Chitosan Coating Incorporated with Carvacrol Improves Postharvest Guava (Psidium guajava) Quality
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Fruit Materials
2.2. Preparation of Coating Solutions
2.3. Coating Treatment and Storage Condition
2.4. Characterization of Coating Solution
2.5. Color Parameters
2.6. Firmness
2.7. Total Soluble Solid Content and Titratable Acidity
2.8. Weight Loss
2.9. Total Phenolic Content
2.10. Statistical Analysis
3. Results and Discussion
3.1. Particle Size, Zeta Potential, and Polydispersity Index of Coating Solution
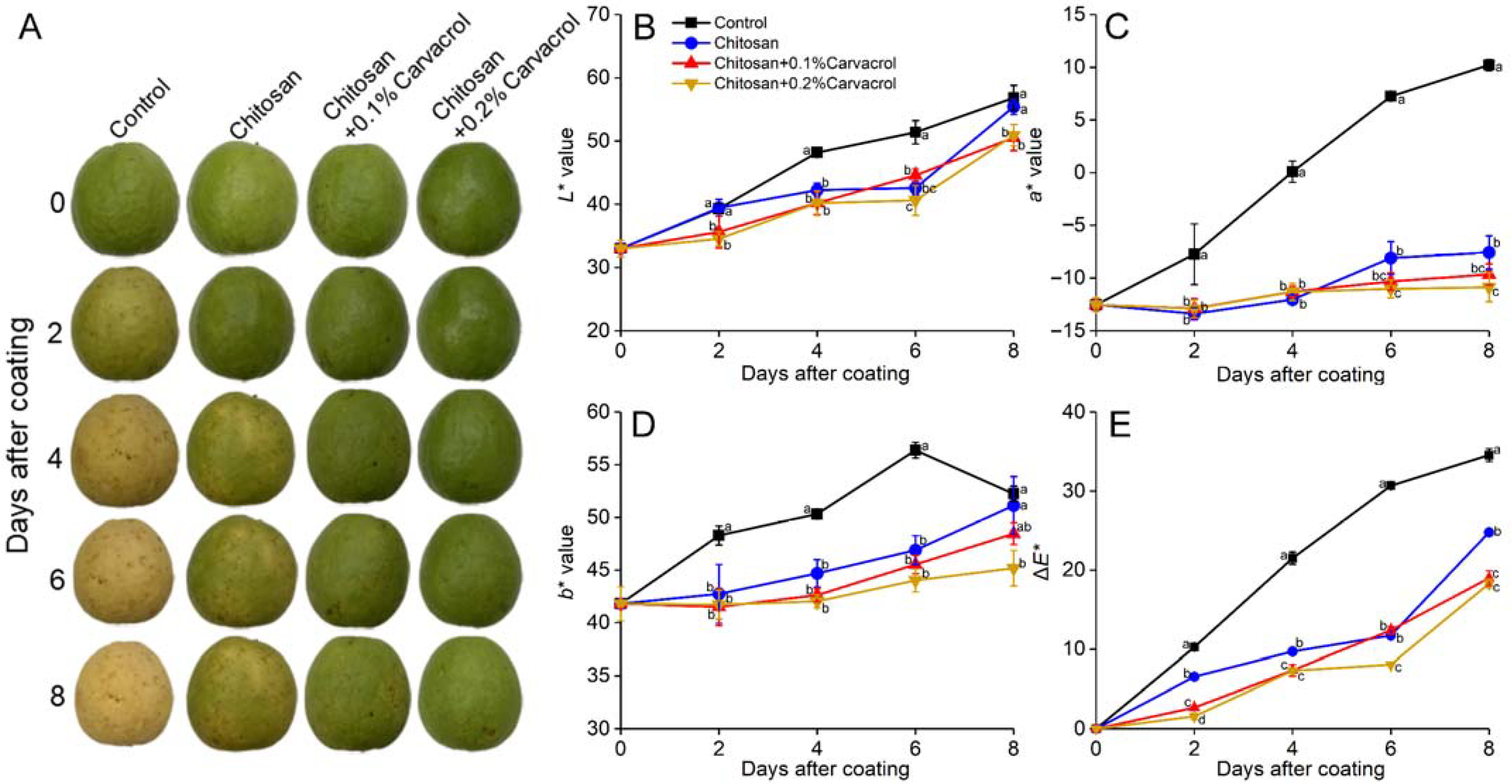
3.2. Fruit Appearance and Color Change
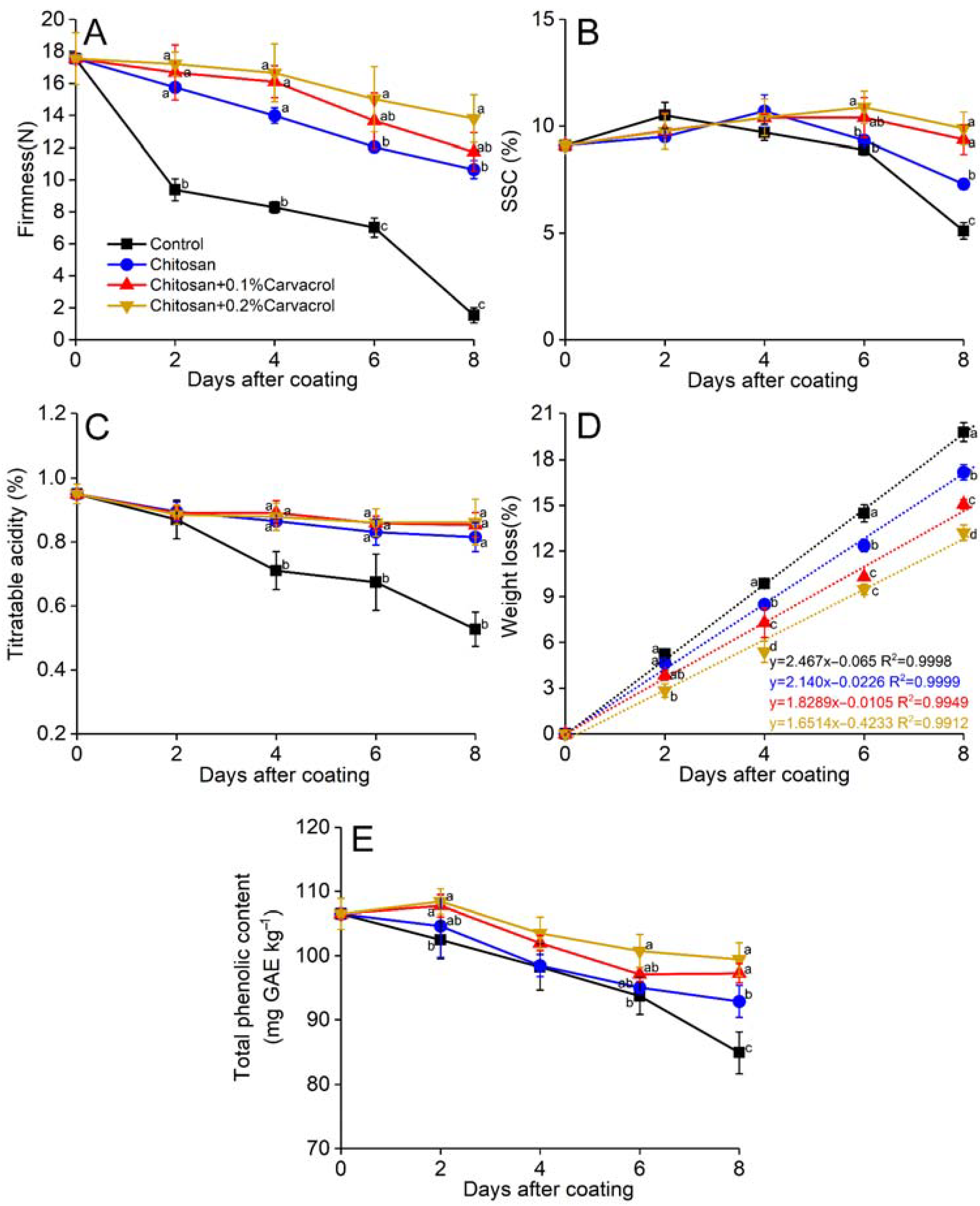
3.3. Fruit Firmness, Soluble Solid Content, Titratable Acidity, and Weight Loss
3.4. Total Phenolic Content
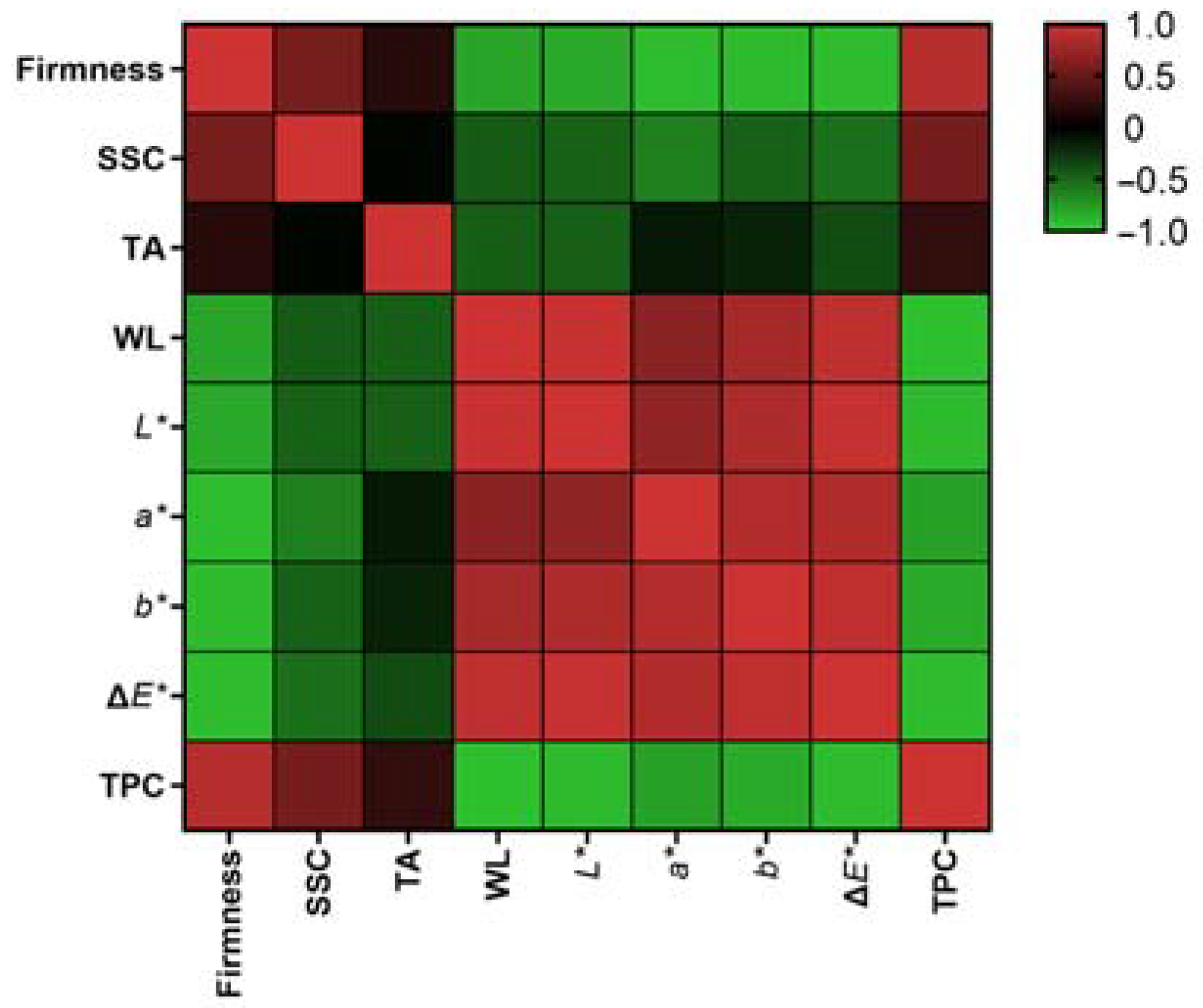
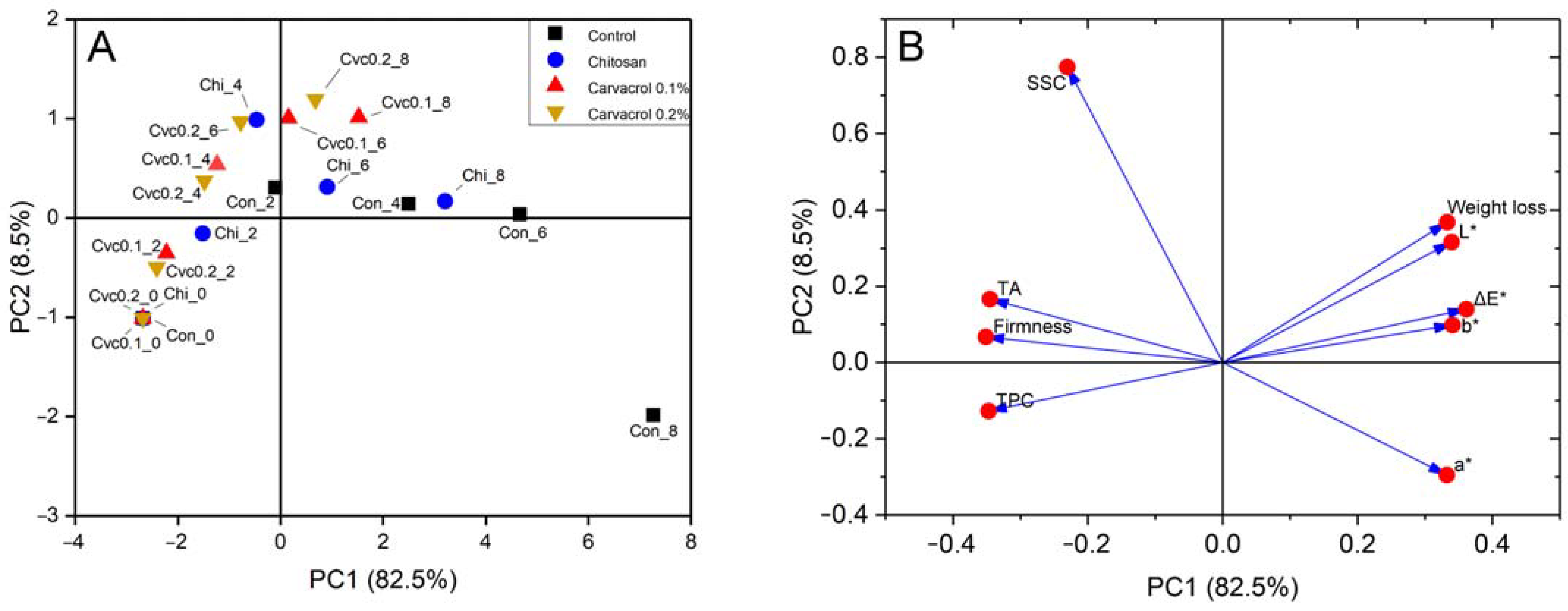
3.5. Correlation and Principal Component Analysis of Fruit Quality Attribute Response to Chitosan–Carvacrol Coatings
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Angulo-López, J.E.; Flores-Gallegos, A.C.; Torres-León, C.; Ramírez-Guzmán, K.N.; Martínez, G.A.; Aguilar, C.N. Guava (Psidium guajava L.) Fruit and Valorization of Industrialization By-Products. Processes 2021, 9, 1075. [Google Scholar] [CrossRef]
- Jamieson, S.; Wallace, C.E.; Das, N.; Bhattacharyya, P.; Bishayee, A. Guava (Psidium guajava L.): A Glorious Plant with Cancer Preventive and Therapeutic Potential. Crit. Rev. Food Sci. Nutr. 2023, 63, 192–223. [Google Scholar] [CrossRef] [PubMed]
- Hong, K.; Xie, J.; Zhang, L.; Sun, D.; Gong, D. Effects of Chitosan Coating on Postharvest Life and Quality of Guava (Psidium guajava L.) Fruit during Cold Storage. Sci. Hortic. 2012, 144, 172–178. [Google Scholar] [CrossRef]
- González-Aguilar, G.A.; Tiznado-Hernández, M.E.; Zavaleta-Gatica, R.; Martínez-Téllez, M.A. Methyl Jasmonate Treatments Reduce Chilling Injury and Activate the Defense Response of Guava Fruits. Biochem. Biophys. Res. Commun. 2004, 313, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Chaiwong, S.; Saengrayap, R.; Rattanakaran, J.; Chaithanarueang, A.; Arwatchananukul, S.; Aunsri, N.; Tontiwattanakul, K.; Jitkokkruad, K.; Kitazawa, H.; Trongsatitkul, T. Natural Rubber Latex Cushioning Packaging to Reduce Vibration Damage in Guava during Simulated Transportation. Postharvest Biol. Technol. 2023, 199, 112273. [Google Scholar] [CrossRef]
- Singh, S.P.; Pal, R.K. Response of Climacteric-Type Guava (Psidium guajava L.) to Postharvest Treatment with 1-MCP. Postharvest Biol. Technol. 2008, 47, 307–314. [Google Scholar] [CrossRef]
- Xiao, J.; Gu, C.; Zhu, D.; Chao, H.; Liang, Y.; Quan, S. Near-Freezing Temperature (NFT) Storage Alleviates Chilling Injury by Enhancing Antioxidant Metabolism of Postharvest Guava (Psidium guajava L.). Sci. Hortic. 2022, 305, 111395. [Google Scholar] [CrossRef]
- Singh, S.P.; Pal, R.K. Ionizing Radiation Treatment to Improve Postharvest Life and Maintain Quality of Fresh Guava Fruit. Radiat. Phys. Chem. 2009, 78, 135–140. [Google Scholar] [CrossRef]
- Ortizano, J.; Benitez, M.; Valida, A.; Acedo, A., Jr. Postharvest Quality of Guapple (Psidium guajava L.) as Influenced by Hot Water Treatment and Modified Atmosphere Packaging. Acta Hortic. 2018, 1213, 153–160. [Google Scholar] [CrossRef]
- Singh, S.P. Prospective and retrospective approaches to postharvest quality management of fresh guava (Psidium guajava L.) fruit in supply chain. Fresh Produce 2010, 4, 36–48. [Google Scholar]
- Fan, S.; Xiong, T.; Lei, Q.; Tan, Q.; Cai, J.; Song, Z.; Yang, M.; Chen, W.; Li, X.; Zhu, X. Melatonin Treatment Improves Postharvest Preservation and Resistance of Guava Fruit (Psidium guajava L.). Foods 2022, 11, 262. [Google Scholar] [CrossRef]
- Riva, S.C.; Opara, U.O.; Fawole, O.A. Recent Developments on Postharvest Application of Edible Coatings on Stone Fruit: A Review. Sci. Hortic. 2020, 262, 109074. [Google Scholar] [CrossRef]
- Maqbool, M.; Ali, A.; Alderson, P.G.; Zahid, N.; Siddiqui, Y. Effect of a Novel Edible Composite Coating Based on Gum Arabic and Chitosan on Biochemical and Physiological Responses of Banana Fruits during Cold Storage. J. Agric. Food Chem. 2011, 59, 5474–5482. [Google Scholar] [CrossRef]
- Kumar, S.; Baswal, A.K.; Ramezanian, A.; Gill, K.S.; Mirza, A.A. Impact of Carboxymethyl Cellulose Based Edible Coating on Storage Life and Quality of Guava Fruit Cv. ‘Allahabad Safeda’ under Ambient Storage Conditions. Food Meas. 2021, 15, 4805–4812. [Google Scholar] [CrossRef]
- Zhang, X.; Ismail, B.B.; Cheng, H.; Jin, T.Z.; Qian, M.; Arabi, S.A.; Liu, D.; Guo, M. Emerging Chitosan-Essential Oil Films and Coatings for Food Preservation—A Review of Advances and Applications. Carbohydr. Polym. 2021, 273, 118616. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, F.; Jiang, Y.; Chai, Z.; Li, P.; Cheng, Y.; Jing, H.; Leng, X. Synergistic Antimicrobial Activities of Natural Essential Oils with Chitosan Films. J. Agric. Food Chem. 2011, 59, 12411–12419. [Google Scholar] [CrossRef] [PubMed]
- Choo, K.W.; Lin, M.; Mustapha, A. Chitosan/Acetylated Starch Composite Films Incorporated with Essential Oils: Physiochemical and Antimicrobial Properties. Food Biosci. 2021, 43, 101287. [Google Scholar] [CrossRef]
- Romanazzi, G.; Moumni, M. Chitosan and Other Edible Coatings to Extend Shelf Life, Manage Postharvest Decay, and Reduce Loss and Waste of Fresh Fruits and Vegetables. Curr. Opin. Biotechnol. 2022, 78, 102834. [Google Scholar] [CrossRef]
- Batista Silva, W.; Cosme Silva, G.M.; Santana, D.B.; Salvador, A.R.; Medeiros, D.B.; Belghith, I.; da Silva, N.M.; Cordeiro, M.H.M.; Misobutsi, G.P. Chitosan Delays Ripening and ROS Production in Guava (Psidium guajava L.) Fruit. Food Chem. 2018, 242, 232–238. [Google Scholar] [CrossRef]
- Mi, T.; Luo, D.; Li, J.; Qu, G.; Sun, Y.; Cao, S. Carvacrol Exhibits Direct Antifungal Activity against Stem-End Rot Disease and Induces Disease Resistance to Stem-End Rot Disease in Kiwifruit. Physiol. Mol. Plant Pathol. 2023, 127, 102065. [Google Scholar] [CrossRef]
- Felicia, W.X.L.; Rovina, K.; Vonnie, J.M.; Aqilah, M.N.N.; Erna, K.H.; Mailin, M. Consolidating Plant-Based Essential Oils onto Polysaccharides-Based Coatings: Effect on Mechanisms and Reducing Postharvest Losses of Fruits. Appl. Food Res. 2022, 2, 100226. [Google Scholar] [CrossRef]
- McClements, D.J.; Rao, J. Food-Grade Nanoemulsions: Formulation, Fabrication, Properties, Performance, Biological Fate, and Potential Toxicity. Crit. Rev. Food Sci. Nutr. 2011, 51, 285–330. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.; Gaysinsky, S.; Davidson, M.; McClements, J. CHAPTER 24—Nanostructured Encapsulation Systems: Food Antimicrobials. In Global Issues in Food Science and Technology; Barbosa-Cánovas, G., Mortimer, A., Lineback, D., Spiess, W., Buckle, K., Colonna, P., Eds.; Academic Press: San Diego, CA, USA, 2009; pp. 425–479. ISBN 978-0-12-374124-0. [Google Scholar]
- Sun, X.; Narciso, J.; Wang, Z.; Ference, C.; Bai, J.; Zhou, K. Effects of Chitosan-Essential Oil Coatings on Safety and Quality of Fresh Blueberries. J. Food Sci. 2014, 79, M955–M960. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Cao, J.; Jiang, W. Evaluation and Comparison of Vitamin C, Phenolic Compounds, Antioxidant Properties and Metal Chelating Activity of Pulp and Peel from Selected Peach Cultivars. LWT Food Sci. Technol. 2015, 63, 1042–1048. [Google Scholar] [CrossRef]
- Shu, C.; Cao, J.; Jiang, W. Postharvest Vibration-Induced Apple Quality Deterioration Is Associated with the Energy Dissipation System. Food Chem. 2022, 386, 132767. [Google Scholar] [CrossRef]
- de Oliveira Filho, J.G.; Miranda, M.; Ferreira, M.D.; Plotto, A. Nanoemulsions as Edible Coatings: A Potential Strategy for Fresh Fruits and Vegetables Preservation. Foods 2021, 10, 2438. [Google Scholar] [CrossRef] [PubMed]
- Severino, R.; Ferrari, G.; Vu, K.D.; Donsì, F.; Salmieri, S.; Lacroix, M. Antimicrobial Effects of Modified Chitosan Based Coating Containing Nanoemulsion of Essential Oils, Modified Atmosphere Packaging and Gamma Irradiation against Escherichia coli O157:H7 and Salmonella Typhimurium on Green Beans. Food Control 2015, 50, 215–222. [Google Scholar] [CrossRef]
- Arabpoor, B.; Yousefi, S.; Weisany, W.; Ghasemlou, M. Multifunctional Coating Composed of Eryngium campestre L. Essential Oil Encapsulated in Nano-Chitosan to Prolong the Shelf-Life of Fresh Cherry Fruits. Food Hydrocoll. 2021, 111, 106394. [Google Scholar] [CrossRef]
- Flores, Z.; San Martín, D.; Villalobos-Carvajal, R.; Tabilo-Munizaga, G.; Osorio, F.; Leiva-Vega, J. Physicochemical Characterization of Chitosan-Based Coating-Forming Emulsions: Effect of Homogenization Method and Carvacrol Content. Food Hydrocoll. 2016, 61, 851–857. [Google Scholar] [CrossRef]
- Berne, B.J.; Pecora, R. Dynamic Light Scattering: With Applications to Chemistry, Biology, and Physics; Courier Corporation: North Chelmsford, MA, USA, 2000; ISBN 978-0-486-41155-2. [Google Scholar]
- Chaudhari, A.K.; Das, S.; Singh, B.K.; Kishore Dubey, N. Green Facile Synthesis of Cajuput (Melaleuca cajuputi Powell.) Essential Oil Loaded Chitosan Film and Evaluation of Its Effectiveness on Shelf-Life Extension of White Button Mushroom. Food Chem. 2023, 401, 134114. [Google Scholar] [CrossRef]
- Arroyo, B.J.; Bezerra, A.C.; Oliveira, L.L.; Arroyo, S.J.; de Melo, E.A.; Santos, A.M.P. Antimicrobial Active Edible Coating of Alginate and Chitosan Add ZnO Nanoparticles Applied in Guavas (Psidium guajava L.). Food Chem. 2020, 309, 125566. [Google Scholar] [CrossRef]
- Yuan, G.; Chen, X.; Li, D. Chitosan Films and Coatings Containing Essential Oils: The Antioxidant and Antimicrobial Activity, and Application in Food Systems. Food Res. Int. 2016, 89, 117–128. [Google Scholar] [CrossRef]
- Aghayan, N.S.; Seyfi, J.; Asadollahzadeh, M.J.; Davachi, S.M.; Hasani, M. Developing Multicomponent Edible Films Based on Chitosan, Hybrid of Essential Oils, and Nanofibers: Study on Physicochemical and Antibacterial Properties. Int. J. Biol. Macromol. 2020, 164, 4065–4072. [Google Scholar] [CrossRef]
- Sun, X.; Wall, M.; Follett, P.; Liang, P.; Xu, S.; Zhong, T. Effect of Pectin Coatings Containing Trans-Cinnamaldehyde on the Postharvest Quality of Rambutan. HortScience 2023, 58, 11–15. [Google Scholar] [CrossRef]
- de Oliveira, L.I.G.; de Oliveira, K.Á.R.; de Medeiros, E.S.; Batista, A.U.D.; Madruga, M.S.; dos Santos Lima, M.; de Souza, E.L.; Magnani, M. Characterization and Efficacy of a Composite Coating Containing Chitosan and Lemongrass Essential Oil on Postharvest Quality of Guava. Innov. Food Sci. Emerg. Technol. 2020, 66, 102506. [Google Scholar] [CrossRef]
- Anjum, M.A.; Akram, H.; Zaidi, M.; Ali, S. Effect of Gum Arabic and Aloe Vera Gel Based Edible Coatings in Combination with Plant Extracts on Postharvest Quality and Storability of ‘Gola’ Guava Fruits. Sci. Hortic. 2020, 271, 109506. [Google Scholar] [CrossRef]
- Taştan, Ö.; Pataro, G.; Donsì, F.; Ferrari, G.; Baysal, T. Decontamination of Fresh-Cut Cucumber Slices by a Combination of a Modified Chitosan Coating Containing Carvacrol Nanoemulsions and Pulsed Light. Int. J. Food Microbiol. 2017, 260, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.P.; Pal, R.K. Controlled Atmosphere Storage of Guava (Psidium guajava L.) Fruit. Postharvest Biol. Technol. 2008, 47, 296–306. [Google Scholar] [CrossRef]
- Etemadipoor, R.; Mirzaalian Dastjerdi, A.; Ramezanian, A.; Ehteshami, S. Ameliorative Effect of Gum Arabic, Oleic Acid and/or Cinnamon Essential Oil on Chilling Injury and Quality Loss of Guava Fruit. Sci. Hortic. 2020, 266, 109255. [Google Scholar] [CrossRef]
- Šamec, D.; Karalija, E.; Šola, I.; Vujčić Bok, V.; Salopek-Sondi, B. The Role of Polyphenols in Abiotic Stress Response: The Influence of Molecular Structure. Plants 2021, 10, 118. [Google Scholar] [CrossRef]
- Peng, J.; Zhu, S.; Lin, X.; Wan, X.; Zhang, Q.; Njie, A.; Luo, D.; Long, Y.; Fan, R.; Dong, X. Evaluation of Preharvest Melatonin on Soft Rot and Quality of Kiwifruit Based on Principal Component Analysis. Foods 2023, 12, 1414. [Google Scholar] [CrossRef] [PubMed]
- Kan, C.; Gao, Y.; Wan, C.; Chen, M.; Zhao, X.; Liu, S.; Chen, J. Influence of Different Cold Storage Times on Quality of “Cuiguan” Pear Fruits during Shelf Life. J. Food Process. Preserv. 2019, 43, e14245. [Google Scholar] [CrossRef]
- Xie, J.; Qin, Z.; Pan, J.; Li, J.; Li, X.; Khoo, H.E.; Dong, X. Melatonin Treatment Improves Postharvest Quality and Regulates Reactive Oxygen Species Metabolism in “Feizixiao” Litchi Based on Principal Component Analysis. Front. Plant Sci. 2022, 13, 965345. [Google Scholar] [CrossRef] [PubMed]
| Particle Size (nm) | Zeta Potential (mV) | Polydispersity Index | |
|---|---|---|---|
| Chitosan | 127.3 ± 2.62 c | 55.46 ± 1.736 a | 0.22 ± 0.04 a |
| Chitosan + 0.1% Carvacrol | 144.3 ± 5.62 b | 52.33 ± 2.039 ab | 0.29 ± 0.06 a |
| Chitosan + 0.2% Carvacrol | 186.4 ± 8.80 a | 49.50 ± 3.224 b | 0.31 ± 0.07 a |
| Principle Component | Eigenvalue | Percentage of Variance (%) | Cumulative (%) |
|---|---|---|---|
| 1 | 7.423 | 82.479 | 82.479 |
| 2 | 0.762 | 8.467 | 90.945 |
| 3 | 0.471 | 5.238 | 96.183 |
| 4 | 0.160 | 1.780 | 97.963 |
| 5 | 0.097 | 1.083 | 99.046 |
| Group | Storage Time (d) | Name of Observations | FAC1 | FAC2 | FAC3 | FAC4 | FAC5 | F1 | F2 | F3 | F4 | F5 | F | F Average |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | 0 | Con_0 | −2.680 | −1.010 | −0.086 | 0.234 | −0.104 | −7.299 | −0.880 | −0.059 | 0.094 | −0.031 | −6.153 | 5.253 |
| 2 | Con_2 | −0.094 | 0.304 | 1.245 | 0.485 | −0.483 | −0.255 | 0.265 | 0.854 | 0.194 | −0.145 | −0.185 | ||
| 4 | Con_4 | 2.497 | 0.139 | 1.244 | −0.065 | 0.338 | 6.802 | 0.121 | 0.853 | −0.026 | 0.101 | 5.678 | ||
| 6 | Con_6 | 4.668 | 0.036 | 1.396 | 0.604 | 0.234 | 12.716 | 0.032 | 0.957 | 0.242 | 0.070 | 10.596 | ||
| 8 | Con_8 | 7.265 | −1.990 | −0.449 | −0.763 | −0.087 | 19.790 | −1.735 | −0.308 | −0.305 | −0.026 | 16.329 | ||
| Chitosan | 0 | Chi_0 | −2.680 | −1.010 | −0.086 | 0.234 | −0.104 | −7.299 | −0.880 | −0.059 | 0.094 | −0.031 | −6.153 | −0.243 |
| 2 | Chi_2 | −1.513 | −0.156 | −0.274 | 0.007 | 0.203 | −4.123 | −0.136 | −0.188 | 0.003 | 0.061 | −3.445 | ||
| 4 | Chi_4 | −0.467 | 0.989 | 0.062 | −0.325 | −0.358 | −1.272 | 0.862 | 0.042 | −0.130 | −0.107 | −0.985 | ||
| 6 | Chi_6 | 0.912 | 0.313 | −0.258 | −0.227 | −0.699 | 2.484 | 0.272 | −0.177 | −0.091 | −0.210 | 2.090 | ||
| 8 | Chi_8 | 3.206 | 0.169 | −1.556 | 0.817 | 0.014 | 8.734 | 0.147 | −1.066 | 0.327 | 0.004 | 7.281 | ||
| Chitosan + 0.1% Carvacrol | 0 | Cvc0.1_0 | −2.680 | −1.010 | −0.086 | 0.234 | −0.104 | −7.299 | −0.880 | −0.059 | 0.094 | −0.031 | −6.153 | −2.002 |
| 2 | Cvc0.1_2 | −2.222 | −0.353 | 0.071 | −0.214 | 0.355 | −6.053 | −0.308 | 0.049 | −0.086 | 0.106 | −5.066 | ||
| 4 | Cvc0.1_4 | −1.235 | 0.538 | −0.063 | −0.361 | 0.042 | −3.364 | 0.469 | −0.043 | −0.144 | 0.013 | −2.761 | ||
| 6 | Cvc0.1_6 | 0.159 | 1.007 | −0.107 | −0.241 | −0.294 | 0.433 | 0.877 | −0.074 | −0.097 | −0.088 | 0.435 | ||
| 8 | Cvc0.1_8 | 1.526 | 1.014 | −0.679 | 0.403 | 0.052 | 4.156 | 0.884 | −0.465 | 0.161 | 0.016 | 3.534 | ||
| Chitosan + 0.2% Carvacrol | 0 | Cvc0.2_0 | −2.680 | −1.010 | −0.086 | 0.234 | −0.104 | −7.299 | −0.880 | −0.059 | 0.094 | −0.031 | −6.153 | −3.008 |
| 2 | Cvc0.2_2 | −2.410 | −0.500 | 0.181 | −0.173 | 0.377 | −6.564 | −0.436 | 0.124 | −0.069 | 0.113 | −5.502 | ||
| 4 | Cvc0.2_4 | −1.481 | 0.372 | 0.067 | −0.408 | 0.310 | −4.034 | 0.324 | 0.046 | −0.163 | 0.093 | −3.332 | ||
| 6 | Cvc0.2_6 | −0.775 | 0.967 | 0.206 | −0.470 | −0.111 | −2.111 | 0.843 | 0.141 | −0.188 | −0.033 | −1.686 | ||
| 8 | Cvc0.2_8 | 0.681 | 1.191 | −0.741 | −0.004 | 0.523 | 1.856 | 1.039 | −0.508 | −0.002 | 0.157 | 1.632 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shu, C.; Kim-Lee, B.; Sun, X. Chitosan Coating Incorporated with Carvacrol Improves Postharvest Guava (Psidium guajava) Quality. Horticulturae 2024, 10, 80. https://doi.org/10.3390/horticulturae10010080
Shu C, Kim-Lee B, Sun X. Chitosan Coating Incorporated with Carvacrol Improves Postharvest Guava (Psidium guajava) Quality. Horticulturae. 2024; 10(1):80. https://doi.org/10.3390/horticulturae10010080
Chicago/Turabian StyleShu, Chang, Beatrice Kim-Lee, and Xiuxiu Sun. 2024. "Chitosan Coating Incorporated with Carvacrol Improves Postharvest Guava (Psidium guajava) Quality" Horticulturae 10, no. 1: 80. https://doi.org/10.3390/horticulturae10010080
APA StyleShu, C., Kim-Lee, B., & Sun, X. (2024). Chitosan Coating Incorporated with Carvacrol Improves Postharvest Guava (Psidium guajava) Quality. Horticulturae, 10(1), 80. https://doi.org/10.3390/horticulturae10010080








