Comparative Analysis of Japanese Quince Juice Concentrate as a Substitute for Lemon Juice Concentrate: Functional Applications as a Sweetener, Acidifier, Stabilizer, and Flavoring Agent
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals, Reagents, and Standards
2.2. Sample Information
2.3. Preparation of Japanese Quince Juice and Concentrate
2.4. Evaluation of Nutritional Quality, Energy Value, and Microbiological Safety of Japanese Quince Juice and Lemon Juice Concentrates
2.5. Preparation of Extracts from Japanese Quince and Lemon Juice Concentrates for Determination of Phenolics, Flavonoids, and Antioxidant Activity Using Spectrophotometric Studies
2.6. Spectrophotometric Studies
2.6.1. Determination of Phenolic Content
2.6.2. Determination of Flavonoid Content
2.7. Antiradical Activity of Japanese Quince and Lemon Juice Concentrates
2.7.1. DPPH• Free Radical Scavenging Activity
2.7.2. Ferric-Reducing Antioxidant Power (FRAP)
2.8. Solid-Phase Extraction of Free Phenolics from Japanese Quince and Lemon Juice Concentrates for Analysis by LC-ESI-TQ-MS/MS
2.9. The LC-ESI-TQ-MS/MS Analytical Conditions for Individual Phenolic Compounds
2.10. Sample Preparation for Quantitative Analysis of Saccharides and Organic Acids
2.11. Analytical Conditions of the HPLC-RID System for the Quantitative Analysis of Saccharides
2.12. Analytical Conditions of the HPLC-PDA System for the Quantitative Analysis of Organic Acids
2.13. Statistical Analysis
3. Results and Discussion
3.1. The Nutritional, Energy, Microbiological, and Biochemical Qualities of Japanese Quince Juice and Lemon Juice Concentrates
3.2. Saccharide Profile of Japanese Quince Juice and Lemon Juice Concentrates
3.3. Organic Acid Profiles of Japanese Quince Juice and Lemon Juice Concentrates
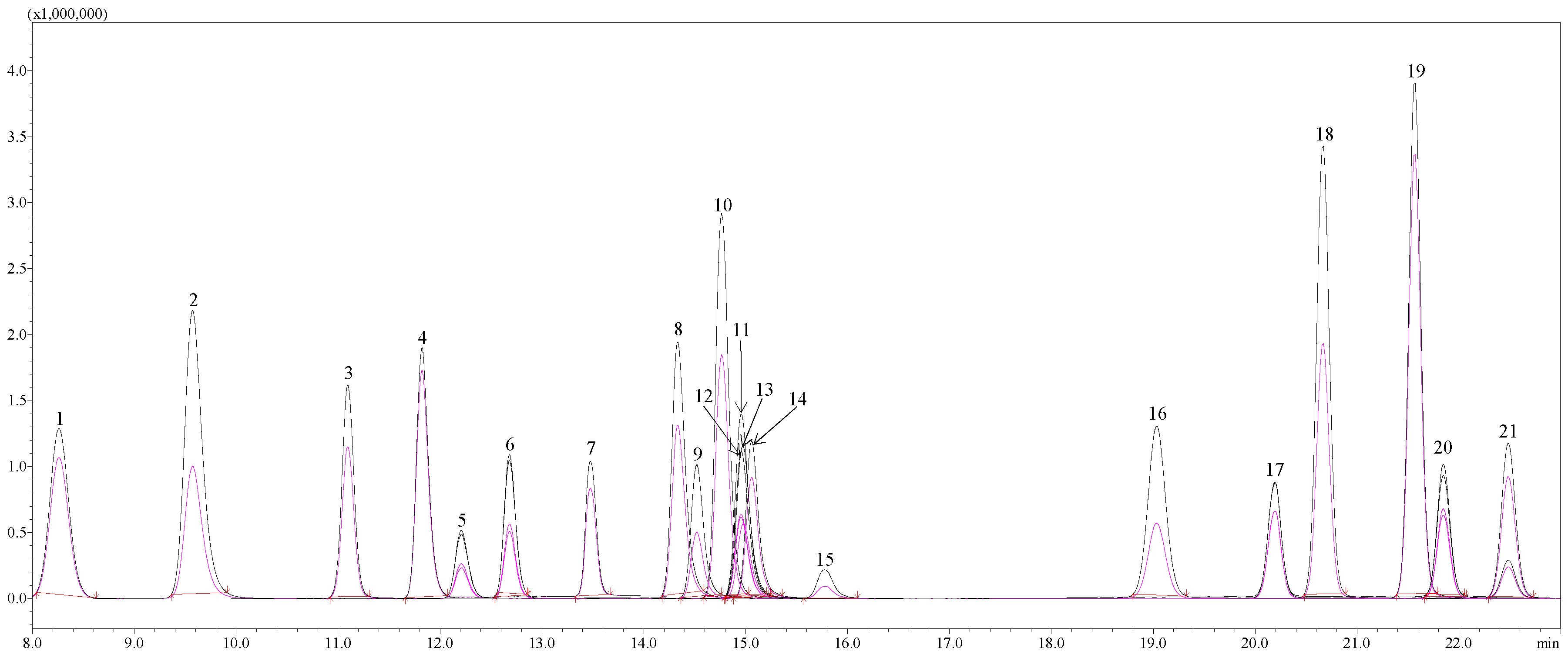
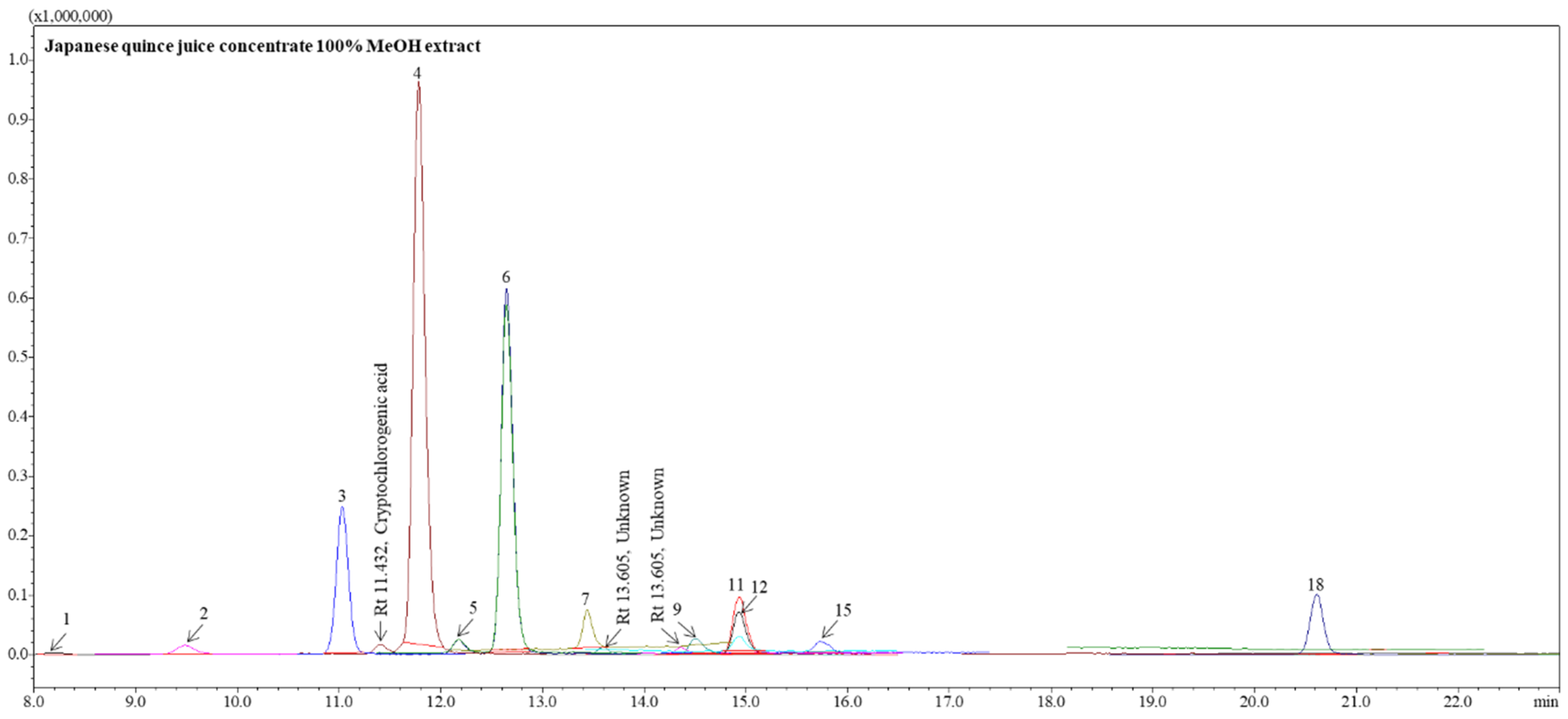
3.4. The Contents of Individual Phenolic Compounds in Japanese Quince Juice and Lemon Juice Concentrates
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adnan, A.; Mushtaq, M.; Islam, T. Fruit Juice Concentrates; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128024911. [Google Scholar]
- Salehi, F. Physicochemical characteristics and rheological behaviour of some fruit juices and their concentrates. J. Food Meas. Charact. 2020, 14, 2472–2488. [Google Scholar] [CrossRef]
- Tiencheu, B.; Nji, D.N.; Achidi, A.U.; Egbe, A.C.; Tenyang, N.; Tiepma Ngongang, E.F.; Djikeng, F.T.; Fossi, B.T. Nutritional, sensory, physico-chemical, phytochemical, microbiological and shelf-life studies of natural fruit juice formulated from orange (Citrus sinensis), lemon (Citrus limon), Honey and Ginger (Zingiber officinale). Heliyon 2021, 7, e07177. [Google Scholar] [CrossRef]
- De Farias Silva, C.E.; Da Silva, I.C.C.; Abud, A.K.D.S. Acidulants in tropical fruit pulp: Physicochemical and sensory changes. Chem. Eng. Trans. 2015, 44, 109–114. [Google Scholar] [CrossRef]
- Chakraborty, S.; Bhattacharjee, P. Design of lemon–mustard nutraceutical beverages based on synergism among antioxidants and in vitro antioxidative, hypoglycaemic and hypocholesterolemic activities: Characterization and shelf life studies. J. Food Meas. Charact. 2018, 12, 2110–2120. [Google Scholar] [CrossRef]
- Saura, D.; Vegara, S.; Martí, N.; Valero, M.; Laencina, J. Non-enzymatic browning due to storage is reduced by using clarified lemon juice as acidifier in industrial-scale production of canned peach halves. J. Food Sci. Technol. 2017, 54, 1873–1881. [Google Scholar] [CrossRef] [PubMed]
- Rasyid, N.; Rahman, F.; Birowo, P.; Widyahening, I.S. Effect of citrus-based products on urine profile: A systematic review and meta-analysis. F1000Research 2017, 6, 220. [Google Scholar] [CrossRef]
- Freitas, D.; Boué, F.; Benallaoua, M.; Airinei, G.; Benamouzig, R.; Le Feunteun, S. Lemon juice, but not tea, reduces the glycemic response to bread in healthy volunteers: A randomized crossover trial. Eur. J. Nutr. 2021, 60, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.H.; Obaid, Q.A.; Awaid, K.G. Lemon juice antioxidant activity against oxidative stress. Baghdad Sci. J. 2020, 17, 207–213. [Google Scholar] [CrossRef]
- Aguilar-Hernández, M.G.; Núñez-Gómez, D.; Forner-Giner, M.Á.; Hernández, F.; Pastor-Pérez, J.J.; Legua, P. Quality parameters of Spanish lemons with commercial interest. Foods 2021, 10, 62. [Google Scholar] [CrossRef]
- Juicehedge Juicehedge: Lemon Juice. Available online: https://www.juicehedge.com/juice/lemon/ (accessed on 15 October 2024).
- Baor Concentrates: Lemon Juice Concentrate for Manufacturers and Distributors. Available online: https://baorproducts.com/lemon-juice-concentrate/ (accessed on 15 October 2024).
- Future Market Insights: Lemon Juice Concentrate Market Outlook from (2023 to 2033). Available online: https://www.futuremarketinsights.com/reports/lemon-juice-concentrate-market (accessed on 15 October 2024).
- Juhnevica-Radenkova, K.; Radenkovs, V.; Krasnova, I. The impact of 1-MCP treatment and controlled atmosphere storage on the postharvest performance of four (Chaenomeles japonica (Thunb.) Lindl. ex Spach) fruit cultivars. J. Food Process. Preserv. 2022, 46, 1–13. [Google Scholar] [CrossRef]
- Rumpunen, K.; Kviklys, D.; Kauppinen, S.; Ruisa, S. Breeding Strategies for the Fruit Crop Japanese Quince (Chaenomeles japonica ). Jpn. Quince—Potential. Fruit. Crop North. Eur. 2003, 59–80. [Google Scholar]
- Ros, J.M.; Laencina, J.; Hellín, P.; Jordán, M.J.; Vila, R.; Rumpunen, K. Characterization of juice in fruits of different Chaenomeles species. LWT—Food Sci. Technol. 2004, 37, 301–307. [Google Scholar] [CrossRef]
- Kaufmane, E.; Segliņa, D.; Górnaś, P. (Chaenomeles japonica)—From field via lab to table: The role of “green” technologies. In Latvian Academy of Sciences Yearbook 2021; Zinātne Ltd.: Riga, Latvia, 2021; pp. 121–123. ISBN 978-9934-599-13-2. [Google Scholar]
- Urbanavičiūtė, I.; Viškelis, P. Biochemical Composition of Japanese Quince (Chaenomeles japonica) and Its Promising Value for Food, Cosmetic, and Pharmaceutical Industries. In Fruit Industry; InTech Open: Rijeka, Croatia, 2022. [Google Scholar] [CrossRef]
- Hellín, P.; Jordán, M.J.; Vila, R.; Gustafsson, M.; Göransson, E.; Åkesson, B.; Gröön, I. Processing and Products of Japanese Quince (Chaenomeles japonica) Fruits. In Japanese Quince—Potential Fruit Crop for Northern Europe; Kimmo, R., Ed.; Swedish University of Agricultural Sciences: Uppsala, Sweden, 2003; pp. 169–176. [Google Scholar]
- Seglina, D.; Krasnova, I.; Heidemane, G.; Ruisa, S. Influence of Drying Technology on the Quality of Dried Candied Chaenomeles japonica during Storage. Latv. J. Agron./Agron. Vestis 2009, 19, 147–152. [Google Scholar]
- Baranowska-Bosiacka, I.; Bosiacka, B.; Rast, J.; Gutowska, I.; Wolska, J.; Rębacz-Maron, E.; Dębia, K.; Janda, K.; Korbecki, J.; Chlubek, D. Macro- and Microelement Content and Other Properties of Chaenomeles japonica L. Fruit and Protective Effects of Its Aqueous Extract on Hepatocyte Metabolism. Biol. Trace Elem. Res. 2017, 178, 327–337. [Google Scholar] [CrossRef][Green Version]
- Du, H.; Wu, J.; Li, H.; Zhong, P.; Xu, Y.; Li, C.; Ji, K.; Wang, L. Polyphenols and triterpenes from Chaenomeles fruits: Chemical analysis and antioxidant activities assessment. Food Chem. 2013, 141, 4260–4268. [Google Scholar] [CrossRef]
- Marat, N.; Danowska-Oziewicz, M.; Narwojsz, A. Chaenomeles Species—Characteristics of Plant, Fruit and Processed Products: A Review. Plants 2022, 11, 3036. [Google Scholar] [CrossRef]
- Hellín, M.P.; Jordán, M.J.; Rumpunen, K.; García, J.M.R. Chromatographic characterization of juice in fruits of different Japanese quince (Chaenomeles japonica L.) genotypes cultivated in Sweden. Emir. J. Food Agric. 2020, 32, 816–825. [Google Scholar] [CrossRef]
- Tarko, T.; Duda-Chodak, A.; Satora, P.; Sroka, P.; Pogoń, P.; Machalica, J. Chaenomeles japonica, Cornus mas, Morus nigra fruits characteristics and their processing potential. J. Food Sci. Technol. 2014, 51, 3934–3941. [Google Scholar] [CrossRef] [PubMed]
- Bieniasz, M.; Dziedzic, E.; Kaczmarczyk, E. The effect of storage and processing on Vitamin C content in Japanese quince fruit. Folia Hortic. 2017, 29, 83–93. [Google Scholar] [CrossRef]
- Thomas, M.; Thibault, J.F. Cell-wall polysaccharides in the fruits of Japanese quince (Chaenomeles japonica): Extraction and preliminary characterisation. Carbohydr. Polym. 2002, 49, 345–355. [Google Scholar] [CrossRef]
- Urbanavičiūte, I.; Liaudanskas, M.; Bobinas, Č.; Šarkinas, A.; Rezgiene, A.; Viskelis, P. Japanese quince (Chaenomeles japonica) as a potential source of phenols: Optimization of the extraction parameters and assessment of antiradical and antimicrobial activities. Foods 2020, 9, 1132. [Google Scholar] [CrossRef]
- Zakłos-Szyda, M.; Pawlik, N. Japanese quince (Chaenomeles japonica L.) fruit polyphenolic extract modulates carbohydrate metabolism in HepG2 cells via AMP-activated protein kinase. Acta Biochim. Pol. 2018, 65, 67–78. [Google Scholar] [CrossRef] [PubMed]
- FAO; World Health Organization. Vitamin and Mineral Requirements in Human Nutrition, 2nd ed.; World Health Organization: Geneva, Switzerland, 1998; pp. 1–20. [Google Scholar]
- Byczkiewicz, S.; Szwajgier, D.; Kobus-Cisowska, J.; Szczepaniak, O.; Szulc, P. Comparative Examination of Bioactive Phytochemicals in Quince (chaenomeles) Fruits and their in Vitro Antioxidant Activity. Emir. J. Food Agric. 2021, 33, 293–302. [Google Scholar] [CrossRef]
- Muñoz-Almagro, N.; Montilla, A.; Villamiel, M. Role of pectin in the current trends towards low-glycaemic food consumption. Food Res. Int. 2021, 140, 109851. [Google Scholar] [CrossRef] [PubMed]
- Klimek-Szczykutowicz, M.; Szopa, A.; Ekiert, H. Citrus limon (Lemon) phenomenon—A review of the chemistry, pharmacological properties, applications in the modern pharmaceutical, food, and cosmetics industries, and biotechnological studies. Plants 2020, 9, 119. [Google Scholar] [CrossRef] [PubMed]
- Lorente, J.; Vegara, S.; Martí, N.; Ibarz, A.; Coll, L.; Hernández, J.; Valero, M.; Saura, D. Chemical guide parameters for Spanish lemon (Citrus limon (L.) Burm.) juices. Food Chem. 2014, 162, 186–191. [Google Scholar] [CrossRef]
- Bhattarai, A.; Shrestha, N.; Shrestha, S. Determination of Ascorbic Acid in Different Citrus Fruits of Kathmandu Valley. J. Med. Biol. Sci. Res. 2016, 2, 9–14. [Google Scholar]
- Hajimahmoodi, M.; Aliabadipoor, M.; Moghaddam, G.; Sadeghi, N.; Oveisi, M.R.; Jannat, B. Evaluation of in vitro antioxidant activities of lemon juice for safety assessment. Am. J. Food Technol. 2012, 7, 708–714. [Google Scholar] [CrossRef]
- Singleton, V.; Orthofer, R.; Maluela-Raventos, R. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Yang, H.; Kim, Y.J.; Shin, Y. Influence of ripening stage and cultivar on physicochemical properties and antioxidant compositions of aronia grown in South Korea. Foods 2019, 8, 598. [Google Scholar] [CrossRef] [PubMed]
- Radenkovs, V.; Püssa, T.; Juhnevica-Radenkova, K.; Kviesis, J.; Salar, F.J.; Moreno, D.A.; Drudze, I. Wild apple (Malus spp.) by-products as a source of phenolic compounds and vitamin C for food applications. Food Biosci. 2020, 38, 100744. [Google Scholar] [CrossRef]
- Radenkovs, V.; Kviesis, J.; Juhnevica-Radenkova, K.; Valdovska, A.; Püssa, T.; Klavins, M.; Drudze, I. Valorization of wild apple (Malus spp.) by-products as a source of essential fatty acids, tocopherols and phytosterols with antimicrobial activity. Plants 2018, 7, 90. [Google Scholar] [CrossRef] [PubMed]
- Juhnevica-Radenkova, K.; Krasnova, I.; Seglina, D.; Kaufmane, E.; Gravite, I.; Valdovska, A.; Radenkovs, V. Biochemical profile and antioxidant activity of dried fruit produced from apricot cultivars grown in Latvia. Horticulturae 2024, 10, 205. [Google Scholar] [CrossRef]
- CoSeteng, M.Y.; McLellan, M.R.; Downing, D.L. Influence of Titratable Acidity and pH on Intensity of Sourness of Citric, Malic, Tartaric, Lactic and Acetic Acids Solutions and on the Overall Acceptability of Imitation Apple Juice. Can. Inst. Food Sci. Technol. J. 1989, 22, 46–51. [Google Scholar] [CrossRef]
- Thomas, M.; Guillemin, F.; Guillon, F.; Thibault, J.F. Pectins in the fruits of Japanese quince (Chaenomeles japonica). Carbohydr. Polym. 2003, 53, 361–372. [Google Scholar] [CrossRef]
- Huang, W.; He, J.; Nisar, M.F.; Li, H.; Wan, C. Phytochemical and Pharmacological Properties of Chaenomeles speciosa: An Edible Medicinal Chinese Mugua. Evid.-Based Complement. Altern. Med. 2018, 2018, 9591845. [Google Scholar] [CrossRef]
- Peterson, J.J.; Beecher, G.R.; Bhagwat, S.A.; Dwyer, J.T.; Gebhardt, S.E.; Haytowitz, D.B.; Holden, J.M. Flavanones in grapefruit, lemons, and limes: A compilation and review of the data from the analytical literature. J. Food Compos. Anal. 2006, 19, 74–80. [Google Scholar] [CrossRef]
- Marín, F.R.; Martinez, M.; Uribesalgo, T.; Castillo, S.; Frutos, M.J. Changes in nutraceutical composition of lemon juices according to different industrial extraction systems. Food Chem. 2002, 78, 319–324. [Google Scholar] [CrossRef]
- Saipin, A.; Athikaphan, P.; Neramittagapong, A.; Neramittagapong, S. Hydrolysis of High Concentration Sucrose Solution into Glucose and Fructose over Amberlyst-15 Catalyst. Nihon Enerugi Gakkaishi/J. Jpn. Inst. Energy 2023, 102, 51–56. [Google Scholar] [CrossRef]
- Rehman, M.A.; Khan, M.R.; Sharif, M.K.; Ahmad, S.; Shah, F.-U.-H. Study on the storage stability of fruit juice concentrates. Pak. J. Food Sci. 2014, 24, 101–107. [Google Scholar]
- Capuano, E.; Fogliano, V. Acrylamide and 5-hydroxymethylfurfural (HMF): A review on metabolism, toxicity, occurrence in food and mitigation strategies. LWT 2011, 44, 793–810. [Google Scholar] [CrossRef]
- Rababah, T.M.; Al-Mahasneh, M.A.; Kilani, I.; Yang, W.; Alhamad, M.N.; Ereifej, K.; Al-u’datt, M. Effect of jam processing and storage on total phenolics, antioxidant activity, and anthocyanins of different fruits. J. Sci. Food Agric. 2011, 91, 1096–1102. [Google Scholar] [CrossRef] [PubMed]
- Godswill Awuchi, C.; Kate Echeta, C.; Godswill, C.; Kate, C. Current Developments in Sugar Alcohols: Chemistry, Nutrition, and Health Concerns of Sorbitol, Xylitol, Glycerol, Arabitol, Inositol, Maltitol, and Lactitol. Int. J. Adv. Acad. Res. 2019, 5, 2488–9849. [Google Scholar]
- Marques, C.; Sotiles, A.R.; Farias, F.O.; Oliveira, G.; Mitterer-Daltoé, M.L.; Masson, M.L. Full physicochemical characterization of malic acid: Emphasis in the potential as food ingredient and application in pectin gels. Arab. J. Chem. 2020, 13, 9118–9129. [Google Scholar] [CrossRef]
- Valcheva-Kuzmanova, S.V.; Denev, P.N.; Ognyanov, M.H. Chemical Composition and Antioxidant Activity of Chaenomeles Maulei Fruit Juice. J. Biomed. Clin. Res. 2018, 11, 41–48. [Google Scholar] [CrossRef]
- Cinkmanis, I.; Augspole, I.; Vucane, S.; Fredijs, D. Analysis of organic acids in herbal and fruit syrups by liquid chromatography. In Proceedings of the FOODBALT 2019 13th Baltic Conference on Food Science and Technology Food, Nutrition, Well-Being, Jelgava, Latvia, 2–3 May 2019; pp. 193–197. [Google Scholar]
- Granados, M.V.; Vila, R.; Laencina, J.; Rumpunen, K.; Ros, J.M. Characteristics and composition of chaenomeles seed oil. In Japanese Quince—Potential Fruit Crop for Northern Europe; Rumpunen, K., Ed.; Swedish University of Agricultural Sciences, Department of Crop Science: Uppsala, Sweden, 2003; pp. 127–139. ISBN 9789163137655. [Google Scholar]
- Rodgers, A.L.; Webber, D.; De Charmoy, R.; Jackson, G.E.; Ravenscroft, N. Malic acid supplementation increases urinary citrate excretion and urinary ph: Implications for the potential treatment of calcium oxalate stone disease. J. Endourol. 2014, 28, 229–236. [Google Scholar] [CrossRef]
- Zhang, P.; Jiang, G.; Wang, Y.; Yan, E.; He, L.; Guo, J.; Yin, J.; Zhang, X. Maternal consumption of L-malic acid enriched diets improves antioxidant capacity and glucose metabolism in offspring by regulating the gut microbiota. Redox Biol. 2023, 67, 102889. [Google Scholar] [CrossRef]
- Rein, D.; Lotito, S.; Holt, R.R.; Keen, C.L.; Schmitz, H.H.; Fraga, C.G. Epicatechin in human plasma: In vivo determination and effect of chocolate consumption on plasma oxidation status. J. Nutr. 2000, 130, 2109–2114. [Google Scholar] [CrossRef]
- Shimura, T.; Koyama, M.; Aono, D.; Kunugita, N. Epicatechin as a promising agent to countermeasure radiation exposure by mitigating mitochondrial damage in human fibroblasts and mouse hematopoietic cells. FASEB J. 2019, 33, 6867–6876. [Google Scholar] [CrossRef] [PubMed]
- Connolly, K.; Batacan, R.; Jackson, D.; Fenning, A.S. Effects of epicatechin on cardiovascular function in middle-aged diet-induced obese rat models of metabolic syndrome. Br. J. Nutr. 2024, 131, 593–605. [Google Scholar] [CrossRef]
- Haskell-Ramsay, C.F.; Schmitt, J.; Actis-Goretta, L. The impact of epicatechin on human cognition: The role of cerebral blood flow. Nutrients 2018, 10, 986. [Google Scholar] [CrossRef] [PubMed]
- Shay, J.; Elbaz, H.A.; Lee, I.; Zielske, S.P.; Malek, M.H.; Hüttemann, M. Molecular mechanisms and therapeutic effects of (−)-epicatechin and other polyphenols in cancer, inflammation, diabetes, and neurodegeneration. Oxid. Med. Cell. Longev. 2015, 2015, 181260. [Google Scholar] [CrossRef]
- Nguyen, V.; Taine, E.G.; Meng, D.; Cui, T.; Tan, W. Chlorogenic Acid: A Systematic Review on the Biological Functions, Mechanistic Actions, and Therapeutic Potentials. Nutrients 2024, 16, 924. [Google Scholar] [CrossRef]
- Kanchanasurakit, S.; Saokaew, S.; Phisalprapa, P.; Duangjai, A. Chlorogenic acid in green bean coffee on body weight: A systematic review and meta-analysis of randomized controlled trials. Syst. Rev. 2023, 12, 163. [Google Scholar] [CrossRef] [PubMed]
- Santana-Gálvez, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Chlorogenic Acid: Recent advances on its dual role as a food additive and a nutraceutical against metabolic syndrome. Molecules 2017, 22, 7–9. [Google Scholar] [CrossRef] [PubMed]
- Antoniewska, A.; Rutkowska, J.; Pineda, M.M. Antioxidative, sensory and volatile profiles of cookies enriched with freeze-dried Japanese quince (Chaenomeles japonica) fruits. Food Chem. 2019, 286, 376–387. [Google Scholar] [CrossRef]
- Kostecka-Gugała, A. Quinces (Cydonia oblonga, Chaenomeles sp., and Pseudocydonia sinensis) as Medicinal Fruits of the Rosaceae Family: Current State of Knowledge on Properties and Use. Antioxidants 2024, 13, 71. [Google Scholar] [CrossRef] [PubMed]
- Turkiewicz, I.P.; Wojdyło, A.; Tkacz, K.; Nowicka, P.; Golis, T.; Bąbelewski, P. ABTS on-line antioxidant, α-amylase, α-glucosidase, pancreatic lipase, acetyl-and butyrylcholinesterase inhibition activity of chaenomeles fruits determined by polyphenols and other chemical compounds. Antioxidants 2020, 9, 60. [Google Scholar] [CrossRef]
- Lee, S.; Khoo, C.S.; Pearson, J.L.; Hennell, J.R.; Bensoussan, A. Liquid chromatographic determination of narirutin and hesperidin in zhi ke (Citrus aurantium L.) in the form of the raw herb and of the dried aqueous extract. J. AOAC Int. 2009, 92, 789–796. [Google Scholar] [CrossRef]
- Spigoni, V.; Mena, P.; Fantuzzi, F.; Tassott, M.; Brighenti, F.; Bonadonna, R.C.; Del Rio, D.; Dei Cas, A. Bioavailability of bergamot (Citrus bergamia) flavanones and biological activity of their circulating metabolites in human pro-angiogenic cells. Nutrients 2017, 9, 1328. [Google Scholar] [CrossRef]
- Xi, W.; Lu, J.; Qun, J.; Jiao, B. Characterization of phenolic profile and antioxidant capacity of different fruit part from lemon (Citrus limon Burm.) cultivars. J. Food Sci. Technol. 2017, 54, 1108–1118. [Google Scholar] [CrossRef] [PubMed]
- Mateus, A.R.S.; Teixeira, J.D.; Barros, S.C.; Almeida, C.; Silva, S.; Sanches-Silva, A. Unlocking the Potential of Citrus medica L.: Antioxidant Capacity and Phenolic Profile across Peel, Pulp, and Seeds. Molecules 2024, 29, 3533. [Google Scholar] [CrossRef]
- Yari, Z.; Movahedian, M.; Imani, H.; Alavian, S.M.; Hedayati, M.; Hekmatdoost, A. The effect of hesperidin supplementation on metabolic profiles in patients with metabolic syndrome: A randomized, double-blind, placebo-controlled clinical trial. Eur. J. Nutr. 2020, 59, 2569–2577. [Google Scholar] [CrossRef]
- Mas-Capdevila, A.; Teichenne, J.; Domenech-Coca, C.; Caimari, A.; Bas, J.M.D.; Escoté, X.; Crescenti, A. Effect of hesperidin on cardiovascular disease risk factors: The role of intestinal microbiota on hesperidin bioavailability. Nutrients 2020, 12, 1488. [Google Scholar] [CrossRef]
- Yao, L.; Liu, W.; Bashir, M.; Nisar, M.F.; Wan, C. (Craig) Eriocitrin: A review of pharmacological effects. Biomed. Pharmacother. 2022, 154, 113563. [Google Scholar] [CrossRef] [PubMed]
- Cesar, T.B.; Ramos, F.M.M.; Ribeiro, C.B. Nutraceutical Eriocitrin (Eriomin) Reduces Hyperglycemia by Increasing Glucagon-Like Peptide 1 and Downregulates Systemic Inflammation: A Crossover-Randomized Clinical Trial. J. Med. Food 2022, 25, 1050–1058. [Google Scholar] [CrossRef]
- Meng, X.; Wu, H.; Xiong, J.; Li, Y.; Chen, L.; Gu, Q.; Li, P. Metabolism of eriocitrin in the gut and its regulation on gut microbiota in mice. Front. Microbiol. 2023, 13, 1111200. [Google Scholar] [CrossRef] [PubMed]
- Ahmad Juanda, N.I.B.; Md Saleh, N.; Yuhana, N.Y.; Asman, S.; Yusoff, F. Analysis of Methylphenol Concentration in Selangor Rivers, Malaysia using Solid Phase Extraction Technique Coupled with UV-Vis Spectroscopy. Sains Malays. 2023, 52, 1453–1468. [Google Scholar] [CrossRef]
- Uchiyama, H.; Tozuka, Y.; Imono, M.; Takeuchi, H. Improvement of dissolution and absorption properties of poorly water-soluble drug by preparing spray-dried powders with α-glucosyl hesperidin. Int. J. Pharm. 2010, 392, 101–106. [Google Scholar] [CrossRef]
- Cao, X.; He, Y.; Kong, Y.; Mei, X.; Huo, Y.; He, Y.; Liu, J. Elucidating the interaction mechanism of eriocitrin with β-casein by multi-spectroscopic and molecular simulation methods. Food Hydrocoll. 2019, 94, 63–70. [Google Scholar] [CrossRef]
- Radenkovs, V.; Püssa, T.; Juhnevica-Radenkova, K.; Anton, D.; Seglina, D. Phytochemical characterization and antimicrobial evaluation of young leaf/shoot and press cake extracts from Hippophae rhamnoides L. Food Biosci. 2018, 24, 56–66. [Google Scholar] [CrossRef]
- Spranger, I.; Sun, B.; Mateus, A.M.; Freitas, V.d.; Ricardo-da-Silva, J.M. Chemical characterization and antioxidant activities of oligomeric and polymeric procyanidin fractions from grape seeds. Food Chem. 2008, 108, 519–532. [Google Scholar] [CrossRef]
- Sheng, Y.; Sun, Y.; Tang, Y.; Yu, Y.; Wang, J.; Zheng, F.; Li, Y.; Sun, Y. Catechins: Protective mechanism of antioxidant stress in atherosclerosis. Front. Pharmacol. 2023, 14, 1144878. [Google Scholar] [CrossRef] [PubMed]



| Item | JQJC | LJC | Percentage Difference, % |
|---|---|---|---|
| Total acidity as citric acid (g CAE 100 g−1 FW) | 28.5 ± 0.0 b | 32.8 ± 0.0 a | 14.0 |
| Total soluble solids (°Brix) | 50.6 ± 0.0 a | 39.8 ± 0.0 b | 23.9 |
| pH | 2.5 ± 0.0 a | 2.3 ± 0.0 a | 8.3 |
| Protein (N × 6.25) (g 100 g−1 FW) | 0.3 ± 0.1 b | 2.0 ± 0.1 a | 147.8 |
| Fat (g 100 g−1 FW)) | 0.1 ± 0.1 b | 0.5 ± 0.1 a | 133.3 |
| Dietary fiber (g 100 g−1 FW) | 0.5 ± 0.1 a | 0.1 ± 0.1 b | 133.3 |
| Density (kg cm3) | 1.2496 ± 0.0250 a | 1.1920 ± 0.0324 a | 4.7 |
| Energy value, kcal/kJ (100 g−1 FW) | 215/919 a | 150/627 b | 35.6 |
| CFU g−1 FW | |||
| Yeasts | <1.0 × 102 | <1.0 × 102 | - |
| Molds | <1.0 × 102 | <1.0 × 102 | - |
| E. coli | n.d. | n.d. | - |
| Total plate count | <1.0 × 102 | <1.0 × 103 | - |
| Item | JQJC | LJC | Percentage Difference, % |
|---|---|---|---|
| TPC (mg GAE 100 g−1 FW) | 2189.6 ± 17.6 a | 262.8 ± 5.6 b | 157.1 |
| TFC (mg CE 100 g−1 FW) | 1791.9 ± 9.1 a | 190.5 ± 3.1 b | 161.6 |
| DPPH• (M TE 100 g−1 FW) | 3.7 ± 0.1 a | 0.4 ± 0.0 b | 161.0 |
| FRAP (mM TE 100 g−1 FW) | 361.3 ± 8.8 a | 37.9 ± 3.0 b | 162.0 |
| Saccharide | JQJC | LJC | Percentage Difference, % |
|---|---|---|---|
| Glycerol | n.d | 1.17 ± 0.01 | - |
| Xylose | 0.31 ± 0.00 | n.d. | - |
| Fructose | 7.20 ± 0.22 a | 3.62 ± 0.03 b | 66.2 |
| Glucose | 1.94 ± 0.07 b | 3.76 ± 0.00 a | 63.9 |
| Sorbitol | 0.78 ± 0.03 | n.d. | - |
| Unknown | n.d. | 0.03 ± 0.00 | - |
| Unknown | n.d. | 0.06 ± 0.00 | - |
| Unknown | 0.14 ± 0.00 b | 0.52 ± 0.01 a | 115.2 |
| Unknown | n.d. | 0.19 ± 0.02 | - |
| Total | 10.36 ± 0.32 a | 9.35 ± 0.07 b | 10.2 |
| Organic acid | |||
| Oxalic acid | 0.21± 0.00 a | 0.12 ± 0.00 a | 54.5 |
| Tartaric acid | 1.08 ± 0.00 a | 0.22 ± 0.01 b | 132.3 |
| Quinic acid | 8.50 ± 0.05 a | 0.45 ± 0.05 b | 179.9 |
| Malic acid | 20.98 ± 0.08 a | 3.14 ± 0.00 b | 147.9 |
| Ascorbic acid | 0.24 ± 0.00 a | 0.03 ± 0.00 b | 155.6 |
| Citric acid | 1.78 ± 0.01 b | 30.86 ± 0.09 a | 178.2 |
| Fumaric acid | n.d. | 0.00 ± 0.00 | - |
| Succinic acid | 0.04 ± 0.00 b | 0.91 ± 0.40 a | 183.2 |
| Total | 32.82 ± 0.15 b | 35.74 ± 0.56 a | 8.5 |
| Phenolic Compound | JQJC | LJC | |||
|---|---|---|---|---|---|
| 100% MeOH | 30% MeOH | 30% MeOH SPE | 100% MeOH | 30% MeOH SPE | |
| Gallic acid | 0.60 ± 0.00 a | 0.60 ± 0.01 a | 0.37 ± 0.00 c | n.d. | n.d. |
| Neochlorogenic acid | 1.11 ± 0.01 a | 1.11 ± 0.04 a | 0.88 ± 0.01 c | n.d. | n.d. |
| Protocatechuic acid | 11.80 ± 0.22 a | 12.11 ± 0.07 a | 11.60 ± 0.26 a | 0.15 ± 0.02 b | 0.15 ± 0.02 b |
| Cryptochlorogenic acid | 0.88 ± 0.04 a | 0.88 ± 0.03 a | 0.84 ± 0.01 a | n.d. | n.d. |
| Chlorogenic acid | 34.75 ± 0.14 a | 35.80 ± 0.50 a | 35.28 ± 0.35 a | n.d. | n.d. |
| (+)-Catechin | 2.96 ± 0.13 a | 2.93 ± 0.08 a | 3.01 ± 0.02 a | n.d. | n.d. |
| (−)-Epicatechin | 50.63 ± 0.95 c | 51.74 ±1.68 b | 55.07 ± 0.52 a | n.d. | n.d. |
| Caffeic acid | 1.04 ± 0.08 a | 1.06 ± 0.06 a | 1.22 ± 0.01 a | n.d. | n.d. |
| Unknown | 1.13 ± 0.02 a | 1.16 ± 0.00 a | 1.25 ± 0.06 a | n.d. | n.d. |
| Hesperidin | n.d. | n.d. | n.d. | 81.81 ± 0.08 a | 29.79 ± 0.04 b |
| Eriocitrin | n.d. | n.d. | n.d. | 18.48 ± 0.16 a | 6.87 ± 0.26 b |
| Unknown | 0.37 ± 0.01 a | 0.38 ± 0.00 a | 0.39 ± 0.00 a | n.d. | n.d. |
| Myricetin-3-O-glucoside | n.d. | n.d. | n.d. | n.d. | n.d. |
| Quercetin-3-O-rutinoside (rutin) | 1.33 ± 0.01 b | 1.30 ± 0.04 b | 1.42 ± 0.02 b | 8.96 ± 0.11 a | 9.06 ± 0.01 a |
| Myricetin-3-O-rhamnoside (myricitrin) | n.d. | n.d. | n.d. | n.d. | n.d. |
| Luteolin-7-O-glucoside (cynaroside) | 0.16 ± 0.01 a | 0.15 ± 0.01 a | 0.16 ± 0.00 a | n.d. | n.d. |
| Quercetin-3-O-galactoside (hyperoside) | 0.48 ± 0.01 a | 0.48 ± 0.03 a | 0.53 ± 0.01 a | 0.06 ± 0.00 b | 0.06 ± 0.00 b |
| Kaempferol-3-O-rutinoside (nicotiflorin) | n.d. | n.d. | n.d. | n.d. | n.d. |
| Quercetin-3-β-glucoside (isoquercitrin) | 1.07 ± 0.00 a | 1.09 ± 0.02 a | 1.16 ± 0.02 a | 0.10 ± 0.01 b | 0.10 ± 0.00 b |
| Quercetin-3-O-rhamnoside (quercitrin) | 0.39 ± 0.04 a | 0.37 ± 0.04 a | 0.48 ± 0.02 a | n.d. | n.d. |
| Myricetin (aglycone) | n.d. | n.d. | n.d. | n.d. | n.d. |
| Luteolin (aglycone) | n.d. | n.d. | n.d. | n.d. | n.d. |
| Quercetin | 0.53 ± 0.09 b | 0.65 ± 0.29 a | 0.45 ± 0.01 b | 0.11 ± 0.06 c | 0.09 ± 0.04 c |
| Kaempferol | n.d. | n.d. | n.d. | n.d. | n.d. |
| Rhamnetin | n.d. | n.d. | n.d. | n.d. | n.d. |
| Isorhamnetin | n.d. | n.d. | n.d. | n.d. | n.d. |
| TOTAL | 109.20 ± 0.78 a | 111.77 ± 1.76 a | 114.07 ± 0.49 a | 109.65 ± 0.04 a | 46.10 ± 0.28 b |
| Variable | TPC | TFC | DPPH• | FRAP | AA | TIP |
|---|---|---|---|---|---|---|
| TPC | 1 | |||||
| TFC | 1 | 1 | ||||
| DPPH• | 0.9997 | 0.9996 | 1 | |||
| FRAP | 0.9995 | 0.9995 | 0.9996 | 1 | ||
| AA | 0.9999 | 0.9999 | 0.9994 | 0.9994 | 1 | |
| TIP | 0.5400 | 0.5364 | 0.5576 | 0.5527 | 0.5312 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radenkovs, V.; Krasnova, I.; Cinkmanis, I.; Juhnevica-Radenkova, K.; Rubauskis, E.; Seglina, D. Comparative Analysis of Japanese Quince Juice Concentrate as a Substitute for Lemon Juice Concentrate: Functional Applications as a Sweetener, Acidifier, Stabilizer, and Flavoring Agent. Horticulturae 2024, 10, 1362. https://doi.org/10.3390/horticulturae10121362
Radenkovs V, Krasnova I, Cinkmanis I, Juhnevica-Radenkova K, Rubauskis E, Seglina D. Comparative Analysis of Japanese Quince Juice Concentrate as a Substitute for Lemon Juice Concentrate: Functional Applications as a Sweetener, Acidifier, Stabilizer, and Flavoring Agent. Horticulturae. 2024; 10(12):1362. https://doi.org/10.3390/horticulturae10121362
Chicago/Turabian StyleRadenkovs, Vitalijs, Inta Krasnova, Ingmars Cinkmanis, Karina Juhnevica-Radenkova, Edgars Rubauskis, and Dalija Seglina. 2024. "Comparative Analysis of Japanese Quince Juice Concentrate as a Substitute for Lemon Juice Concentrate: Functional Applications as a Sweetener, Acidifier, Stabilizer, and Flavoring Agent" Horticulturae 10, no. 12: 1362. https://doi.org/10.3390/horticulturae10121362
APA StyleRadenkovs, V., Krasnova, I., Cinkmanis, I., Juhnevica-Radenkova, K., Rubauskis, E., & Seglina, D. (2024). Comparative Analysis of Japanese Quince Juice Concentrate as a Substitute for Lemon Juice Concentrate: Functional Applications as a Sweetener, Acidifier, Stabilizer, and Flavoring Agent. Horticulturae, 10(12), 1362. https://doi.org/10.3390/horticulturae10121362








