | 4.8 ± 0.8 bA | 4.5 ± 0.2 eC | 37.6 ± 2.4 bNs | 0.44 cC |
| Mi | 81.3 ± 2.6 bA | 101.0 ± 1.4 aA | Nd | 1.0 ± 0.1 dC | 4.1 ± 0.6 eB | 21.6 ± 1.2 dA | 5.7 ± 1.5 aA | 5.9 ± 0.1 dB | 30.9 ± 2.6 c | 0.49 cB | |
| S | 41.2 ± 1.4 fB | 57.1 ± 0.6 dC | Nd | 0.3 ± 0.0 dD | 6.0 ± 0.5 dA | 14.6 ± 0.6 eB | 3.5 ± 0.5 cB | 6.0 ± 0.1 dB | 31.0 ± 2.5 c | 0.33 dC | |
| B | 43.0 ± 1.3 fB | 57.3 ± 1.1 dC | Nd | 2.9 ± 0.6 dB | 4.2 ± 0.4 eB | 14.0 ± 0.6 eB | 3.2 ± 0.4 cB | 7.7 ± 0.5 cA | 31.2 ± 2.2 c | 0.36 dC | |
| Op | 41.2 ± 1.1 fB | 57.4 ± 0.9 dC | Nd | 2.9 ± 0.6 dB | 2.5 ± 0.5 eC | 9.5 ± 0.4 eC | 3.7 ± 0.5 cB | 5.7 ± 0.1 cB | 30.8 ± 2.2 c | 0.48 cB | |
| Owp | 37.8 ± 1.8 fB | 56.7 ± 0.2 dC | Nd | 3.7 ± 0.5 dA | 1.8 ± 0.6 eD | 6.1 ± 0.2 eD | 3.6 ± 0.1 cB | 4.8 ± 0.5 dC | 31.2 ± 0.1 c | 0.60 bA | |
| 5623 | Crude | 7.3 ± 2.4 hD | 36.2 ± 0.2 eC | Nd | 0.1 ± 0.0 dC | 4.7 ± 0.4 eC | 19.6 ± 0.3 dC | 2.7 ± 0.2 cC | 6.6 ± 0.1 dC | 37.7 ± 0.5 bB | 0.13 fC |
| Mi | 102.6 ± 2.1 bA | 84.0 ± 0.3 bB | Nd | 0.1 ± 0.0 dC | 11.2 ± 0.0 cA | 57.1 ± 0.1 cA | 2.3 ± 0.2 dC | 5.9 ± 0.2 dD | 42.1 ± 2.6 aA | 0.24 eB | |
| S | 96.3 ± 1.4 bB | 98.3 ± 0.1 aA | Nd | 1.3 ± 0.4 dA | 8.2 ± 0.5 dB | 31.3 ± 0.1 dB | 3.9 ± 0.2 bB | 8.9 ± 0.2 cA | 41.8 ± 1.1 aA | 0.37 dA | |
| B | 36.2 ± 1.1 fC | 30.8 ± 0.3 fC | Nd | 0.5 ± 0.0 dB | 6.4 ± 0.5 dB | 9.3 ± 0.4 eD | 5.0 ± 0.1 bA | 7.1 ± 0.1 cC | 38.1 ± 0.4 bB | 0.24 eB | |
| Op | 86.4 ± 1.8 bB | 23.9 ± 0.5 fD | Nd | 1.1 ± 0.3 dA | 13.1 ± 0.6 cA | 6.7 ± 0.6 eD | 3.2 ± 0.1 cB | 6.8 ± 0.6 dC | 29.5 ± 2.1 cC | 0.37 dA | |
| Owp | 114.1 ± 1.6 aA | 28.8 ± 1.2 fD | Nd | 1.5 ± 0.1 dA | 15.3 ± 0.4 cA | 17.8 ± 0.4 eC | 5.0 ± 0.5 bA | 7.9 ± 0.5 cB | 43.3 ± 1.8 aA | 0.31 dA | |
| 2306 | Crude | 37.1 ± 0.2 fD | 34.3 ± 0.4 eD | Nd | 1.1 ± 0.2 dA | 2.7 ± 0.3 eC | 36.7 ± 0.4 dB | 4.4 ± 0.6 bA | 5.0 ± 0.1 eB | 25.4 ± 1.6 dNs | 0.15 fC |
| Mi | 95.2 ± 4.2 aA | 67.9 ± 0.6 cB | Nd | 0.6 ± 0.0 dB | 8.9 ± 0.1 dB | 73.8 ± 0.6 cA | 3.0 ± 0.1 cB | 4.2 ± 0.2 eC | 27.5 ± 1.4 c | 0.18 eC | |
| S | 82.8 ± 1.6 bB | 60.5 ± 0.5 dC | Nd | 0.7 ± 0.0 dB | 8.3 ± 1.2 dB | 19.7 ± 0.3 dD | 3.2 ± 0.6 cB | 3.9 ± 0.1 eD | 23.4 ± 1.5 d | 0.41 cA | |
| B | 88.3 ± 2.3 bB | 73.7 ± 0.3 cA | Nd | 0.4 ± 0.0 dB | 10.8 ± 1.5 cA | 29.1 ± 0.4 dC | 3.7 ± 0.4 cB | 5.4 ± 0.2 eB | 20.1 ± 2.4 e | 0.33 dB | |
| Op | 57.6 ± 2.5 dC | 68.1 ± 0.2 cB | Nd | 1.1 ± 0.5 dA | 12.4 ± 1.8 cA | 25.8 ± 0.4 dC | 2.1 ± 0.5 dC | 5.6 ± 0.1 dB | 23.8 ± 1.6 d | 0.28 eB | |
| Owp | 52.2 ± 5.1 eC | 34.7 ± 0.1 eD | Nd | 0.9 ± 0.0 dA | 2.1 ± 1.1 eC | 22.8 ± 0.8 dC | 0.6 ± 0.0 eD | 6.2 ± 0.5 dA | 26.3 ± 0.5 d | 0.28 eB | |
| Beaudegard | Crude | 56.7 ± 4.3 dA | 36.9 ± 0.8 eA | Nd | 0.4 ± 0.0 dE | 14.5 ± 1.6 cC | 1.9 ± 0.4 eE | 1.3 ± 0.1 eD | 5.0 ± 0.4 eC | 28.9 ± 0.4 cNs | 0.42 cB |
| Mi | 14.1 ± 1.2 hD | 5.1 ± 0.9 hD | Nd | 12.7 ± 1.0 bB | 15.3 ± 2.4 cC | 58.7 ± 1.3 cB | 2.1 ± 0.2 dC | 23.6 ± 0.2 aA | 30.0 ± 0.5 c | 0.03 gC | |
| S | 21.7 ± 1.8 gC | 4.0 ± 0.20 hD | Nd | 6.5 ± 0.6 cD | 15.6 ± 1.4 cC | 28.6 ± 1.2 dD | 5.1 ± 0.6 bA | 19.0 ± 0.1 bB | 33.1 ± 0.1 c | 0.05 gB | |
| B | 19.3 ± 2.1 gC | 24.8 ± 0.4 fB | Nd | 9.9 ± 0.2 bC | 13.4 ± 1.5 cC | 37.5 ± 1.2 dC | 3.9 ± 0.4 bB | 3.2 ± 0.5 eD | 30.9 ± 0.5 c | 0.09 gA | |
| Op | 26.0 ± 2.3 gB | 29.5 ± 1.2 fB | Nd | 12.3 ± 0.2 bB | 26.2 ± 2.1 aA | 87.0 ± 2.6 bA | 4.2 ± 0.2 bB | 6.9 ± 0.6 dC | 33.1 ± 1.3 c | 0.05 gB | |
| Owp | 23.0 ± 2.2 gB | 16.7 ± 0.6 gC | Nd | 31.3 ± 1.5 aA | 20.3 ± 2.5 bB | 23.6 ± 2.4 dD | 4.2 ± 0.2 bB | 15.4 ± 0.5 bB | 30.8 ± 1.4 c | 0.11 fA | |
| 3513 | Crude | 48.9 ± 1.0 eD | 82.0 ± 1.6 bC | Nd | 1.3 ± 0.6 dB | 4.8 ± 1.1 eC | 1.9 ± 0.1 eE | 5.1 ± 0.1 bB | 6.3 ± 0.5 dA | 29.3 ± 2.4 cB | 0.73 aA |
| Mi | 97.8 ± 0.3 aA | 92.0 ± 1.5 aB | Nd | 2.1 ± 0.1 dA | 3.5 ± 0.5 eC | 44.0 ± 1.2 dC | 6.6 ± 0.2 aA | 4.9 ± 0.4 B | 37.8 ± 3.1 bA | 0.33 dB | |
| S | 84.6 ± 3.0 bB | 85.5 ± 1.1 bC | Nd | 1.4 ± 0.1 dB | 1.3 ± 0.4 eD | 68.8 ± 2.3 cC | 4.7 ± 0.1 bB | 3.7 ± 0.1 eC | 38.2 ± 2.6 bA | 0.22 eC | |
| B | 87.3 ± 1.8 bB | 102.7 ± 2.0 aA | Nd | 1.0 ± 0.1 dB | 10.9 ± 1.3 cB | 95.7 ± 2.1 bB | 6.2 ± 0.1 aA | 4.7 ± 0.2 eB | 32.5 ± 7.8 cB | 0.16 fD | |
| Op | 11.5 ± 0.5 hE | 23.9 ± 2.3 fD | Nd | Nd | 3.5 ± 0.4 eC | 3.4 ± 0.9 eD | 2.7 ± 0.0 cC | 1.8 ± 0.1 eD | 16.1 ± 0.5 eC | 0.31 dB | |
| Owp | 67.0 ± 5.1 cC | 95.0 ± 5.1 aB | Nd | 2.3 ± 0.1 dA | 19.5 ± 0.5 bA | 162.2 ± 8.5 aA | 2.9 ± 0.1 cC | 4.7 ± 0.1 eB | 38.1 ± 2.4 bA | 0.09 gD | |
| Purple pulp | |||||||||||
| JNRX1 | Crude | 20.6 ± 0.4 dE | 7.8 ± 1.4 hD | 5.3 ± 0.4 fD | 0.5 ± 0.0 bC | 3.8 ± 0.5 cD | 3.7 ± 0.1 eC | 1.4 ± 0.3 cB | 2.3 ± 0.5 fD | 22.8 ± 0.3 eB | 0.30 eD |
| Mi | 87.3 ± 2.8 cB | 83.5 ± 1.8 bA | 55.3 ± 1.4 eB | 0.4 ± 0.0 bC | 11.1 ± 0.4 aB | 2.6 ± 0.1 eC | 0.9 ± 0.0 cC | 5.3 ± 0.3 eC | 22.8 ± 0.5 eB | 1.24 aA | |
| S | 75.4 ± 3.0 cC | 22.8 ± 2.0 fB | 47.8 ± 0.0 eB | 1.8 ± 0.1 bB | 11.0 ± 0.7 aB | 10.5 ± 1.5 eB | 2.4 ± 0.8 cA | 7.1 ± 0.0 dB | 21.7 ± 0.1 eC | 0.48 dC | |
| B | 116.6 ± 2.4 bA | 49.5 ± 2.1 dA | 101.3 ± 1.9 bA | 0.9 ± 0.0 bB | 7.7 ± 0.3 bC | 10.4 ± 0.3 eB | 0.9 ± 0.1 cC | 7.4 ± 0.0 dB | 17.1 ± 0.6 fD | 1.01 aA | |
| Op | 86.8 ± 1.0 cB | 49.1 ± 3.9 dA | 98.0 ± 71 bA | 1.2 ± 0.2 bB | 12.8 ± 1.2 aA | 12.6 ± 1.8 eA | 2.3 ± 0.9 cA | 8.4 ± 0.4 dA | 62.2 ± 3.8 aA | 0.65 cB | |
| Owp | 45.2 ± 1.6 dD | 10.2 ± 2.0 hC | 26.3 ± 0.3 eC | 3.1 ± 0.5 bA | 9.1 ± 0.5 bB | 1.0 ± 0.0 eD | 1.6 ± 0.3 cB | 4.4 ± 0.0 eC | 20.2 ± 0.2 fC | 0.52 dC | |
| JNRX2 | Crude | 70.7 ± 2.1 cC | 44.8 ± 1.5 dD | Nd | 4.0 ± 1.1 aC | 12.6 ± 0.8 aB | 57.9 ± 1.1 cC | 4.7 ± 0.6 bB | 9.6 ± 1.1 dC | 29.0 ± 0.9 cC | 0.14 eB |
| Mi | 107.2 ± 0.6 bB | 68.8 ± 0.6 cC | Nd | 3.2 ± 0.6 bD | 12.9 ± 0.2 aB | 56.0 ± 0.5 cC | 7.5 ± 1.3 aA | 13.8 ± 0.2 bB | 29.0 ± 0.3 cC | 0.20 eB | |
| S | 111.9 ± 1.3 bB | 73.7 ± 0.4 bC | Nd | 4.5 ± 0.5 aC | 12.4 ± 0.1 aB | 88.2 ± 0.5 aA | 4.4 ± 0.9 bB | 13.7 ± 0.9 bB | 35.3 ± 0.3 bA | 0.16 eB | |
| B | 184.6 ± 4.3 aA | 114.3 ± 3.9 aA | Nd | 6.7 ± 0.3 aA | 12.6 ± 0.3 aB | 72.6 ± 1.6 bB | 1.5 ± 0.2 C | 16.7 ± 0.6 aA | 31.9 ± 0.1 bB | 0.29 eA | |
| Op | 133.3 ± 1.1 bB | 82.7 ± 0.8 bB | Nd | 6.5 ± 0.2 aA | 14.2 ± 0.3 aA | 51.0 ± 0.5 dC | 0.5 ± 0.0 cD | 15.3 ± 1.8 aA | 35.3 ± 1.5 bA | 0.28 eA | |
| Owp | 89.7 ± 0.7 cC | 57.3 ± 0.7 cD | Nd | 5.4 ± 0.2 aB | 10.4 ± 0.3 aC | 47.8 ± 0.4 dC | 0.4 ± 0.0 cD | 16.2 ± 0.0 aA | 29.6 ± 0.1 c eC | 0.20 eB | |
| JNRX7 | Crude | 22.7 ± 0.3 dD | 12.0 ± 1.4 gC | 21.2 ± 0.2 eD | 4.6 ± 0.4 aB | 8.1 ± 0.4 bD | 7.7 ± 0.2 eC | 0.8 ± 0.1 cC | 6.6 ± 1.4 dC | 21.6 ± 2.8 eC | 0.26 eD |
| Mi | 84.5 ± 1.6 cB | 54.0 ± 0.1 dB | 63.3 ± 0.4 dA | 5.5 ± 0.3 aA | 14.2 ± 1.2 aB | 9.2 ± 0.1 eB | 1. 3 ± 0.1 cB | 12.3 ± 1.7 eA | 19.4 ± 0.1 fC | 0.56 dB | |
| S | 70.8 ± 0.4 cC | 8.6 ± 0.6 hD | 43.1 ± 0.6 eB | 0.5 ± 0.0 bE | 10.7 ± 0.4 aC | 7.3 ± 0.3 eC | 2.1 ± 0.1 cA | 7.9 ± 1.1 dB | 23.1 ± 0.0 eB | 0.35 eC | |
| B | 92.6 ± 1.0 cA | 61.4 ± 0.6 cA | 74.3 ± 0.5 dA | 1.8 ± 0.2 bD | 12.4 ± 0.5 aC | 6.0 ± 0.1 eD | 0.9 ± 0.3 cC | 8.4 ± 0.2 dB | 23.1 ± 1.1 eB | 0.70 cA | |
| Op | 24.1 ± 2.2 dD | 16.7 ± 2.7 gC | 19.5 ± 1.7 eD | 2.5 ± 0.3 bC | 17.3 ± 0.2 aA | 11.3 ± 0.2 eA | 1.6 ± 0.0 cB | 4.9 ± 0.9 eC | 25.6 ± 0.4 eA | 0.28 eD | |
| Owp | 17.8 ± 3.1 dD | 7.9 ± 1.8 hD | 29.6 ± 0.2 eC | 0.8 ± 0.0 bD | 7.4 ± 0.3 bD | 7.0 ± 0.2 eC | 0.7 ± 0.1 cC | 5.6 ± 0.0 eC | 21.3 ± 1.9 eC | 0.27 eD | |
| JNRX12 | Crude | 118.1 ± 2.1 bB | 30.3 ± 2.5 eB | 100.5 ± 1.0 bB | 4.9 ± 0.8 aA | 7.9 ± 0.9 bC | 6.0 ± 0.5 eB | 0.4 ± 0.1 cB | 6.3 ± 0.0 eC | 18.2 ± 0.8 fNs | 0.89 bA |
| Mi | 145.9 ± 1.3 bA | 25.1 ± 0.1 fC | 135.1 ± 1.7 aA | 2.0 ± 0.4 bB | 11.1 ± 1.0 aB | 6.2 ± 0.2 eB | 1.7 ± 0.1 cA | 7.4 ± 0.5 dB | 21.7 ± 0.3 e | 0.87 bA | |
| S | 109.2 ± 1.2 bB | 20.7 ± 1.2 fC | 103.1 ± 0.7 bB | 2.4 ± 0.0 bB | 13.4 ± 0.3 aB | 8.4 ± 0.0 eA | 1.2 ± 0.2 cA | 11.0 ± 0.9 cA | 19.4 ± 1.6 f | 0.63 dB | |
| B | 104.9 ± 4.5 bB | 36.5 ± 2.1 eA | 111.2 ± 1.3 bB | 0.9 ± 0.1 bC | 10.6 ± 0.4 aB | 5.6 ± 0.8 eC | 1.6 ± 0.1 cA | 5.9 ± 0.2 eC | 18.6 ± 0.1 f | 0.80 bA | |
| Op | 78.5 ± 4.3 cC | 29.9 ± 3.4 eB | 84.8 ± 1.4 cC | 1.6 ± 0.1 bB | 15.2 ± 0.2 aA | 6.5 ± 0.5 eB | 1.4 ± 0.0 cA | 6.1 ± 0.4 eC | 22.8 ± 0.6 e | 0.67 cB | |
| Owp | 114.9 ± 6.7 bB | 30.3 ± 1.4 eB | 101.3 ± 3.1 bB | 5.0 ± 0.2 aA | 11.5 ± 0.1 aB | 7.3 ± 0.7 eB | 1.6 ± 0.2 cA | 8.3 ± 0.3 dB | 21.5 ± 0.5 e | 0.74 cB | |
| Trabuca | Crude | 10.2 ± 0.3 dC | 11.0 ± 3.0 hB | Nd | 0.5 ± 0.0 bD | 1.1 ± 0.0 cE | 1.4 ± 0.0 eD | 0.5 ± 0.0 cB | 1.3 ± 0.4 fD | 24.4 ± 0.1 dNs | 0.51 dB |
| Mi | 29.4 ± 0.9 dB | 24.8 ± 1.0 fA | 29.0 ± 0.4 eC | 6.4 ± 0.6 aA | 11.5 ± 0.8 aB | 10.6 ± 0.2 eB | 0.6 ± 0.0 cB | 7.5 ± 1.2 dB | 24.3 ± 1.0 d | 0.30 eD | |
| S | 109.1 ± 4.2 bA | 6.3 ± 1.2 hC | 87.7 ± 2.2 cA | 0.9 ± 0.4 bC | 9.2 ± 0.3 bB | 13.3 ± 0.7 eA | 0.6 ± 0.0 cB | 5.5 ± 0.6 eC | 23.5 ± 0.5 e | 0.71 cA | |
| B | 38.1 ± 1.3 dB | 24.0 ± 0.7 fA | 32.6 ± 1.7 eC | 0.9 ± 0.2 bC | 6.5 ± 0.6 bC | 9.9 ± 0.3 eB | 1.1 ± 0.1 cA | 5.9 ± 1.3 eC | 22.5 ± 0.5 e | 0.41 dC | |
| Op | 30.0 ± 0.6 dB | 24.3 ± 2.6 fA | 40.2 ± 3.7 eB | 2.6 ± 0.1 bB | 13.1 ± 0.3 aA | 13.2 ± 0.3 eA | 1.3 ± 0.2 cA | 9.0 ± 0.8 dA | 24.8 ± 0.0 d | 0.27 eD | |
| Owp | 22.5 ± 0.5 dB | 14.5 ± 2.6 gB | 14.5 ± 1.2 fD | 1.2 ± 0.4 bC | 4.2 ± 0.1 cD | 7.6 ± 0.2 eC | 0.9 ± 0.0 cA | 5.6 ± 1.4 eC | 23.4 ± 0.2 e | 0.29 eD | |
| Genotypes | Cooking | 5-Hydroxytryptophan (mg/100 g) | Tryptophan (mg/100 g) | L-Dopa (µg/100 g) |
|---|---|---|---|---|
| Orange pulp | ||||
| LLR15 * | Crude | 694.0 ± 4.0 aA * | 1192.6 ± 27 aA | 88.8 ± 2.7 dB |
| Mi | 36.3 ± 6.1 dD | 1212.8 ± 57 aA | 91.2 ± 3.4 dA | |
| S | 39.6 ± 1.0 dD | 979.9 ± 6.3 bC | 72.4 ± 2.5 dC | |
| B | 18.4 ± 3.9 eE | 929.0 ± 1.6 bC | 73.8 ± 2.1 dC | |
| Op | 68.3 ± 0.2 dC | 1010.4 ± 24 aB | 72.7 ± 2.2 dC | |
| Owp | 148.9 ± 3.5 cB | 1097.1 ± 12 aB | 75.5 ± 2.1 dC | |
| 5623 | Crude | 596.1 ± 2.0 bA | 540.8 ± 0.4 dA | 36.9 ± 1.5 eD |
| Mi | 44.6 ± 0.4 dB | 651.6 ± 6.4 cA | 29.6 ± 0.4 eD | |
| S | 45.7 ± 0.9 dB | 394.1 ± 7.8 eC | 171.9 ± 0.5 cA | |
| B | 36.5 ± 0.7 dC | 508.0 ± 2.5 dB | 92.1 ± 0.6 dC | |
| Op | 57.6 ± 2.8 dB | 399.7 ± 1.5 dC | 81.7 ± 0.1 dC | |
| Owp | 66.9 ± 1.7 dB | 605.3 ± 11 cA | 133.5 ± 0.2 cB | |
| 2306 | Crude | 644.2 ± 5.6 bA | 838.8 ± 11 bA | 24.7 ± 0.6 eC |
| Mi | 35.6 ± 1.3 dC | 517.7 ± 16 dC | 16.4 ± 0.4 eD | |
| S | 57.4 ± 1.8 dB | 672.9 ± 14 cB | 23.0 ± 1.1 eC | |
| B | 41.7 ± 0.6 dB | 765.4 ± 25 cB | 25.5 ± 1.1 eC | |
| Op | 6.8 ± 0.4 eD | 541.7 ± 26 dC | 36.6 ± 0.5 eB | |
| Owp | 56.3 ± 0.3 dB | 806.4 ± 1.1 bA | 45.8 ± 0.4 eA | |
| Beaudegard | Crude | 605.1 ± 0.1 bA | 633.0 ± 1.1 cA | 23.7 ± 0.5 eE |
| Mi | 33.3 ± 1.2 dD | 691.1 ± 24 cA | 313.6 ± 6.4 bB | |
| S | 30.6 ± 1.6 eD | 558.4 ± 14 dB | 328.5 ± 5.4 bB | |
| B | 24.3 ± 0.9 eD | 508.4 ± 25 eB | 67.7 ± 0.5 dD | |
| Op | 116.8 ± 1.9 cB | 529.7 ± 26 dB | 182.0 ± 0.6 cC | |
| Owp | 50.4 ± 4.3 dC | 670.4 ± 25 cA | 412.3 ± 4.7 aA | |
| 3513 | Crude | 637.5 ± 8.1 bA | 1154.8 ± 35 aA | 119.2 ± 5.4 cB |
| Mi | 54.3 ± 5.1 dB | 1023.4 ± 36 aA | 146.2 ± 1.1 cA | |
| S | 53.6 ± 4.5 dB | 752.9 ± 28 cB | 45.1 ± 0.5 eC | |
| B | 46.6 ± 1.2 dB | 846.6 ± 24 bB | 53.7 ± 0.1 eC | |
| Op | 41.8 ± 7.1 dB | 284.6 ± 11 eC | 15.4 ± 0.3 eD | |
| Owp | 87.2 ± 3.5 dB | 690.7 ± 23 cB | 38.8 ± 5.1 eC | |
| Purple pulp | ||||
| JNRX1 | Crude | 225.9 ± 3.2 dD | 692.8 ± 7.6 bA | 33.8 ± 0.8 cE |
| Mi | 425.1 ± 5.1 cC | 715.4 ± 4.1 bA | 26.9 ± 0.9 cE | |
| S | 554.3 ± 1.9 bB | 627.4 ± 1.7 cB | 87.0 ± 2.3 bB | |
| B | 503.5 ± 4.6 bC | 409.5 ± 7.6 dC | 52.4 ± 0.1 cC | |
| Op | 679.5 ± 4.2 aA | 541.9 ± 4.7 dB | 100.5 ± 0.5 bA | |
| Owp | 559.2 ± 6.2 bB | 398.3 ± 1.4 dD | 41.0 ± 0.8 cD | |
| JNRX2 | Crude | 375.6 ± 0.7 cA | 361.8 ± 1.2 eA | 77.5 ± 1.8 bD |
| Mi | 11.2 ± 0.3 eD | 359.4 ± 2.1 eA | 87.8 ± 3.7 bC | |
| S | 67.7 ± 0.4 eC | 248.5 ± 0.8 eB | 87.5 ± 1.1 bC | |
| B | 89.6 ± 0.6 eB | 255.6 ± 3.8 eB | 94.8 ± 1.1 bB | |
| Op | 10.1 ± 0.1 eD | 253.2 ± 0.5 eB | 107.2 ± 2.5 bA | |
| Owp | 15.2 ± 0.5 eD | 259.1 ± 3.1 eB | 109.6 ± 3.1 bA | |
| JNRX7 | Crude | 456.3 ± 5.0 cB | 848.9 ± 1.2 aA | 114.6 ± 3.5 bB |
| Mi | 341.5 ± 4.6 dC | 855.7 ± 6.1 aA | 200.2 ± 2.9 aA | |
| S | 258.0 ± 3.7 dD | 673.1 ± 4.4 cD | 68.8 ± 1.6 bC | |
| B | 563.3 ± 4.2 bB | 819.5 ± 1.3 aB | 55.9 ± 0.9 cD | |
| Op | 701.2 ± 2.3 aA | 822.4 ± 6.7 aB | 45.6 ± 0.8 cE | |
| Owp | 263.6 ± 4.4 dD | 716.5 ± 4.2 bC | 56.5 ± 0.2 cD | |
| JNRX12 | Crude | 557.2 ± 3.7 bA | 979.9 ± 2.9 aA | 55.3 ± 0.4 cD |
| Mi | 562.5 ± 1.2 bA | 865.5 ± 1.9 aC | 35.2 ± 1.0 cD | |
| S | 429.6 ± 1.0 cB | 762.6 ± 2.3 bD | 147.2 ± 2.6 aB | |
| B | 487.7 ± 1.8 cB | 835.3 ± 5.7 aC | 155.8 ± 2.4 aB | |
| Op | 561.6 ± 1.3 bA | 893.9 ± 3.2 aB | 93.7 ± 4.8 bC | |
| Owp | 370.1 ± 1.4 cC | 898.7 ± 1.8 aB | 192.6 ± 4.1 aA | |
| Trabuca | Crude | 294.2 ± 4.8 dB | 474.4 ± 8.3 dA | 20.5 ± 0.3 cC |
| Mi | 252.4 ± 1.9 dC | 326.5 ± 8.3 eB | 98.4 ± 0.7 bA | |
| S | 272.4 ± 5.3 dC | 331.9 ± 4.2 eB | 12.5 ± 0.5 cD | |
| B | 304.3 ± 5.3 dB | 363.9 ± 7.5 eB | 33.7 ± 1.2 cC | |
| Op | 380.1 ± 3.4 cA | 331.0 ± 7.9 eB | 178.0 ± 8.1 aA | |
| Owp | 233.7 ± 1.1 dC | 350.5 ± 8.8 eB | 92.3 ± 1.7 bB | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basílio, L.S.P.; Nunes, A.; Minatel, I.O.; Diamante, M.S.; Di Lázaro, C.B.; Silva, A.C.A.F.e.; Vargas, P.F.; Vianello, F.; Maraschin, M.; Lima, G.P.P. The Phytochemical Profile and Antioxidant Activity of Thermally Processed Colorful Sweet Potatoes. Horticulturae 2024, 10, 18. https://doi.org/10.3390/horticulturae10010018
Basílio LSP, Nunes A, Minatel IO, Diamante MS, Di Lázaro CB, Silva ACAFe, Vargas PF, Vianello F, Maraschin M, Lima GPP. The Phytochemical Profile and Antioxidant Activity of Thermally Processed Colorful Sweet Potatoes. Horticulturae. 2024; 10(1):18. https://doi.org/10.3390/horticulturae10010018
Chicago/Turabian StyleBasílio, Letícia Silva Pereira, Aline Nunes, Igor Otavio Minatel, Marla Sílvia Diamante, Carla Beatriz Di Lázaro, Anna Carolina Abreu Francisco e Silva, Pablo Forlan Vargas, Fabio Vianello, Marcelo Maraschin, and Giuseppina Pace Pereira Lima. 2024. "The Phytochemical Profile and Antioxidant Activity of Thermally Processed Colorful Sweet Potatoes" Horticulturae 10, no. 1: 18. https://doi.org/10.3390/horticulturae10010018






