Integrated Transcriptome and Metabolome Analysis Revealed Mechanism Underlying Anthocyanin Biosynthesis During Flower Color Formation in Lagerstroemia indica
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Detection of Total Flavonoids in Lagerstroemia indica Petals for Metabolomic Analysis
2.3. Transcriptome Analysis Method for Lagerstroemia indica
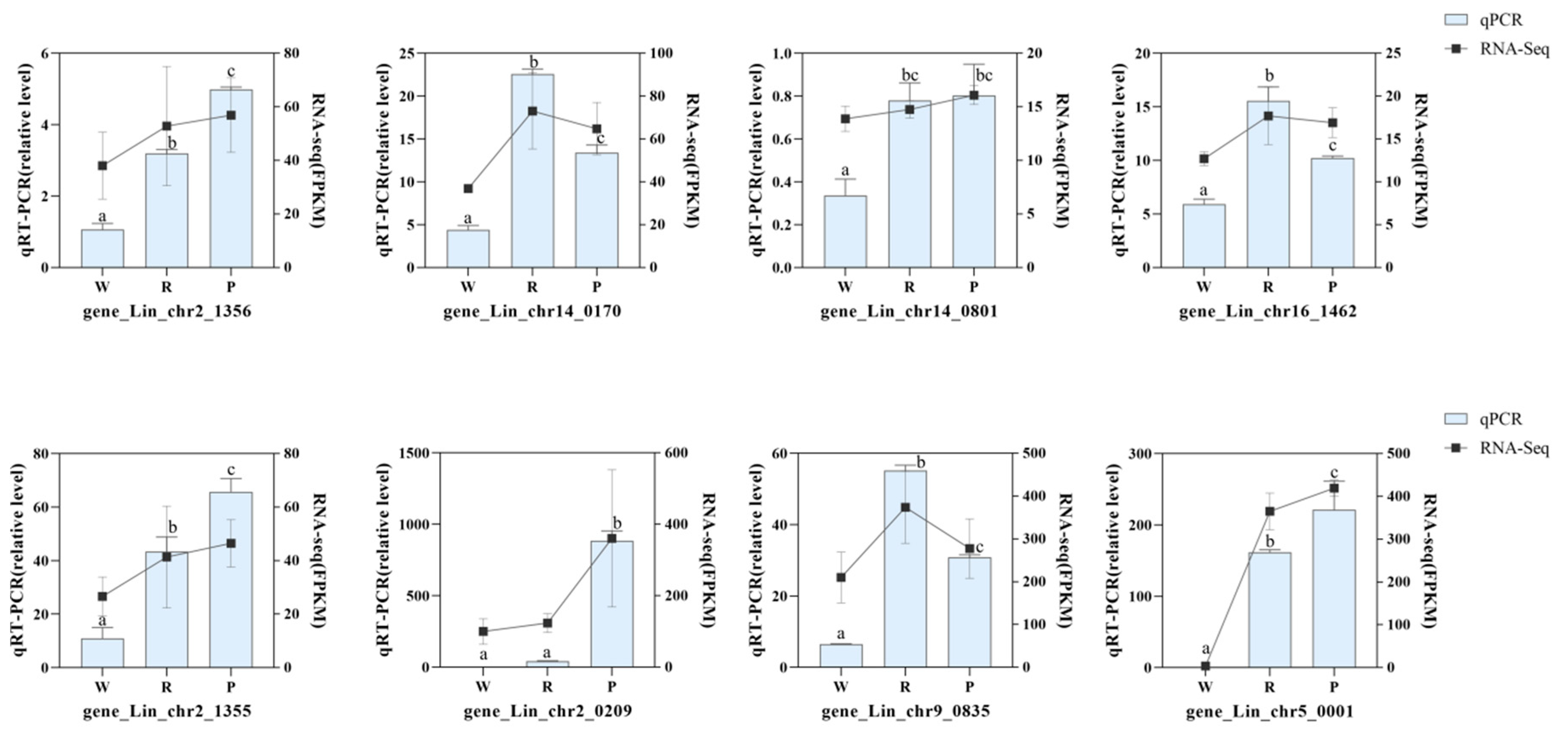
2.4. Quantitative Real-Time PCR (qRT-PCR)
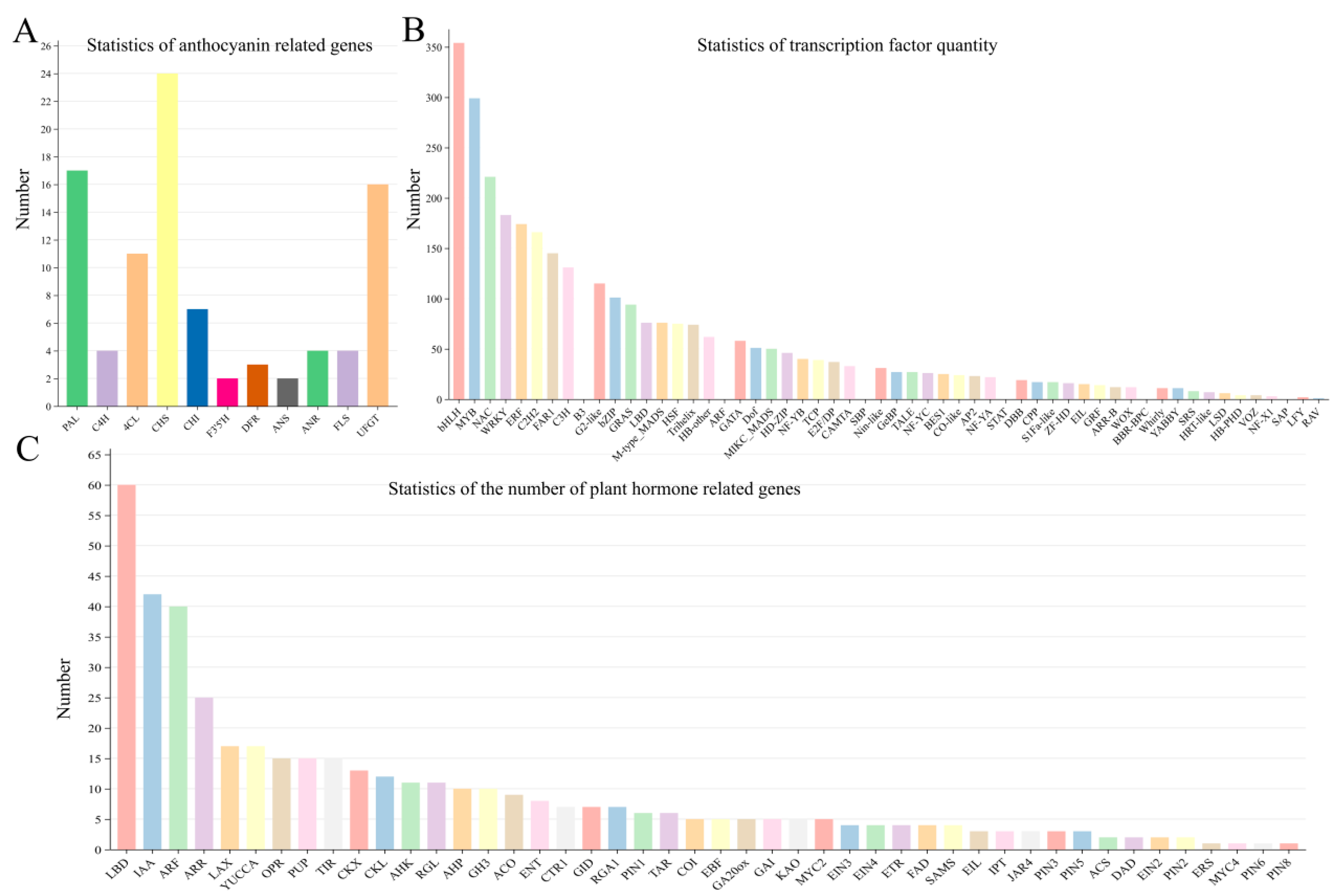
2.5. Transcription Factors Analysis
2.6. Statistical Analysis
3. Results
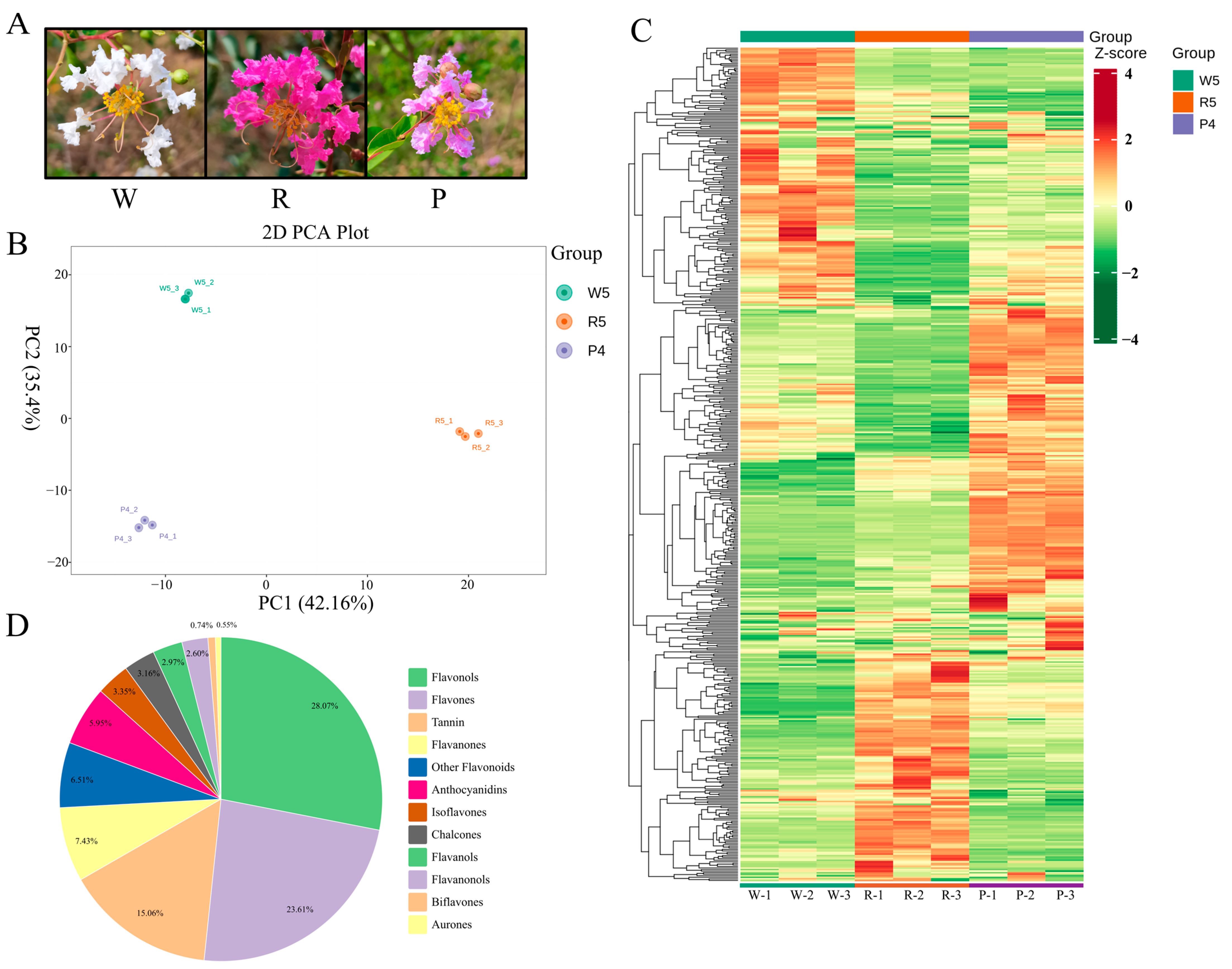
3.1. Principal Component Analysis of Metabolites
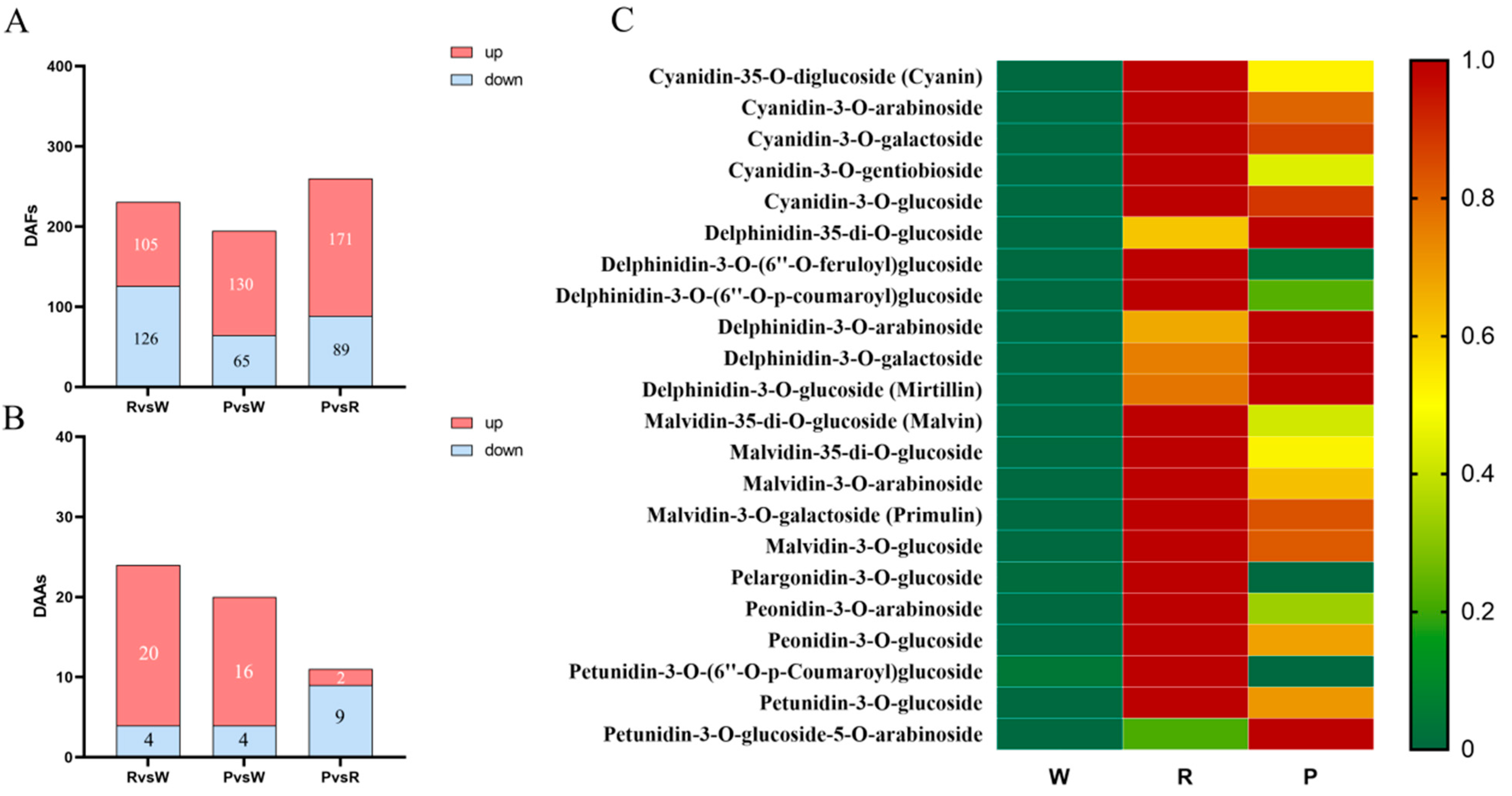
3.2. Analysis of Differential Metabolites
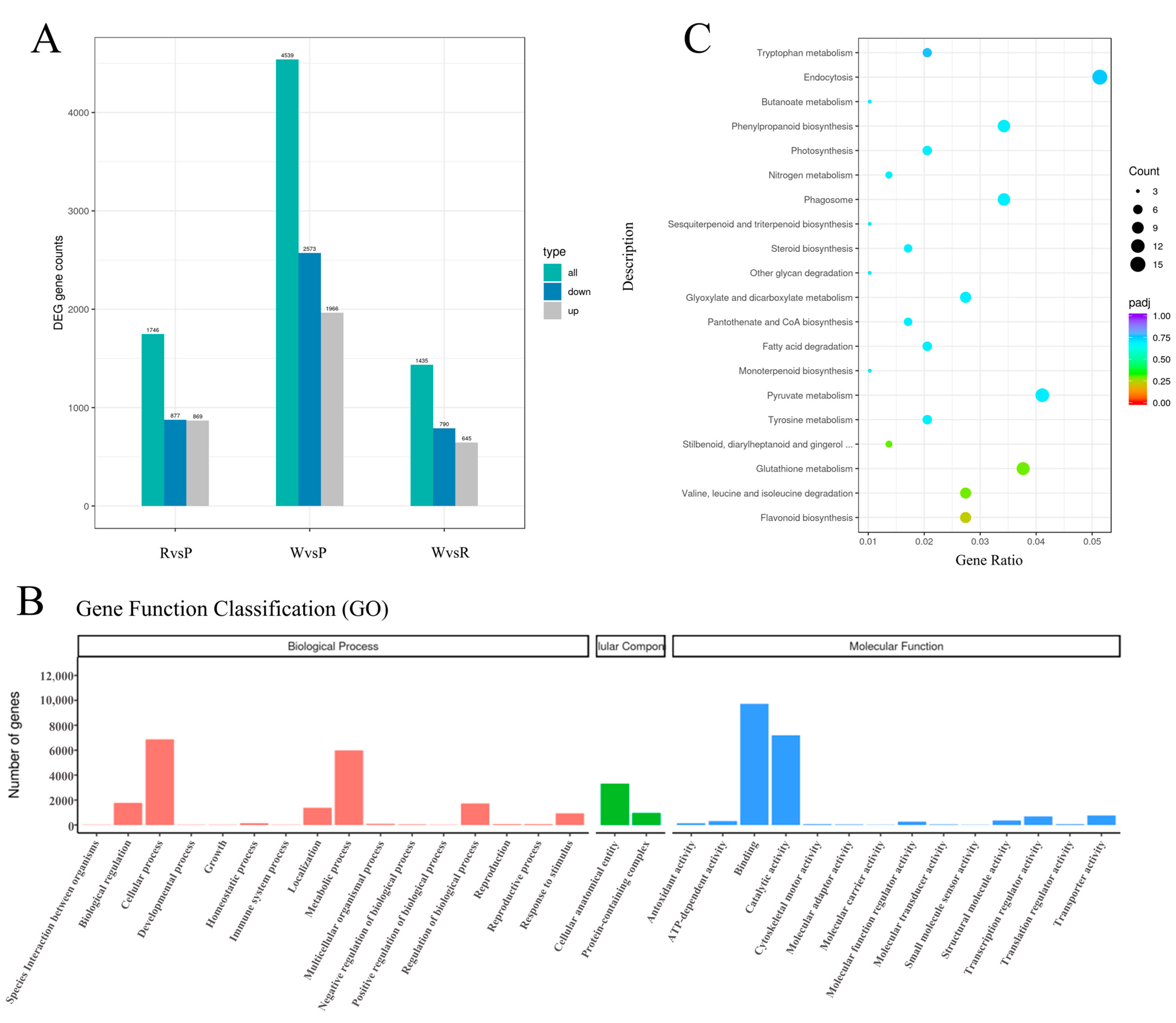
3.3. Transcriptome Sequencing and Analysis
3.4. Identification and Enrichment of DEGs
3.5. DEGs Involved in the Anthocyanin Synthesis Pathway
3.6. Models of Anthocyanin Biosynthetic Pathways and qRT-PCR Verification
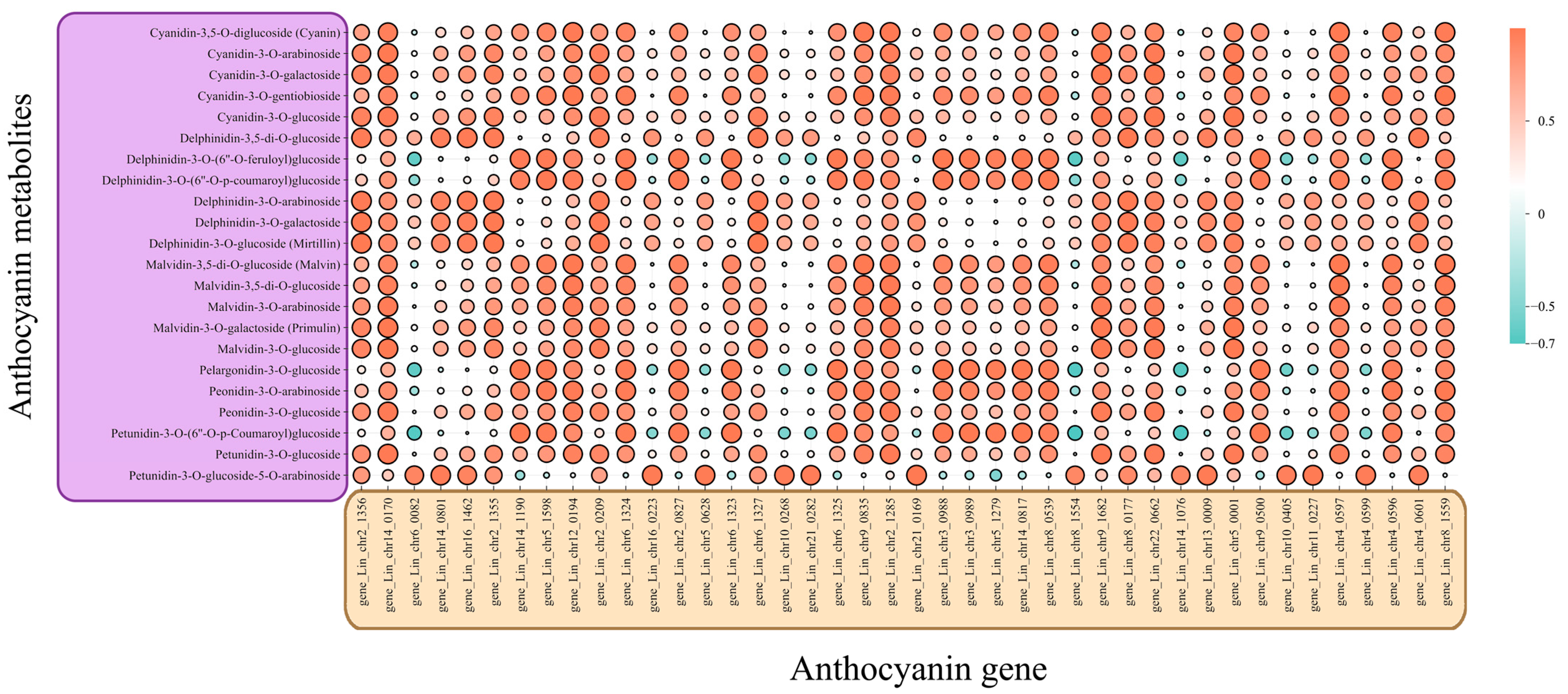
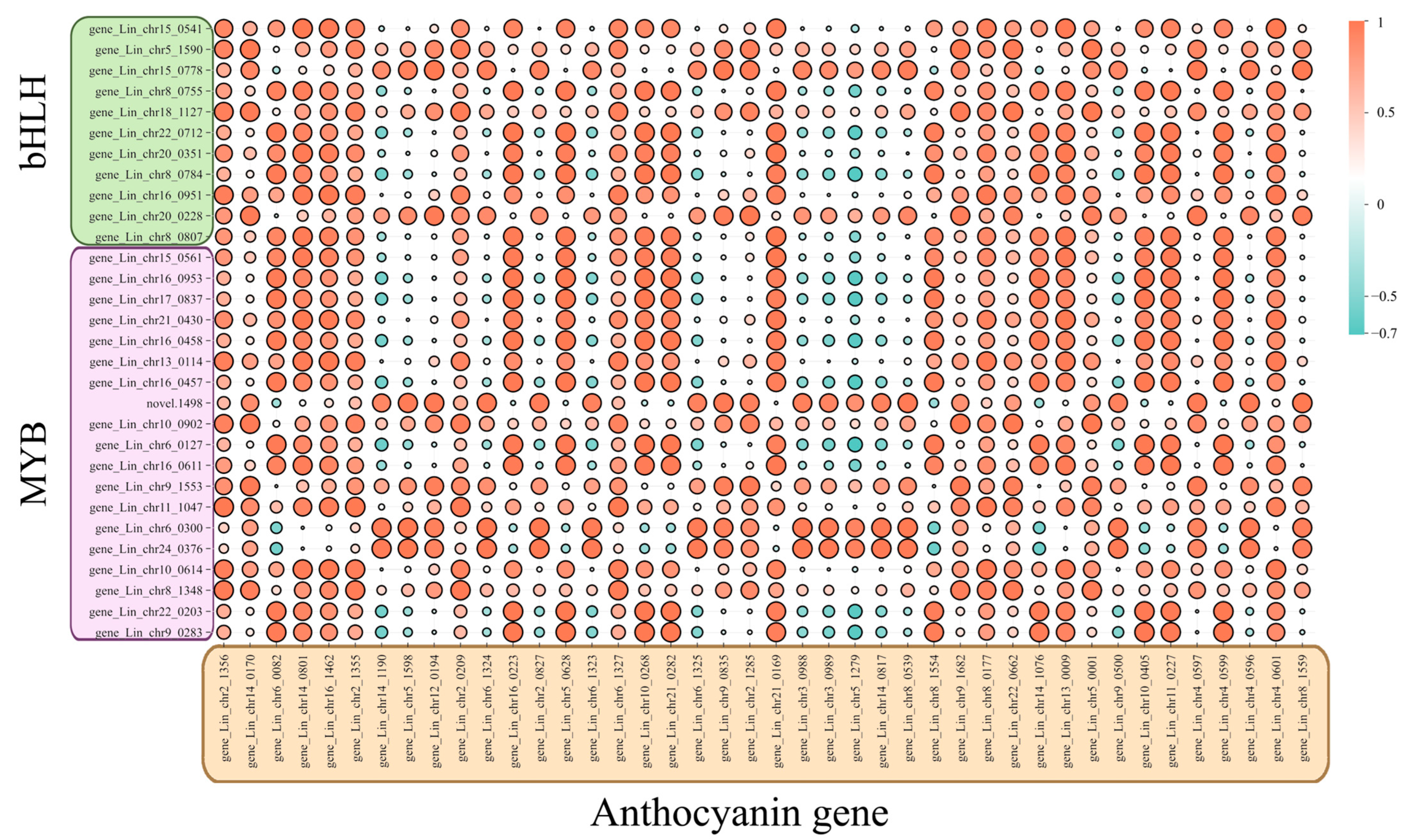
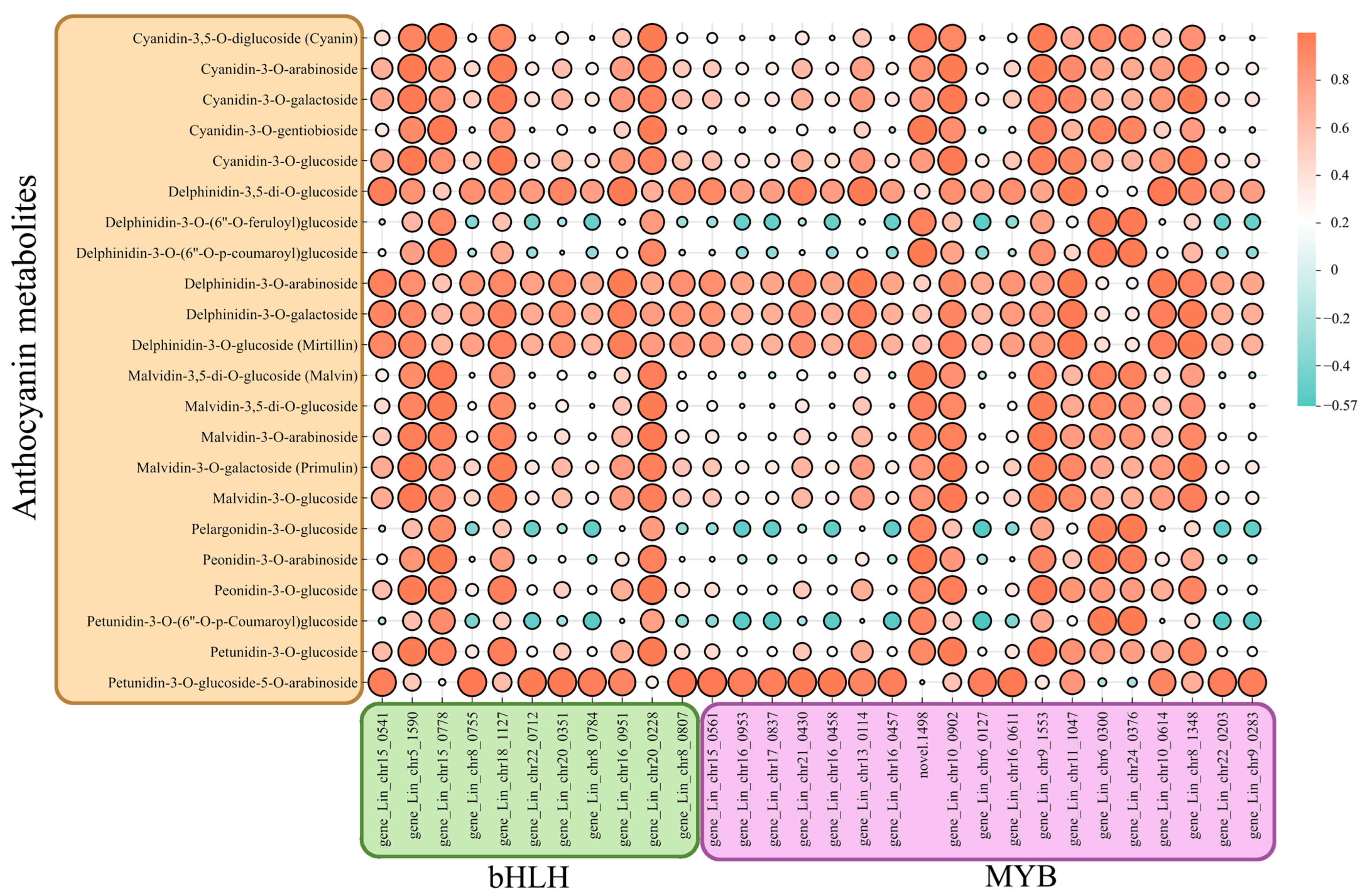
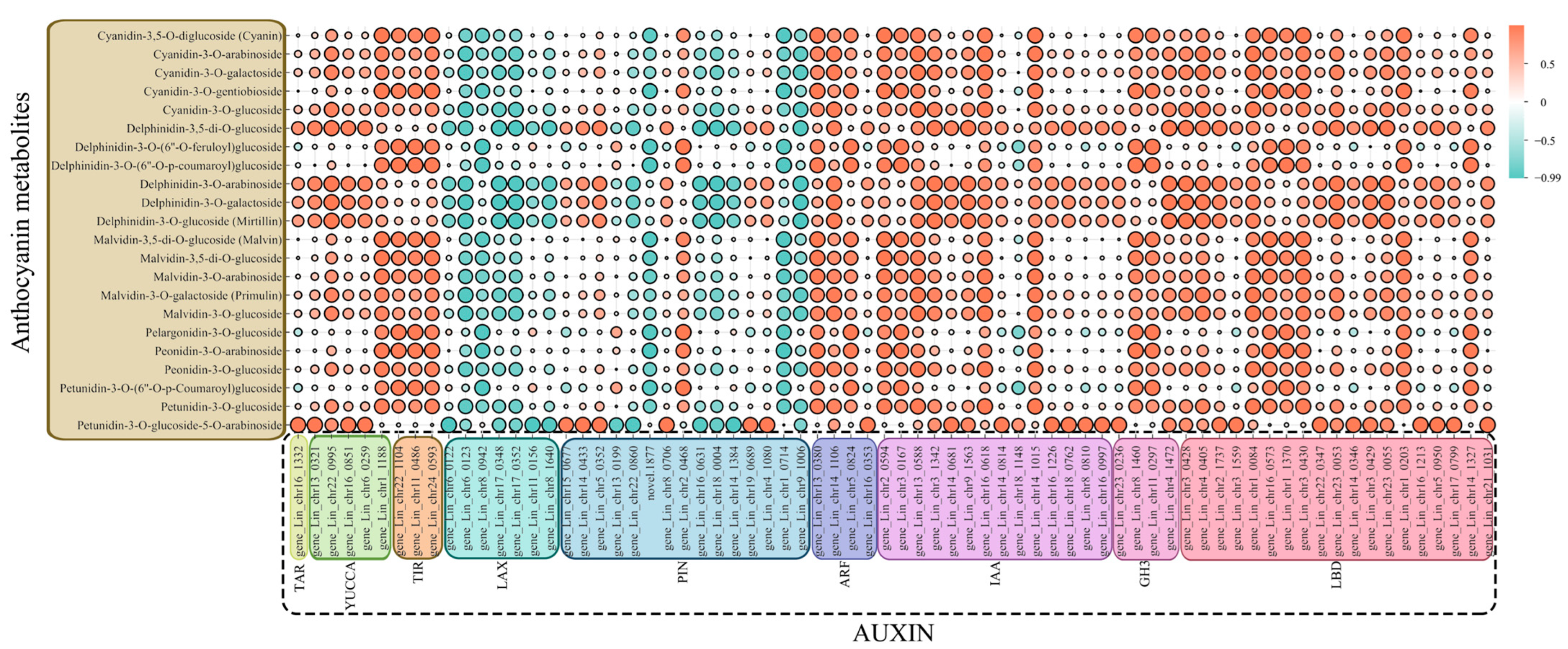
3.7. Correlation Analysis of Anthocyanin Synthesis Pathways
4. Discussion
4.1. Structural Gene Analysis of Anthocyanin Synthesis Pathway
4.2. Correlation of Transcription Factors Involved in Anthocyanin Synthesis in L. indica
4.3. Auxins May Be Involved in the Regulation of Anthocyanin Synthesis in L. indica
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhao, D.; Tao, J. Recent Advances on the Development and Regulation of Flower Color in Ornamental Plants. Front. Plant Sci. 2015, 6, 261. [Google Scholar] [CrossRef]
- Ahmed, N.U.; Park, J.-I.; Jung, H.-J.; Hur, Y.; Nou, I.-S. Anthocyanin Biosynthesis for Cold and Freezing Stress Tolerance and Desirable Color in Brassica Rapa. Funct. Integr. Genom. 2015, 15, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, K.; Makino, K.; Iwadate, E.; Deguchi, Y.; Ishikawa, F. Anthocyanins from Purple Sweet Potato Ipomoea Batatas Cultivar Ayamurasaki Suppress the Development of Atherosclerotic Lesions and Both Enhancements of Oxidative Stress and Soluble Vascular Cell Adhesion Molecule-1 in Apolipoprotein E-Deficient Mice. J. Agric. Food Chem. 2008, 56, 11485–11492. [Google Scholar] [CrossRef] [PubMed]
- Hichri, I.; Barrieu, F.; Bogs, J.; Kappel, C.; Delrot, S.; Lauvergeat, V. Recent Advances in the Transcriptional Regulation of the Flavonoid Biosynthetic Pathway. J. Exp. Bot. 2011, 62, 2465–2483. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Mori, M.; Kondo, T. Blue Flower Color Development by Anthocyanins: From Chemical Structure to Cell Physiology. Nat Prod. Rep. 2009, 26, 884–915. [Google Scholar] [CrossRef]
- Tanaka, Y.; Sasaki, N.; Ohmiya, A. Biosynthesis of Plant Pigments: Anthocyanins, Betalains and Carotenoids. Plant J. 2008, 54, 733–749. [Google Scholar] [CrossRef]
- Yoshida, K.; Oyama, K.; Kondo, T. Insight into Chemical Mechanisms of Sepal Color Development and Variation in Hydrangea. Proc. Jpn. Acad. Ser. B 2021, 97, 51–68. [Google Scholar] [CrossRef]
- Liu, W.; Feng, Y.; Yu, S.; Fan, Z.; Li, X.; Li, J.; Yin, H. The Flavonoid Biosynthesis Network in Plants. Int. J. Mol.Sci. 2021, 22, 12824. [Google Scholar] [CrossRef]
- Winkel-Shirley, B. Flavonoid Biosynthesis. A Colorful Model for Genetics, Biochemistry, Cell Biology, and Biotechnology. Plant Physiol. 2001, 126, 485–493. [Google Scholar] [CrossRef]
- Dong, N.; Lin, H. Contribution of Phenylpropanoid Metabolism to Plant Development and Plant–Environment Interactions. J. Integr. Plant Biol. 2021, 63, 180–209. [Google Scholar] [CrossRef]
- Zhao, M.; Li, J.; Zhu, L.; Chang, P.; Li, L.; Zhang, L. Identification and Characterization of MYB-bHLH-WD40 Regulatory Complex Members Controlling Anthocyanidin Biosynthesis in Blueberry Fruits Development. Genes 2019, 10, 496. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Deng, R.; Bai, Y.; Wu, H.; Li, C.; Wu, Q.; Zhao, H. Tartary Buckwheat R2R3-MYB Gene FtMYB3 Negatively Regulates Anthocyanin and Proanthocyanin Biosynthesis. Int. J. Mol. Sci. 2022, 23, 2775. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Man, Y.; Wang, Y. Light- and Temperature-Induced Expression of an R2R3-MYB Gene Regulates Anthocyanin Biosynthesis in Red-Fleshed Kiwifruit. Int. J. Mol. Sci. 2019, 20, 5228. [Google Scholar] [CrossRef] [PubMed]
- Albert, N.W.; Butelli, E.; Moss, S.M.A.; Piazza, P.; Waite, C.N.; Schwinn, K.E.; Davies, K.M.; Martin, C. Discrete bHLH Transcription Factors Play Functionally Overlapping Roles in Pigmentation Patterning in Flowers of Antirrhinum Majus. New Phytol. 2021, 231, 849–863. [Google Scholar] [CrossRef] [PubMed]
- Moro, L. Postharvest Auxin and Methyl Jasmonate Effect on Anthocyanin Biosynthesis in Red Raspberry (Rubus idaeus L.). J. Plant Growth Regul. 2017, 36, 773–782. [Google Scholar] [CrossRef]
- Clayton-Cuch, D.; Yu, L.; Shirley, N.; Bradley, D.; Bulone, V.; Böttcher, C. Auxin Treatment Enhances Anthocyanin Production in the Non-Climacteric Sweet Cherry (Prunus avium L.). Int. J.Mol. Sci. 2021, 22, 10760. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Z.; Wang, P.; Wang, S.; Ma, L.; Li, L.; Yang, R.; Ma, Y.; Wang, Q. Comprehensive Transcriptome Analysis Discovers Novel Candidate Genes Related to Leaf Color in a Lagerstroemia indica Yellow Leaf Mutant. Genes Genom. 2015, 37, 851–863. [Google Scholar] [CrossRef]
- Zetter, R.; Ferguson, D.K. Lagerstroemia (Lythraceae) Pollen from the Miocene of Eastern China. Grana 2008, 47, 262–271. [Google Scholar]
- Esmail Al-Snafi, A. A Review on Lagerstroemia Indica: A Potential Medicinal Plant. IOSR J. Pharm. 2019, 9, 6–42. [Google Scholar]
- Jin-fen, W. Research Progress in Breeding of Lagerstroemia Plant. Acta Hortic. Sin. 2013, 40, 1795–1804. [Google Scholar]
- Wang, A.; Li, R.; Ren, L.; Gao, X.; Zhang, Y.; Ma, Z.; Ma, D.; Luo, Y. A Comparative Metabolomics Study of Flavonoids in Sweet Potato with Different Flesh Colors (Ipomoea batatas (L.) Lam). Food Chem. 2018, 260, 124–134. [Google Scholar] [CrossRef]
- Chen, W.; Gong, L.; Guo, Z.; Wang, W.; Zhang, H.; Liu, X.; Yu, S.; Xiong, L.; Luo, J. A Novel Integrated Method for Large-Scale Detection, Identification, and Quantification of Widely Targeted Metabolites: Application in the Study of Rice Metabolomics. Mol Plant. 2013, 6, 1769–1780. [Google Scholar] [CrossRef] [PubMed]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.-C.; Mendell, J.T.; Salzberg, S.L. StringTie Enables Improved Reconstruction of a Transcriptome from RNA-Seq Reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Tu, H.; Wan, J.; Chen, W.; Liu, X.; Luo, J.; Xu, J.; Zhang, H. Spatio-Temporal Distribution and Natural Variation of Metabolites in Citrus Fruits. Food Chem. 2016, 199, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Liu, T.; Liu, J.; Cai, M.; Cheng, T.; Wang, J.; Zhang, Q.; Pan, H. Flavonoids Composition and Content in Petals of Lagerstroemia and Heimia Species and Cultivars. Acta Hortic. Sin. 2021, 48, 1956–1968. [Google Scholar]
- Zhang, J.; Wang, L.-S.; Gao, J.-M.; Shu, Q.-Y.; Li, C.-H.; Yao, J.; Hao, Q.; Zhang, J.-J. Determination of Anthocyanins and Exploration of Relationship between Their Composition and Petal Coloration in Crape Myrtle (Lagerstroemia Hybrid). J. Integr. Plant Biol. 2008, 50, 581–588. [Google Scholar] [CrossRef]
- Xia, Y.; Chen, W.; Xiang, W.; Wang, D.; Xue, B.; Liu, X.; Xing, L.; Wu, D.; Wang, S.; Guo, Q. Integrated Metabolic Profiling and Transcriptome Analysis of Pigment Accumulation in Lonicera Japonica Flower Petals during Colour-Transition. BMC. Plant Biol. 2021, 21, 98. [Google Scholar] [CrossRef]
- Morita, Y.; Hoshino, A.; Kikuchi, Y.; Okuhara, H.; Ono, E.; Tanaka, Y.; Fukui, Y.; Saito, N.; Nitasaka, E.; Noguchi, H. Japanese Morning Glory Dusky Mutants Displaying Reddish-brown or Purplish-gray Flowers Are Deficient in a Novel Glycosylation Enzyme for Anthocyanin Biosynthesis, UDP-glucose: Anthocyanidin 3-O-glucoside-2″-O-glucosyltransferase, Due to 4-bp Insertions in the Gene. Plant J. 2005, 42, 353–363. [Google Scholar]
- Gu, Z.; Zhu, J.; Hao, Q.; Yuan, Y.-W.; Duan, Y.-W.; Men, S.; Wang, Q.; Hou, Q.; Liu, Z.-A.; Shu, Q. A Novel R2R3-MYB Transcription Factor Contributes to Petal Blotch Formation by Regulating Organ-Specific Expression of PsCHS in Tree Peony (Paeonia suffruticosa). Plant Cell Physiol. 2019, 60, 599–611. [Google Scholar] [CrossRef]
- Sun, X. Anthocyanins: From Biosynthesis Regulation to Crop Improvement. Bot. Lett. 2021, 168, 1–12. [Google Scholar] [CrossRef]
- Deng, X.; Bashandy, H.; Ainasoja, M.; Kontturi, J.; Pieti, M.; Albert, V.A.; Valkonen, J.P.T.; Elomaa, P.; Teeri, T.H. Functional Diversification of Duplicated Chalcone Synthase Genes in Anthocyanin Biosynthesis of Gerbera Hybrida. New Phytol. 2014, 201, 1469–1483. [Google Scholar] [CrossRef] [PubMed]
- Feng, X. The Dihydroflavonol 4-Reductase BoDFR1 Drives Anthocyanin Accumulation in Pink-Leaved Ornamental Kale. Theor. Appl. Genet. 2021, 134, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, W.; Zhang, P.; Pan, Q.; Zhan, J.; Huang, W. Gene Transcript Accumulation, Tissue and Subcellular Localization of Anthocyanidin Synthase (ANS) in Developing Grape Berries. Plant Sci. 2010, 179, 103–113. [Google Scholar] [CrossRef]
- Xue, L.; Huang, X.; Zhang, Z.; Lin, Q.; Zhong, Q.; Zhao, Y.; Gao, Z.; Xu, C. An Anthocyanin-Related Glutathione S-Transferase, MrGST1, Plays an Essential Role in Fruit Coloration in Chinese Bayberry (Morella rubra). Front. Plant Sci. 2022, 13, 903333. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lu, W.; Ran, L.; Dou, L.; Yao, S.; Hu, J.; Fan, D.; Li, C.; Luo, K. R2R3- MYB Transcription Factor MYB 6 Promotes Anthocyanin and Proanthocyanidin Biosynthesis but Inhibits Secondary Cell Wall Formation in Populus tomentosa. Plant J. 2019, 99, 733–751. [Google Scholar] [CrossRef]
- Jin, W.; Wang, H.; Li, M.; Wang, J.; Yang, Y.; Zhang, X.; Yan, G.; Zhang, H.; Liu, J.; Zhang, K. The R2R3 MYB Transcription Factor PavMYB10.1 Involves in Anthocyanin Biosynthesis and Determines Fruit Skin Colour in Sweet Cherry (Prunus avium L.). Plant Biotechnol. J. 2016, 14, 2120–2133. [Google Scholar] [CrossRef]
- Koes, R.; Verweij, W.; Quattrocchio, F. Flavonoids: A Colorful Model for the Regulation and Evolution of Biochemical Pathways. Trends Plant Sci. 2005, 10, 236–242. [Google Scholar] [CrossRef]
- Stracke, R.; Ishihara, H.; Huep, G.; Barsch, A.; Mehrtens, F.; Niehaus, K.; Weisshaar, B. Differential Regulation of Closely Related R2R3-MYB Transcription Factors Controls Flavonol Accumulation in Different Parts of the Arabidopsis thaliana Seedling. Plant J. 2007, 50, 660–677. [Google Scholar] [CrossRef]
- Li, Y.; Shan, X.; Gao, R.; Han, T.; Zhang, J.; Wang, Y.; Kimani, S.; Wang, L.; Gao, X. MYB Repressors and MBW Activation Complex Collaborate to Fine-Tune Flower Coloration in Freesia Hybrida. Commun. Biol. 2020, 3, 396. [Google Scholar] [CrossRef]
- Totsuka, A.; Okamoto, E.; Miyahara, T.; Kouno, T.; Cano, E.A.; Sasaki, N.; Watanabe, A.; Tasaki, K.; Nishihara, M.; Ozeki, Y. Repressed Expression of a Gene for a Basic Helix-Loop-Helix Protein Causes a White Flower Phenotype in Carnation. Breed Sci. 2018, 68, 139–143. [Google Scholar] [CrossRef]
- Jia, N.; Wang, J.-J.; Liu, J.; Jiang, J.; Sun, J.; Yan, P.; Sun, Y.; Wan, P.; Ye, W.; Fan, B. DcTT8, a bHLH Transcription Factor, Regulates Anthocyanin Biosynthesis in Dendrobium Candidum. Plant Physiol. Biochem. 2021, 162, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, S.; Shi, Y.; Lv, S.; Zhu, C.; Xu, C.; Zhang, B.; Allan, A.C.; Grierson, D.; Chen, K. The R2R3 MYB Ruby1 Is Activated by Two Cold Responsive Ethylene Response Factors, via the Retrotransposon in Its Promoter, to Positively Regulate Anthocyanin Biosynthesis in Citrus. Plant J. 2024, 119, 1433–1448. [Google Scholar] [CrossRef] [PubMed]
- Jaakola, L. New Insights into the Regulation of Anthocyanin Biosynthesis in Fruits. Trends Plant Sci. 2013, 18, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D.R.; Ramirez, M.V.; Miller, N.D.; Vallabhaneni, P.; Ray, W.K.; Helm, R.F.; Winkel, B.S.J.; Muday, G.K. Auxin and Ethylene Induce Flavonol Accumulation through Distinct Transcriptional Networks. Plant Physiol. 2011, 156, 144–164. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, N.; Xu, H.; Jiang, S.; Fang, H.; Su, M.; Zhang, Z.; Zhang, T.; Chen, X. Auxin Regulates Anthocyanin Biosynthesis through the Aux/IAA–ARF Signaling Pathway in Apple. Hortic. Res. 2018, 5, 59. [Google Scholar] [CrossRef]
- Li, L. Co-Regulation of Auxin and Cytokinin in Anthocyanin Accumulation During Natural Development of Purple Wheat Grains. J. Plant Growth Regul. 2021, 40, 1881–1893. [Google Scholar] [CrossRef]
- Chen, J.; Mao, L.; Lu, W.; Ying, T.; Luo, Z. Transcriptome Profiling of Postharvest Strawberry Fruit in Response to Exogenous Auxin and Abscisic Acid. Planta 2016, 243, 183–197. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Z.; Chen, Z.; Wang, J.; Liu, W. Integrated Transcriptome and Metabolome Analysis Revealed Mechanism Underlying Anthocyanin Biosynthesis During Flower Color Formation in Lagerstroemia indica. Horticulturae 2024, 10, 1229. https://doi.org/10.3390/horticulturae10111229
Gao Z, Chen Z, Wang J, Liu W. Integrated Transcriptome and Metabolome Analysis Revealed Mechanism Underlying Anthocyanin Biosynthesis During Flower Color Formation in Lagerstroemia indica. Horticulturae. 2024; 10(11):1229. https://doi.org/10.3390/horticulturae10111229
Chicago/Turabian StyleGao, Zilong, Zhuomei Chen, Jinfeng Wang, and Weixin Liu. 2024. "Integrated Transcriptome and Metabolome Analysis Revealed Mechanism Underlying Anthocyanin Biosynthesis During Flower Color Formation in Lagerstroemia indica" Horticulturae 10, no. 11: 1229. https://doi.org/10.3390/horticulturae10111229
APA StyleGao, Z., Chen, Z., Wang, J., & Liu, W. (2024). Integrated Transcriptome and Metabolome Analysis Revealed Mechanism Underlying Anthocyanin Biosynthesis During Flower Color Formation in Lagerstroemia indica. Horticulturae, 10(11), 1229. https://doi.org/10.3390/horticulturae10111229






