The Role of Brassinosteroids and Nano-Encapsulated Brassinosteroids in Capsicum Pepper Growth and Physiological Adaptations to High-Temperature Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Plantation
2.2. Brassinosteroid Production in Nano-Capsules and Their Physicochemical Properties
2.3. Growth and Physiological Parameters
2.4. Determination of Proline, Antioxidants, and Lipid Peroxidation
2.5. Glucose and Fructose Content
2.6. Total Protein and Fatty Acids Content
2.7. Statistical Analysis
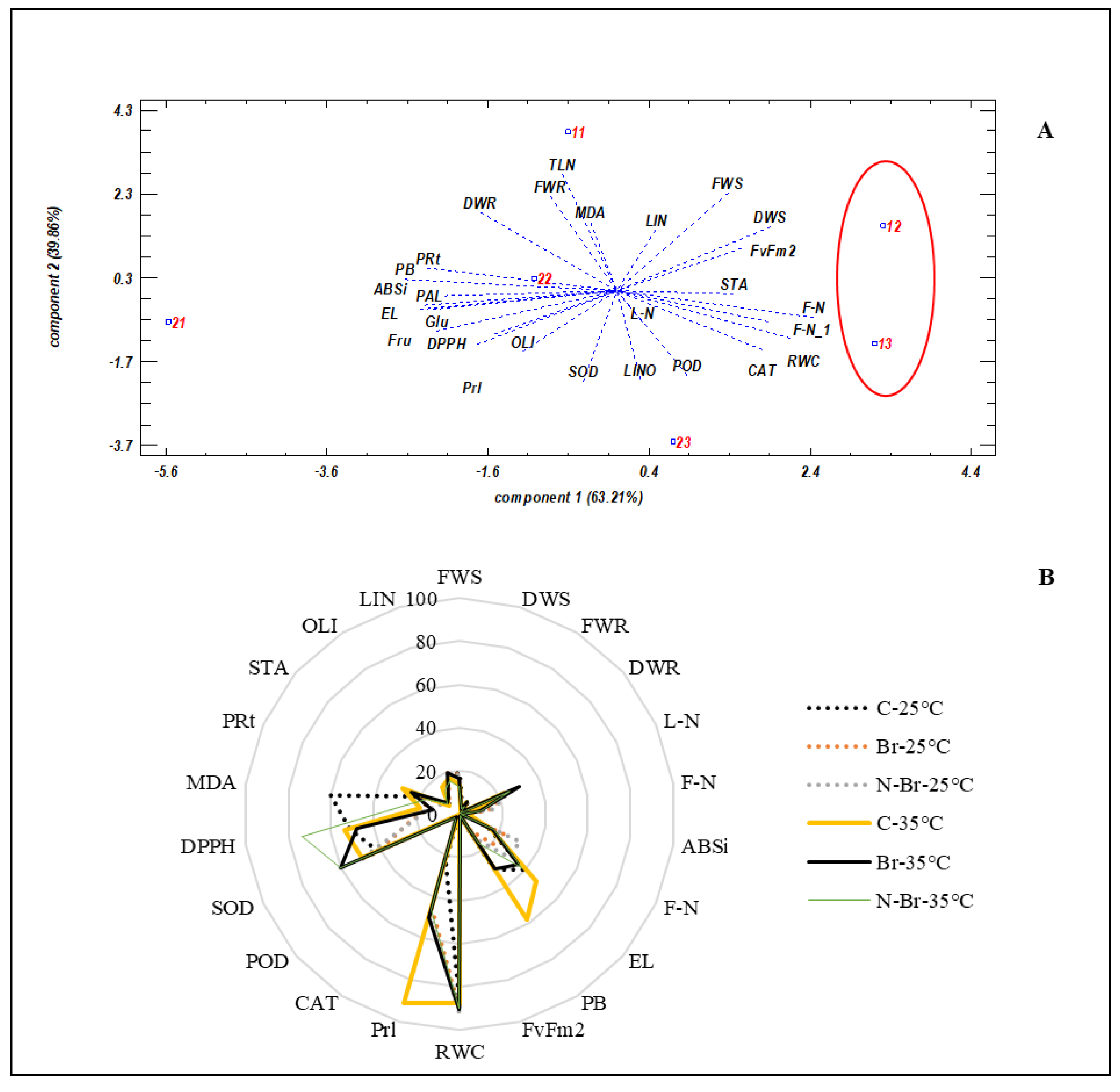
3. Results
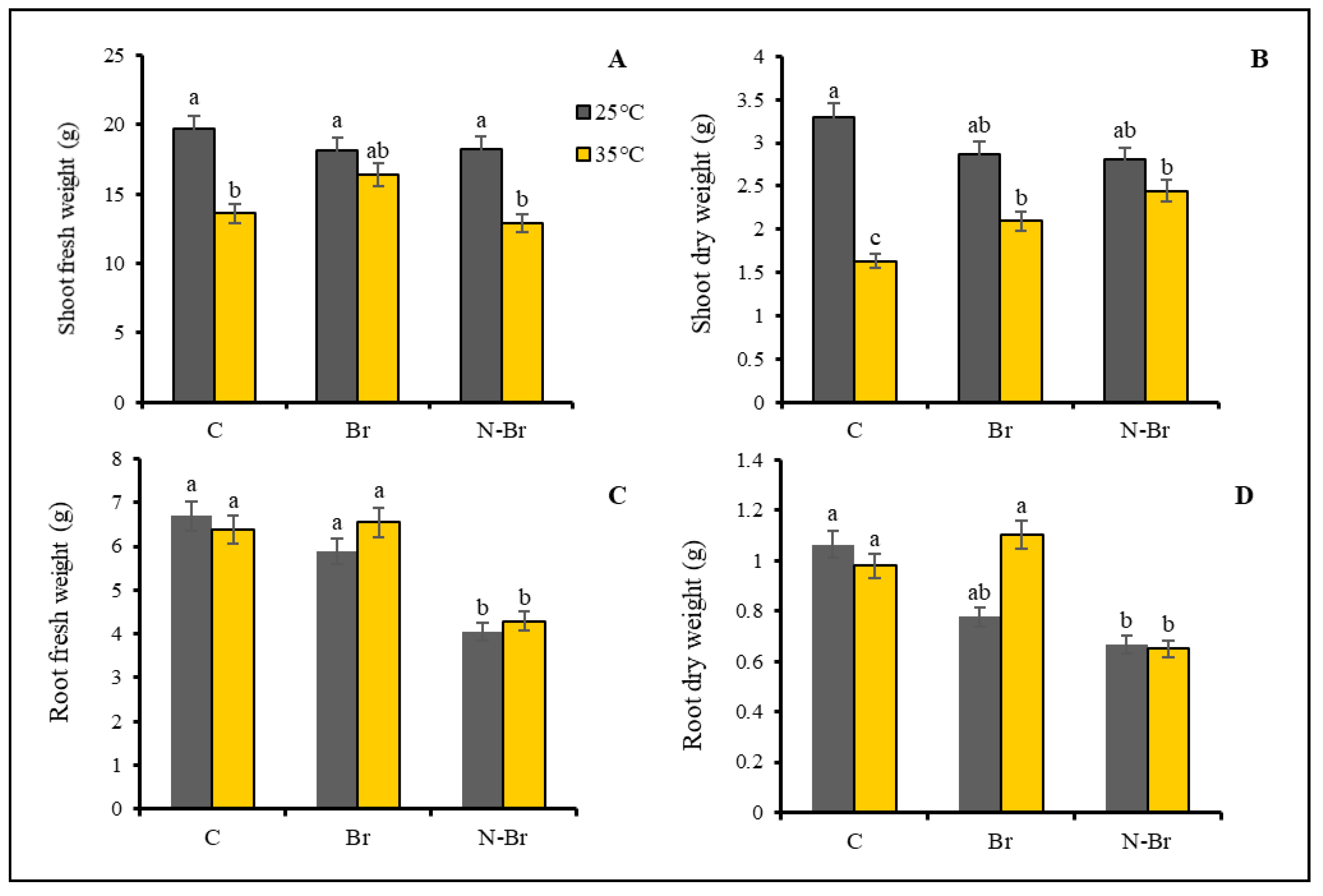
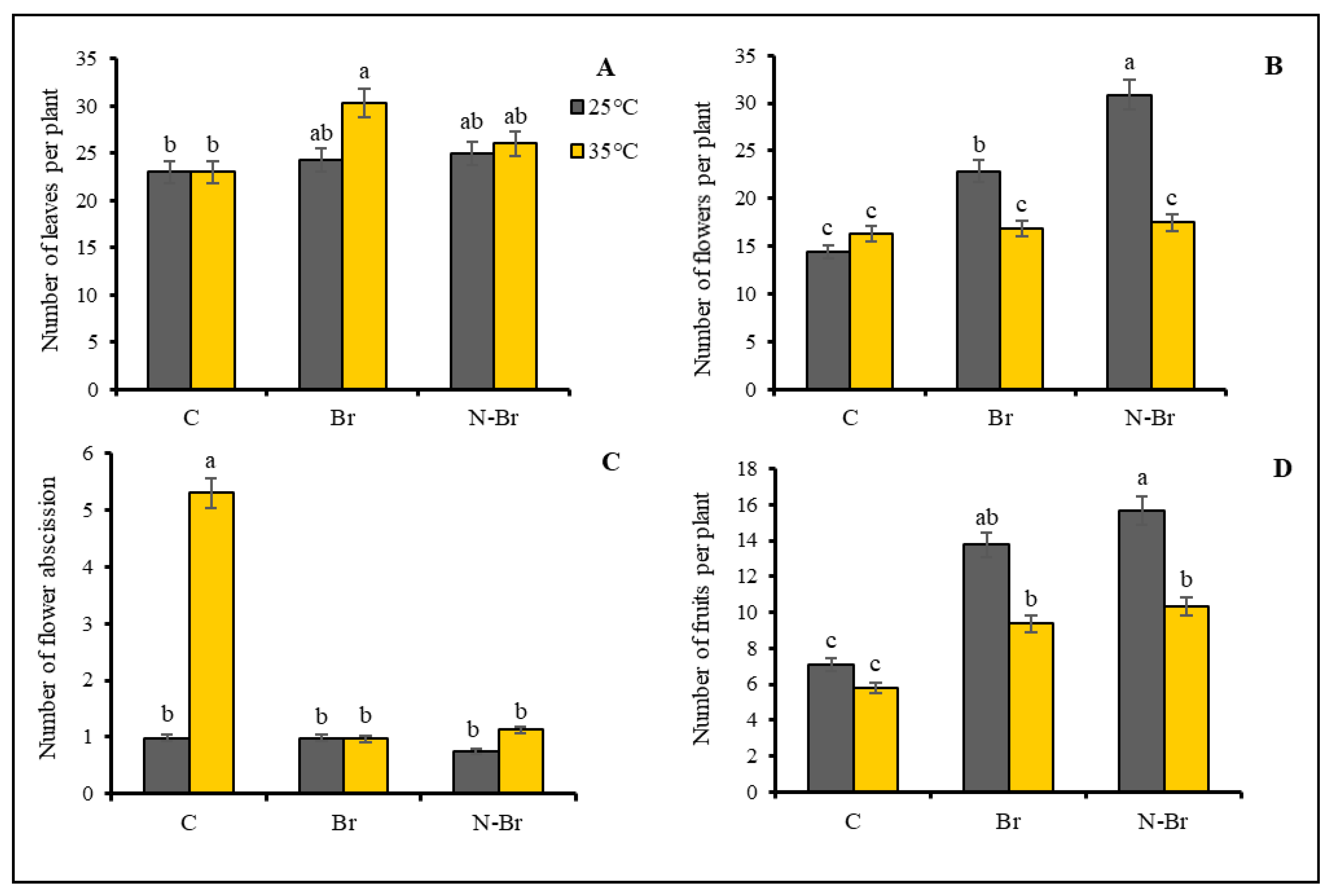
3.1. Growth and Yield Parameters
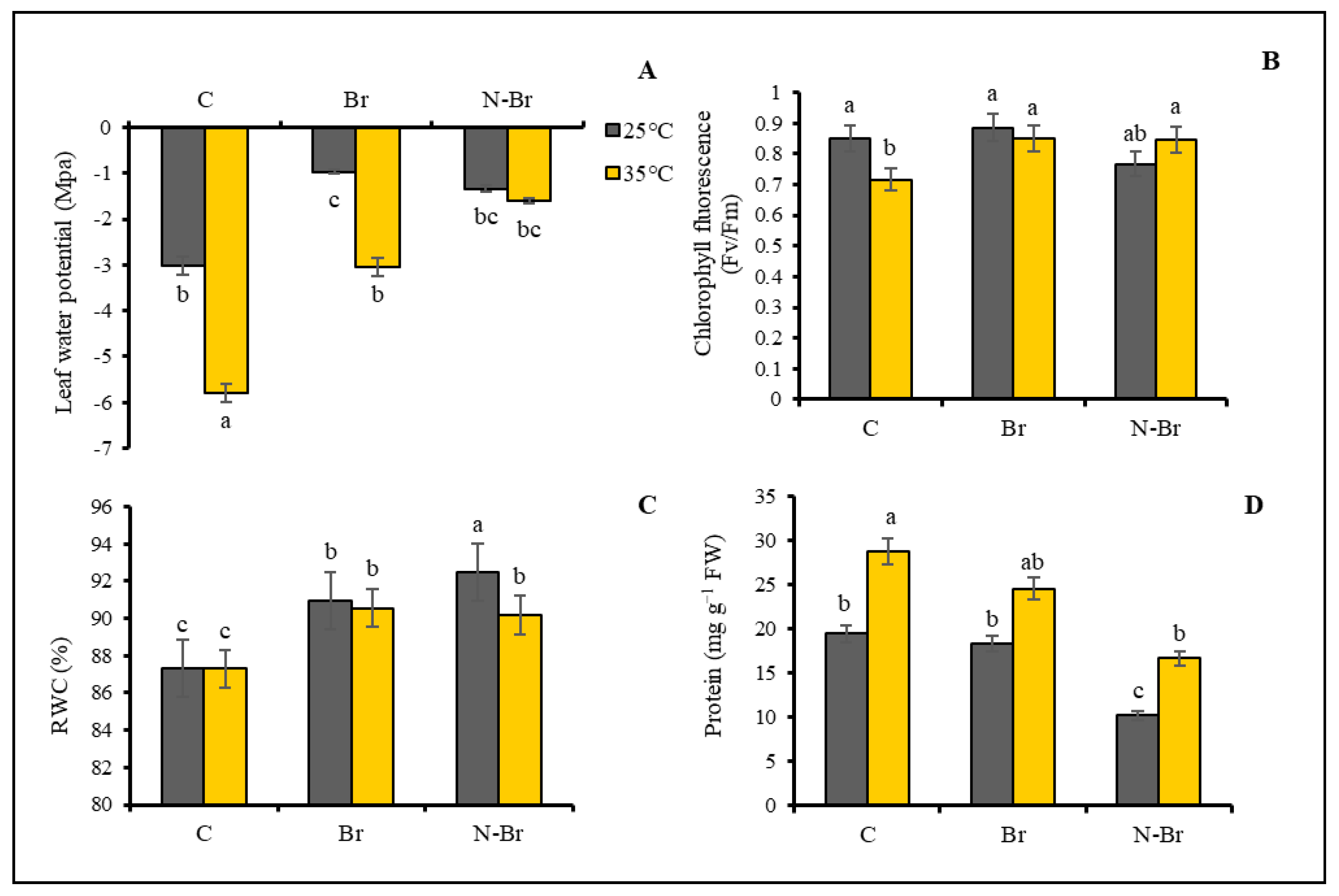
3.2. Physiological Parameters
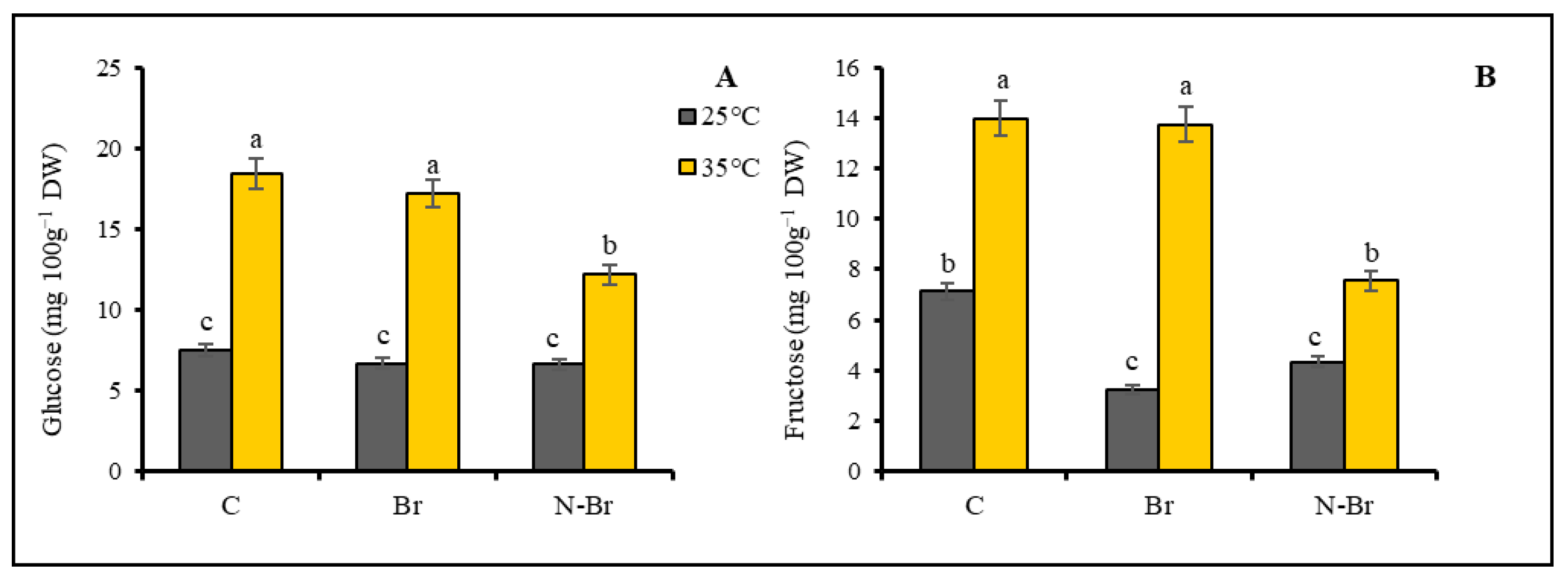
3.3. Sugars and Fatty Acids
4. Discussion
4.1. The Interacting Effect of Br and N-Br on Morphological Characteristics of Bell Pepper
4.2. The Interaction Effect of Br and N-Br on Physiological Characteristics of Bell Pepper
4.3. The Interacting Effect of Br and N-Br on Antioxidant Enzyme Activities, MDA, and Proline Content of Bell Pepper
4.4. The Interacting Effect of Br and N-Br on Glucose and Fructose Content of Bell Pepper
4.5. The Interaction Effect of Br and N-Br on Saturated and Unsaturated Fatty Acids of Pepper
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fan, R.; Tao, X.Y.; Xia, Z.Q.; Sim, S.; Hu, L.S.; Wu, B.D.; Wang, Q.H.; Hao, C.Y. Comparative transcriptome and metabolome analysis of resistant and susceptible piper species upon infection by the oomycete phytophthora capsici. Front. Plant Sci. 2022, 13, 864927. [Google Scholar] [CrossRef] [PubMed]
- Nolan, R.H.; Collins, L.; Leigh, A.; Ooi, M.K.J.; Curran, T.J.; Fairman, T.A.; Resco de Dios, V.; Bradstock, R. Limits to post-fire vegetation recovery under climate change. Plant Cell Environ. 2021, 44, 3471–3489. [Google Scholar] [CrossRef]
- Ahmed, W.; Xia, Y.; Li, R.; Bai, G.; Siddique, K.H.M.; Guo, P. Non-coding RNAs: Functional roles in the regulation of stress response in Brassica crops. Genomics 2020, 112, 1419–1424. [Google Scholar] [CrossRef] [PubMed]
- Taheri, M.; Haghighi, M. Benzyl adenine is more effective than potassium silicate on decreasing the detrimental effects of heat stress in pepper (Capsicum annum cv. PS301). Iran Agric. Res. 2018, 37, 89–98. [Google Scholar] [CrossRef]
- Pagamas, P.; Nawata, E. Sensitive stages of fruit and seed development of chili pepper (Capsicum annuum L. var. Shishito) exposed to high-temperature stress. Scie Hortic. 2008, 117, 21–25. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.M.; Roychowdhury, R.; Fujita, M. Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int. J. Mol. Sci. 2013, 14, 9643–9684. [Google Scholar] [CrossRef]
- Shabala, S.; Cuin, T.A. Potassium transport and plant salt tolerance. Physiol. Plant. 2008, 133, 651–669. [Google Scholar] [CrossRef] [PubMed]
- Jogawat, A. Osmolytes and their role in abiotic stress tolerance in plants. In Molecular Plant Abiotic Stress: Biology and Biotechnology; Wiley: London, UK, 2019; pp. 91–104. [Google Scholar] [CrossRef]
- Raza, A.; Razzaq, A.; Mehmood, S.S.; Hussain, M.A.; Wei, S.; He, H. Omics: The way forward to enhance abiotic stress tolerance in Brassica napus L. GM Crops Food 2021, 12, 251–281. [Google Scholar] [CrossRef]
- Saddiq, M.S.; Afzal, I.; Iqbal, S.; Hafeez, M.B.; Raza, A. Low sodium content in leaves improves grain yield and physiological performance of wheat genotypes in saline- sodic soil. Trop. Agric. Res. Pesqu. Agropec. Trop. 2021, 51, e67663. [Google Scholar] [CrossRef]
- dos Santos, T.B.; Ribas, A.F.; de Souza, S.G.H.; Budzinski, I.G.F.; Domingues, D.S. Physiological responses to drought, salinity, and heat stress in plants: A review. Stresses 2022, 2, 113–135. [Google Scholar] [CrossRef]
- Raza, A. Metabolomics: A systems biology approach for enhancing heat stress tolerance in plants. Plant Cell Rep. 2020, 41, 741–763. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Parvin, K.; Bardhan, K.; Nahar, K.; Anee, T.I.; Masud, A.A.C.; Fotopoulos, V. Biostimulants for the regulation of reactive oxygen species metabolism in plants under abiotic stress. Cells 2021, 10, 2537. [Google Scholar] [CrossRef] [PubMed]
- Napieraj, N.; Janicka, M.; Reda, M. Interactions of polyamines and phytohormones in plant response to abiotic stress. Plants 2023, 12, 1159. [Google Scholar] [CrossRef]
- Sarraf, M.; Janeeshma, E.; Arif, N.; QudratUllahFarooqi, M.; Kumar, V.; Ansari, N.A.; Ghani, M.I.; Ahanger, M.A.; Hasanuzzaman, M. Understanding the role of beneficial elements in developing plant stress resilience: Signalling and crosstalk with phytohormones and microbes. Plant Stress 2023, 10, 100224. [Google Scholar] [CrossRef]
- Manghwar, H.; Hussain, A.; Ali, Q.; Liu, F. Brassinosteroids (BRs) role in plant development and coping with different stresses. Int. J. Mol. Sci. 2022, 23, 1012. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.P.S.D.; Alencar, A.A.d.S.; Sudré, C.P.; Araújo, M.d.S.B.d.; Lobato, A.K.d.S. Brassinosteroids: Relevant evidence related to mitigation of abiotic and biotic stresses in plants. Agronomy 2024, 14, 840. [Google Scholar] [CrossRef]
- Peres, A.L.G.L.; Soares, J.S.; Tavares, R.G.; Righetto, G.; Zullo, M.A.T.; Mandava, N.B.; Menossi, M. Brassinosteroids, the sixth class of phytohormones: A molecular view from the discovery to hormonal interactions in plant development and stress adaptation. Int. J. Mol. Sci. 2019, 20, 331. [Google Scholar] [CrossRef]
- Miao, R.; Li, C.; Liu, Z.; Zhou, X.; Chen, S.; Zhang, D.; Luo, J.; Tang, W.; Wang, C.; Wu, J.; et al. The role of endogenous brassinosteroids in the mechanisms regulating plant reactions to various abiotic stresses. Agronomy 2024, 14, 356. [Google Scholar] [CrossRef]
- Basit, F.; Liu, J.; An, J.; Chen, M.; He, C.; Zhu, X.; Li, Z.; Hu, J.; Guan, Y. Brassinosteroids as a multidimensional regulator of plant physiological and molecular responses under various environmental stresses. Environ. Sci. Pollut. Res. 2021, 28, 44768–44779. [Google Scholar] [CrossRef]
- Wu, W.; Zhang, Q.; Ervin, E.H.; Yang, Z.; Zhang, X. Physiological mechanism of enhancing salt stress tolerance of perennial ryegrass by 24-epibrassinolide. Front. Plant Sci. 2017, 8, 1017–1026. [Google Scholar] [CrossRef]
- Abbas, S.; Latif, H.H.; Elsherbiny, E.A. Effect of 24-epibrassinolide on the physiological and genetic changes on two varieties of pepper under salt stress conditions. Pak. J. Bot. 2013, 45, 1273–1284. [Google Scholar]
- Zulfiqar, F.; Nafees, M.; Chen, J.; Darras, A.; Ferrante, A.; Hancock, J.T.; Ashraf, M.; Zaid, A.; Latif, N.; Corpas, F.J.; et al. Chemical priming enhances plant tolerance to salt stress. Front. Plant Sci. 2022, 13, 2883. [Google Scholar] [CrossRef] [PubMed]
- Ogweno, O.; Otieno, J.; Shun Song, X.; Shi, K.; Hu, W.H.; Mao, W.H.; Hong Zhou, Y.; Yu, J.Q.; Nogués, S. Brassinosteroids alleviate heat-induced inhibition of photosynthesis by increasing carboxylation efficiency and enhancing antioxidant systems in Lycopersicon esculentum. J. Plant Growth Regul. 2008, 27, 49–57. [Google Scholar] [CrossRef]
- Wang, W.; Xie, Y.; Liu, C.; Jiang, H. The exogenous application of brassinosteroids confers tolerance to heat stress by increasing antioxidant capacity in soybeans. Agriculture 2022, 12, 1095. [Google Scholar] [CrossRef]
- Eremina, M.; Unterholzner, S.J.; Rathnayake, A.I.; Castellanos, M.; Khan, M.; Kugler, K.G.; May, S.T.; Mayer, K.F.; Rozhon, W.; Poppenberger, B. Brassinosteroids participate in the control of basal and acquired freezing tolerance of plants. Proc. Natl. Acad. Sci. USA 2016, 113, E5982–E5991. [Google Scholar] [CrossRef]
- Yang, P.; Wang, Y.; Li, J.; Bian, Z. Effects of brassinosteroids on photosynthetic performance and nitrogen metabolism in pepper seedlings under chilling stress. Agronomy 2019, 9, 839. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, Q.; Wang, X.; Xi, Z. Brassinosteroid stimulates hydrogen peroxide biosynthesis and reduces the effect of cold stress. J. Plant Growth Regul. 2023, 42, 3757–3769. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Ashraf, M. Nanoparticles potentially mediate salt stress tolerance in plants. Plant Physiol. Biochem. 2021, 160, 257–268. [Google Scholar] [CrossRef]
- Hamed, R.; Jodeh, S.; Alkowni, R. Nano bio fertilizer capsules for sustainable agriculture. Sci Rep. 2024, 14, 13646. [Google Scholar] [CrossRef]
- Zahra, Z.; Habib, Z.; Hyun, H.; Shahzad, H.M.A. Overview on recent developments in the design, application, and impacts of nanofertilizers in agriculture. Sustainability 2022, 14, 9397. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Navarro, M.; Ashraf, M.; Akram, N.A.; Munne-Bosch, S. Nanofertilizer use for sustainable agriculture: Advantages and limitations. Plant Sci. 2019, 289, 110270. [Google Scholar] [CrossRef] [PubMed]
- Garg, D.; Sridhar, K.; Stephen Inbaraj, B.; Chawla, P.; Tripathi, M.; Sharma, M. Nano-biofertilizer formulations for agriculture: A systematic review on recent advances and prospective applications. Bioengineering 2023, 10, 1010. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.; Yadav, K.; Abd-Elsalam, K.A. Nanofertilizers: Types, delivery and advantages in agricultural sustainability. Agrochemicals 2023, 2, 296–336. [Google Scholar] [CrossRef]
- Hong, J.; Wang, C.; Wagner, D.C.; Gardea-Torresdey, J.L.; He, F.; Rico, C.M. Foliar application of nanoparticles: Mechanisms of absorption, transfer, and multiple impacts. Environ. Sci. Nano 2021, 8, 1196–1210. [Google Scholar] [CrossRef]
- Abbasifar, A.; Shahrabadi, F.; Valizadehkaji, B. Effects of green synthesized zinc and copper nano- fertilizers on the morphological and biochemical attributes of basil plant. J. Plant Nutr. 2020, 43, 1104–1118. [Google Scholar] [CrossRef]
- Jones, J.B. Hydroponics: A Practical Guide for the Soilless Grower; CRC Press: New York, NY, USA, 2016. [Google Scholar]
- Mozafari, R.; Johnson, M.; Hatziantoniou, C.; Demetzos, S.C. Nanoliposomes and their applications in food nanotechnology. J. Liposome Res. 2008, 18, 309–327. [Google Scholar] [CrossRef]
- Hallaji, B.; Haghighi, M.; Abolghasemi, R.; Mozafarian, M. Effect of foliar applications of aminolevulinic acid (bulk and nano-encapsulated) on bell pepper under heat stress. Plant Stress 2024, 12, 100477. [Google Scholar] [CrossRef]
- Seetapan, N.; Bejrapha, P.; Srinuanchai, W.; Ruktanonchai, U.R. Rheological and morphological characterizations on physical stability of gamma-oryzanol-loaded solid lipid nanoparticles (SLNs). Micron 2010, 41, 51–58. [Google Scholar] [CrossRef]
- Rodriguez-Dominguez, C.M.; Forner, A.; Martorell, S.; Choat, B.; Lopez, R.; Peters, J.M.R.; Pfautsch, S.; Mayr, S.; Carins-Murphy, M.R.; McAdam, S.A.M.; et al. Leaf water potential measurements using the pressure chamber: Synthetic testing of assumptions towards best practices for precision and accuracy. Plant Cell Environ. 2022, 45, 037–2061. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence a practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Dhopte, A.M.; Livera, M.M. Principles and Techniques for Plant Scientist; Agrobios: Chopasani, India, 2002. [Google Scholar]
- Lutts, S.; Kinet, J.; Bouharmont, J. NaCl-induced senescence in leaves of rice (Oryza sativa L.) cultivars differing in salinity tolerate. Ann. Bot. 1996, 78, 389–398. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Koleva, I.I.; Van Beek, T.A.; Linssen, J.P.; Groot, A.D.; Evstatieva, L.N. Screening of plant extracts for antioxidant activity: A comparative study on three testing methods. Phytochem Anal. 2002, 13, 8–17. [Google Scholar] [CrossRef]
- Tabatabaei, S.; Ehsanzadeh, P. Photosynthetic pigments, ionic and antioxidative behaviour of hulled tetraploid wheat in response to NaCl. Photosynthetica 2016, 54, 340–350. [Google Scholar] [CrossRef]
- Khoshbakht, D.; Asghari, M.R.; Haghighi, M. Effects of foliar applications of nitric oxide and spermidine on chlorophyll fluorescence, photosynthesis and antioxidant enzyme activities of citrus seedlings under salinity stress. Photosynthetica 2018, 56, 1313–1325. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Jiang, M.; Zhang, J. Effect of abscisic acid on active oxygen species, antioxidative defence system and oxidative damage in leaves of maize seedlings. Plant Cell Physiol. 2001, 42, 1265–1273. [Google Scholar] [CrossRef]
- Adams, S.H.; Stanhope, K.L.; Grant, R.W.; Cummings, B.P.; Havel, P.J. Metabolic and endocrine profiles in response to systemic infusion of fructose and glucose in rhesus macaques. Endocrinology 2008, 149, 3002–3008. [Google Scholar] [CrossRef][Green Version]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Goli, S.A.H.; Kadivar, M.; Keramat, J.; Fazilati, M. Conjugated linoleic acid (ClA) production and lipase-catalyzed interesterification of purified ClA with canola oil. Eur. J. Lipid Sci. Technol. 2008, 110, 400–404. [Google Scholar] [CrossRef]
- Abdelmageed, A.H.A.; Gruda, N. Influence of high temperatures on gas exchange rate and growth of eight tomato cultivars under controlled heat stress conditions. Eur. J. Hortic. Sci. 2009, 74, 152–162. [Google Scholar]
- Niu, J.H.; Anjum, A.S.; Wang, R.; Li, J.H.; Liu, M.R.; Song, J.X.; Zohaib, A.; Lv, J.; Wang, S.G.; Zong, X.F. Exogenous application of brassinolide can alter morphological and physiological traits of Leymus chinensis (Trin) Tzvelev under room and high temperatures. Chil. J. Agric. Res. 2016, 76, 27–33. [Google Scholar] [CrossRef]
- Li, J.; Yang, P.; Kang, J.; Gan, Y.; Yu, J.; Calderon-Urrea, A.; Jian, L.; Zhang, G.; Feng, Z.; Xie, J. Transcriptome analysis of pepper revealed a role of 24-epibrassinolide in response to chilling. Front. Plant Sci. 2016, 7, 1281. [Google Scholar] [CrossRef] [PubMed]
- Shu, S.; Tang, Y.; Yuan, Y.; Sun, J.; Zhong, M.; Guo, S. The role of 24-epibrassinolide in the regulation of photosynthetic characteristics and nitrogen metabolism of tomato seedlings under a combined low temperature and weak light stress. Plant Physiol. Biochem. 2016, 7, 344–353. [Google Scholar] [CrossRef]
- Wu, Z.; Gu, S.; Gu, H.; Cheng, D.; Li, L.; Guo, X.; Wang, M.; He, S.; Li, M.; Chen, J. Physiological and transcriptomic analyses of brassinosteroid function in kiwifruit root Zhiyong. Environ. Exp. Bot. 2022, 194, 104685. [Google Scholar] [CrossRef]
- Tong, H.; Xiao, Y.; Liu, D.; Gao, S.; Liu, L.; Yin, Y.; Jin, Y.; Qian, Q.; Chu, C. Brassinosteroid regulates cell elongation by modulating gibberellin metabolism in rice. Plant Cell 2014, 26, 4376–4393. [Google Scholar] [CrossRef]
- Upreti, K.K.; Srinivasa Rao, N.K.; Jayaram, H.L. Floral abscission in capsicum under high temperature: Role of endogenous hormones and polyamines. Indian J. Plant Physiol. 2012, 17, 207–214. [Google Scholar]
- Dutta, S.K.; Gurung, G.; Yadav, A.; Laha, R.; Mishra, V.K. Factors associated with citrus fruit abscission and management strategies developed so far: A review. New Zealand J. Crop. Hortic. Sci. 2022, 51, 467–488. [Google Scholar] [CrossRef]
- Shi, Y.; Song, B.; Liang, Q.; Su, D.; Lu, W.; Liu, Y.; Li, Z. Molecular regulatory events of flower and fruit abscission in horticultural plants. Hortic. Plant J. 2023, 9, 867–883. [Google Scholar] [CrossRef]
- Ma, X.; Yuan, Y.; Li, C.; Wu, Q.; He, Z.; Li, J.; Zhao, M. Brassinosteroids suppress ethylene-induced fruitlet abscission through LcBZR1/2-mediated transcriptional repression of LcACS1/4 and LcACO2/3 in litchi. Hortic. Res. 2021, 8, 105. [Google Scholar] [CrossRef]
- Lv, B.; Tian, H.; Zhang, F.; Liu, J.; Lu, S.; Bai, M.; Li, C.; Ding, Z. Brassinosteroids regulate root growth by controlling reactive oxygen species homeostasis and dual effect on ethylene synthesis in Arabidopsis. PLoS Genet. 2018, 14, e1007144. [Google Scholar] [CrossRef] [PubMed]
- Furio, R.; Salazar, S.; Mariotti-Martínez, J.; Martínez-Zamora, G.; Coll, Y.; Díaz-Ricci, J. Brassinosteroid applications enhance the tolerance to abiotic stresses, production and quality of strawberry fruits. Horticulturae 2022, 8, 572. [Google Scholar] [CrossRef]
- Ji, W.; Luo, H.; Song, Y.; Hong, E.; Li, Z.; Lin, B.; Fan, C.; Wang, H.; Song, X.; Jin, S.; et al. Changes in photosynthetic characteristics of paeonia suffruticosa under high temperature stress. Agronomy 2022, 12, 1203. [Google Scholar] [CrossRef]
- Sadura, I.; Janeczko, A. Brassinosteroids and the tolerance of cereals to low and high temperature stress: Photosynthesis and the physicochemical properties of cell membranes. Int. J. Mol. Sci. 2022, 23, 342. [Google Scholar] [CrossRef]
- Gudesblat, G.E.; Schneider-Pizon, J.; Betti, C.; Mayerhofer, J.; Vanhoutte, I.; Dongen, W. Speechless integrates brassinosteroid and stomata signalling pathways. Nat. Cell Biol. 2012, 14, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Daszkowska-Golec, A.; Szarejko, I. Open or close the gate—Stomata action under the control of phytohormones in drought stress conditions. Front. Plant Sci. 2013, 4, 138. [Google Scholar] [CrossRef]
- Sharma, I.; Ching, E.; Saini, S.; Bhardwaj, R.; Pati, P.K. Exogenous application of brassinosteroid offers tolerance to salinity by altering stress responses in rice variety Pusa Basmati-1. Plant Physiol. Biochem. 2013, 69, 17–26. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Mir, R.A.; Alyemeni, M.N.; Ahmad, P. Combined effects of brassinosteroid and kinetin mitigates salinity stress in tomato through the modulation of antioxidant and osmolyte metabolism. Plant Physiol. Biochem. 2020, 147, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Neha; Twinkle; Mohapatra, S.; Sirhindi, G.; Dogra, V. Seed priming with brassinolides improves growth and reinforces antioxidative defenses under normal and heat stress conditions in seedlings of Brassica juncea. Physiol. Plant. 2022, 174, e13814. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Y.; Chen, H.; Xiang, J.; Zhang, Y.; Wang, Z.; Zhu, D.; Zhang, Y. Brassinosteroids mediate endogenous phytohormone metabolism to alleviate high temperature injury at panicle initiation stage in rice. Rice Sci. 2023, 30, 70–86. [Google Scholar] [CrossRef]
- Nazir, F.; Jahan, B.; Kumari, S.; Iqbal, N.; Albaqami, M.; Sofo, A.; Khan, M.I.R. Brassinosteroid modulates ethylene synthesis and antioxidant metabolism to protect rice (Oryza sativa) against heat stress-induced inhibition of source—Sink capacity and photosynthetic and growth attributes. J. Plant Physiol. 2023, 289, 154096. [Google Scholar] [CrossRef] [PubMed]
- Hafeez, M.B.; Zahra, N.; Zahra, K.; Raza, A.; Khan, A.; Shaukat, K.; Khan, S. Brassinosteroids: Molecular and physiological responses in plant growth and abiotic stresses. Plant Stress 2021, 2, 100029. [Google Scholar] [CrossRef]
- Camejo, D.; Rodríguez, P.; Morales, M.A.; Dell’Amico, J.M.; Torrecillas, A.; Alarcón, J.J. High temperature effects on photosynthetic activity of two tomato cultivars with different heat susceptibility. J. Plant Physiol. 2005, 162, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.P.; Huang, L.F.; Cheng, F.; Zhou, Y.H.; Xia, X.J.; Mao, W.H.; Shi, K.; Yu, J. Brassinosteroids accelerate recovery of photosynthetic apparatus from cold stress bybalancing the electron partitioning, carboxylation and redox homeostasis in cucumber. Physiol. Plant. 2013, 148, 133–145. [Google Scholar] [CrossRef]
- Lee, H.J.; Lee, J.H.; Lee, S.G.; An, S.; Lee, H.S.; Choi, C.K.; Kim, S.K. Foliar application of biostimulants affects physiological responses and improves heat stress tolerance in Kimchi cabbage. Hortic. Environ. Biotech. 2020, 61, 207. [Google Scholar] [CrossRef]
- Arief, M.A.A.; Kim, H.; Kurniawan, H.; Nugroho, A.P.; Kim, T.; Cho, B.-K. Chlorophyll fluorescence imaging for early detection of drought and heat stress in strawberry plants. Plants 2023, 12, 1387. [Google Scholar] [CrossRef]
- Bhattarai, S.; Harvey, J.T.; Djidonou, D.; Leskovar, D.I. Exploring morpho-physiological variation for heat stress tolerance in tomato. Plants 2021, 10, 347. [Google Scholar] [CrossRef]
- Thussagunpanit, J.; Jutamanee, K.; Sonjaroon, W.; Kaveeta, L.; Chai-Arree, W.; Pankean, P.; Suksamrarn, A. Effects of brassinosteroid and brassinosteroid mimic on photosynthetic efficiency and rice yield under heat stress. Photosynthetica 2015, 53, 312–320. [Google Scholar] [CrossRef]
- Adams, S.R.; Cockshull, K.E.; Cave, C.R.J. Effect of temperature on the growth and development of tomato fruits. Ann. Bot. 2001, 88, 869–877. [Google Scholar] [CrossRef]
- Motamedi, M.; Haghighi, M.; Goli, A. Physiological changes of sweet and hot peppers in vegetative and reproductive growth stages treated by Ca and H2O2 under unforeseen heat stresses. Sci. Hortic. 2019, 249, 306–313. [Google Scholar] [CrossRef]
- Sepuveda, G.; Kliewer, W.M. Effect of high temperature on grapevines (Vitis vinifera L.). II. Distribution of soluble sugars. Am. J. Enol. Vitic. 1986, 37, 20–25. [Google Scholar] [CrossRef]
- Barros, M.H.; Bandy, B.; Tahara, E.B.; Kowaltowski, A.J. Higher respiratory activity decreases mitochondrial reactive oxygen release and increases life span in Saccharomyces cerevisiae. J. Biol. Chem. 2004, 279, 49883–49888. [Google Scholar] [CrossRef] [PubMed]
- Thussagunpanit, J.; Jutamanee, K.; Kaveeta, L.; Chai-arree, W.; Pankean, P.; Homvisasevongsa, S.; Suksamrarn, A. Comparative effects of brassinosteroid and brassinosteroid mimic on improving photosynthesis, lipid peroxidation and rice seed set under heat stress. J. Plant Growth Regul. 2015, 34, 320–331. [Google Scholar] [CrossRef]
- Raison, J.K.; Roberts, J.K.; Berry, J.A. Correlations between the thermal stability of chloroplast (thylakoid) membranes and the composition and fluidity of their polar lipids upon acclimation of the higher plant, Nerium oleander, to growth temperature. Biochim. Biophys. Acta. 1982, 688, 218–228. [Google Scholar] [CrossRef]
- Combos, Z.; Wada, H.; Hideg, E.; Murata, N. The unsaturation of membrane lipids stabilizes photosynthesis against heat stress. Plant. Physiol. 1994, 104, 563–567. [Google Scholar] [CrossRef]
- Quinn, P.J.; Joo, F.; Vigh, L. The role of unsaturated lipids in membrane structure and stability. Prog. Biophys. Mol. 1989, 53, 71–103. [Google Scholar] [CrossRef] [PubMed]
- Saidi, Y.; Peter, M.; Finka, A.; Cicekli, C.; Vigh, L.; Goloubinoff, P. Membrane lipid composition affects plant heat sensing and modulates Ca2+-dependent heat shock response. Plant Signal. Behav. 2010, 5, 1530–1533. [Google Scholar] [CrossRef]
- Li, B.; Zhang, C.; Cao, B.; Qin, G.; Wang, W.; Tian, S. Brassinolide enhances cold stress tolerance of fruit by regulating plasma membrane proteins and lipids. Amino Acids 2012, 43, 2469–2480. [Google Scholar] [CrossRef]
- Zafari, M.; Ebadi, A.; Sedghi, M.; Jahanbakhsh, S.; Miransari, M.J. Alleviating effect of 24-epibrassinolide on seed oil content and fatty acid composition under drought stress in safflower. J. Food Compost. Anal. 2020, 92, 103544. [Google Scholar] [CrossRef]

| 25 °C | 35 °C | |||||
|---|---|---|---|---|---|---|
| C | Br | N-Br | C | Br | N-Br | |
| Palmitic acid | 19.514 ± 0.645 ab | 19.913 ± 0.11 ab | 18.449 ± 0.128 b | 23.520 ± 0.71 a | 19.082 ± 0.25 ab | 24.66 ± 0.50 a |
| Stearic acid | 4.769 ± 0.654 c | 5.850 ± 0.62 c | 9.386 ± 0.165 c | 14.135 ± 0.45 b | 15.216 ± 55 b | 18.751 ± 0.38 a |
| Oleic acid | 19.340 ± 0.746 a | 16.057 ± 0.91 b | 19.73 ± 0.163 a | 13.298 ± 0.43 c | 19.47 ± 0.74 a | 17.788 ± 0.44 b |
| Linolelaidic acid | 15.462 ± 0.937 b | 10.079 ± 0.61 b | 18.609 ± 0.109 ab | 18.643 ± 0.09 ab | 19.057 ± 0.31 a | 21.414 ± 0.34 a |
| α-Linoleic acid | 8.959 ± 0.639 c | 9.952 ± 0.710 bc | 7.836 ± 0.262 c | 16.057 ± 0.41 a | 10.079 ± 0.59 b | 10.544 ± 0.51 b |
| Linoleic acid | 15.665 ± 0.1823 b | 16.55 ± 0.16 b | 17.854 ± 0.28 ab | 10.079 ± 0.61 c | 18.117 ± 0.37 a | 17.907 ± 0.21 ab |
| Saturated % | 24.28 | 25.76 | 27.83 | 37.65 | 34.30 | 43.42 |
| Unsaturated % | 59.43 | 52.64 | 64.03 | 58.08 | 66.72 | 67.65 |
| Saturated/unsaturated | 0.41 | 0.49 | 0.43 | 0.65 | 0.51 | 0.64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Halaji, B.; Haghighi, M.; Kovács, G.P.; Mirmazloum, I.; Szegő, A. The Role of Brassinosteroids and Nano-Encapsulated Brassinosteroids in Capsicum Pepper Growth and Physiological Adaptations to High-Temperature Stress. Horticulturae 2024, 10, 1062. https://doi.org/10.3390/horticulturae10101062
Halaji B, Haghighi M, Kovács GP, Mirmazloum I, Szegő A. The Role of Brassinosteroids and Nano-Encapsulated Brassinosteroids in Capsicum Pepper Growth and Physiological Adaptations to High-Temperature Stress. Horticulturae. 2024; 10(10):1062. https://doi.org/10.3390/horticulturae10101062
Chicago/Turabian StyleHalaji, Behnaz, Maryam Haghighi, Gergő Péter Kovács, Iman Mirmazloum, and Anita Szegő. 2024. "The Role of Brassinosteroids and Nano-Encapsulated Brassinosteroids in Capsicum Pepper Growth and Physiological Adaptations to High-Temperature Stress" Horticulturae 10, no. 10: 1062. https://doi.org/10.3390/horticulturae10101062
APA StyleHalaji, B., Haghighi, M., Kovács, G. P., Mirmazloum, I., & Szegő, A. (2024). The Role of Brassinosteroids and Nano-Encapsulated Brassinosteroids in Capsicum Pepper Growth and Physiological Adaptations to High-Temperature Stress. Horticulturae, 10(10), 1062. https://doi.org/10.3390/horticulturae10101062







