Identification of the LH2 Locus for Prostrate Hair Density in Grapevine
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. DNA Extraction
2.3. Target Site Selection for GBTS and Probe Design
2.4. Library Construction, Probe Hybridization, and Sequencing
2.5. Phenotyping of Prostrate Hair Density
2.6. Map Construction and QTL Mapping
2.7. GWAS Analysis
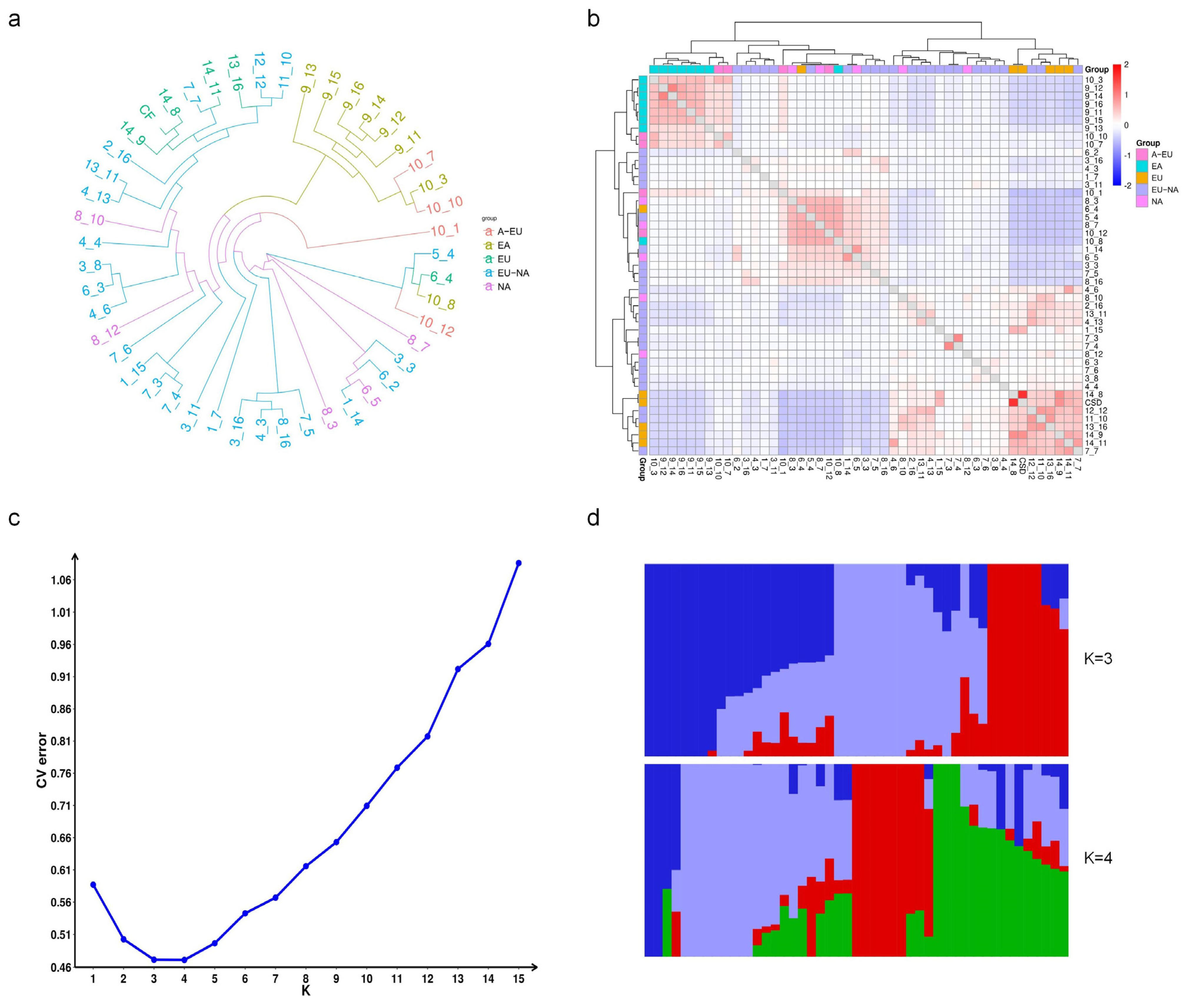
2.8. Genetic Relationship Data Analysis
3. Results
3.1. Phenotypic Analysis
3.2. QTL Mapping
3.3. GWAS Analysis
3.4. Joint Analysis and Gene Clustering
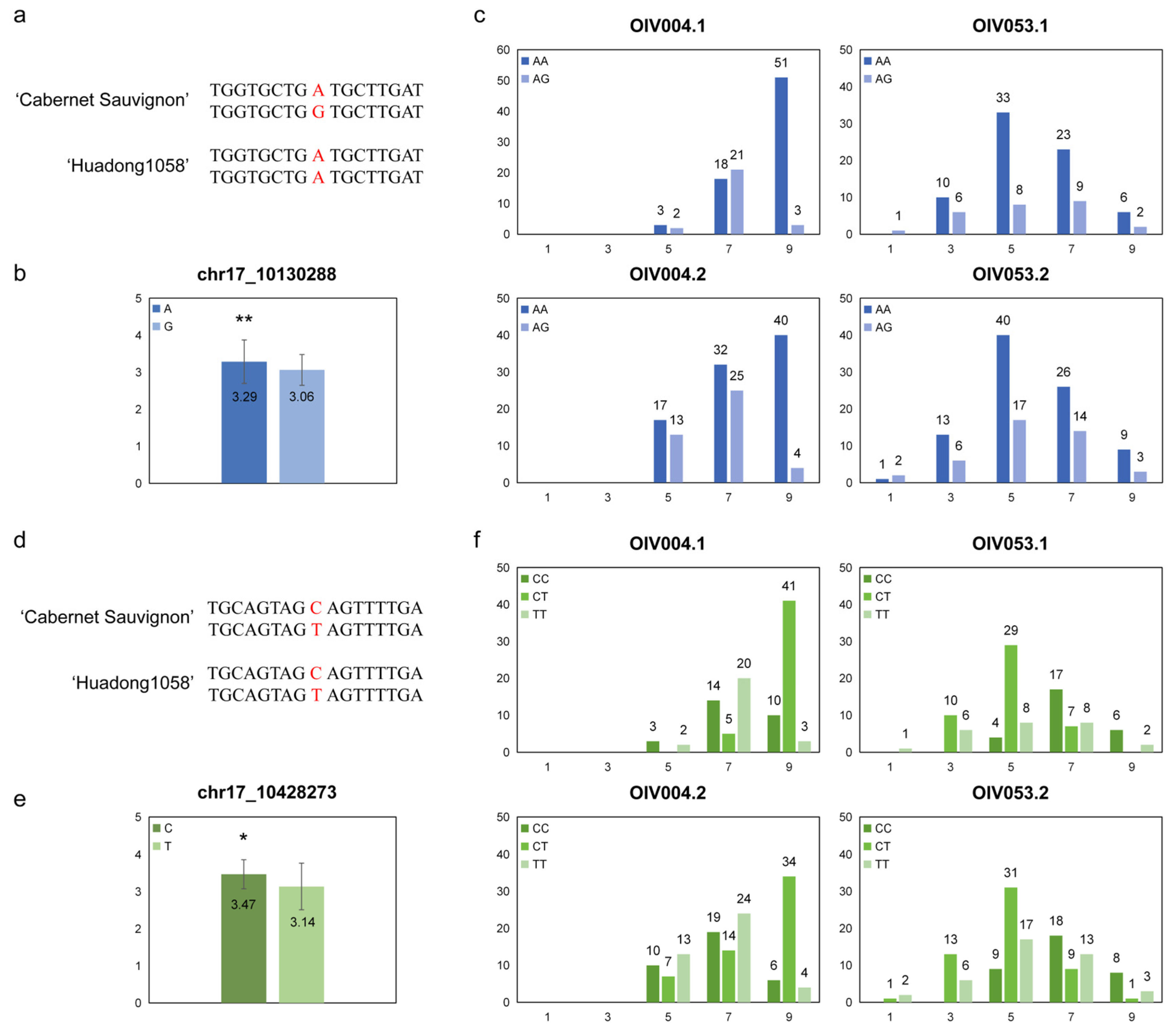
3.5. Correlation Between Prostrate Hair Density and the Linkage SNPs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yang, B.H.; He, S.; Liu, Y.; Liu, B.C.; Ju, Y.L.; Kang, D.Z.; Sun, X.; Fang, Y. Transcriptomics integrated with metabolomics reveals the effect of regulated deficit irrigation on anthocyanin biosynthesis in Cabernet Sauvignon grape berries. Food Chem. 2020, 314, 126170. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.W.; Jiang, J.F.; Fan, X.C.; Zhang, Y.; Zhang, G.H.; Liu, C.H. Diversity analysis of Chinese wild grape species. J. Plant Genet. Resour. 2013, 14, 996–1012. [Google Scholar]
- Ma, Z.Y.; Wen, J.; Ickert-Bond, S.M.; Chen, L.Q.; Liu, X.Q. Morphology, structure, and ontogeny of trichomes of the grape genus (Vitis, Vitaceae). Front. Plant Sci. 2016, 7, 704. [Google Scholar] [CrossRef] [PubMed]
- Gago, P.; Conejero, G.; Martinez, M.C.; Boso, S.; This, P.; Verdeil, J.L. Microanatomy of leaf trichomes: Opportunities for improved ampelographic discrimination of grapevine (Vitis vinifera L.) cultivars. Aust. J. Grape Wine Res. 2016, 22, 494–503. [Google Scholar] [CrossRef]
- Schmidt, R.A. Leaf structures affect predatory mites (Acari: Phytoseiidae) and biological control: A review. Exp. Appl. Acarol. 2013, 62, 1–17. [Google Scholar] [CrossRef]
- Yin, L.; Karn, A.; Cadle-Davidson, L.; Zou, C.; Underhill, A.; Atkins, P.; Treiber, E.; Voytas, D.; Clark, M. Fine mapping of leaf trichome density revealed a 747-kb region on chromosome 1 in cold-hardy hybrid wine grape populations. Front. Plant Sci. 2021, 12, 587640. [Google Scholar] [CrossRef]
- Levin, D.A. The role of trichomes in plant defense. Q. Rev. Biol. 1973, 48, 3–15. [Google Scholar] [CrossRef]
- Ågren, J.; Schemske, D. Artificial selection on trichome number in Brassica rapa. Theor. Appl. Genet. 1992, 83, 673–678. [Google Scholar] [CrossRef]
- Grover, G.; Kaur, B.; Pathak, D.; Kumar, V. Genetic variation for leaf trichome density and its association with sucking insect-pests incidence in Asiatic cotton. Indian J. Genet. Plant Breed 2016, 76, 365–368. [Google Scholar] [CrossRef]
- Sato, Y.; Kudoh, H. Tests of associational defence provided by hairy plants for glabrous plants of Arabidopsis halleri subsp. gemmifera against insect herbivores. Ecol. Entomol. 2015, 40, 269–279. [Google Scholar] [CrossRef]
- Grebe, M. The patterning of epidermal hairs in Arabidopsis—Updated. Curr. Opin. Plant Biol. 2012, 15, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Kono, A.; Shimizu, T. Leaf trichomes as an effective structure for disease resistance: The case of grapevine downy mildew. Jpn. Agric. Res. Q. 2020, 54, 293–298. [Google Scholar] [CrossRef]
- Staudt, G.; Kassemeyer, H. Evaluation of downy mildew resistance in various accessions of wild Vitis species. Vitis 1995, 34, 225–228. [Google Scholar]
- Kortekamp, A.; Wind, R.; Zyprian, E. The role of hairs on the wettability of grapevine (Vitis spp.) leaves. Vitis 1999, 38, 101–106. [Google Scholar]
- Kortekamp, A.; Zyprian, E. Leaf hairs as a basic protective barrier against downy mildew of grape. J. Phytopathol. 2008, 147, 453–459. [Google Scholar] [CrossRef]
- Barba, P.; Loughner, R.; Wentworth, K.; Nyrop, J.P.; Loeb, G.M.; Reisch, B.I. A QTL associated with leaf trichome traits has a major influence on the abundance of the predatory mite Typhlodromus pyri in a hybrid grapevine population. Hortic. Res. 2019, 6, 87. [Google Scholar] [CrossRef]
- Yang, L.; Li, M.; Shen, M.; Bu, S.; Zhu, B.; He, F.; Zhang, X.; Gao, X.; Xiao, J. Chromosome-level genome assembly and annotation of the native Chinese wild blueberry Vaccinium bracteatum. Fruit Res. 2022, 2, 8. [Google Scholar] [CrossRef]
- Fu, P.N.; Wu, W.; Lai, G.T.; Li, R.F.; Peng, Y.C.; Yang, B.H.; Wang, B.; Yin, L.; Qu, J.; Song, S.; et al. Identifying Plasmopara viticola resistance Loci in grapevine (Vitis amurensis) via genotyping-by-sequencing-based QTL mapping. Plant Physiol. Biochem. 2020, 154, 75–84. [Google Scholar] [CrossRef]
- Fu, P.N.; Tian, Q.Y.; Lai, G.T.; Li, R.F.; Song, S.R.; Lu, J. Cgr1, a ripe rot resistance QTL in Vitis amurensis ‘Shuang Hong’ grapevine. Hortic. Res. 2019, 6, 67. [Google Scholar] [CrossRef]
- Plant and Fungi Data Integration. GrapeReSeq_Illumina_20K. 2018. Available online: https://urgi.versailles.inra.fr/Species/Vitis/GrapeReSeq_Illumina_20K (accessed on 10 September 2020).
- Sun, Y.; Yang, B.H.; Li, M.M.; Liu, C.J.; Yin, Y.G.; Jia, N.; Wang, X.Y.; Zeng, Q.M.; Guo, Y.; Wang, Y.J.; et al. Rpv34: The noval Plasmopara viticola resistance locus in ‘Moldova’. Fruit Res. 2024, 4, e024. [Google Scholar] [CrossRef]
- Coombe, B.G. Adoption of a system for identifying grapevine growth stages. Aust. J. Grape Wine Res. 1995, 1, 104–110. [Google Scholar] [CrossRef]
- OIV. Descriptor List for Grape Varieties and Vitis Species, 2nd ed.; International Organization of Vine and Wine: Paris, France, 2009. [Google Scholar]
- Wang, J.B.; Zhang, Z.W. GAPIT Version 3: Boosting Power and Accuracy for Genomic Association and Prediction. Genom. Proteom. Bioinform. 2021, 19, 629–640. [Google Scholar] [CrossRef]
- Yang, B.H.; Wu, W.; Lv, J.L.; Li, J.Y.; Xu, Y.F.; Yin, L.; Lu, J.; Fu, P. Identification of sex determination locus and development of marker combination in Vitis based on genotyping by target sequencing. Fruit Res. 2023, 3, 31. [Google Scholar] [CrossRef]
- Divilov, K.; Barba, P.; Cadle-Davidson, L.; Reisch, B.I. Single and multiple phenotype QTL analyses of downy mildew resistance in interspecific grapevines. Theor. Appl. Genet. 2018, 131, 1133–1143. [Google Scholar] [CrossRef]
- Cadle-Davidson, L. Variation within and between Vitis spp. for foliar resistance to the downy mildew pathogen Plasmopara viticola. Plant Dis. 2008, 92, 1577–1584. [Google Scholar] [CrossRef] [PubMed]
- Peressotti, E.; Wiedemann-Merdinoglu, S.; Delmotte, F.; Bellin, D.; Gaspero, G.; Testolin, R.; Merdinoglu, D.; Mestre, P. Breakdown of resistance to grapevine downy mildew upon limited deployment of a resistant variety. BMC Plant Biol. 2010, 10, 147. [Google Scholar] [CrossRef] [PubMed]
- Chitwood, D.H.; Ranjan, A.; Martinez, C.C.; Headland, L.R.; Thiem, T.; Kumar, R.; Covington, M.F.; Hatcher, T.; Naylor, D.T.; Zimmerman, S.; et al. A modern ampelography: A genetic basis for leaf shape and venation patterning in grape. Plant Physiol. 2013, 164, 259–272. [Google Scholar] [CrossRef]
- Karabourniotis, G.; Liakopoulos, G.; Nikolopoulos, D.; Bresta, P. Protective and defensive roles of non-glandular trichomes against multiple stresses: Structure–function coordination. J. For. Res. 2020, 31, 1–12. [Google Scholar] [CrossRef]
- Wang, X.J.; Shen, C.; Meng, P.H.; Tan, G.F.; Lv, L.T. Analysis and review of trichomes in plants. BMC Plant Biol. 2021, 21, 70. [Google Scholar] [CrossRef]
- Kono, A.; Ban, Y.; Mitani, N.; Fujii, H.; Sato, S.; Suzaki, K.; Azuma, A.; Onoue, N.; Sato, A. Development of SSR markers linked to QTL reducing leaf hair density and grapevine downy mildew resistance in Vitis vinifera. Mol. Breed. 2018, 38, 138. [Google Scholar] [CrossRef]
- Gerrath, J.; Posluszny, U.; Melville, L. Taming the Wild Grape: Botany and Horticulture in the Vitaceae; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Poland, J.A.; Balint-Kurti, P.J.; Wisser, R.J.; Pratt, R.C.; Nelson, R.J. Shades of gray: The world of quantitative disease resistance. Trends Plant Sci. 2009, 14, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Payne, T.; Clement, J.; Arnold, D.; Lloyd, A. Heterologous myb genes distinct from GL1 enhance trichome production when overexpressed in Nicotiana tabacum. Development 1999, 126, 671–682. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.W.; Wang, T.; Bartholomew, E.; Black, K.; Dong, M.M.; Zhang, Y.; Yang, S.; Cai, Y.; Xue, S.; Weng, Q.; et al. Comprehensive analysis of NAC transcription factors and their expression during fruit spine development in cucumber (Cucumis sativus L.). Hortic. Res. 2018, 5, 31. [Google Scholar] [CrossRef] [PubMed]

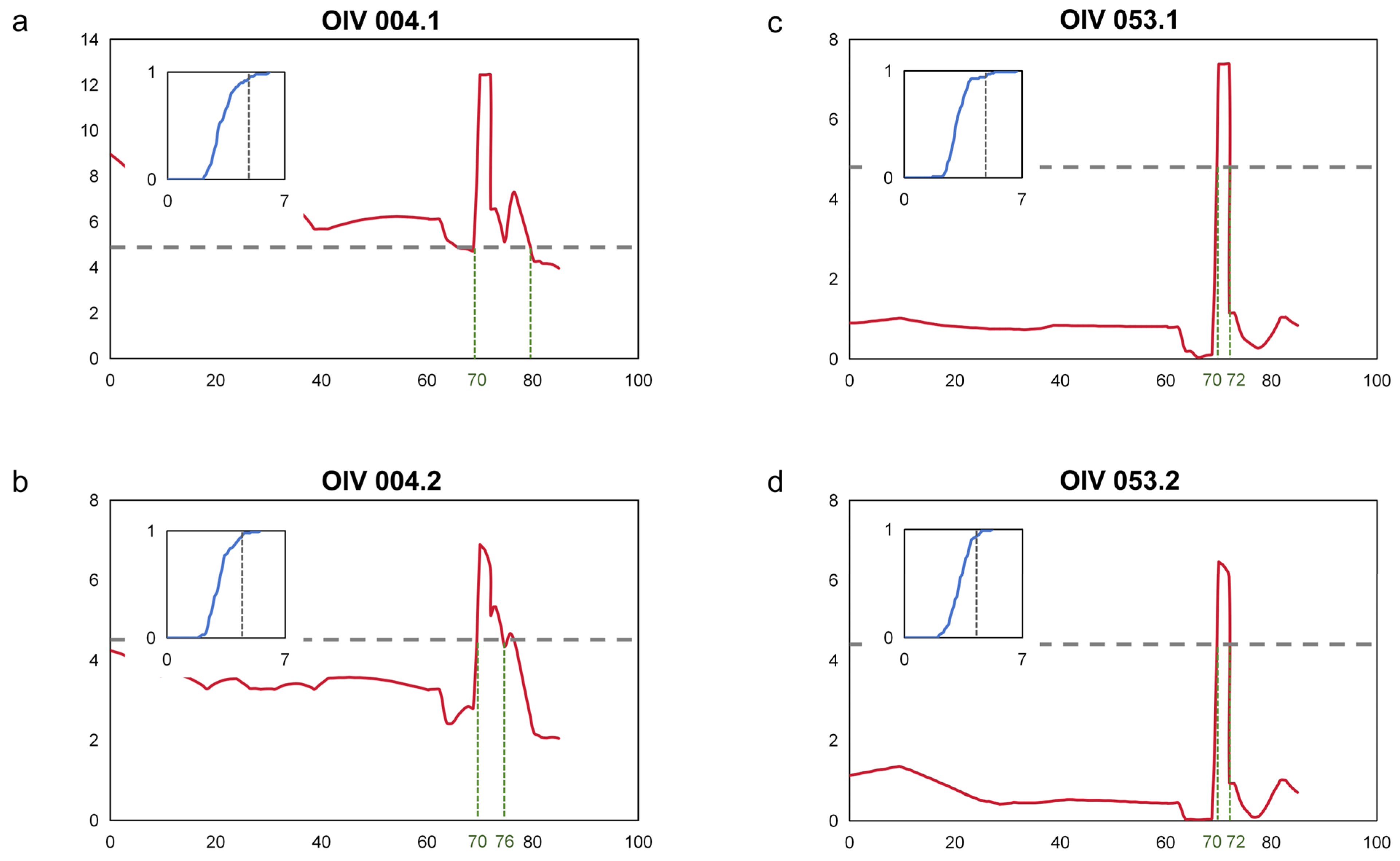
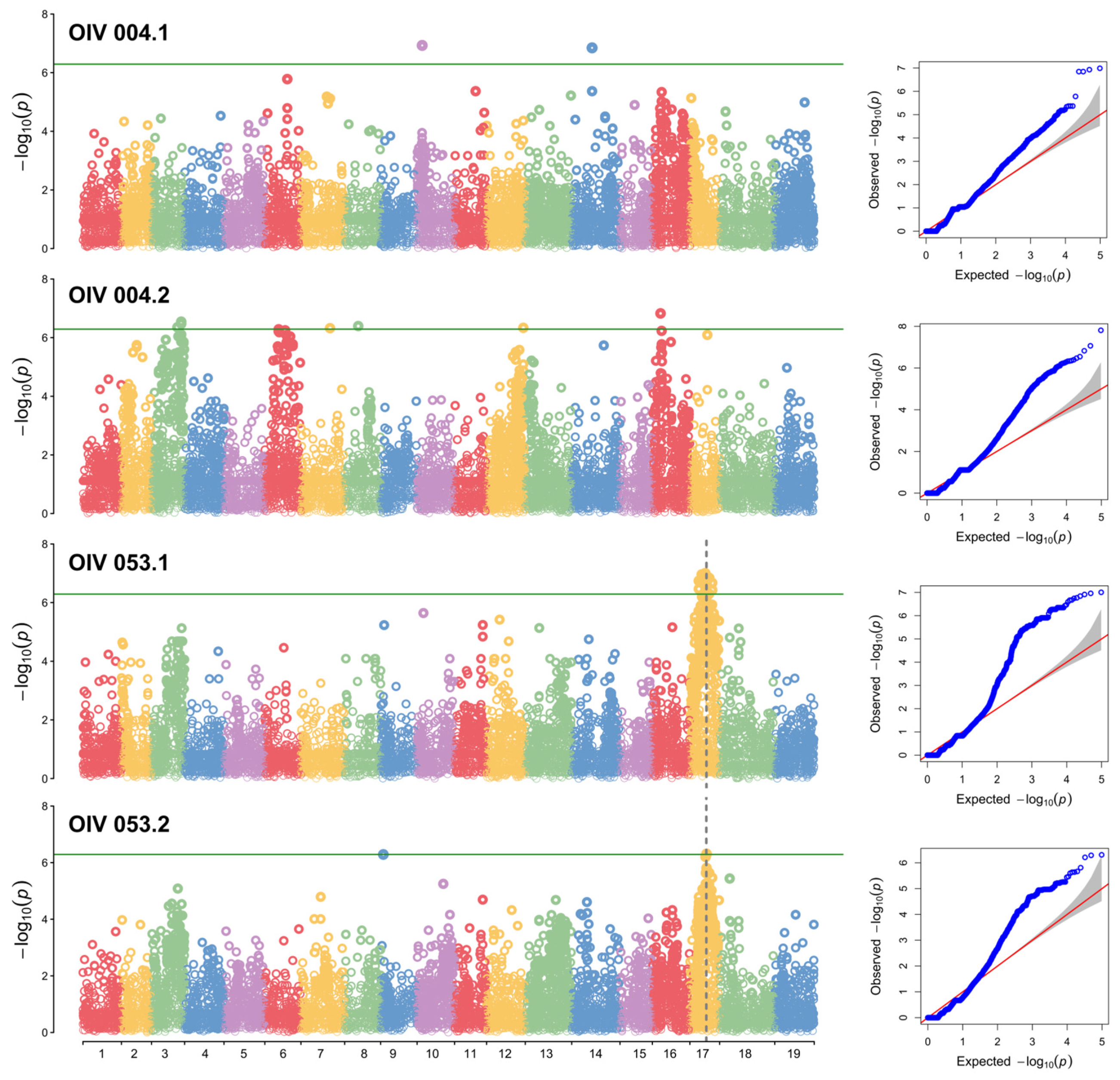
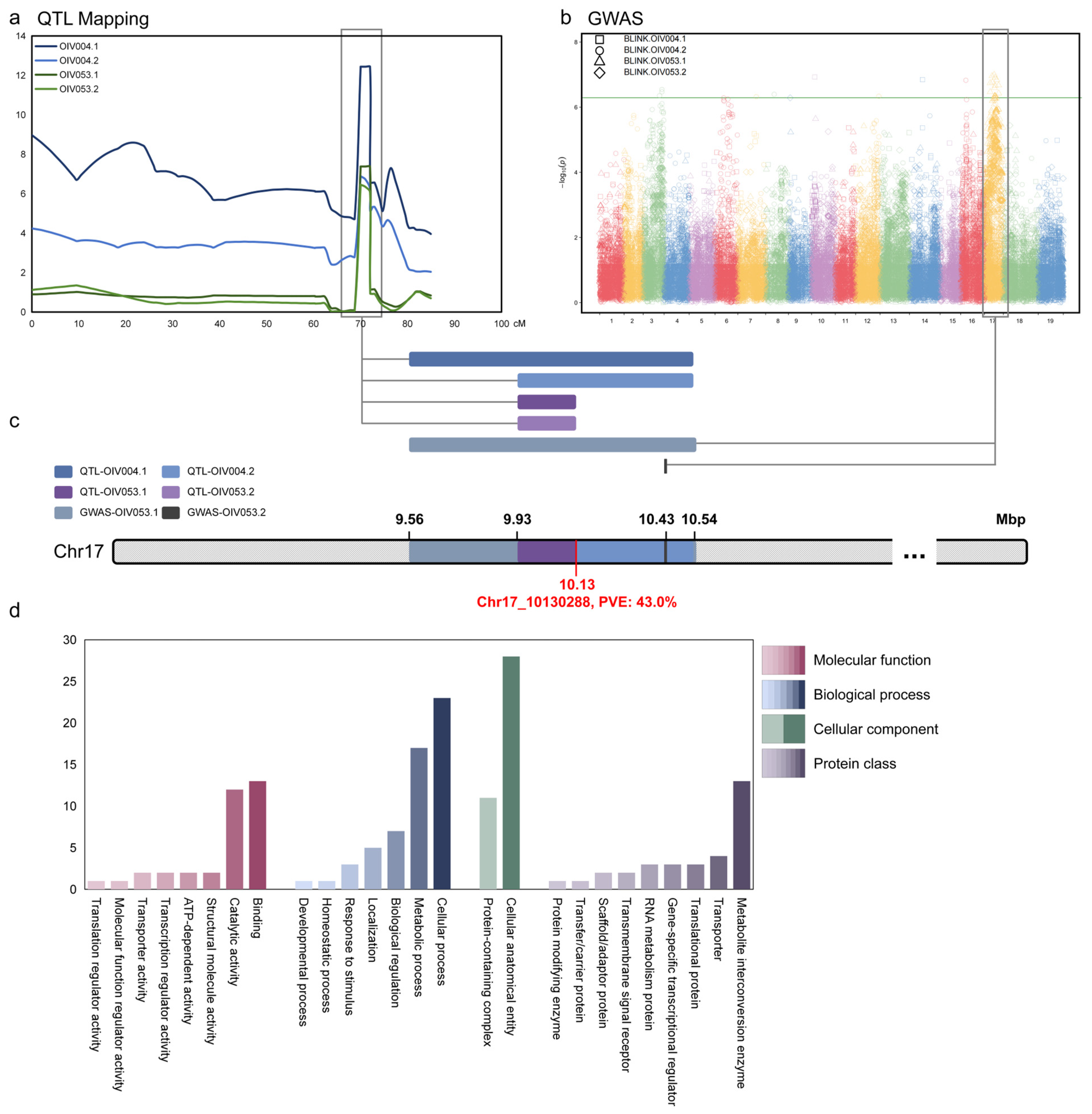
| LG | Threshold | LODmax of QTL/-log10(p)max | Peak (Mbp) | Start (Mbp) | End (Mbp) | PVE (%) | |
|---|---|---|---|---|---|---|---|
| QTL-OIV004.1 | 17 | 4.9 | 12.44 | 10.13 | 9.56 | 10.53 | 43.0 |
| QTL-OIV004.2 | 17 | 4.5 | 6.89 | 10.13 | 9.93 | 10.53 | 20.7 |
| QTL-OIV053.1 | 17 | 4.8 | 7.38 | 10.13 | 9.93 | 10.13 | 28.3 |
| QTL-OIV053.2 | 17 | 4.4 | 6.46 | 10.13 | 9.93 | 10.13 | 19.5 |
| GWAS-OIV053.1 | 17 | 6.3 | 8.77 | 10.43 | 9.56 | 10.54 | — |
| GWAS-OIV053.2 | 17 | 6.3 | 11.59 | 10.43 | — | — | — |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, B.; Liu, J.; Gu, Q.; Xu, Z.; Yao, X.; Liang, J.; Xu, M.; Lu, J.; Fu, P. Identification of the LH2 Locus for Prostrate Hair Density in Grapevine. Horticulturae 2024, 10, 1309. https://doi.org/10.3390/horticulturae10121309
Yang B, Liu J, Gu Q, Xu Z, Yao X, Liang J, Xu M, Lu J, Fu P. Identification of the LH2 Locus for Prostrate Hair Density in Grapevine. Horticulturae. 2024; 10(12):1309. https://doi.org/10.3390/horticulturae10121309
Chicago/Turabian StyleYang, Bohan, Jiaqi Liu, Qinqin Gu, Zhizhuo Xu, Xiukun Yao, Jianxiang Liang, Menghao Xu, Jiang Lu, and Peining Fu. 2024. "Identification of the LH2 Locus for Prostrate Hair Density in Grapevine" Horticulturae 10, no. 12: 1309. https://doi.org/10.3390/horticulturae10121309
APA StyleYang, B., Liu, J., Gu, Q., Xu, Z., Yao, X., Liang, J., Xu, M., Lu, J., & Fu, P. (2024). Identification of the LH2 Locus for Prostrate Hair Density in Grapevine. Horticulturae, 10(12), 1309. https://doi.org/10.3390/horticulturae10121309





