Sweet Cherry Fruit Firmness Evaluation Using Compression Distance Methods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Sampling Process
2.2. Textural Properties
2.3. Firmness Assessment by Panelist
2.4. Evaluation of the Fruit’s Cellular Damage
2.5. Fruit Weight Loss and Respiration Rate
2.6. Quantification of Primary Metabolites
2.7. Statistical Analysis
3. Results
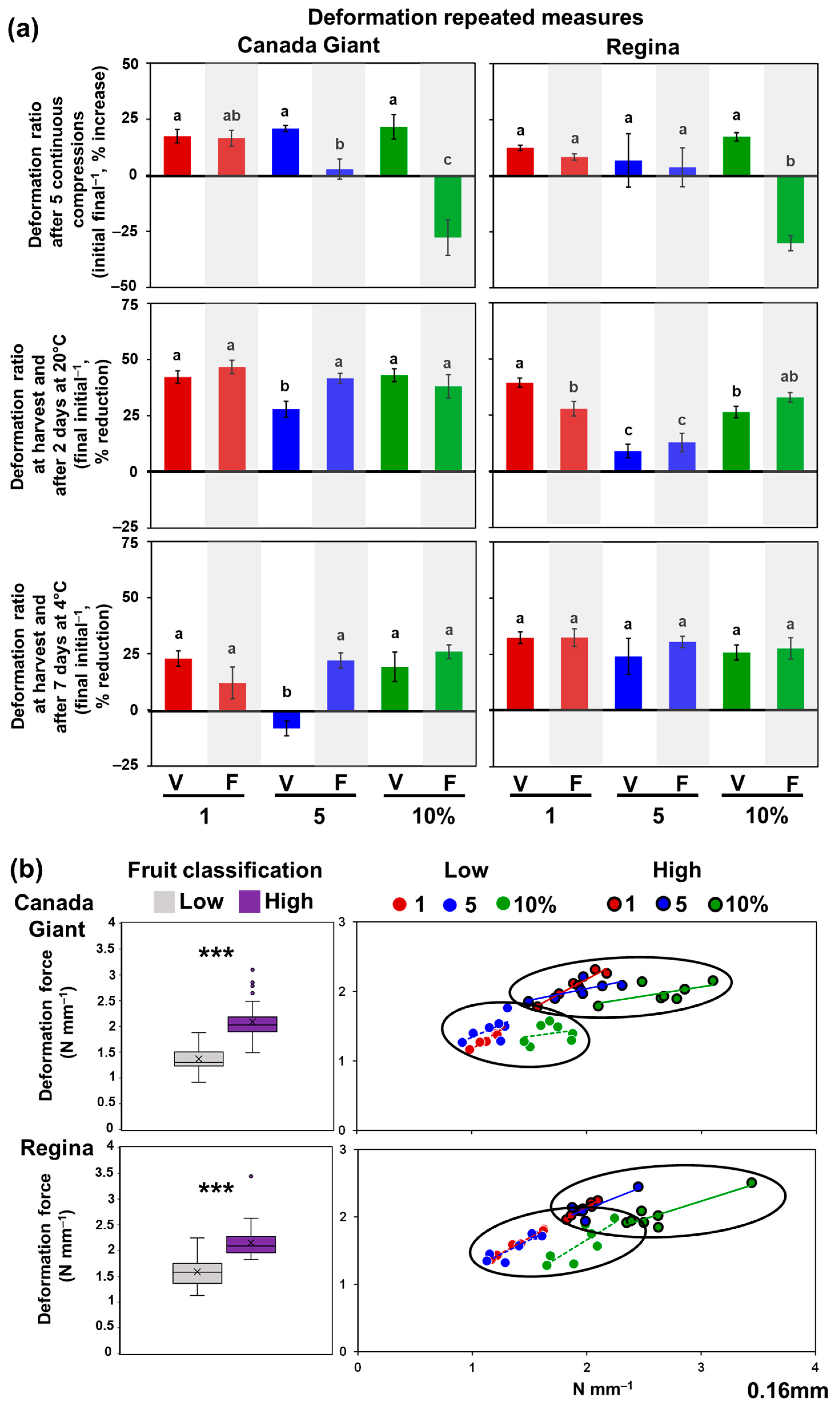
3.1. Flesh Firmness Test in Sweet Cherries
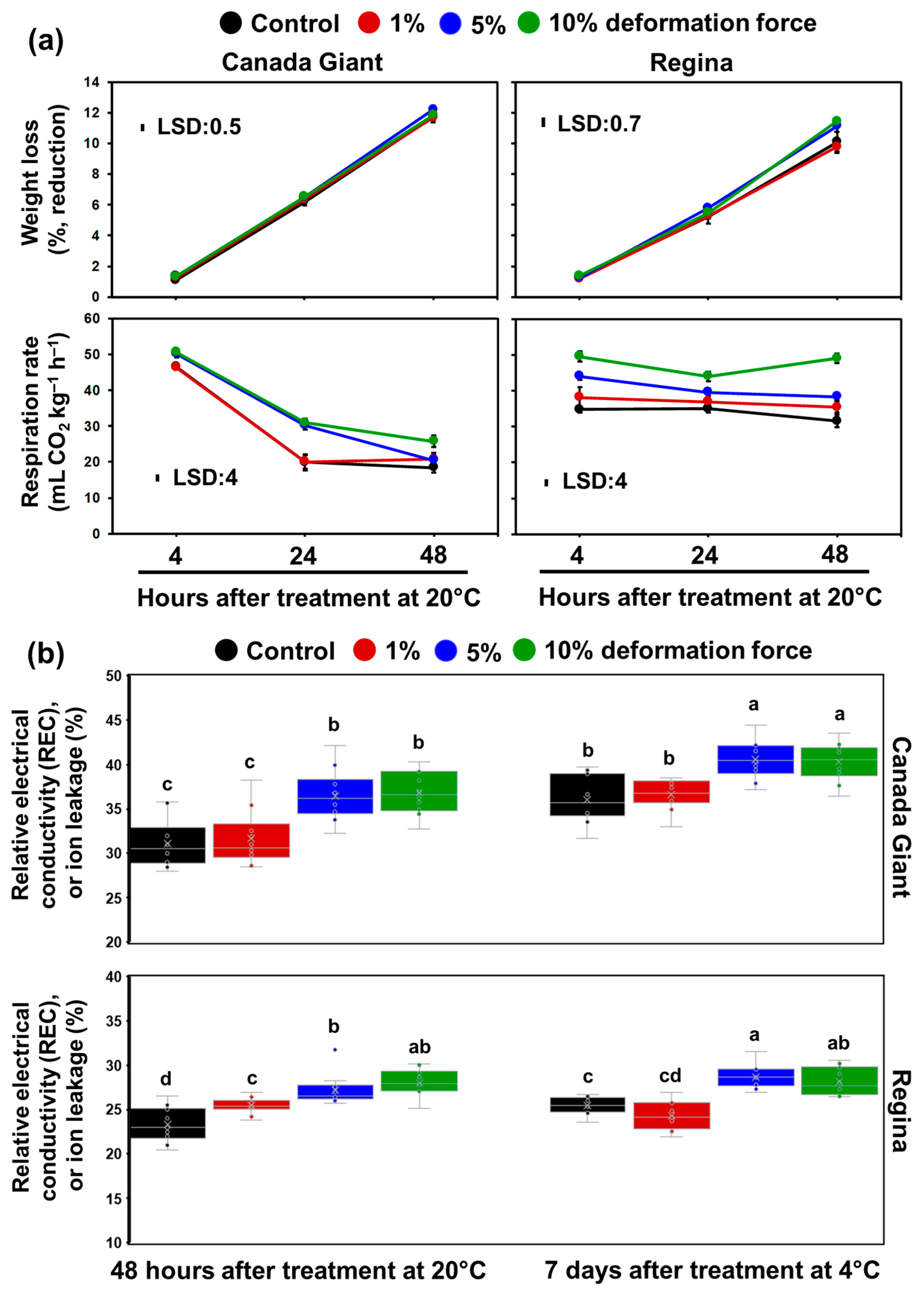
3.2. Monitoring Sweet Cherry Quality Traits
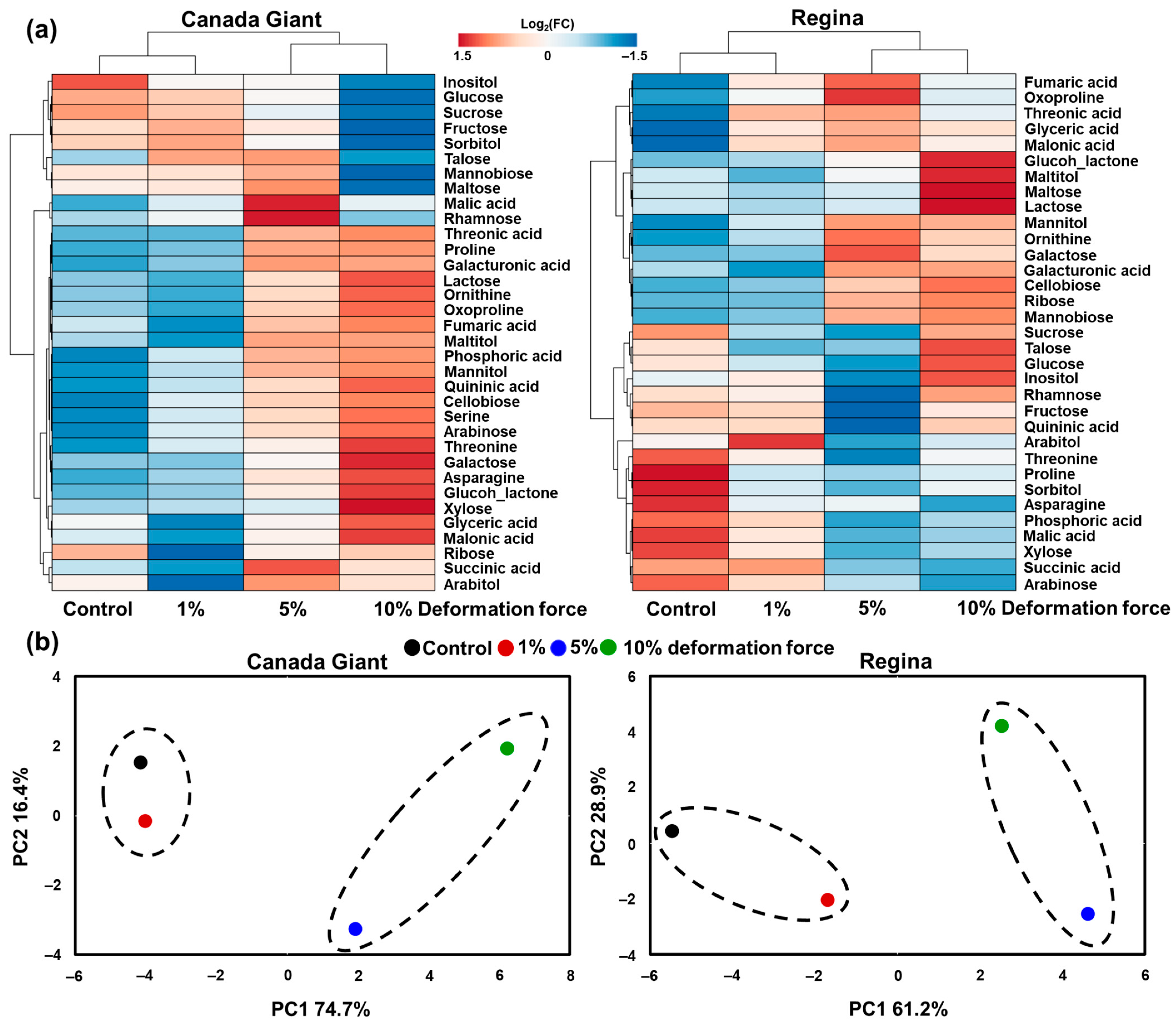
3.3. Primary Metabolic Profile of Sweet Cherry Fruit Exposed to Deformation Experiments
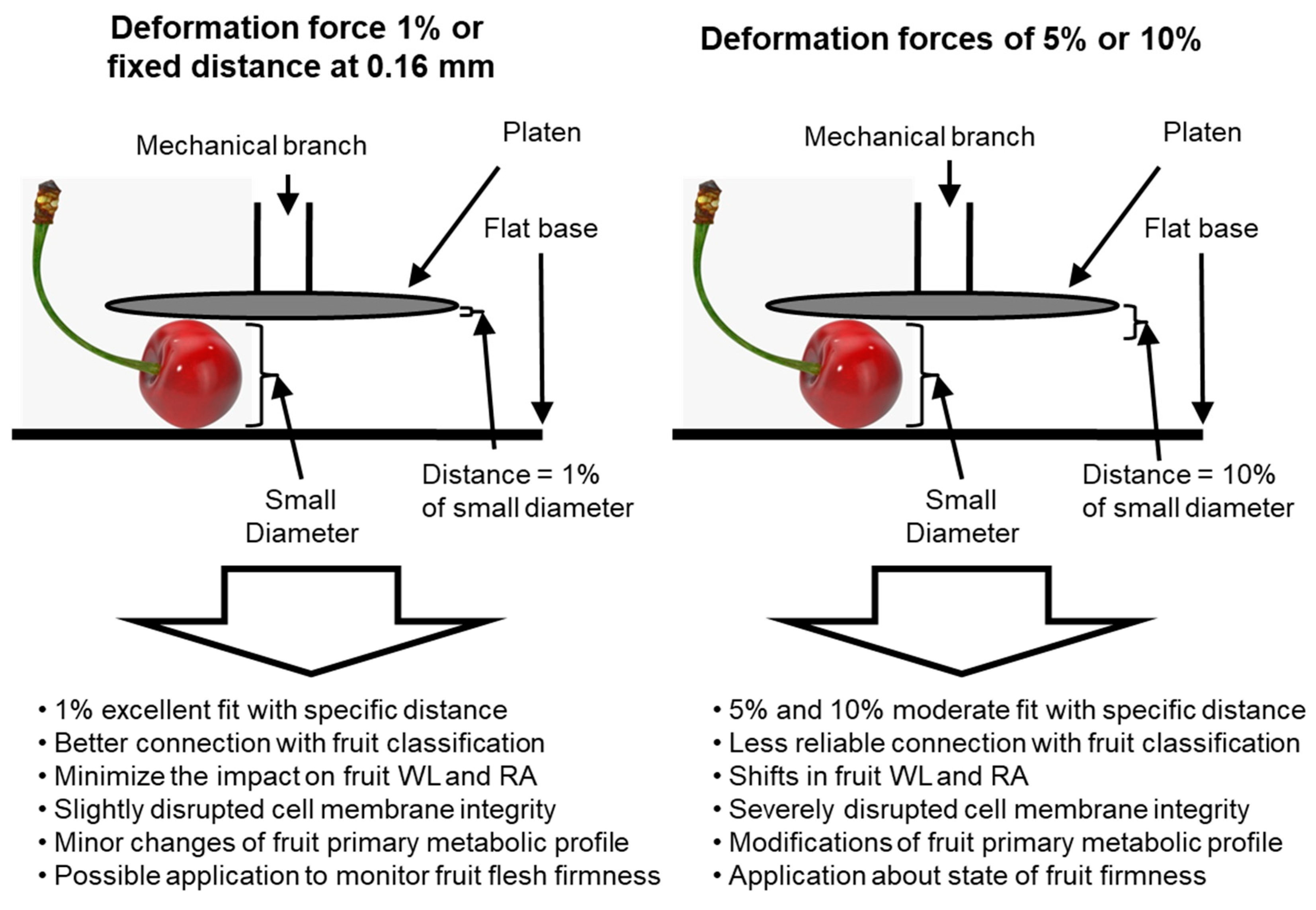
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chockchaisawasdee, S.; Golding, J.B.; Vuong, Q.V.; Papoutsis, K.; Stathopoulos, C.E. Sweet cherry: Composition, postharvest preservation, processing and trends for its future use. Trends Food Sci. Technol. 2016, 55, 72–83. [Google Scholar] [CrossRef]
- Kelley, D.S.; Adkins, Y.; Laugero, K.D. A review of the health benefits of cherries. Nutrients 2018, 10, 368. [Google Scholar] [CrossRef] [PubMed]
- Michailidis, M.; Karagiannis, E.; Tanou, G.; Sarrou, E.; Karamanoli, K.; Lazaridou, A.; Martens, S.; Molassiotis, A. Sweet cherry fruit cracking: Follow-up testing methods and cultivar-metabolic screening. Plant Methods 2020, 16, 51. [Google Scholar] [CrossRef] [PubMed]
- Michailidis, M.; Karagiannis, E.; Polychroniadou, C.; Tanou, G.; Karamanoli, K.; Molassiotis, A. Metabolic features underlying the response of sweet cherry fruit to postharvest UV-C irradiation. Plant Physiol. Biochem. 2019, 144, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Ganopoulos, I.; Farsakoglou, A.M.; Aravanopoulos, F.; Molassiotis, A.; Michailidis, M.; Malliarou, E.; Avramidou, E.; Tsaftaris, A.; Osanthanunkul, M.; Madesis, P.; et al. Towards sweet cherry (Prunus avium L.) breeding: Phenotyping evaluation of newly developed hybrids. Euphytica 2018, 214, 99. [Google Scholar] [CrossRef]
- Correia, S.; Schouten, R.; Silva, A.P.; Gonçalves, B. Factors Affecting Quality and Health Promoting Compounds during Growth and Postharvest Life of Sweet Cherry (Prunus avium L.). Front. Plant Sci. 2017, 8, 2166. [Google Scholar] [CrossRef] [PubMed]
- Hampson, C.R.; Stanich, K.; McKenzie, D.L.; Herbert, L.; Lu, R.; Li, J.; Cliff, M.A. Determining the optimum firmness for sweet cherries using Just-About-Right sensory methodology. Postharvest Biol. Technol. 2014, 91, 104–111. [Google Scholar] [CrossRef]
- Ross, C.F.; Chauvin, M.A.; Whiting, M. Firmness evaluation of sweet cherries by a trained and consumer sensory panel. J. Texture Stud. 2009, 40, 554–570. [Google Scholar] [CrossRef]
- Gallardo, R.K.; Li, H.; Mccracken, V.; Yue, C.; Luby, J.; Mcferson, J.R. Market intermediaries’ willingness to pay for apple, peach, cherry, and strawberry quality attributes. Agribusiness 2015, 31, 259–280. [Google Scholar] [CrossRef]
- Michailidis, M.; Karagiannis, E.; Tanou, G.; Sarrou, E.; Stavridou, E.; Ganopoulos, I.; Karamanoli, K.; Madesis, P.; Martens, S.; Molassiotis, A. An integrated metabolomic and gene expression analysis identifies heat and calcium metabolic networks underlying postharvest sweet cherry fruit senescence. Planta 2019, 250, 2009–2022. [Google Scholar] [CrossRef]
- Michailidis, M.; Karagiannis, E.; Tanou, G.; Samiotaki, M.; Sarrou, E.; Karamanoli, K.; Lazaridou, A.; Martens, S.; Molassiotis, A. Proteomic and metabolic analysis reveals novel sweet cherry fruit development regulatory points influenced by girdling. Plant Physiol. Biochem. 2020, 149, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Michailidis, M.; Tanou, G.; Sarrou, E.; Karagiannis, E.; Ganopoulos, I.; Martens, S.; Molassiotis, A. Pre- and Post-harvest Melatonin Application Boosted Phenolic Compounds Accumulation and Altered Respiratory Characters in Sweet Cherry Fruit. Front. Nutr. 2021, 8, 306. [Google Scholar] [CrossRef] [PubMed]
- Basanta, M.F.; Ponce, N.M.A.; Salum, M.L.; Raffo, M.D.; Vicente, A.R.; Erra-Balsells, R.; Stortz, C.A. Compositional changes in cell wall polysaccharides from five sweet cherry (Prunus avium L.) cultivars during on-tree ripening. J. Agric. Food Chem. 2014, 62, 12418–12427. [Google Scholar] [CrossRef] [PubMed]
- Belge, B.; Comabella, E.; Graell, J.; Lara, I. Post-storage cell wall metabolism in two sweet cherry (Prunus avium L.) cultivars displaying different postharvest performance. Food Sci. Technol. Int. 2015, 21, 416–427. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Liu, C.; Song, L.; Li, M. PaMADS7, a MADS-box transcription factor, regulates sweet cherry fruit ripening and softening. Plant Sci. 2020, 301, 110634. [Google Scholar] [CrossRef] [PubMed]
- Calle, A.; Balas, F.; Cai, L.; Iezzoni, A.; López-Corrales, M.; Serradilla, M.J.; Wünsch, A. Fruit size and firmness QTL alleles of breeding interest identified in a sweet cherry ‘Ambrunés’ × ‘Sweetheart’ population. Mol. Breed. 2020, 40, 1–17. [Google Scholar] [CrossRef]
- Goulas, V.; Minas, I.S.; Kourdoulas, P.M.; Lazaridou, A.; Molassiotis, A.N.; Gerothanassis, I.P.; Manganaris, G.A. 1H NMR Metabolic Fingerprinting to Probe Temporal Postharvest Changes on Qualitative Attributes and Phytochemical Profile of Sweet Cherry Fruit. Front. Plant Sci. 2015, 6, 959. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Romero, D.; Alburquerque, N.; Valverde, J.M.; Guillén, F.; Castillo, S.; Valero, D.; Serrano, M. Postharvest sweet cherry quality and safety maintenance by Aloe vera treatment: A new edible coating. Postharvest Biol. Technol. 2006, 39, 93–100. [Google Scholar] [CrossRef]
- Karagiannis, E.; Sarrou, E.; Michailidis, M.; Tanou, G.; Ganopoulos, I.; Bazakos, C.; Kazantzis, K.; Martens, S.; Xanthopoulou, A.; Molassiotis, A. Fruit quality trait discovery and metabolic profiling in sweet cherry genebank collection in Greece. Food Chem. 2021, 342, 128315. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Bai, J.; Long, L.E. Quality and physiological responses of two late-season sweet cherry cultivars “Lapins” and “Skeena” to modified atmosphere packaging (MAP) during simulated long distance ocean shipping. Postharvest Biol. Technol. 2015, 110, 1–8. [Google Scholar] [CrossRef]
- Ricardo-Rodrigues, S.; Laranjo, M.; Agulheiro-Santos, A.C. Methods for quality evaluation of sweet cherry. J. Sci. Food Agric. 2023, 103, 463–478. [Google Scholar] [CrossRef]
- Belge, B.; Goulao, L.F.; Comabella, E.; Graell, J.; Lara, I. Refrigerated storage and calcium dips of ripe ‘Celeste’ sweet cherry fruit: Combined effects on cell wall metabolism. Sci. Hortic. 2017, 219, 182–190. [Google Scholar] [CrossRef]
- Zhu, D.; Liang, J.; Liu, H.; Cao, X.; Ge, Y.; Li, J. Sweet cherry softening accompanied with moisture migration and loss during low-temperature storage. J. Sci. Food Agric. 2018, 98, 3651–3658. [Google Scholar] [CrossRef] [PubMed]
- Kurlus, R.; Rutkowski, K.; Łysiak, G.P. Improving of cherry fruit quality and bearing regularity by chemical thinning with fertilizer. Agronomy 2020, 10, 1281. [Google Scholar] [CrossRef]
- Marquina, P.L.; Burgos, J.; Oria, R. Application of a compression-relaxation test for the characterization of Burlat sweet cherry. J. Texture Stud. 2001, 32, 15–30. [Google Scholar] [CrossRef]
- Valero, D.; Díaz-Mula, H.M.; Zapata, P.J.; Castillo, S.; Guillén, F.; Martínez-Romero, D.; Serrano, M. Postharvest treatments with salicylic acid, acetylsalicylic acid or oxalic acid delayed ripening and enhanced bioactive compounds and antioxidant capacity in Sweet cherry. J. Agric. Food Chem. 2011, 59, 5483–5489. [Google Scholar] [CrossRef] [PubMed]
- Overbeck, V.; Schmitz, M.; Blanke, M. Non-Destructive Sensor-Based Prediction of Maturity and Optimum Harvest Date of Sweet Cherry Fruit. Sensors 2017, 17, 277. [Google Scholar] [CrossRef] [PubMed]
- Lu, R. Predicting firmness and sugar content of sweet cherries using near-infrared diffuse reflectance spectroscopy. Trans. Am. Soc. Agric. Eng. 2001, 44, 1265–1271. [Google Scholar] [CrossRef]
- Sun, J.; Künnemeyer, R.; McGlone, A. Optical Methods for Firmness Assessment of Fresh Produce: A Review. In Postharvest Handling; Academic Press: Cambridge, MA, USA, 2017. [Google Scholar] [CrossRef]
- Li, Z.; Yang, H.; Li, P.; Liu, J.; Wang, J.; Xu, Y. Fruit biomechanics based on anatomy: A review. Int. Agrophysics 2013, 27, 97–106. [Google Scholar] [CrossRef]
- Papapetros, S.; Louppis, A.; Kosma, I.; Kontakos, S.; Badeka, A.; Papastephanou, C.; Kontominas, M.G. Physicochemical, spectroscopic and chromatographic analyses in combination with chemometrics for the discrimination of four sweet cherry cultivars grown in northern Greece. Foods 2019, 8, 442. [Google Scholar] [CrossRef] [PubMed]
- Michailidis, M.; Bazakos, C.; Kollaros, M.; Adamakis, I.D.S.; Ganopoulos, I.; Molassiotis, A.; Tanou, G. Boron stimulates fruit formation and reprograms developmental metabolism in sweet cherry. Physiol. Plant. 2023, 175, e13946. [Google Scholar] [CrossRef] [PubMed]
- Michailidis, M.; Titeli, V.S.; Karagiannis, E.; Feidaki, K.; Ganopoulos, I.; Tanou, G.; Argiriou, A.; Molassiotis, A. Tissue-specific transcriptional analysis outlines calcium-induced core metabolic changes in sweet cherry fruit. Plant Physiol. Biochem. 2022, 189, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Hummel, J.; Strehmel, N.; Selbig, J.; Walther, D.; Kopka, J. Decision tree supported substructure prediction of metabolites from GC-MS profiles. Metabolomics 2010, 6, 322–333. [Google Scholar] [CrossRef] [PubMed]
- Metsalu, T.; Vilo, J. ClustVis: A web tool for visualizing clustering of multivariate data using Principal Component Analysis and heatmap. Nucleic Acids Res. 2015, 43, W566–W570. [Google Scholar] [CrossRef] [PubMed]
- Cappai, F.; Benevenuto, J.; Ferrão, L.F.V.; Munoz, P. Molecular and genetic bases of fruit firmness variation in blueberry—A review. Agronomy 2018, 8, 174. [Google Scholar] [CrossRef]
- Karagiannis, E.; Michailidis, M.; Karamanoli, K.; Lazaridou, A.; Minas, I.S.; Molassiotis, A. Postharvest responses of sweet cherry fruit and stem tissues revealed by metabolomic profiling. Plant Physiol. Biochem. 2018, 127, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Özkaya, O.; Şener, A.; Saridaş, M.A.; Ünal, Ü.; Valizadeh, A.; Dündar, Ö. Influence of Fast Cold Chain and Modified Atmosphere Packaging Storage on Postharvest Quality of Early Season-Harvested Sweet Cherries. J. Food Process. Preserv. 2015, 39, 2119–2128. [Google Scholar] [CrossRef]
- An, X.; Li, Z.; Zude-Sasse, M.; Tchuenbou-Magaia, F.; Yang, Y. Characterization of textural failure mechanics of strawberry fruit. J. Food Eng. 2020, 282, 110016. [Google Scholar] [CrossRef]
- Li, Z.; Miao, F.; Andrews, J. Mechanical Models of Compression and Impact on Fresh Fruits. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1296–1312. [Google Scholar] [CrossRef] [PubMed]
- Lien, C.C.; Ay, C.; Ting, C.H. Non-destructive impact test for assessment of tomato maturity. J. Food Eng. 2009, 91, 402–407. [Google Scholar] [CrossRef]
- Tian, S.; Xu, H. Mechanical-based and optical-based methods for nondestructive evaluation of fruit firmness. Food Rev. Int. 2023, 39, 4009–4039. [Google Scholar] [CrossRef]
- Tziotzios, G.; Pantazi, X.E.; Paraskevas, C.; Tsitsopoulos, C.; Valasiadis, D.; Nasiopoulou, E.; Michailidis, M.; Molassiotis, A. Non-Destructive Quality Estimation Using a Machine Learning-Based Spectroscopic Approach in Kiwifruits. Horticulturae 2024, 10, 251. [Google Scholar] [CrossRef]
- Brüggenwirth, M.; Knoche, M. Factors affecting mechanical properties of the skin of sweet cherry fruit. J. Am. Soc. Hortic. Sci. 2016, 141, 45–53. [Google Scholar] [CrossRef]
- Chauvin, M.A.; Whiting, M.; Ross, C.F. The influence of harvest time on sensory properties and consumer acceptance of sweet cherries. Horttechnology 2009, 19, 748–754. [Google Scholar] [CrossRef]
- Martínez-Romero, D.; Castillo, S.; Valero, D. Forced-air cooling applied before fruit handling to prevent mechanical damage of plums (Prunus salicina Lindl.). Postharvest Biol. Technol. 2003, 28, 135–142. [Google Scholar] [CrossRef]
- Wang, Y.; Long, L.E. Respiration and quality responses of sweet cherry to different atmospheres during cold storage and shipping. Postharvest Biol. Technol. 2014, 92, 62–69. [Google Scholar] [CrossRef]
- Jiang, Y.; Shiina, T.; Nakamura, N.; Nakahara, A. Electrical conductivity evaluation of postharvest strawberry damage. J. Food Sci. 2001, 66, 1392–1395. [Google Scholar] [CrossRef]
- Ali, Z.M.; Chin, L.H.; Lazan, H. A comparative study on wall degrading enzymes, pectin modifications and softening during ripening of selected tropical fruits. Plant Sci. 2004, 167, 317–327. [Google Scholar] [CrossRef]
- Wang, L.; Jin, P.; Wang, J.; Jiang, L.; Shan, T.; Zheng, Y. Effect of β-aminobutyric acid on cell wall modification and senescence in sweet cherry during storage at 20 °C. Food Chem. 2015, 175, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Merchant, A.; Richter, A.A. Polyols as biomarkers and bioindicators for 21st century plant breeding. Funct. Plant Biol. 2011, 38, 934–940. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Guo, X.; Guo, X.; Zhao, D.; Zhao, W.; Chen, J.; Li, T. PacMYBA, a sweet cherry R2R3-MYB transcription factor, is a positive regulator of salt stress tolerance and pathogen resistance. Plant Physiol. Biochem. 2017, 112, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Hatoum, D.; Annaratone, C.; Hertog, M.L.A.T.M.; Geeraerd, A.H.; Nicolai, B.M. Targeted metabolomics study of “Braeburn” apples during long-term storage. Postharvest Biol. Technol. 2014, 96, 33–41. [Google Scholar] [CrossRef]
- Lisec, J.; Schauer, N.; Kopka, J.; Willmitzer, L.; Fernie, A.R. Gas chromatography mass spectrometry-based metabolite profiling in plants. Nat. Protoc. 2006, 1, 387–396. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karageorgiadou, M.; Rodovitou, M.; Nasiopoulou, E.; Titeli, V.S.; Michailidis, M. Sweet Cherry Fruit Firmness Evaluation Using Compression Distance Methods. Horticulturae 2024, 10, 435. https://doi.org/10.3390/horticulturae10050435
Karageorgiadou M, Rodovitou M, Nasiopoulou E, Titeli VS, Michailidis M. Sweet Cherry Fruit Firmness Evaluation Using Compression Distance Methods. Horticulturae. 2024; 10(5):435. https://doi.org/10.3390/horticulturae10050435
Chicago/Turabian StyleKarageorgiadou, Maria, Maria Rodovitou, Elpida Nasiopoulou, Vaia Styliani Titeli, and Michail Michailidis. 2024. "Sweet Cherry Fruit Firmness Evaluation Using Compression Distance Methods" Horticulturae 10, no. 5: 435. https://doi.org/10.3390/horticulturae10050435
APA StyleKarageorgiadou, M., Rodovitou, M., Nasiopoulou, E., Titeli, V. S., & Michailidis, M. (2024). Sweet Cherry Fruit Firmness Evaluation Using Compression Distance Methods. Horticulturae, 10(5), 435. https://doi.org/10.3390/horticulturae10050435







