Mediator Subunit RhMED15a Regulates Drought Tolerance in Rose
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Treatments
2.2. Sequence Analysis of RhMED15a
2.3. RNA Extraction and Quantitative RT-PCR
2.4. Protein Subcellular Localization Assays
2.5. Virus-Induced Gene Silencing
2.6. Malondialdehyde (MDA) Content Determination
2.7. Statistical Analysis
3. Results
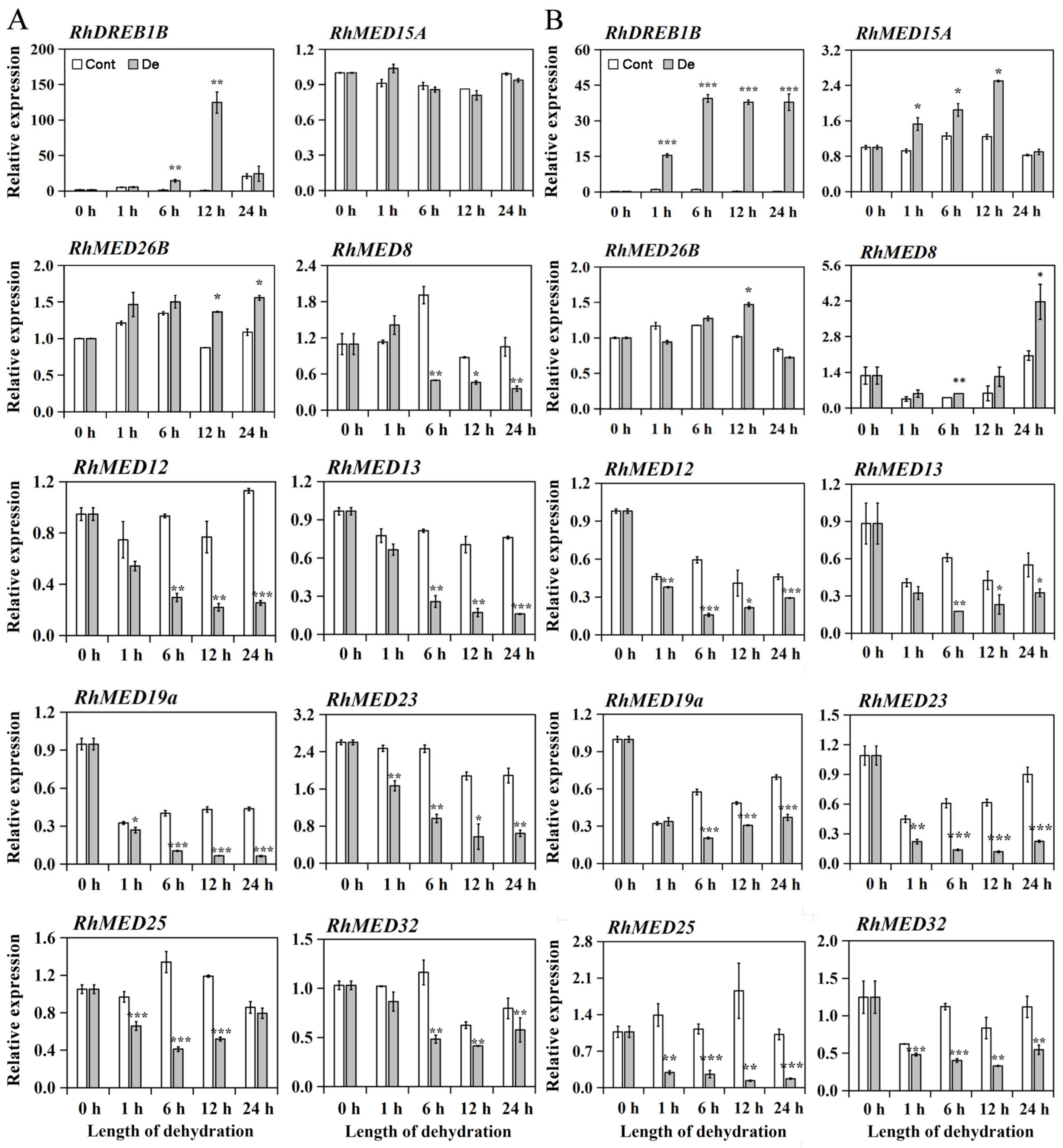
3.1. RhMED15a Was Induced by Dehydration Treatment in Roses
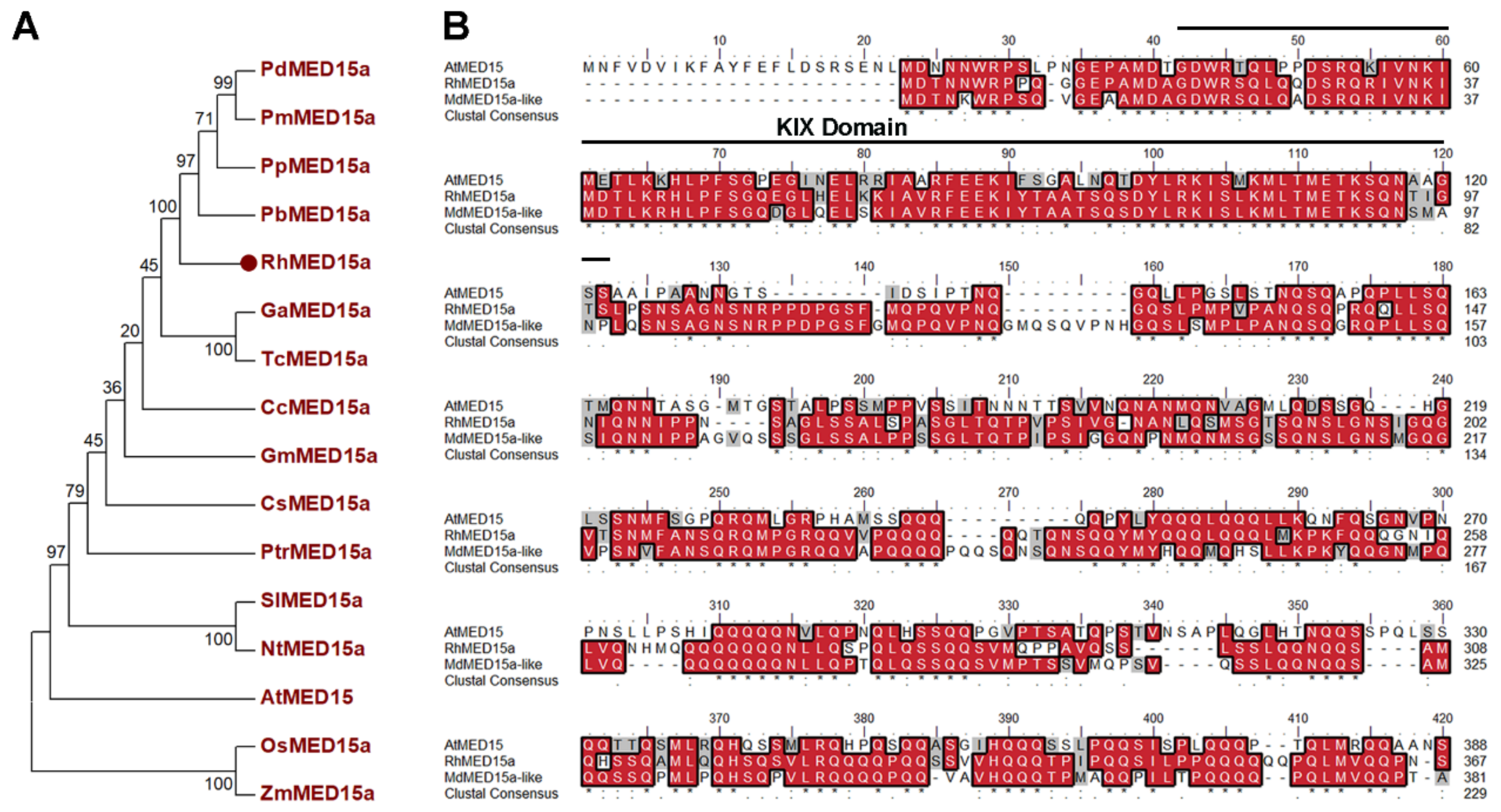
3.2. Isolation and Sequence Analysis of RhMED15a
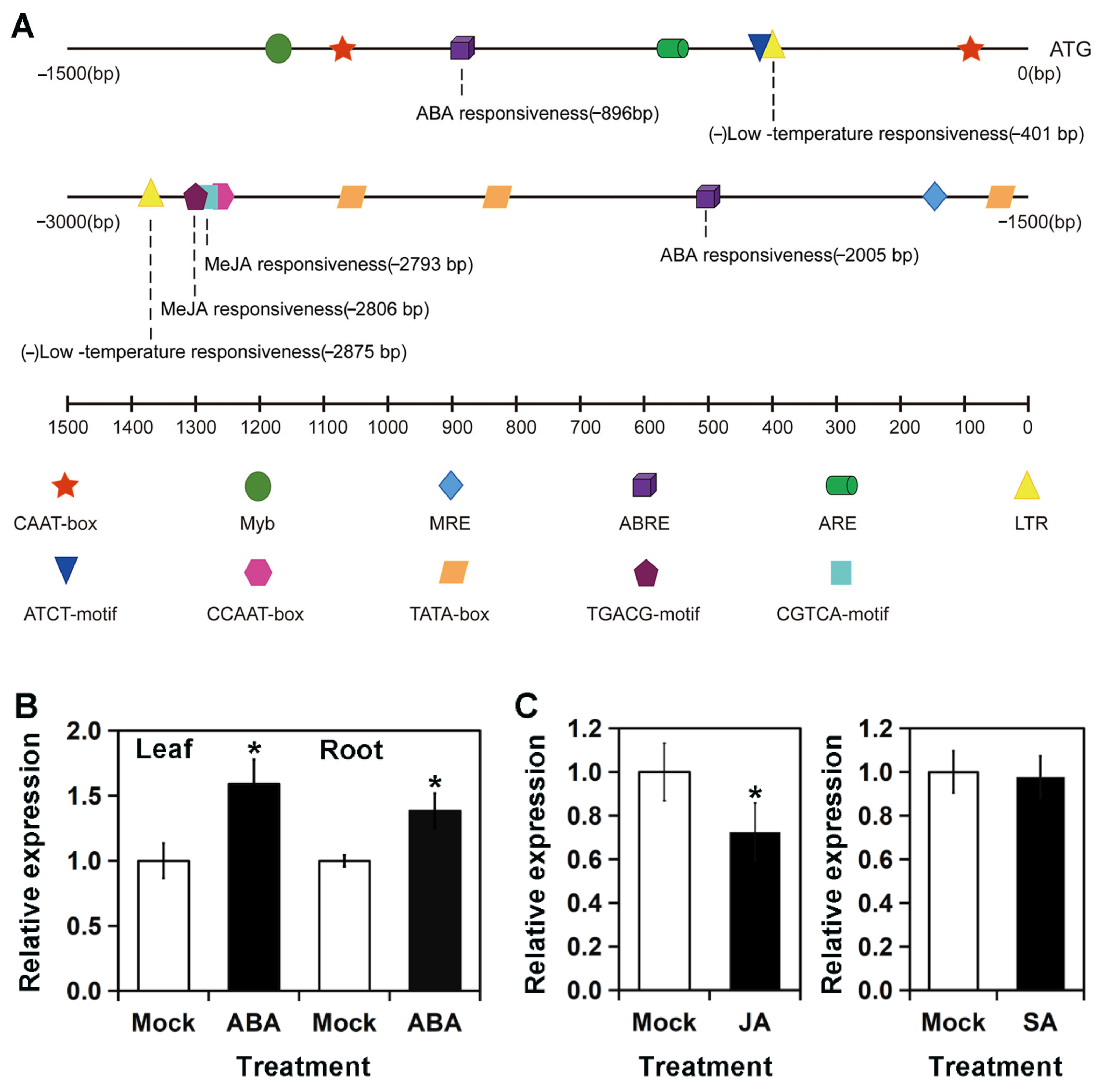
3.3. Expression of RhMED15a Was Significantly Up-Regulated by ABA but Down-Regulated by MeJA
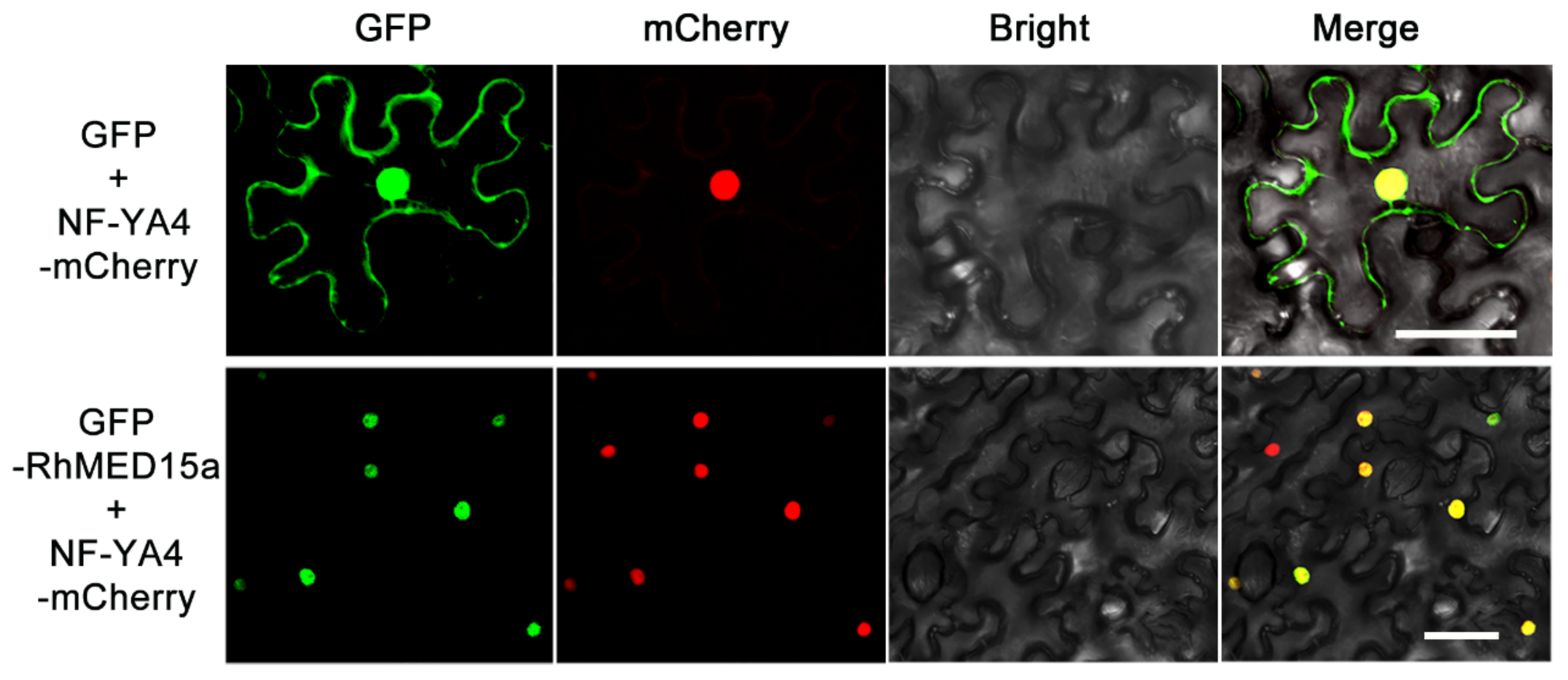
3.4. RhMED15a Was Localized in the Nucleus
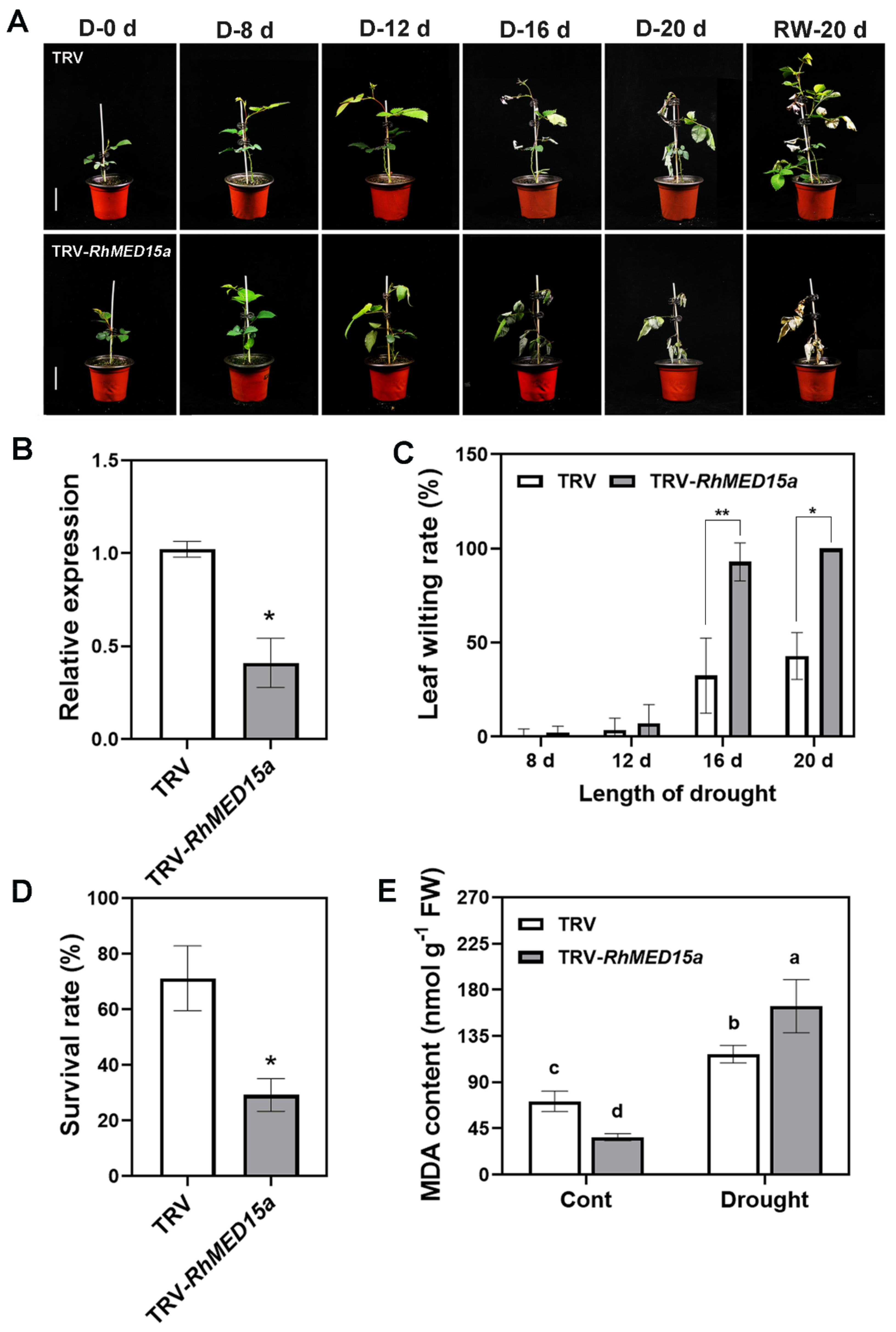
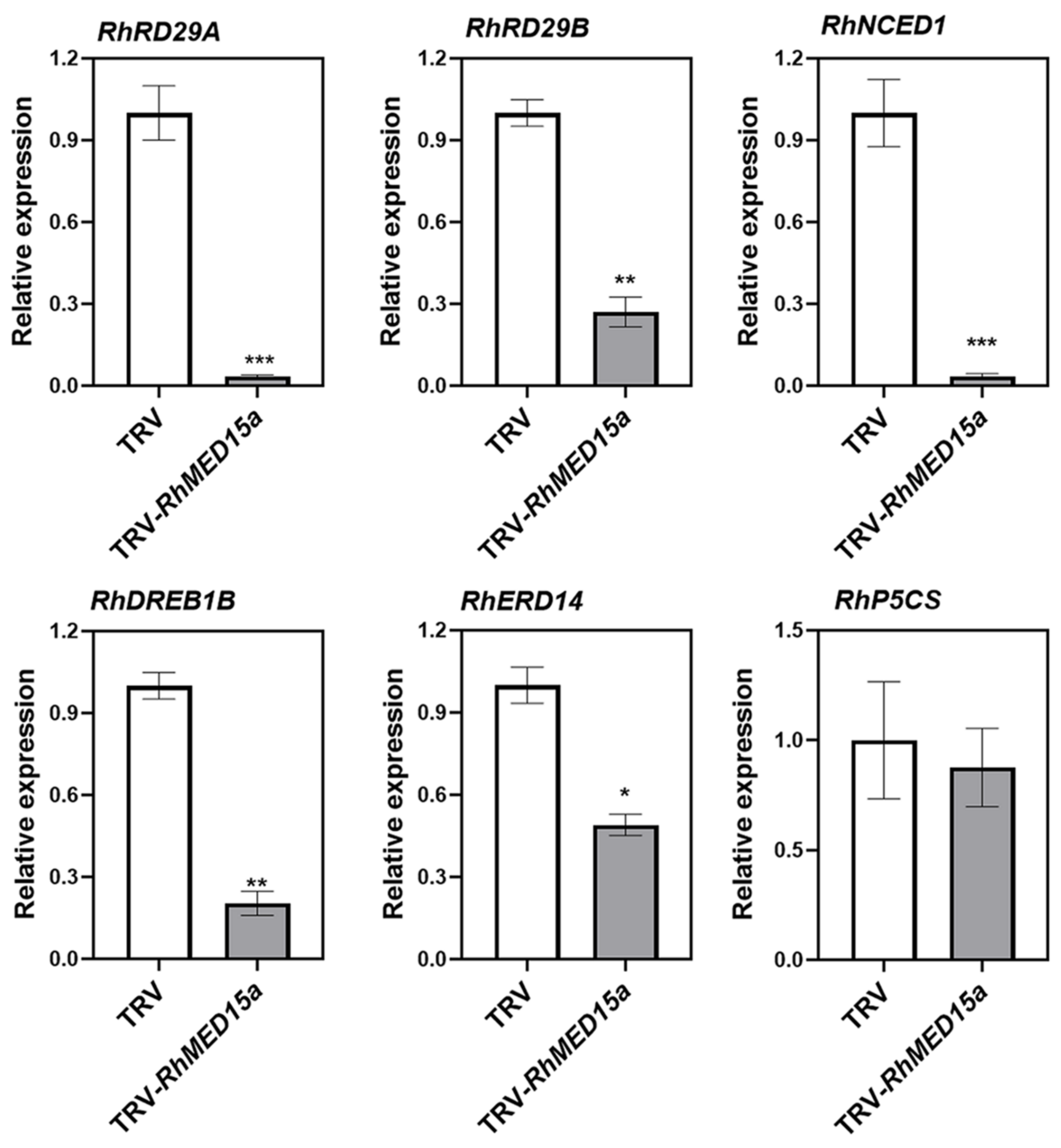
3.5. Silencing of RhMED15a Decreased Drought Tolerance in Rose Plants
4. Discussion
4.1. Dehydration Stress Responsive Mediator Subunits in Roses
4.2. Insights into the Role of RhMED15a in Drought Stress Responses in Roses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gleick, P.H. The world’s water, 2000–2001: The biennial report on freshwater resources. Electron. Green J. 2002, 1, 210–212. [Google Scholar] [CrossRef]
- Gupta, A.; Rico-Medina, A.; Caño-Delgado, A.I. The physiology of plant responses to drought. Science 2020, 368, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Cao, B.; Zhao, S.; Wang, X.; Zhang, H.; Gao, D.; Duan, Y. Selection and evaluation of suitable tree species in dry and dusty mining areas of Northwest China. J. For. Res. 2022, 33, 1817–1828. [Google Scholar] [CrossRef]
- Li, W.; Fu, L.; Geng, Z.; Zhao, X.; Liu, Q.; Jiang, X. Physiological characteristic changes and full-length transcriptome of rose (Rosa chinensis) roots and leaves in response to drought stress. Plant Cell Physiol. 2021, 61, 2153–2166. [Google Scholar] [CrossRef] [PubMed]
- Dinneny, J.R. Developmental responses to water and salinity in root systems. Annu. Rev. Cell Dev. Biol. 2019, 35, 239–257. [Google Scholar] [CrossRef]
- Rellán-Álvarez, R.; Lobet, G.; Dinneny, J.R. Environmental control of root system biology. Annu. Rev. Plant Biol. 2016, 67, 619–642. [Google Scholar] [CrossRef] [PubMed]
- Bailey-Serres, J.; Parker, J.E.; Ainsworth, E.A.; Oldroyd, G.E.D.; Schroeder, J.I. Genetic strategies for improving crop yields. Nature 2019, 575, 109–118. [Google Scholar] [CrossRef]
- Sah, S.K.; Reddy, K.R.; Li, J. Abscisic acid and abiotic stress tolerance in crop plants. Front Plant Sci. 2016, 7, 571. [Google Scholar] [CrossRef]
- Fan, Y.; Liu, J.; Zou, J.; Zhang, X.; Jiang, L.; Liu, K.; Lü, P.; Gao, J.; Zhang, C. The RhHB1/RhLOX4 module affects the dehydration tolerance of rose flowers (Rosa hybrida) by fine-tuning jasmonic acid levels. Hortic. Res. 2020, 7, 74. [Google Scholar] [CrossRef]
- Llanes, A.S.; Andrade, A.M.; Alemano, S.G.; Luna, M.V. Alterations of endogenous hormonal levels in plants under drought and salinity. Am. J. Plant Sci. 2016, 7, 1357–1371. [Google Scholar] [CrossRef]
- Riechmann, J.L.; Heard, J.; Martin, G.; Reuber, L.; Jiang, C.; Keddie, J.; Adam, L.; Pineda, O.; Ratcliffe, O.J.; Samaha, R.R.; et al. Arabidopsis transcription factors: Genome-wide comparative analysis among eukaryotes. Science 2000, 290, 2105–2110. [Google Scholar] [CrossRef] [PubMed]
- Crawford, T.; Karamat, F.; Lehotai, N.; Rentoft, M.; Blomberg, J.; Strand, A.; Björklund, S. Specific functions for Mediator complex subunits from different modules in the transcriptional response of Arabidopsis thaliana to abiotic stress. Sci. Rep. 2020, 10, 5073. [Google Scholar] [CrossRef] [PubMed]
- Allen, B.L.; Taatjes, D.J. The Mediator complex: A central integrator of transcription. Nat. Rev. Mol. Cell Biol. 2015, 16, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Buendía-Monreal, M.; Gillmor, C.S. Mediator: A key regulator of plant development. Dev. Biol. 2016, 419, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Samanta, S.; Thakur, J.K. Importance of Mediator complex in the regulation and integration of diverse signaling pathways in plants. Front Plant Sci. 2015, 6, 757. [Google Scholar] [CrossRef] [PubMed]
- Robinson, P.J.; Trnka, M.J.; Bushnell, D.A.; Davis, R.E.; Mattei, P.J.; Burlingame, A.L.; Kornberg, R.D. Structure of a complete mediator-RNA polymerase II pre-Initiation complex. Cell 2016, 166, 1411–1422. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, L.; Qu, L.J. Plant Mediator complex and its critical functions in transcription regulation. J. Integr. Plant Biol. 2016, 58, 106–118. [Google Scholar] [CrossRef]
- Chen, J.; Yang, S.; Fan, B.; Zhu, C.; Chen, Z. The Mediator Complex: A central coordinator of plant adaptive responses to environmental stresses. Int. J. Mol. Sci. 2022, 23, 6170. [Google Scholar] [CrossRef]
- Elfving, N.; Davoine, C.; Benlloch, R.; Blomberg, J.; Brännström, K.; Müller, D.; Nilsson, A.; Ulfstedt, M.; Ronne, H.; Wingsle, G.; et al. The Arabidopsis thaliana Med25 mediator subunit integrates environmental cues to control plant development. Proc. Natl. Acad. Sci. USA 2011, 108, 8245–8250. [Google Scholar] [CrossRef]
- An, C.; Li, L.; Zhai, Q.; You, Y.; Deng, L.; Wu, F.; Chen, R.; Jiang, H.; Wang, H.; Chen, Q.; et al. Mediator subunit MED25 links the jasmonate receptor to transcriptionally active chromatin. Proc. Natl. Acad. Sci. USA 2017, 114, E8930–E8939. [Google Scholar] [CrossRef]
- Liu, Y.; Du, M.; Deng, L.; Shen, J.; Fang, M.; Chen, Q.; Lu, Y.; Wang, Q.; Li, C.; Zhai, Q. MYC2 regulates the termination of jasmonate signaling via an autoregulatory negative feedback loop. Plant Cell 2019, 31, 106–127. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Jiang, H.; Li, L.; Zhai, Q.; Qi, L.; Zhou, W.; Liu, X.; Li, H.; Zheng, W.; Sun, J.; et al. The Arabidopsis mediator subunit MED25 differentially regulates jasmonate and abscisic acid signaling through interacting with the MYC2 and ABI5 transcription factors. Plant Cell 2012, 24, 2898–2916. [Google Scholar] [CrossRef] [PubMed]
- Knight, H.; Veale, E.L.; Warren, G.J.; Knight, M.R. The sfr6 mutation in Arabidopsis suppresses low-temperature induction of genes dependent on the CRT/DRE sequence motif. Plant Cell 1999, 11, 875–886. [Google Scholar] [CrossRef] [PubMed]
- Knight, H.; Mugford, S.G.; Ulker, B.; Gao, D.; Thorlby, G.; Knight, M.R. Identification of SFR6, a key component in cold acclimation acting post-translationally on CBF function. Plant J. 2009, 58, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Boyce, J.M.; Knight, H.; Deyholos, M.; Openshaw, M.R.; Galbraith, D.W.; Warren, G.; Knight, M.R. The sfr6 mutant of Arabidopsis is defective in transcriptional activation via CBF/DREB1 and DREB2 and shows sensitivity to osmotic stress. Plant J. 2003, 34, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Hemsley, P.A.; Hurst, C.H.; Kaliyadasa, E.; Lamb, R.; Knight, M.R.; De Cothi, E.A.; Steele, J.F.; Knight, H. The Arabidopsis mediator complex subunits MED16, MED14, and MED2 regulate mediator and RNA polymerase II recruitment to CBF-responsive cold-regulated genes. Plant Cell 2014, 26, 465–484. [Google Scholar] [CrossRef]
- Ohama, N.; Moo, T.L.; Chua, N.H. Differential requirement of MED14/17 recruitment for activation of heat inducible genes. New Phytol. 2020, 229, 3360–3376. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, H.; Wang, N.; Fan, H.; Chen, C.; Cui, Y.; Liu, H.; Ling, H.Q. Mediator subunit 16 functions in the regulation of iron uptake gene expression in Arabidopsis. New Phytol. 2014, 203, 770–783. [Google Scholar] [CrossRef]
- Yang, Y.; Ou, B.; Zhang, J.; Si, W.; Gu, H.; Qin, G.; Qu, L.J. The Arabidopsis Mediator subunit MED16 regulates iron homeostasis by associating with EIN3/EIL1 through subunit MED25. Plant J. 2014, 77, 838–851. [Google Scholar] [CrossRef]
- He, H.; Denecker, J.; Van Der Kelen, K.; Willems, P.; Pottie, R.; Phua, S.Y.; Hannah, M.A.; Vertommen, D.; Van Breusegem, F.; Mhamdi, A. The Arabidopsis mediator complex subunit 8 regulates oxidative stress responses. Plant Cell 2021, 33, 2032–2057. [Google Scholar] [CrossRef]
- Zhu, Y.; Huang, P.; Guo, P.; Chong, L.; Yu, G.; Sun, X.; Hu, T.; Li, Y.; Hsu, C.C.; Tang, K.; et al. CDK8 is associated with RAP2.6 and SnRK2.6 and positively modulates abscisic acid signaling and drought response in Arabidopsis. New Phytol. 2020, 228, 1573–1590. [Google Scholar] [CrossRef] [PubMed]
- Dai, F.; Zhang, C.; Jiang, X.; Kang, M.; Yin, X.; Lü, P.; Zhang, X.; Zheng, Y.; Gao, J. RhNAC2 and RhEXPA4 are involved in the regulation of dehydration tolerance during the expansion of rose petals. Plant Physiol. 2012, 160, 2064–2082. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Zhang, C.; Lü, P.; Jiang, G.; Liu, X.; Dai, F.; Gao, J. RhNAC3, a stress-associated NAC transcription factor, has a role in dehydration tolerance through regulating osmotic stress-related genes in rose petals. Plant Biotechnol. J. 2014, 12, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Fan, Y.; Zou, J.; Fang, Y.; Wang, L.; Wang, M.; Jiang, X.; Liu, Y.; Gao, J.; Zhang, C. A RhABF2/Ferritin module affects rose (Rosa hybrida) petal dehydration tolerance and senescence by modulating iron levels. Plant J. 2017, 92, 1157–1169. [Google Scholar] [CrossRef]
- Zhang, S.; Feng, M.; Chen, W.; Zhou, X.; Lu, J.; Wang, Y.; Li, Y.; Jiang, C.Z.; Gan, S.S.; Ma, N.; et al. In rose, transcription factor PTM balances growth and drought survival via PIP2;1 aquaporin. Nat. Plants 2019, 5, 290–299. [Google Scholar] [CrossRef]
- Wang, T.; Tong, Z.; Ma, N.; Gao, J. Isolation and expression analysis of Rh-DREB1s gene in cut roses (Rosa hybrida) under ethylene treatment and water deficit stress. Acta Hortic. Sin. 2009, 36, 65–72. [Google Scholar] [CrossRef]
- Kumar, V.; Waseem, M.; Dwivedi, N.; Maji, S.; Kumar, A.; Thakur, J.K. KIX domain of AtMed15a, a Mediator subunit of Arabidopsis, is required for its interaction with different proteins. Plant Signal. Behav. 2018, 13, e1428514. [Google Scholar] [CrossRef]
- Verma, V.; Ravindran, P.; Kumar, P.P. Plant hormone-mediated regulation of stress responses. BMC Plant Biol. 2016, 16, 86. [Google Scholar] [CrossRef]
- Canet, J.V.; Dobón, A.; Tornero, P. Non-recognition-of-BTH4, an Arabidopsis mediator subunit homolog, is necessary for development and response to salicylic acid. Plant Cell 2012, 24, 4220–4235. [Google Scholar] [CrossRef]
- Kim, M.J.; Jang, I.C.; Chua, N.H. The Mediator complex MED15 subunit mediates activation of downstream lipid-related genes by the WRINKLED1 transcription factor. Plant Physiol. 2016, 171, 1951–1964. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 1–26. [Google Scholar] [CrossRef]
- Yamaguchi-Shinozaki, K.; Shinozaki, K. A novel cis-acting element in an Arabidopsis gene is involved in responsiveness to drought, low-temperature, or high-salt stress. Plant Cell 1994, 6, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Kasuga, M.; Sakuma, Y.; Abe, H.; Miura, S.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought- and low-temperature-responsive gene expression, respectively, in Arabidopsis. Plant Cell 1998, 10, 1391–1406. [Google Scholar] [CrossRef]
- Narusaka, Y.; Nakashima, K.; Shinwari, Z.K.; Sakuma, Y.; Furihata, T.; Abe, H.; Narusaka, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Interaction between two cis-acting elements, ABRE and DRE, in ABA-dependent expression of Arabidopsis rd29A gene in response to dehydration and high-salinity stresses. Plant J. 2003, 34, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Lehr, P.P.; Hernández-Montes, E.; Ludwig-Müller, J.; Keller, M.; Zrb, C. Abscisic acid and proline are not equivalent markers for heat, drought and combined stress in grapevines. Aust. J. Grape Wine Res. 2022, 28, 119–130. [Google Scholar] [CrossRef]
- Mathur, S.; Vyas, S.; Kapoor, S.; Tyagi, A.K. The Mediator complex in plants: Structure, phylogeny, and expression profiling of representative genes in a dicot (Arabidopsis) and a monocot (rice) during reproduction and abiotic stress. Plant Physiol. 2011, 157, 1609–1627. [Google Scholar] [CrossRef]
- Larivière, L.; Seizl, M.; Cramer, P. A structural perspective on Mediator function. Curr. Opin. Cell Biol. 2012, 24, 305–313. [Google Scholar] [CrossRef]
- Bäckström, S.; Elfving, N.; Nilsson, R.; Wingsle, G.; Björklund, S. Purification of a plant mediator from Arabidopsis thaliana identifies PFT1 as the Med25 subunit. Mol. Cell 2007, 26, 717–729. [Google Scholar] [CrossRef]
- Li, X.; Yang, R.; Gong, Y.; Chen, H. The Arabidopsis mediator complex subunit MED19a is involved in ABI5-mediated ABA responses. J. Plant Biol. 2018, 61, 97–110. [Google Scholar] [CrossRef]
- Wang, Y.; Liang, H.; Chen, G.; Liao, C.; Wang, Y.; Hu, Z.; Xie, Q. Molecular and phylogenetic analyses of the mediator subunit genes in Solanum lycopersicum. Front. Genet. 2019, 10, 1222. [Google Scholar] [CrossRef]
- Floris, M.; Mahgoub, H.; Lanet, E.; Robaglia, C.; Menand, B. Post-transcriptional regulation of gene expression in plants during abiotic stress. Int. J. Mol. Sci. 2009, 10, 3168–3185. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Wang, S.; Valerius, O.; Hall, H.; Zeng, Q.; Li, J.F.; Weston, D.J.; Ellis, B.E.; Chen, J.G. Involvement of Arabidopsis RACK1 in protein translation and its regulation by abscisic acid. Plant Physiol. 2011, 155, 370–383. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Du, X.; Mou, Z. The mediator complex subunits MED14, MED15, and MED16 are involved in defense signaling crosstalk in Arabidopsis. Front Plant Sci. 2016, 7, 1947. [Google Scholar] [CrossRef]
- Dwivedi, N.; Maji, S.; Waseem, M.; Thakur, P.; Kumar, V.; Parida, S.K.; Thakur, J.K. The mediator subunit OsMED15a is a transcriptional co-regulator of seed size/weight-modulating genes in rice. BBA Gene Regul. Mech. 2019, 1862, 194432. [Google Scholar] [CrossRef]
- Wei, T.; Deng, K.; Gao, Y.; Liu, Y.; Yang, M.; Zhang, L.; Zheng, X.; Wang, C.; Song, W.; Chen, C.; et al. Arabidopsis DREB1B in transgenic Salvia miltiorrhiza increased tolerance to drought stress without stunting growth. Plant Physiol. Biochem. 2016, 104, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Kiyosue, T.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Characterization of two cDNAs (ERD10 and ERD14) corresponding to genes that respond rapidly to dehydration stress in Arabidopsis thaliana. Plant Cell Physiol. 1994, 35, 225–231. [Google Scholar] [CrossRef]
- Msanne, J.; Lin, J.; Stone, J.M.; Awada, T. Characterization of abiotic stress-responsive Arabidopsis thaliana RD29A and RD29B genes and evaluation of transgenes. Planta 2011, 234, 97–107. [Google Scholar] [CrossRef]
- Lee, S.U.; Mun, B.G.; Bae, E.K.; Kim, J.Y.; Kim, H.H.; Shahid, M.; Choi, Y.I.; Hussain, A.; Yun, B.W. Drought stress-mediated transcriptome profile reveals NCED as a key player modulating drought tolerance in Populus davidiana. Front. Plant Sci. 2021, 12, 755539. [Google Scholar] [CrossRef]
- De Ollas, C.; Arbona, V.; Gómez-Cadenas, A.; Dodd, I.C. Attenuated accumulation of jasmonates modifies stomatal responses to water deficit. J. Exp. Bot. 2018, 69, 2103–2116. [Google Scholar] [CrossRef]
- Seki, M.; Umezawa, T.; Urano, K.; Shinozaki, K. Regulatory metabolic networks in drought stress responses. Curr. Opin. Plant Biol. 2007, 10, 296–302. [Google Scholar] [CrossRef]
- Chen, K.; Li, G.J.; Bressan, R.A.; Song, C.P.; Zhu, J.K.; Zhao, Y. Abscisic acid dynamics, signaling, and functions in plants. J. Integr. Plant Biol. 2020, 62, 25–54. [Google Scholar] [CrossRef] [PubMed]
- Howe, G.A.; Major, I.T.; Koo, A.J. Modularity in jasmonate signaling for multistress resilience. Annu. Rev. Plant Biol. 2018, 69, 387–415. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Song, L.; Gong, X.; Xu, J.; Li, M. Functions of jasmonic acid in plant regulation and response to abiotic stress. Int. J. Mol. Sci. 2020, 21, 1446. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Q.; Deng, L.; Li, C. Mediator subunit MED25: At the nexus of jasmonate signaling. Curr. Opin. Plant Biol. 2020, 57, 78–86. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shang, X.; Xie, N.; Li, Y.; Zhao, Z.; Luo, P.; Cui, Y.; Rao, X.; Chen, W. Mediator Subunit RhMED15a Regulates Drought Tolerance in Rose. Horticulturae 2024, 10, 84. https://doi.org/10.3390/horticulturae10010084
Shang X, Xie N, Li Y, Zhao Z, Luo P, Cui Y, Rao X, Chen W. Mediator Subunit RhMED15a Regulates Drought Tolerance in Rose. Horticulturae. 2024; 10(1):84. https://doi.org/10.3390/horticulturae10010084
Chicago/Turabian StyleShang, Xiaoman, Nanxin Xie, Yalin Li, Zixin Zhao, Ping Luo, Yongyi Cui, Xianlong Rao, and Wen Chen. 2024. "Mediator Subunit RhMED15a Regulates Drought Tolerance in Rose" Horticulturae 10, no. 1: 84. https://doi.org/10.3390/horticulturae10010084
APA StyleShang, X., Xie, N., Li, Y., Zhao, Z., Luo, P., Cui, Y., Rao, X., & Chen, W. (2024). Mediator Subunit RhMED15a Regulates Drought Tolerance in Rose. Horticulturae, 10(1), 84. https://doi.org/10.3390/horticulturae10010084






