Short-Term Evaluation of Woodland Strawberry in Response to Melatonin Treatment under Low Light Environment
Abstract
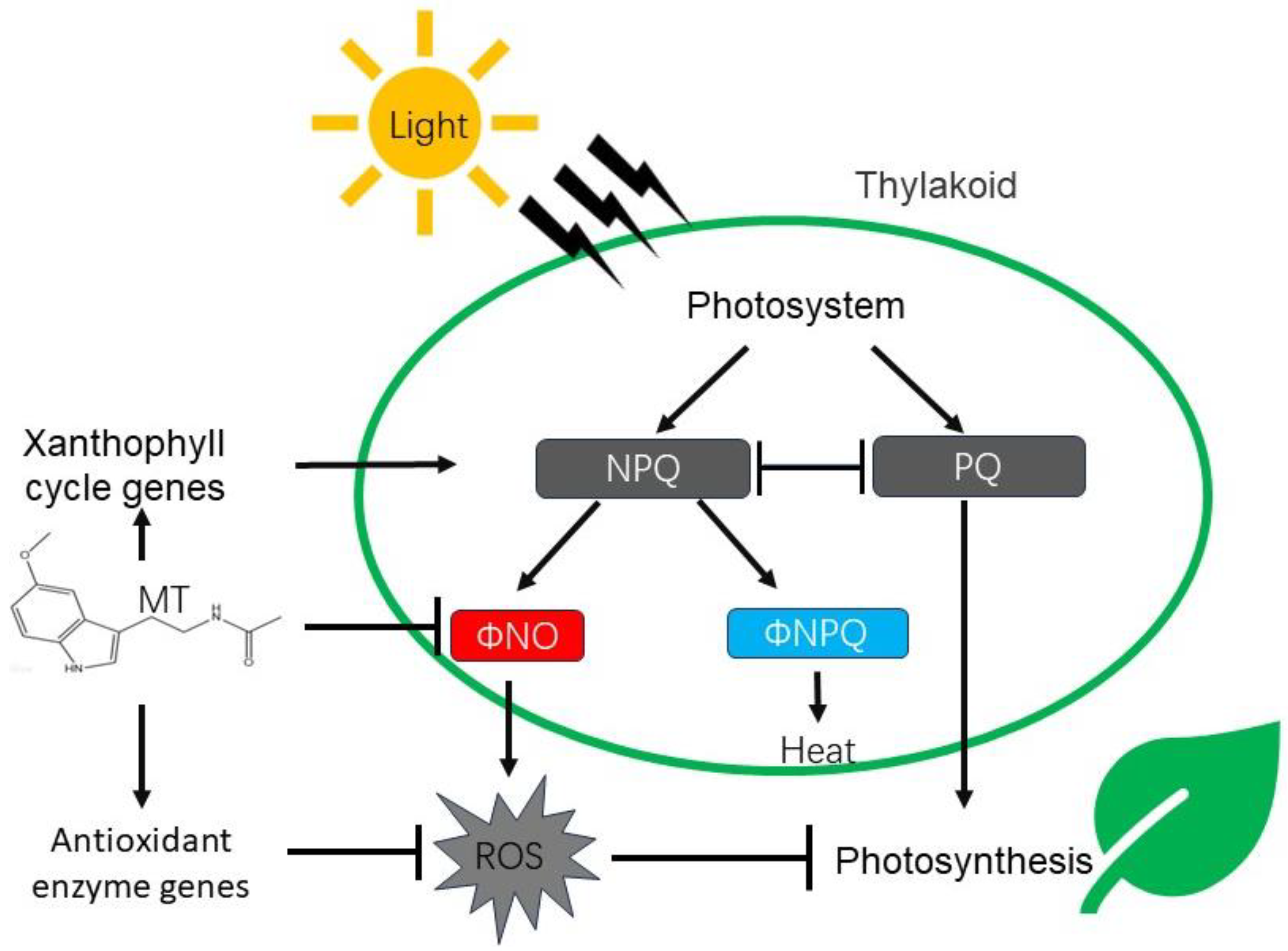
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Treatment
2.2. Gas Exchange and Chlorophyll Fluorescence Measurements
2.3. RNA Isolation and cDNA Synthesis
2.4. RNA Isolation and Quantitative RT-PCR
2.5. Statistical Analysis
3. Results
3.1. Effect of Melatonin Treatment on Photosynthetic Parameters
3.2. Effects of Melatonin on Chlorophyll Fluorescence Parameters
3.3. Effect of Melatonin on Photochemical Efficiency
3.4. Gene Expression Analysis of Xanthophyll Cycle Genes
3.5. Effect of Melatonin on Gene Expression of the Antioxidant Enzyme System
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ashraf, M.; Harris, P.J.C. Photosynthesis under Stressful Environments: An Overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- Long, S.P.; Zhu, X.G.; Naidu, S.L.; Ort, D.R. Can Improvement in Photosynthesis Increase Crop Yields? Plant Cell Environ. 2010, 29, 315–330. [Google Scholar] [CrossRef]
- Ort, D.R.; Merchant, S.S.; Alric, J.; Barkan, A.; Blankenship, R.E.; Bock, R.; Croce, R.; Hanson, M.R.; Hibberd, J.M.; Long, S.P.; et al. Bock Redesigning Photosynthesis to Sustainably Meet Global Food and Bioenergy Demand. Proc. Natl. Acad. Sci. USA 2015, 112, 8529–8536. [Google Scholar] [CrossRef] [PubMed]
- Erdal, S.; Turk, H. Cysteine-Induced Upregulation of Nitrogen Metabolism-Related Genes and Enzyme Activities Enhance Tolerance of Maize Seedlings to Cadmium Stress. Environ. Exp. Bot. 2016, 132, 92–99. [Google Scholar] [CrossRef]
- Duan, W.; Wang, Q.; Zhang, H.; Xie, B.; Li, A.; Hou, F.; Dong, S.; Wang, B.; Qin, Z.; Zhang, L. Comparative Study on Carbon–Nitrogen Metabolism and Endogenous Hormone Contents in Normal and Overgrown Sweetpotato. S. Afr. J. Bot. 2018, 115, 199–207. [Google Scholar] [CrossRef]
- Khan, M.N.; Zhang, J.; Luo, T.; Liu, J.; Rizwan, M.; Fahad, S.; Xu, Z.; Hu, L. Seed Priming with Melatonin Coping Drought Stress in Rapeseed by Regulating Reactive Oxygen Species Detoxification: Antioxidant Defense System, Osmotic Adjustment, Stomatal Traits and Chloroplast Ultrastructure Perseveration. Ind. Crops Prod. 2019, 140, 111597. [Google Scholar] [CrossRef]
- Xiong, D.; Flexas, J. From One Side to Two Sides: The Effects of Stomatal Distribution on Photosynthesis. New Phytol. 2020, 228, 1754–1766. [Google Scholar] [CrossRef]
- Allen, D.J.; Ort, D.R. Impacts of Chilling Temperatures on Photosynthesis in Warm-Climate Plants. Trends Plant Sci. 2001, 6, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Apel, K. Reactive Oxygen Species: Metabolism, Oxidative Stress, and Signal Transduction. Annu. Rev. Plant Biol 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Ding, F.; Wang, M.; Zhang, S. Overexpression of a Calvin Cycle Enzyme SBPase Improves Tolerance to Chilling-Induced Oxidative Stress in Tomato Plants. Sci. Hortic. 2017, 214, 27–33. [Google Scholar] [CrossRef]
- Ding, F.; Wang, M.; Zhang, S.; Ai, X. Changes in SBPase Activity Influence Photosynthetic Capacity, Growth, and Tolerance to Chilling Stress in Transgenic Tomato Plants. Sci. Rep. 2016, 6, 32741. [Google Scholar] [CrossRef]
- Long, S.; Liu, B.; Gong, J.; Wang, R.; Gao, S.; Zhu, T.; Guo, H.; Liu, T.; Xu, Y. 5-Aminolevulinic Acid Promotes Low-Light Tolerance by Regulating Chloroplast Ultrastructure, Photosynthesis, and Antioxidant Capacity in Tall Fescue. Plant Physiol. Biochem. 2022, 190, 248–261. [Google Scholar] [CrossRef]
- Brilli, F.; Pignattelli, S.; Baraldi, R.; Neri, L.; Pollastri, S.; Gonnelli, C.; Giovannelli, A.; Loreto, F.; Cocozza, C. Root Exposure to 5-Aminolevulinic Acid (ALA) Affects Leaf Element Accumulation, Isoprene Emission, Phytohormonal Balance, and Photosynthesis of Salt-Stressed Arundo Donax. Int. J. Mol. Sci. 2022, 23, 4311. [Google Scholar] [CrossRef]
- Li, L.; Gu, W.; Li, J.; Li, C.; Xie, T.; Qu, D.; Meng, Y.; Li, C.; Wei, S. Exogenously Applied Spermidine Alleviates Photosynthetic Inhibition under Drought Stress in Maize (Zea mays L.) Seedlings Associated with Changes in Endogenous Polyamines and Phytohormones. Plant Physiol. Biochem. 2018, 129, 35–55. [Google Scholar] [CrossRef]
- Jiang, D.; Lu, B.; Liu, L.; Duan, W.; Meng, Y.; Li, J.; Zhang, K.; Sun, H.; Zhang, Y.; Dong, H.; et al. Exogenous Melatonin Improves the Salt Tolerance of Cotton by Removing Active Oxygen and Protecting Photosynthetic Organs. BMC Plant Biol. 2021, 21, 1–19. [Google Scholar] [CrossRef]
- Yan, F.; Zhang, J.; Li, W.; Ding, Y.; Zhong, Q.; Xu, X.; Wei, H.; Li, G. Exogenous Melatonin Alleviates Salt Stress by Improving Leaf Photosynthesis in Rice Seedlings. Plant Physiol. Biochem. 2021, 163, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.K.; Xing, Q.-F.; Zhou, C.-Y.; Wang, K.-X.; Xu, T.; Yang, P.; Qi, Z.-Y.; Shao, S.-J.; Ahammed, G.J.; Zhou, J. Melatonin Mediates Elevated Carbon Dioxide-Induced Photosynthesis and Thermotolerance in Tomato. J. Pineal Res. 2023, 74, e12858. [Google Scholar] [CrossRef] [PubMed]
- Murch, S.J.; Saxena, P.K. Mammalian Neurohormones: Potential Significance in Reproductive Physiology of St. John’s Wort (Hypericum perforatum L.)? Naturwissenschaften 2002, 89, 555–560. [Google Scholar] [CrossRef]
- Pelagio-Flores, R.; Muñoz-Parra, E.; Ortiz-Castro, R.; López-Bucio, J. Melatonin Regulates Arabidopsis Root System Architecture Likely Acting Independently of Auxin Signaling. J. Pineal Res. 2012, 53, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.X.; Galano, A. Melatonin: Exceeding Expectations. Physiology 2014, 29, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Lerner, A.B.; Case, J.D.; Takahashi, Y.; Lee, T.H.; Mori, W. ISOLATION OF MELATONIN, THE PINEAL GLAND FACTOR THAT LIGHTENS MELANOCYTES1. J. Am. Chem. Soc. 1958, 80, 2587. [Google Scholar] [CrossRef]
- Vantassel, D.; Li, J.; Oneill, S. Melatonin-Identification of a Potential Dark Signal in Plants. Plant Physiol. 1993, 102, 117. [Google Scholar]
- Katarzyna, S.; Reiter, R.J.; Posmyk, M.M. Melatonin Improves the Photosynthetic Apparatus in Pea Leaves Stressed by Paraquat via Chlorophyll Breakdown Regulation and Its Accelerated de Novo Synthesis. Front. Plant Sci. 2017, 8, 878. [Google Scholar]
- Shah, M.; Guo, S.; Baloch, A.R.; Sun, J.; Kabir, K. Melatonin Alleviates Nickel Phytotoxicity by Improving Photosynthesis, Secondary Metabolism and Oxidative Stress Tolerance in Tomato Seedlings. Ecotoxicol. Environ. Saf. 2020, 197, 110593. [Google Scholar]
- Ayyaz, A.; Amir, M.; Umer, S.; Iqbal, M.; Bano, H.; Gul, H.S.; Noor, Y.; Kanwal, A.; Khalid, A.; Javed, M.; et al. Melatonin Induced Changes in Photosynthetic Efficiency as Probed by OJIP Associated with Improved Chromium Stress Tolerance in Canola (Brassica napus L.). Heliyon 2020, 6, e04364. [Google Scholar] [CrossRef] [PubMed]
- Can, H. Melatonin Application at Different Doses Changes the Physiological Responses in Favor of Cabbage Seedlings (Brassica oleracea var. Capitata) Against Flooding Stress. Gesunde Pflanz. 2023, 75, 2733–2745. [Google Scholar] [CrossRef]
- Kurt-Celebi, A.; Colak, N.; Torun, H.; Dosedělová, V.; Tarkowski, P.; Ayaz, F.A. Exogenous Melatonin Ameliorates Ionizing Radiation-Induced Damage by Modulating Growth, Osmotic Adjustment and Photosynthetic Capacity in Wheat Seedlings. Plant Physiol. Biochem. 2022, 187, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Wang, M.; Liu, B.; Zhang, S. Exogenous Melatonin Mitigates Photoinhibition by Accelerating Non-Photochemical Quenching in Tomato Seedlings Exposed to Moderate Light during Chilling. Front. Plant Sci. 2017, 8, 244. [Google Scholar] [CrossRef]
- Zhao, H.; Su, T.; Huo, L.; Wei, H.; Jiang, Y.; Xu, L.; Ma, F. Unveiling the Mechanism of Melatonin Impacts on Maize Seedling Growth: Sugar Metabolism as a Case. J. Pineal Res. 2015, 59, 255–266. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, C.; Wang, Z.; Sun, S.; Zhan, R.; Zhao, Y.; Ma, B.; Ma, F.; Li, M. Melatonin-Mediated Sugar Accumulation and Growth Inhibition in Apple Plants Involves Down-Regulation of Fructokinase 2 Expression and Activity. Front. Plant Sci. 2019, 10, 150. [Google Scholar] [CrossRef]
- Lee, H.Y.; Back, K. Melatonin Induction and Its Role in High Light Stress Tolerance in Arabidopsis Thaliana. J. Pineal Res. 2018, 65, e12504. [Google Scholar] [CrossRef]
- Yang, S.-J.; Huang, B.; Zhao, Y.-Q.; Hu, D.; Chen, T.; Ding, C.-B.; Chen, Y.-E.; Yuan, S.; Yuan, M. Melatonin Enhanced the Tolerance of Arabidopsis Thaliana to High Light Through Improving Anti-Oxidative System and Photosynthesis. Front. Plant Sci. 2021, 12, 752584. [Google Scholar] [CrossRef]
- Manafi, H.; Baninasab, B.; Gholami, M.; Talebi, M.; Khanizadeh, S. Exogenous Melatonin Alleviates Heat-Induced Oxidative Damage in Strawberry (Fragaria × ananassa Duch. cv. Ventana) Plant. J. Plant Growth Regul. 2022, 41, 52–64. [Google Scholar] [CrossRef]
- Zhou, X.; Zhao, H.; Cao, K.; Hu, L.; Du, T.; Baluška, F.; Zou, Z. Beneficial Roles of Melatonin on Redox Regulation of Photosynthetic Electron Transport and Synthesis of D1 Protein in Tomato Seedlings under Salt Stress. Front. Plant Sci. 2016, 7, 1823. [Google Scholar] [CrossRef]
- Choi, H.G. Correlation among Phenotypic Parameters Related to the Growth and Photosynthesis of Strawberry (Fragaria × ananassa Duch.) Grown under Various Light Intensity Conditions. Front. Plant Sci. 2021, 12, 647585. [Google Scholar] [CrossRef]
- Kouřil, R.; Wientjes, E.; Bultema, J.B.; Croce, R.; Boekema, E.J. High-Light vs. Low-Light: Effect of Light Acclimation on Photosystem II Composition and Organization in Arabidopsis Thaliana. Biochim. Biophys. Acta (BBA)-Bioenerg. 2013, 1827, 411–419. [Google Scholar] [CrossRef]
- Cheng, J.-S.; Duan, W.; Tang, X.-L.; Zhang, Y.-G.; Li, B.; Wang, Y.-J.; Yang, C.-X.; Song, Z.-Z.; Wang, L.-J.; Yang, J.; et al. Low Sink Demand Caused Net Photosynthetic Rate Decrease Is Closely Related to the Irrecoverable Damage of Oxygen-Releasing Complex and Electron Receptor in Peach Trees. J. Plant Physiol. 2021, 266, 153510. [Google Scholar] [CrossRef]
- Kramer, D.M.; Johnson, G.; Kiirats, O.; Edwards, G.E. New Fluorescence Parameters for the Determination of Q A Redox State and Excitation Energy Fluxes. Photosynth. Res. 2004, 79, 209–218. [Google Scholar] [CrossRef]
- Tietz, S.; Hall, C.C.; Cruz, J.A.; Kramer, D.M. NPQ (T): A Chlorophyll Fluorescence Parameter for Rapid Estimation and Imaging of Non-Photochemical Quenching of Excitons in Photosystem-II-Associated Antenna Complexes: New, Rapid Probe of Non-Photochemical Quenching. Plant Cell Environ. 2017, 40, 1243–1255. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Adams, W.W. Photoprotection and Other Responses of Plants to High Light Stress. Ann. Rev. Plant Physiol. Plant Mol. Biol 1992, 43, 599–626. [Google Scholar] [CrossRef]
- Müller, P.; Li, X.P.; Niyogi, K.K. Non-Photochemical Quenching. A Response to Excess Light Energy. Plant Physiol. 2001, 125, 1558–1566. [Google Scholar] [CrossRef]
- Crofts, A.R.; Yerkes, C.T. A Molecular Mechanism for qE-Quenching. Febs Lett. 1994, 352, 265–270. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Adams III, W.W. Photoprotection in an Ecological Context: The Remarkable Complexity of Thermal Energy Dissipation. New Phytol. 2006, 172, 11–21. [Google Scholar] [CrossRef]
- Takahashi, S.; Badger, M.R. Photoprotection in Plants: A New Light on Photosystem II Damage. Trends Plant Sci. 2011, 16, 53–60. [Google Scholar] [CrossRef]
- Smirnoff, N. Ascorbate Biosynthesis and Function in Photoprotection. Philos. Trans. R. Soc. London. Ser. B Biol. Sci. 2000, 355, 1455–1464. [Google Scholar] [CrossRef]
- Smirnoff, N. Ascorbic Acid: Metabolism and Functions of a Multi-Facetted Molecule. Curr. Opin. Plant Biol. 2000, 3, 229–235. [Google Scholar] [CrossRef]
- Hager, A.; Holocher, K. Localization of the Xanthophyll-Cycle Enzyme Violaxanthin de-Epoxidase within the Thylakoid Lumen and Abolition of Its Mobility by a (Light-Dependent) pH Decrease. Planta 1994, 192, 581–589. [Google Scholar] [CrossRef]
- Li, X.-P.; Müller-Moulé, P.; Gilmore, A.M.; Niyogi, K.K. PsbS-Dependent Enhancement of Feedback de-Excitation Protects Photosystem II from Photoinhibition. Proc. Natl. Acad. Sci. USA 2002, 99, 15222–15227. [Google Scholar] [CrossRef]
- Wu, X.; Ren, J.; Huang, X.; Zheng, X.; Tian, Y.; Shi, L.; Dong, P.; Li, Z. Melatonin: Biosynthesis, Content, and Function in Horticultural Plants and Potential Application. Sci. Hortic. 2021, 288, 110392. [Google Scholar] [CrossRef]
- Li, M.; Li, X.; Li, J.; Ji, Y.; Han, C.; Jin, P.; Zheng, Y. Responses of Fresh-Cut Strawberries to Ethanol Vapor Pretreatment: Improved Quality Maintenance and Associated Antioxidant Metabolism in Gene Expression and Enzyme Activity Levels. J. Agric. Food Chem. 2018, 66, 8382–8390. [Google Scholar] [CrossRef]
- Messant, M.; Krieger-Liszkay, A.; Shimakawa, G. Dynamic Changes in Protein-Membrane Association for Regulating Photosynthetic Electron Transport. Cells 2021, 10, 1216. [Google Scholar] [CrossRef]
- Allan, A.C.; Zuo, B.; Zhao, Y.; Reiter, R.J.; Wang, L.; Wang, Z.; Guo, Y.; Zhou, J.; Shan, D.; Li, Q.; et al. Chloroplastic Biosynthesis of Melatonin and Its Involvement in Protection of Plants from Salt Stress. Sci. Rep. 2017, 7, 41236. [Google Scholar] [CrossRef]
- Han, Q.-H.; Huang, B.; Ding, C.-B.; Zhang, Z.-W.; Chen, Y.-E.; Hu, C.; Zhou, L.-J.; Huang, Y.; Liao, J.-Q.; Yuan, S.; et al. Effects of Melatonin on Anti-Oxidative Systems and Photosystem II in Cold-Stressed Rice Seedlings. Front. Plant Sci. 2017, 8, 785. [Google Scholar] [CrossRef]
- Ding, F.; Wang, G.; Zhang, S. Exogenous Melatonin Mitigates Methyl Viologen-Triggered Oxidative Stress in Poplar Leaf. Molecules 2018, 23, 2852. [Google Scholar] [CrossRef]
- Cao, Y.-Y.; Qi, C.-D.; Li, S.; Wang, Z.; Wang, X.; Wang, J.; Ren, S.; Li, X.; Zhang, N.; Guo, Y.-D. Melatonin Alleviates Copper Toxicity via Improving Copper Sequestration and ROS Scavenging in Cucumber. Plant Cell Physiol. 2019, 60, 562–574. [Google Scholar] [CrossRef]
- Park, H.-S.; Kazerooni, E.A.; Kang, S.-M.; Al-Sadi, A.M.; Lee, I.-J. Melatonin Enhances the Tolerance and Recovery Mechanisms in Brassica Juncea (L.) Czern. Under Saline Conditions. Front. Plant Sci. 2021, 12, 593717. [Google Scholar] [CrossRef]
- Cui, G.; Sun, F.; Gao, X.; Xie, K.; Zhang, C.; Liu, S.; Xi, Y. Proteomic Analysis of Melatonin-Mediated Osmotic Tolerance by Improving Energy Metabolism and Autophagy in Wheat (Triticum aestivum L.). Planta 2018, 248, 69–87. [Google Scholar] [CrossRef]
- Li, H.; Chang, J.; Zheng, J.; Dong, Y.; Liu, Q.; Yang, X.; Wei, C.; Zhang, Y.; Ma, J.; Zhang, X. Local Melatonin Application Induces Cold Tolerance in Distant Organs of Citrullus lanatus L. via Long Distance Transport. Sci. Rep. 2017, 7, 40858. [Google Scholar] [CrossRef]
- Li, C.; Tan, D.-X.; Liang, D.; Chang, C.; Jia, D.; Ma, F. Melatonin Mediates the Regulation of ABA Metabolism, Free-Radical Scavenging, and Stomatal Behaviour in Two Malus Species under Drought Stress. J. Exp. Bot. 2015, 66, 669–680. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, V.; Shahzad, B.; Ramakrishnan, M.; Singh Sidhu, G.P.; Bali, A.S.; Handa, N.; Kapoor, D.; Yadav, P.; Khanna, K.; et al. Photosynthetic Response of Plants under Different Abiotic Stresses: A Review. J. Plant Growth Regul. 2020, 39, 509–531. [Google Scholar] [CrossRef]
- Jahan, M.S.; Guo, S.; Sun, J.; Shu, S.; Wang, Y.; El-Yazied, A.A.; Alabdallah, N.M.; Hikal, M.; Mohamed, M.H.M.; Ibrahim, M.F.M.; et al. Melatonin-Mediated Photosynthetic Performance of Tomato Seedlings under High-Temperature Stress. Plant Physiol. Biochem. 2021, 167, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Zahedi, S.M.; Hosseini, M.S.; Abadía, J.; Marjani, M. Melatonin Foliar Sprays Elicit Salinity Stress Tolerance and Enhance Fruit Yield and Quality in Strawberry (Fragaria × ananassa Duch.). Plant Physiol. Biochem. 2020, 149, 313–323. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll Fluorescence—A Practical Guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Mallick, N.; Mohn, F.H. Use of Chlorophyll Fluorescence in Metal-Stress Research: A Case Study with the Green Microalga Scenedesmus. Ecotoxicol. Environ. Saf. 2003, 55, 64–69. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Y.; Fan, X.; Sun, Y.; Yu, Z.; Huang, Y.; Li, D.; Song, Z.; Zhang, K.; Zhang, H. Short-Term Evaluation of Woodland Strawberry in Response to Melatonin Treatment under Low Light Environment. Horticulturae 2024, 10, 118. https://doi.org/10.3390/horticulturae10020118
Shi Y, Fan X, Sun Y, Yu Z, Huang Y, Li D, Song Z, Zhang K, Zhang H. Short-Term Evaluation of Woodland Strawberry in Response to Melatonin Treatment under Low Light Environment. Horticulturae. 2024; 10(2):118. https://doi.org/10.3390/horticulturae10020118
Chicago/Turabian StyleShi, Yunlong, Xiaobin Fan, Yahan Sun, Zhiru Yu, Yan Huang, Danlei Li, Zhizhong Song, Kai Zhang, and Hongxia Zhang. 2024. "Short-Term Evaluation of Woodland Strawberry in Response to Melatonin Treatment under Low Light Environment" Horticulturae 10, no. 2: 118. https://doi.org/10.3390/horticulturae10020118




