Abstract
Fusarium wilt caused by Fusarium oxysporum f. sp. cubense (Foc) is one of the most destructive diseases in banana farming worldwide. Knowledge of the factors of genetic diversity and virulence of the pathogen contributes to the development of resistant cultivars and management strategies based on exclusion. In this study, phenotypic traits such as virulence and aggressiveness in a sample of 52 Foc isolates were analyzed and their relationship to the presence of putative effectors of gene SIX (Secreted in Xylem) pathogenicity homologs was verified. The similarity matrix revealed three isolates that were closest to the standard Foc race 1 strain. Isolates 229A and 218A were selected according to their aggressiveness profile in ‘Grand Naine’ and ‘Prata-Anã’, respectively, to replace the standard isolate of race 1 in the resistance screening process carried out by the breeding program. Two homologs of the SIX8 gene, SIX8a and SIX8b, are present in isolates of Foc from Brazil, and the SIX8b gene correlates with avirulence in the cultivar ‘Grand Naine’ (Cavendish). These results are important to support the banana genetic breeding program by identifying sources of resistance to Foc and contributing to the establishment of the function of SIX effector proteins.
1. Introduction
The banana is a staple food for most tropical and subtropical regions worldwide, considered one of the most relevant agricultural activities in developing countries, with economic and social impact especially in Asia, Africa, Latin America, and the Caribbean [1]. There are more than 50 banana subgroups, of which the “Cavendish” subgroup accounts for approximately 90% of the fruit’s international trade, followed by plantains with 21% of the market share [2,3,4]. In Brazil, approximately 60% of banana production is based on the “Prata” subgroup, with cultivars ‘Prata-Anã’ and ‘Pacovan’ being the most representative. In the south and southeastern regions of the country, the cultivars of the “Cavendish” subgroup have a well-established consumer market, whereas the cultivation of plantains is mostly appreciated in the north and northeast regions [5].
One of the main limitations on current banana production is Fusarium wilt, considered the most destructive disease of the crop, and it is caused by the fungus Fusarium oxysporum f. sp. cubense (Foc). In addition to the “forma specialis” classification, where each isolate has a unique and normally related host range, Foc isolates can be also classified according to their race, meaning their ability to infect particular bananas cultivars. Thus, Foc is classified into three physiological races according to its virulence to the following cultivars: Race 1: isolates virulent to the cultivars ‘Gros Michel’ and ‘Maçã’ (syn: Silk); Race 2: isolates virulent to ‘Bluggoe’ and other cooking bananas (plantains); Race 4: isolates virulent to cultivars of the “Cavendish” subgroup [3,6,7,8]. Races 1 and 2 are present in Brazil, with greater relevance for race 1. Race 3 is not a pathogen of Musa spp. and has ambiguity in its taxonomy since this race causes symptoms in heliconias, a wild relative of bananas [9].
Foc race 4 is subdivided into subtropical (ST4) and tropical (TR4) following the usual nomenclature adopted in recent research and official nomenclature database of the fungus. ST4 causes production losses in the subtropical regions of Australia, South Africa, and the Canary Islands, commonly associated with plantations exposed to abiotic stresses [3]. TR4 is found in many countries in Asia, such as India, China, Israel, Jordan, Laos, Lebanon, Malaysia, Myanmar, Pakistan, Philippines, Sulawesi, Sumatra, Vietnam, Turkey, Mayotte, and Indonesia [3,8,10,11,12,13,14,15]; in Africa, in Mozambique; and Oceania, in Australia, particularly Queensland [16].
TR4 has not yet been reported in Brazil and is considered a quarantine pest by the Brazilian Ministry of Agriculture and Livestock. However, the disease was officially identified in Latin America in August 2019 in Colombia, in April 2021 in Peru, and in early 2023 in Venezuela [17,18,19,20].
For Foc disease, the classification into physiological races does not correlate with the genetic variability and also with existing lineages and phylogenetic groups, but is extremely important for management purposes [15]. In an attempt to obtain a reliable molecular diagnosis capable of distinguishing several races of the pathogen, a family of effector genes known as “SIX” (secreted in xylem), which are reported as virulence factors, previously used successfully to differentiate the three races of F. oxysporum f. sp. lycopersici (Fol) x tomato (Solanum lycopersicum) [21], were also used to distinguish Foc isolates in bananas [22,23,24,25].
In these studies, the SIX7 and SIX8 homologs were detected only in the Foc race 4 isolates (TR4 and ST4), while Foc-SIX8a was present in all TR4 isolates; Foc-SIX8b was present in all ST4 isolates, but was not detected in TR4 isolates [22,23]. Likewise, a set of assays was able to distinguish relevant Foc races and VCGs, in which sets of primers specifically amplified regions of the SIX6 gene in the Foc race 1, SIX1 gene in TR4, SIX8 in the subtropical race 4, SIX9/SIX10 in the Foc VCG 0121, and SIX13 in the Foc VCG 0122 [25]. To investigate the evolution of SIX genes, whole genome sequencing data generated for 23 Foc genetic lineages were queried for 14 known SIX genes (SIX1–SIX14), where SIX gene profile variation, including the presence of SIX-specific homologs, correlated with race structure and evidence of horizontal transmission of SIX genes in Foc, was identified [24].
In Brazil, the genetic variability of Foc was studied by means of a representative survey of Foc isolates from the main banana production regions and their levels of variability and aggressiveness were estimated; in addition, the first detailed map demonstrating the diversity among isolates of the distribution of Foc was elaborated’, especially as to their distribution and ability to infect different banana cultivars [26]. In our work, the 214 isolates analyzed by SSR markers were separated into 52 distinct haplotypes and distributed in different banana-producing regions of Brazil, but no Foc population structure, based on host or geographic regions, was found. Recently, Batista et al. [27] quantified the genetic variability present in Foc isolates from the Brazilian population to investigate the presence of TR4 and to determine the genetic structure based on the SIX gene profile, VCG tests, IGS PCR-RFLP, and SSR markers. In their study, the authors showed that Brazil is, in fact, free of Foc TR4 isolates. In addition, this study identified a high diversity of VCG compared to some Asian countries and a trend of association between the profile of SIX, clades, and VCG genes.
The objective of this study was to characterize a sample of 52 Foc isolates for virulence and aggressiveness in banana plants of the Cavendish (Grand Naine-AAA) and Prata/Pome (Prata-Anã-AAB) subgroups. In addition, the relationship of these traits with the presence of putative effector genes of pathogenicity SIX was verified. New collections of Foc isolates were carried out in the production areas of “Cavendish” and “Prata-Anã”, especially in the south and southeast regions of Brazil. The phenotypic characteristics of aggressiveness and virulence were analyzed by evaluating the symptomatology after inoculation of the isolates and verification of the internal disease index (IDI). Multivariate statistical analyses were used to analyze the results and express the similarity between the 52 isolates by pooling the IDIs of both cultivars. To verify the correspondence between aggressiveness and virulence and the presence of PCR amplification bands of the SIX genes for each isolate, a correspondence analysis, was used. In addition, these analyses were also used to identify a Foc pathotype with virulence and aggressiveness for both cultures that could replace the standard isolate (Foc 0801, race 1) for the selection of banana genotypes resistant to Fusarium wilt by Embrapa’s banana genetic improvement program.
2. Materials and Methods
2.1. Foc Isolates
The regions of São Paulo and Santa Catarina were selected because they are large banana producers in Brazil. After empirical research at the site, an increase in the incidence of Fusarium wilt was found in cultivars of the ‘Cavendish’ subgroup, previously unaffected by the disease. Thus, the cultivars ‘Cavendish’ and ‘Prata-Anã’ that showed symptoms of Fusarium wilt in the field were selected for sampling.
The description of sampling and conservation of the isolates followed the same protocols performed by Costa et al. [26]. At least three samples were collected per field in four plantations sampled by municipality in each state (Table S1). For indirect isolation, fragments of stems infected with Foc (pseudoestemics) were disinfected and then deposited in potato dextrose agar (PDA) culture medium. The isolations were incubated at 25 °C for eight days. At the end, the isolates obtained were cataloged and preserved in sterile distilled water, and also on filter paper with silica gel, and became part of Embrapa’s collection of Foc isolates.
The isolate Foc 0801, characterized as race 1, previously used as a standard of aggressiveness by Embrapa’s banana genetic improvement program for the selection of genotypes resistant to Foc [28,29], and the isolate Foc 218A, characterized as subtropical race 4 (ST4) [30], were selected for this study (Table S1).
2.2. Bioassay
The virulence/aggressiveness trials were conducted at the Plant Pathology Laboratory and under greenhouse conditions at Embrapa Mandioca and Fruticultura, located in Cruz das Almas, Bahia, Brazil. For the inoculation trials, two cultivars with known behavior to Foc “race 1” were used: ‘Grand Naine’ (Cavendish, resistant) and ‘Prata-Anã’ (Prata, moderately susceptible). The plantlets were obtained by in vitro culture and acclimated on commercial substrate and coconut fiber (5:1) for 60 days. After this period, they were transferred to polyethylene bags with dimensions of 20 × 30 cm until reaching 50 cm in height for the bioassay.
For the inoculation of plantlets of Grand Naine and Prata-Anã, the 52 isolates of Foc were individually grown in substrate sand/cornmeal. To produce the substrate, washed sand and cornmeal (5:1 ratio) were used in addition to 150 mL of distilled water for each 300 g of substrate. The mixture was added to plastic bags and autoclaved at 120 °C for 20 min. Foc mycelia disks were transferred to the substrate and incubated in a growth chamber at 25 °C for 30 days. Serial dilutions were performed to quantify the colonies on the substrate. The dilutions 104 and 105 were plated in BDA culture medium and after 48 h the colony-forming units were counted. Subsequently, the inoculum was adjusted to 106 colony-forming units per gram of substrate (UFC g-1). For the inoculation, 10 g of the inoculum were distributed in four holes around the plantlets at a depth of 10 cm. The control treatment was performed with 10 g of the sand/cornmeal per orifice, without the presence of Foc [31].
A trial of completely randomized design with factorial scheme (52 × 2) was used for the greenhouse experiment, totaling 104 treatments, being 52 isolates of Foc and two banana genotypes (‘Grand Naine’ and ‘Prata-Anã’). For each isolate, 5 replicates were used. The incubation period was considered as the time elapsed between the inoculation and the appearance of the first external symptoms.
At 85 DAI, the plants were removed from the substrate and evaluated for internal symptoms by cross-section of the rhizome, following the scale proposed by Dita et al. [32]: (0) No symptoms; (1) Initial rhizome necrosis; (2) Mild necrosis on the rhizome; (3) Intense necrosis symptoms on the rhizome; (4) Rhizome with most internal tissues showing necrosis; (5) Totally necrotic rhizome.
The disease index (DI) for internal symptoms (IDI) was calculated according to the formula proposed by McKinney [33], being DI (%) = 100.∑[(f.v)/(n.x)], where, f is the number of plants with the same score; v is the observed score; n is the number of plants evaluated; and x is the maximum score from the scale.
2.3. Root Bleaching and Coloring
At 85 days after inoculation, after assessing the internal symptoms of the disease, the root fragments were collected and washed and taken immediately to the laboratory to begin the whitening process. The root clarification technique was used to analyze ‘Grand Naine’ plants inoculated with isolates 218A and 0801. Root clarification and staining techniques were used according to Phillips and Haymann [34] and Brundrett et al. [35]. Clarification was performed by immersing the root system cuttings of approximately 2 cm length in a 10% KOH solution for 48 h. Afterwards, the KOH was discarded, washed with water to remove all solution, and the samples neutralized in 1% HCl for 5 min. The staining was conducted with trypan blue in 0.05% solution of lactoglycerol (2:1:1 lactic acid, glycerol, water) at room temperature for 1 h. After this period, the dye was discarded and the roots stored in lactoglycerol solution until observation under light microscope.
2.4. DNA Extraction
The Foc isolates used in this study were grown in BDA medium for seven days. After growth, the mycelium was collected, dried at room temperature and macerated in liquid nitrogen into a fine powder using a mortar and pestle. The DNA was extracted according to the methodology described by Doyle and Doyle [36] with modifications by addition of 700 μL of CTAB extraction buffer (1% CTAB, 0.7 mM NaCl, 20 mM Tris-HCl, 10 mM EDTA, 1% 2-mercaptoethanol) previously heated at 60–65 °C for 45 min.
The samples were then incubated in a water bath (60–65 °C) for one hour, vortexed every 10 min. Subsequently, 500 μL of chloroform: alcohol isoamylic solution (24:1) was added, centrifuged at 10,000 rpm for sedimentation for 10 min, and the supernatant transferred to new microtubes containing 500 μL isopropanol and 200 μL 3M sodium acetate. The DNA was pelleted by centrifugation on 10,000 rpm for 12 min, followed by two washes with 500 µL of ethanol (70%). The pellets were dried at room temperature for 30 min and resuspended in 100 µL of TE buffer (Tris-HCl 10 mM, 1 mMEDTA, pH 8.0) with RNase A (Invitrogen, Waltham, MA, USA) and incubated at 37 °C for 30 min. The amount of gDNA was checked by 1% agarose gel electrophoresis, using ethidium bromide and UV-light.
The DNA of isolate 701 of Tropical Race 4 (TR4) was kindly granted by Plant Pathology Department at the Universidade Federal de Viçosa (UFV), Viçosa-MG, Brazil, and was used as a positive control in this study.
2.5. PCR Analysis
The 52 Foc isolates from this study were used for PCR analysis using the SIX1-F/R (AJ608702), SIX2 (GQ268949), SIX3 (AM234063), SIX4 (HQ260602), SIX5 (FJ767863), SIX6 (FJ755835), SIX7 (FJ755836), and SIX8 (FJ755837) primer set according to SIX gene data published in GenBank [22] (Table 1). Additionally, the new set of primers for the SIX8 homologs, SIX8a-F/R (KF548063) and SIX8b-F/R (KF58064), was used [23]. A set of primers which amplify a region of the intergenic spacer (IGS) region of the nuclear ribosomal operon generated by Dita et al. [37] to specifically detect Tropical Race 4 (TR4) was also used. In addition, primers Foc-1/Foc-2 (EU379562) developed from a random amplified polymorphic (RAPD) DNA marker reported for specific detection of the breed Foc 4 have been tested [38]. To evaluate the quality of the Foc DNA, the translation elongation factor 1a (TEF-1a) gene was also used with the primers EF1 and EF2 [39] (Table 1).

Table 1.
Primer sequences used for PCR analysis in this study. Primers SIX1 to SIX8 were based on the GenBank sequence.
PCR parameters were as follows: denaturation at 94 °C for 10 min, followed by 35 cycles of denaturation at 94 °C for 45 s; annealing at 60 °C for 45 s; and extension at 72 °C for 1 min, with a final extension at 72 °C for 10 min in a thermocycler (Applied Biosystems, San Francisco, CA, USA). The final volume of the reactions was 25 μL consisting of 1 x GoTaq DNA Polymerase (Promega, Corporation, Madison, WI, USA), 10 nmoles dNTPs (Promega), 4 μM of each primer, and 10 ng of DNA. The resulting PCR products were visualized on 1% agarose using ethidium bromide and UV-light. For PCR with the TR4-specific primer, the following program was used: 95 °C for 2 min and 35 cycles of 95 °C for 30 s, 60 °C for 30 s and 72 °C for 1 min, followed by an additional extension time for 10 min at 72 °C [37].
2.6. Data Analysis
The disease index data were submitted to analysis of variance at the 5% significance level and for the factors that presented significant differences, the interactions unfolded by the Skott–Knott test at 5% significance.
The similarity between the 52 isolates in the cultivars Grand Naine and Prata-Anã was calculated from the IDIs using the complete linkage cluster to calculate the dendrograms, and as similarity measures the Euclidean distances were mused based on the same IDIs between IDI columns. These data were expressed by a heatmap obtained by the heatmap.2 function of the ‘gplots’ package in the R software 4.3.2 [40]. Thus, it was possible to group the isolates into different classes, from high aggressiveness and virulence to low aggressiveness and virulence or avirulence. Likewise, we used these methods to compare the profile of bands (pb—base pair) present in the PCR analysis with the primers related to the SIX genes. For the construction of the dendrogram, a binary matrix was established for the presence (1) and absence (0) of the gene, and it was possible to group the isolates into different haplotypes. Thus, two heatmaps were constructed for each case.
The correlation among the different parameters such as internal disease indexes (IDI) and the presence of band patterns associated with presence of different SIX genes was evaluated based on multiple factorial analysis (MFA), for the mixed analysis of qualitative and quantitative data obtained using the ‘FactoMiner‘package implemented in R software. In this case, we used the analysis of similarity between the IDIs and cluster of isolates to correlate with the presence of the amplified bands. A derivative analysis of PCA was used for the comparison of genotype vs. genotype (G × G) interaction, and performed to explain the variation in the isolates in the cultivars ‘Prata-Anã’ and ‘Grand Naine’, using the ‘GGEbiplot’ package in the R software.
3. Results
3.1. Evaluation of Disease Indexes
Based on the external and internal symptoms of Foc in the ‘Grand Naine’ and ‘Prata-Anã’ cultivars, the levels of aggressiveness and virulence of each isolate were estimated according to the scale proposed by Dita et al. [32]. Internal symptoms were evident with a reddish-brown coloration on the rhizome, partially or totally reaching the central cylinder (Figure 1). Thus, it was possible to compare the virulent or avirulent behavior of the same isolate in the different cultivars. We will take the concepts of virulence as the ability of a race to be pathogenic only in certain genotypes of the host, and of aggressiveness as the ability of the isolate to grow vigorously in or on its host; that is, the more aggressive the isolate, the more tissue it can invade in its host at a certain period of time [41].
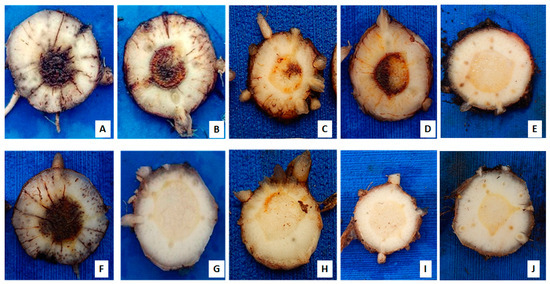
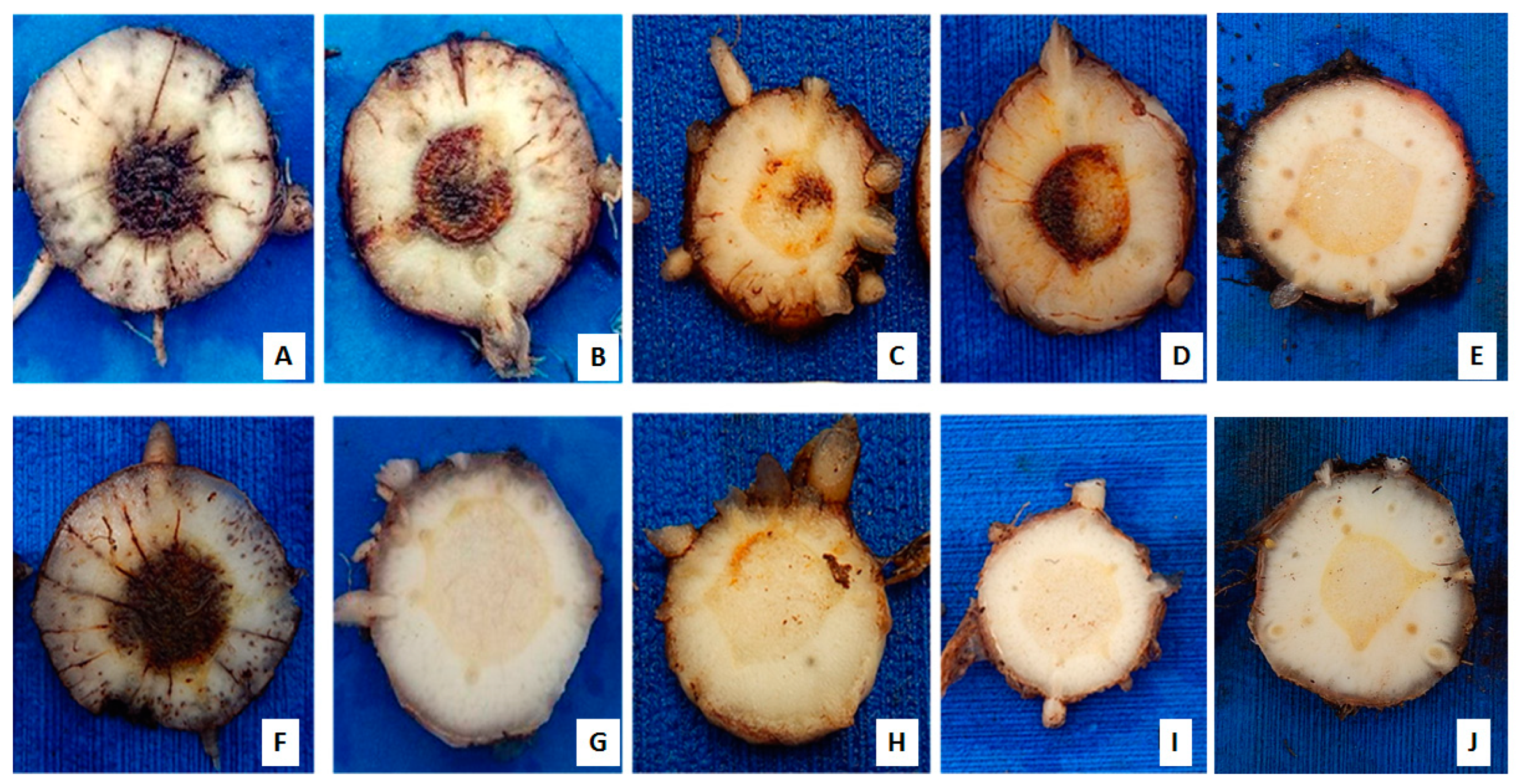
Figure 1.
Internal symptoms of Fusarium oxysporum f. sp. cubense (Foc) in banana rhizomes ‘Prata Anã’ (top) and ‘Grand Naine’ (bottom), 85 days after inoculation in a greenhouse condition using Foc isolates from Brazil. (A,F) isolate 218A; (B,G) isolate 0801; (C,H) isolate 225B; (D,I) isolate 221A; and (E,J) controls without inoculation.
Isolate 218A was virulent to ‘Prata-Anã’ in the same manner as the standard isolate 0801 (race 1), but the aggressiveness of isolate 218A was notorious for the shortened time-period for the manifestation of external symptoms (incubation period) and death of the plants throughout the evaluation (Figure 1A,F). In the ‘Grand Naine’ cultivar, isolate 0801 behaved as avirulent, and there was no manifestation of symptoms, contrary to isolate 218A that was virulent and aggressive toward it (Figure 1B,G). Isolate 225B was virulent to both cultivars, but with less aggressiveness than isolate 218A (Figure 1C,H). Isolate 221A was virulent only for cultivar ‘Prata-Anã’ and internal infection symptoms were not seen in ‘Grand Naine’ (Figure 1D,I).
There were significant differences by the analysis of variance both within and between cultivars and isolates (Table S2). Thus, the Scott–Knott test allowed grouping the isolates in each cultivar in a similar way as for the heatmap clustering (Table S3).
Based on the cluster analysis from the ID and by the Scott–Knott test of the 52 Foc isolates in ‘Grand Naine’ and ‘Prata-Anã’ cultivars, there were significant differences in the aggressiveness and virulence of the isolates (Figure 2, Table S2). These differences were represented according to a color scale in relation to the percentage of disease caused by each isolate (Figure 2).
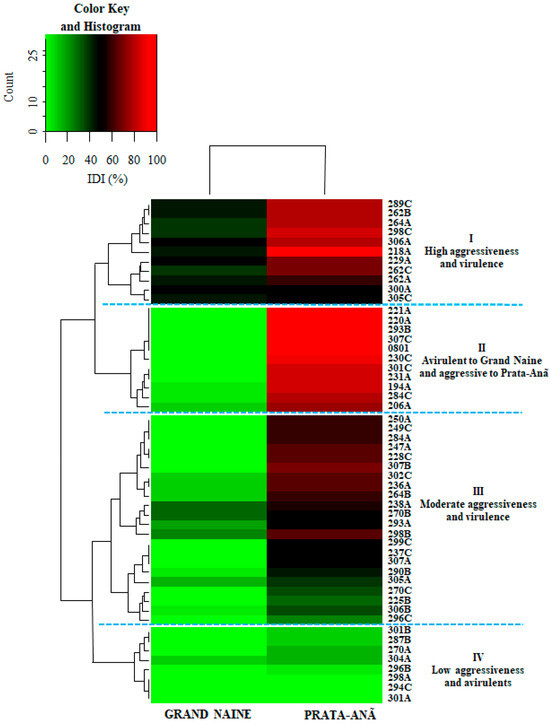
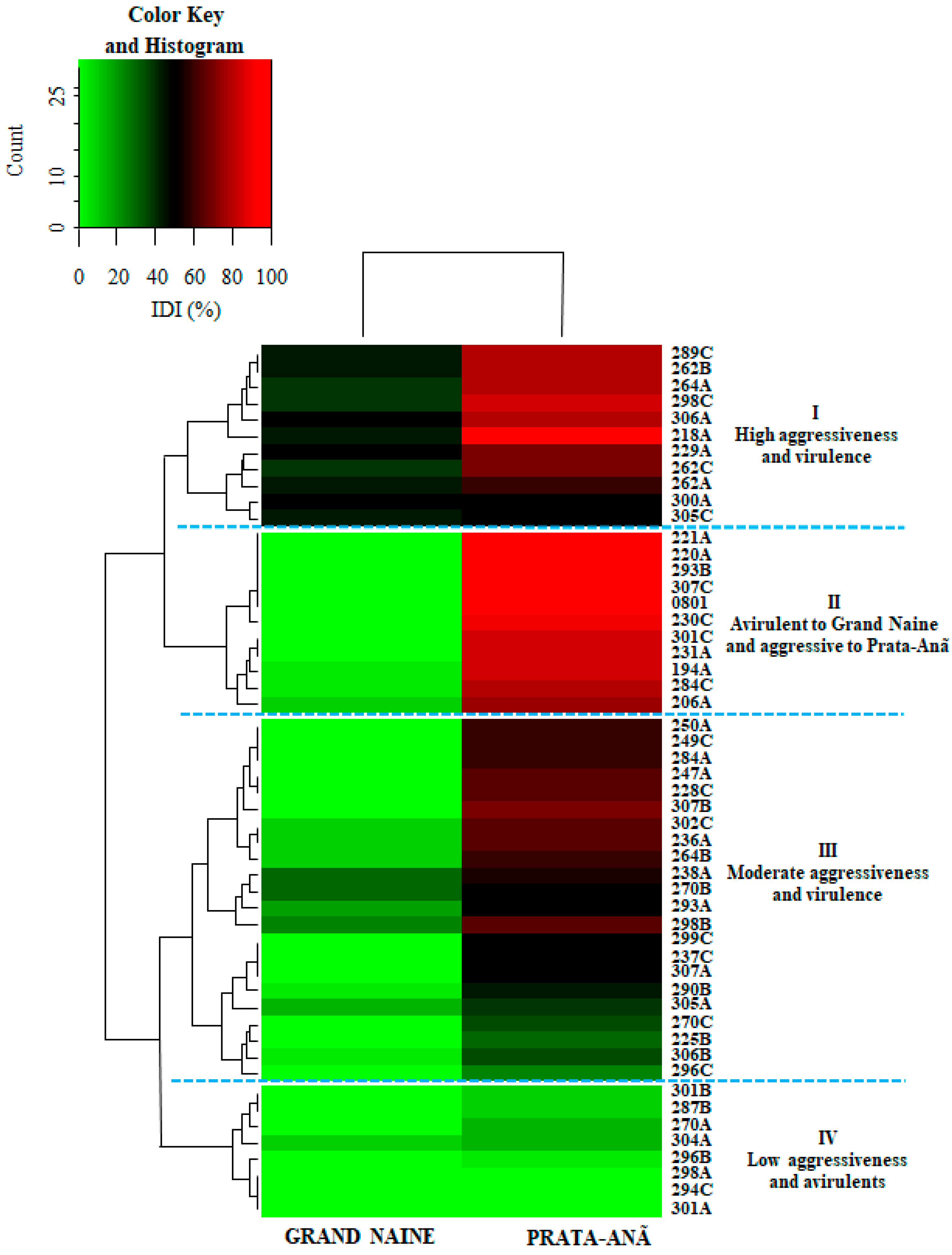
Figure 2.
Hierarchical clustering for internal disease indexes (IDI) of 52 Fusarium oxysporum f. sp. cubense isolates inoculated on cultivars ‘Grand Naine’ and ‘Prata-Anã’. The highest rates of aggressiveness and virulence were located within the range corresponding to the different shades of green with 60 to 100% disease variation. Red tones are represented by the isolates with less aggressiveness with variations from 0 to 40% of disease. The dark interim from 41% to 59% represents medium aggressive isolates. The heatmap is drawn using the heatmap.2 function of the gplots package, and dendrogram distances are based on Euclidean distance.
The isolates that caused disease on both cultivars concomitantly, classified as more aggressive and virulent, are represented in the first cluster (I): 289C, 262B, 264A, 298C, 306A, 218A, 229A, 262A, and 262C. The second cluster (II) was formed by isolates virulent only to cultivar ‘Prata-Anã’, but with high DI values. This cluster includes the standard isolate ‘0801’ (Figure 2). The third cluster (III) showed virulence for both cultivars, but with moderate DI values for both ‘Grand Naine’ and ‘Prata-Anã’ (Figure 2). The last cluster (IV) includes isolates avirulent for both cultivars, such as isolates 294C, 298A, and 301A, and isolates with low DI values (Figure 2).
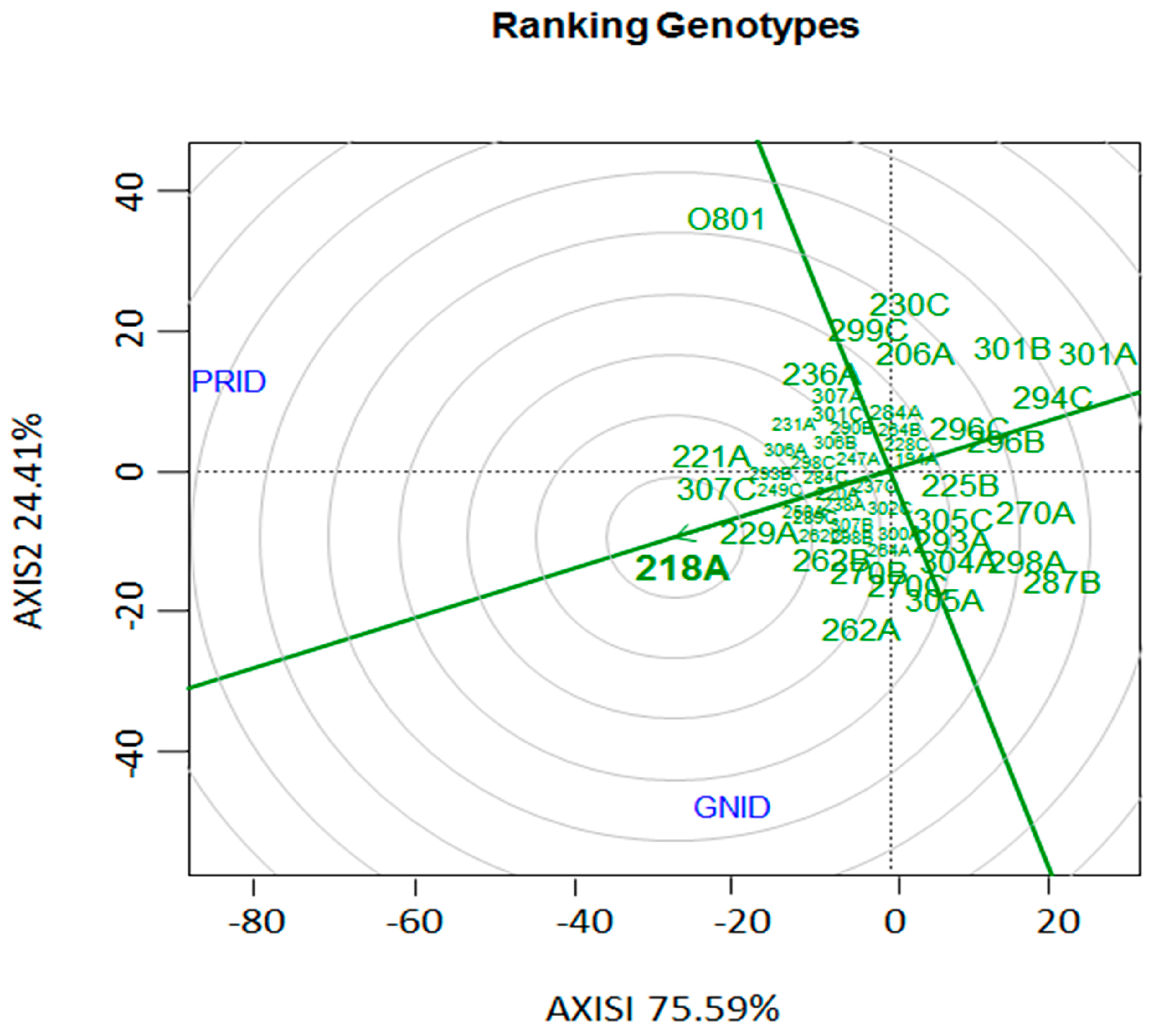
The ‘genotype vs. genotype’ interaction for the relationship among the banana cultivars and 22 Foc isolates based on aggressiveness and virulence, showed a considerable interaction effect and the presence of different pathotypes within the evaluated isolates as well as a good discrimination among them. Thus, isolates 218A, 221A, 307C, 229A, 262B, 270B, and 262A, are the most outstanding regarding aggressiveness and virulence in the cultivars. Isolate 218A can be considered among those evaluated in the collection as the ideal pathotype for use in the Embrapa Banana Breeding Program aiming for resistance to Foc, since it occupied the central (bullseye) position in the graph, followed by isolate 229A (Figure 3).
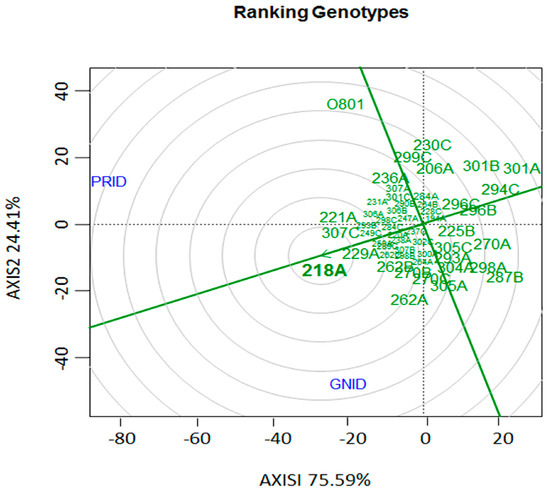
Figure 3.
“Genotype vs. Genotype” interaction (G × G) biplot based on the internal disease indexes (IDI) of 52 Fusarium oxysporum f. sp. cubense isolates in relation to two banana cultivars. PRID: ‘Prata-Anã’ internal disease index; GNID: ‘Grand Naine’ internal disease index. In the center of the “dart board” (bullseye), the isolate with highest virulence and aggressiveness to both varieties is located.
3.2. Root Clarification and Staining of Fungal Structures
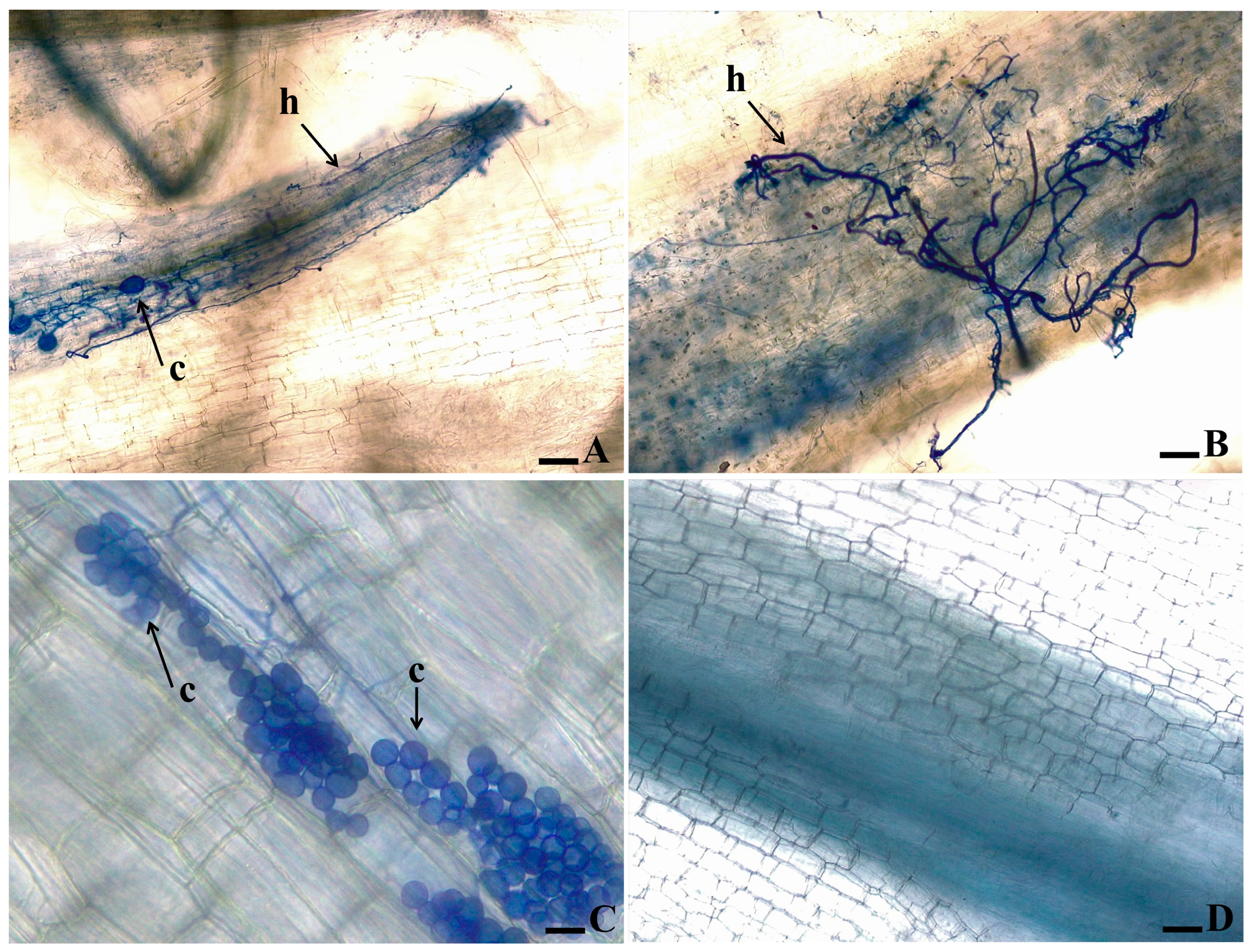
As to the banana’s root clarification and staining of fungal structures in the cultivars ‘Grand Naine’ and ‘Prata-Anã’ inoculated with isolates 218A and 0801, the formation of fungal structures inside the tissue was noticed for isolate 218A in ‘Grand Naine’, which could rapidly colonize the vascular tissues of the plant with intense production of hyphae and chlamydospores (Figure 4A,B). In contrast, isolate 0801 failed to colonize the plant tissues, producing only groups of chlamydospores without hyphae colonization in the vascular system (Figure 4C).
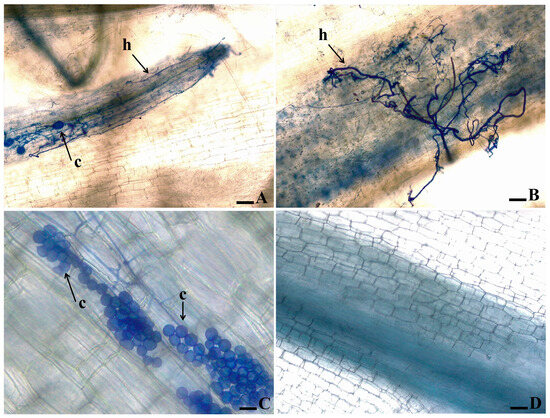
Figure 4.
Microscopic observation of the root tissue of “Grand Naine” banana plants inoculated with two isolates of Fusarium oxysporum f. sp. cubense. The roots were clarified and the pathogen structures were stained with Trypan Blue. (A,B) Isolate 218A; (C) Isolate 0801; (D) Control plant, not inoculated. The arrows indicate the presence of chlamydospores (c) and hyphae (h).
3.3. Presence of SIX Genes by PCR Analysis
The PCR study for the presence of genes related to pathogenicity in Foc revealed differences as to the presence of bands in different isolates (Figure S1). All isolates amplified for a 650 bp band exhibited for the elongation factor gene TEF-1α, ensuring the quality of the DNA extraction and positive control for the PCR reactions (Figure S1). Likewise, all isolates exhibited a 260 bp band for the SIX1 gene amplification (Figure S1). The SIX2, SIX3, SIX4, SIX5, and SIX6 genes, and the IGS primer used to detect TR4 did not amplify for any of the tested isolates (Figure S1). The Foc-1/Foc-2 primer, a marker RAPD set by Lin et al. [38] and Carvalhais et al. [25], amplified bands for most of the isolates, except for 220A, 221A, 287B, 294C, 296B, 296C, 304A, and 0801. These same isolates did not present bands for the SIX7 and SIX8 genes.
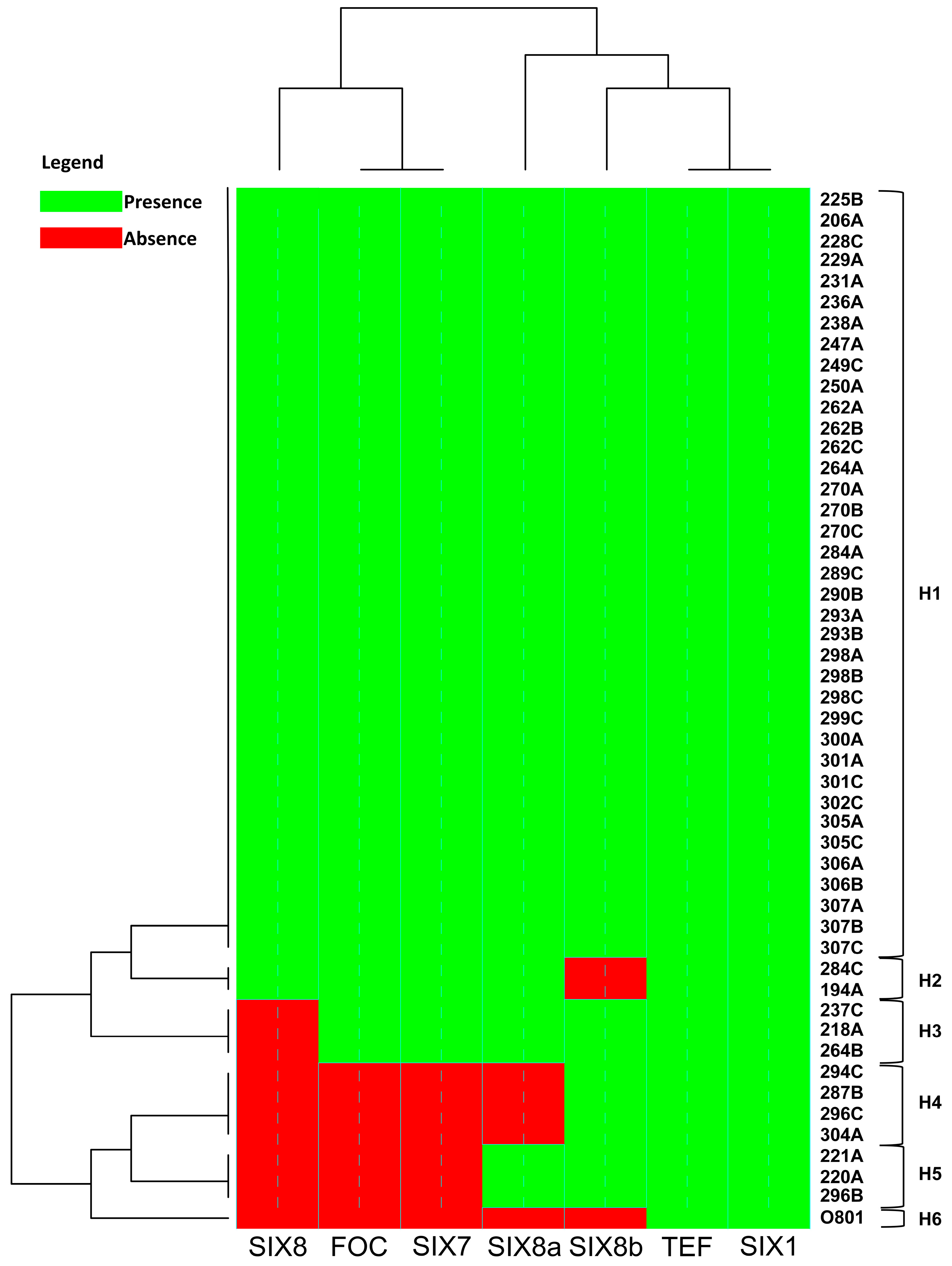
Isolate 218A presented a band for all genes studied except for the SIX8 gene, whereas the 0801 isolate presented a band only for the TEF- α elongation factor of 650 bp and for the SIX 1 gene of 260 bp (Figure S1). Based on the groups formed by cluster analysis for the presence or absence of the bands associated with each gene, the SIX8 gene was absent in a larger number, differing significantly in prevalence when compared to the other regions. The SIX8a gene differed only in five isolates and SIX8b is closer to the SIX1, since it is present in almost all isolates, and absent in only three isolates (Figure 5).
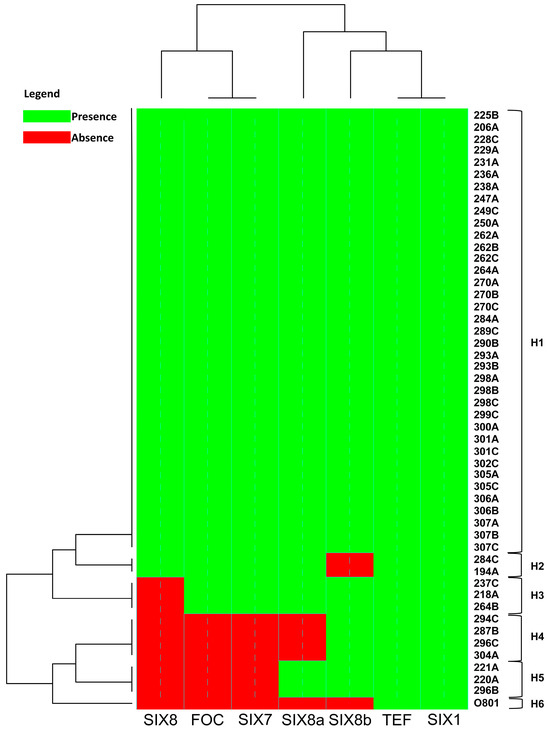
Figure 5.
Heatmap analysis showing the comparison of the absence (Red) and/or presence (Green) of sequence of specific primers to detect race 4 (FOC) and putative pathogenicity genes associated with different isolates of Fusarium oxysporum f. sp. cubense. The heatmap is drawn using the heatmap.2 function of the gplots package, and dendrogram distances are based on Euclidean distance. H: Haplotype.
For the total evaluated isolates, six different haplotypes were found, of which only one was single (H6) representing the isolate 0801, originally from the State of Bahia, Brazil (Table S1). The H1 haplotype comprised a larger number of isolates where all the analyzed genes were present (Figure 5).
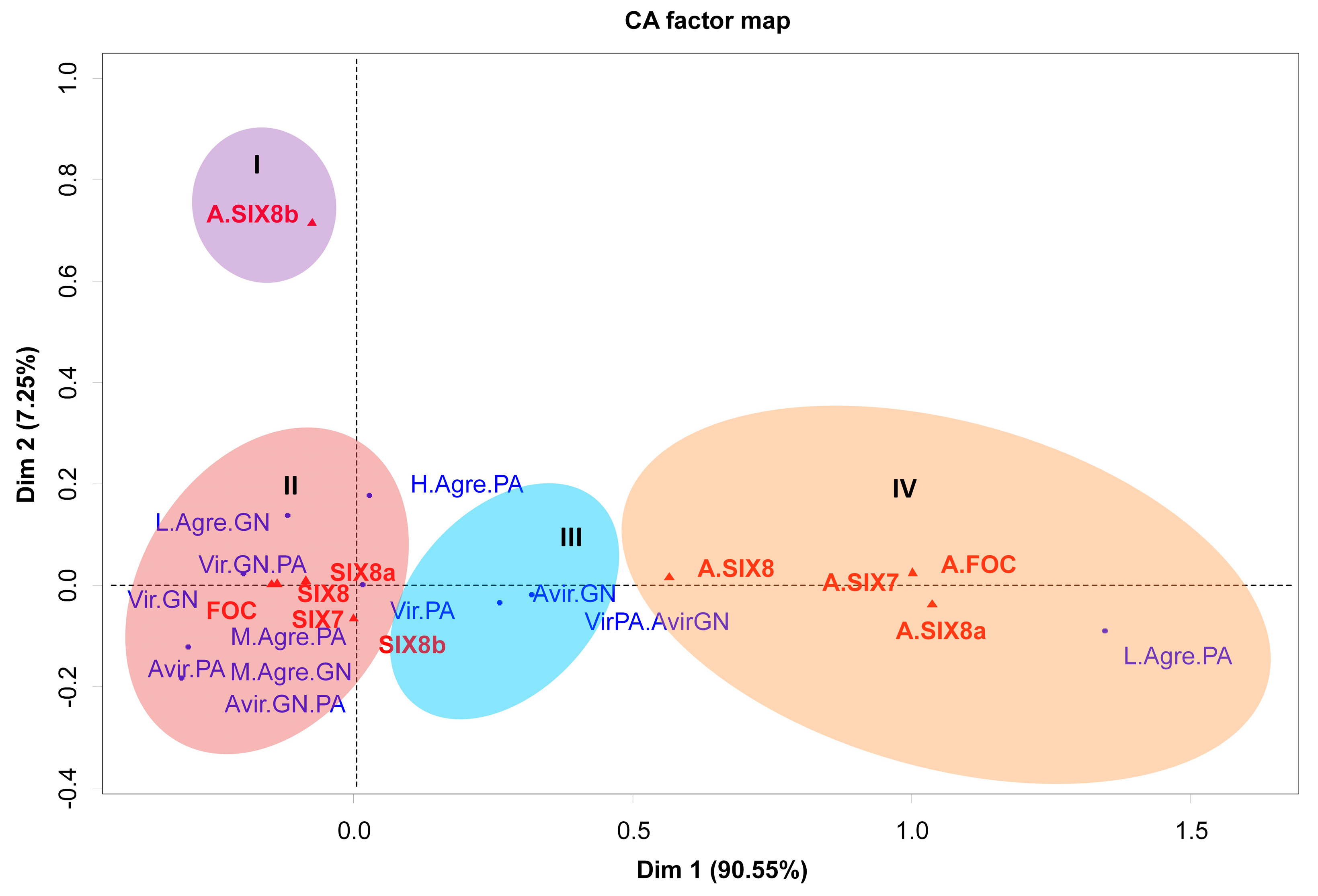
Correspondence analysis (CA) was used to correlate the presence or absence of primers (FOC, SIX7, SIX8a, and SIX8b) with virulence and aggressiveness traits for the two principal components of the data. The CA analysis indicated the formation of four clusters regarding the aggressiveness and virulence pattern of isolates, and four clusters were formed (Figure 6). The correspondence group ‘I’ shows the lack of association of the absence of bands for the SIX8b gene and any pathogenicity trait. However, contrasting results were noticed as to the presence/absence of the other genes studied.
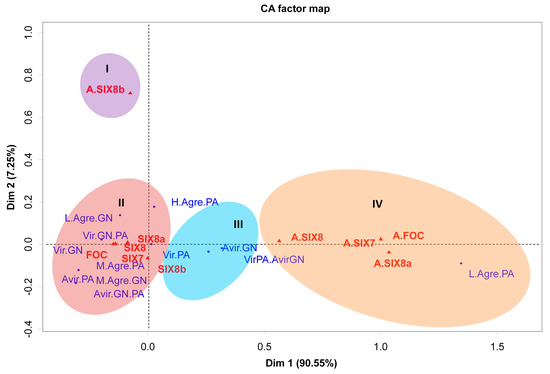
Figure 6.
Clustering of genes present and absent in 50 isolates of Foc in Brazil, based on analysis of correlation for parameters related to virulence and aggressiveness in two banana cultivars. Each circle indicates a group. Red triangle: genes, blue dots: pathogenicity traits associated with virulence or aggressiveness. Caption: A.SIX8a = missing SIX8a gene; A.SIX8 = absence of the SIX8 gene; A.SIX7 = absence of the SIX7 gene; A.SIX1 = absent FOC gene; L.Agre.GN = low aggressiveness in ‘Grand Naine’; GN. PA = Virulence for ‘Grand Naine’ and ‘Prata-Anã’; GN = Virulence for ‘Grand Naine’; M.Agre.PA = Moderate aggressiveness for ‘PrataAnã’; Avir.PA = Avirulence for ‘Prata-Anã’; M.Agre.GN = Moderate aggressiveness for ‘Grand Naine’; GN.PA = Avirulence for ‘Grand Naine’ and ‘Prata-Anã’; PA = Virulence for ‘Prata-Anã’; To come. GN = Avirulence for ‘Grand Naine’; H.Agre.PA = High aggressiveness for ‘Prata-Anã’; Vir.PA.Avir.GN = Virulence for ‘Prata-Anã’ and Avirulence for ‘Grand Naine’; L.Agre.PA = Low aggressiveness for ‘Prata-Anã’. Embrapa, Brazil, 2019. CA = correlation (correspondence) analysis was obtained by the ‘factoextra’, ‘gplots’, and ‘FactoMineR’ packages in the R software.
In the correspondence group ‘II’, a high and positive correlation was noticed between the presence of the FOC, SIX7, SIX8, and SIX8a and the avirulence or moderate to high aggressiveness of the isolates to the cultivar ‘Prata-Anã’, in contrast to the virulence and low to moderate aggressiveness to cultivar ‘Grand Naine’. All isolates that have these gene combinations were virulent in cultivar ‘Grand Naine’ (Figure 6).
A different result was found for the correspondence group ‘III’, where the presence of the SIX8b gene was highly correlated with the virulence to cultivar ‘Prata-Anã’ and avirulence to cultivar ‘Grand Naine’, presenting the exact opposite behavior found in the correspondence group ‘2’. In contrast, the isolates without bands related to the genes SIX7 SIX8, and SIX8a, were associated with low aggressiveness in the cultivar ‘Prata-Anã’ (Figure 6).
4. Discussion
According to a study of a population of Foc isolates in Brazil carried out by Costa et al. [26], the population structure of Foc was characterized by high genetic variability. Although a sexual phase is not known in the reproductive structure of the Fusarium oxysporum species, other evolutionary mechanisms may be related, leading to the emergence of more aggressive variants capable of infecting resistant cultivars [26]. In addition to the existence of physiological races and vegetative compatibility groups, the occurrence of cryptic recombination and the presence of mobile chromosomes that allow selective pathogenicity, have been proven [42]. In the present study, some isolates of Foc were virulent when cultivating ‘Grand Naine’, belonging to the Cavendish subgroup, and a significant number were extremely aggressive in the cultivation of ‘Prata-Anã’; while isolate 218A was more aggressive in both cultivars, which reinforces the idea that this is a new Foc pathotype in Brazil [26,28,30,43].
The phylogenetic basis which explains the variations in the genetic structure of Foc regarding pathogenic specificity has been associated with horizontal gene transfer [24,39,44]. The data suggest that chromosome 14 of lineage-specific genomic regions (LS) harbors genes responsible for pathogenicity that are dispensable and can be transferred laterally between pathogens, including several polyphyletic clonal lines within the species complex [42] (although, to date, there is no published evidence that chromosome 14 exists in Foc). Recent studies indicate that many genes are required for general pathogenicity in relation to plants, and they are widely distributed throughout the F. oxysporum species complex, but the specific pathogenicity of the Foc host can be attributed to fungal effectors such as SIX genes [25,45,46]. Furthermore, a study has stated that the presence of the SIX1a allele is linked to the virulence of Foc ‘TR4’ in bananas from the Cavendish subgroup based on mutagenesis analyses [47]. Other studies reported that the effector gene SIX8 is required for the virulence of Foc ‘TR4’ to the Cavendish banana [48].
In this study, the SIX1 gene was present in all tested isolates, including avirulent isolates for both cultivars (Figure S1). In a similar study, Czislowski et al. [24] identified the diversity of the same gene and found nine types of sequences in total, even though their role in pathogenicity is still unknown. Meldrum et al. [22] and Fraser-Smith et al. [23], assessing the presence of putative genes in pathogenic Foc isolates, showed that the SIX7 and SIX8 genes were present only in race 4 isolates and absent in race 1 and 2; similarly, the homologous gene SIX8 (TR4) differed from subtropical race 4 (ST4) in that the SIX8a gene was present only in TR4 isolates, and the SIX8b gene only in ST4 isolates. Furthermore, a comparison of SIX8a with SIX8b revealed 95% identity. However, in this study, new perspectives on the role of SIX genes in Foc virulence were noticed.
PCR amplification with the set of primers SIX7, SIX8, SIX8a, and SIX8b showed a band for most of the evaluated isolates, but there was no presence of amplification of bands for the set of specific primers of TR4. Based on PCR reactions, we confirmed the absence of TR4 strains in our sample of isolates from different regions of Brazil. In addition, the results confront the proposition that ‘Foc-SIX8′ is a suitable candidate for the molecular differentiation of isolates of race ‘4’ among races ‘1’ and ‘2’, as well as the differentiation of the tropical race 4 (TR4) and subtropical race 4 (ST4) as stated by Meldrum et al. [22], Fraser-Smith et al. [23], and An et al. [48]. Thus, it is assumed that copies of the SIX8 gene may perform different virulence functions in these isolates, since unlike other SIX genes, which are found as single copies, there are nine copies of SIX8 and four copies of SIX8b in the genome of Fol 4287 [22,42]. In general, one must take into account the non-specificity of the primers used to evaluate these genes.
The presence of an association between virulence and/or aggressiveness parameters, and the specific presence of some of the SIX genes, were noticed in this study, since the presence of the SIX8b allele was strongly related to the behavior of avirulence in the cultivar ‘Grand Naine’ (Cavendish subgroup) (Figure 6). It therefore may be inferred that this gene possibly performs functions related to specificity in relation to the host.
The understanding of the host–pathogen interaction is essential to explain the aspects of variation in the virulence and aggressiveness of the isolates in the cultivars ‘Prata-Anã’ and ’Grand Naine’ in this study. Both the virulent isolate 218A and the avirulent isolate 0801 were thus present within the tissues, but the structures produced by an isolate in which the cultivar is resistant were predominantly chlamydospores, which are structures of resistance, indicating that barriers have been imposed to prevent the advancement of hyphae and colonization of the tissue and therefore, the pathogen needed to produce resistance structures. The formation of chlamydospores is correlated with the survival capacity of the fungus as a result of plant defense. This intense production of chlamydospores occurs as defense of the pathogen in relation to the plant, since these structures can guarantee the survival of the fungus for many years [3,8,49].
In another study, the infection process of two commercial Australian cotton varieties differed in susceptibility to F. oxysporum f. sp. vasinfectum (Fov), and the appearance of Fov chlamydospores in the tissues of the susceptible strain was observed, which was associated with significant degradation of the host tissue [50]. Likewise, work with a Foc ST4 line labeled with green fluorescent protein (GFP) verified the path of colonization after infection of susceptible cultivars, and results showed the production of chlamydospores as soon as the host was invaded and prior to expression of external symptoms [51,52].
The overcoming of genetic resistance is a constant threat to field management of Fusarium wilt and this problem can be closely related to the high variability of the fungus [26,27,53,54,55]. According to Buddenhagen [11], tropical race 4 (TR4) evolution was associated with the emergence of the ability to cause disease in banana cultivars of the Cavendish subgroup from spontaneous mutations that generated variants with potential to colonize and cause damage to this subgroup. Thus, the different strains of the pathogen can adapt to different conditions, especially during the extensive use of resistant cultivars since the presence of resistance genes in the plant leads to a directional seal in populations of pathogens, culminating in the increase in the frequency of individuals capable of overcoming plant resistance [26,56].
Based on our findings, it is possible to infer that the most aggressive isolates, identified in the cultivar ‘Grand Naine’, are probably new variants of race 1 isolates, a fact that requires extensive study in order to confirm this hypothesis. Similar results were found by Ribeiro et al. [28] and Rocha et al. [30] for the diversity among Foc isolates, where the 218A isolate was the most virulent, being selected as representative of the most aggressive of the analyzed isolates compared to the standard ‘0801 isolate’. Rocha et al. [43], evaluating a set of genotypes through histological and histochemical analyses, and gene expression, observed that the isolate 229A was associated with the suppression of defense responses in cultivars resistant to isolates 218A and 0801, indicating it was therefore more virulent.
In addition to the emergence of new and more aggressive variants of F. oxysporum f. sp. cubense (Foc), adverse environmental conditions also contribute to the incidence of Foc in banana-producing areas in Brazil. In the latest study on the population structure of Foc in Brazil, the presence of TR4 was investigated in a collection of isolates and the genetic structure was determined based on an expanded set of markers [27]. In this study, the Foc population appears to have low to moderate genetic variability given the high clonal fraction.
Among the isolates evaluated, there was an extensive variation considering virulence and aggressiveness in the cultivars ‘Prata-Anã’ and ‘Grand Naine’ (Figure 2). In a similar study, Cunha et al. [57] collected Foc isolates in banana production areas in the state of Santa Catarina with cultivars of the Cavendish and Prata subgroups, aiming to estimate the diversity of the pathogen, and stated that 78% of the isolates considered as more aggressive were collected in the ‘Grand Naine’ (Cavendish subgroup). In the present study, it is also shown that the majority of the more aggressive/virulent isolates (68%) were also collected in Cavendish cultivars, especially in the state of São Paulo (Table S1). The presented data reinforce the existence of variations in the virulence profile of some isolates and may be associated with the occurrence and widespread distribution of ST4 among Foc isolates in Brazil under favorable environmental conditions in different regions of the country [27].
Given the recent examination concerning the relationship between pathogenicity, avirulence genes, and aggressiveness of Foc in bananas, it is crucial to frame these discoveries within the wider scientific context. A set of interconnected inquiries has shed light on the complex interplay among soil characteristics [58,59], microbial behavior [60,61], and banana yield [62]. Research conducted by Olivares et al. [60] and Olivares et al. [63] has illustrated the connection between soil morphology and microbial behavior with banana yield, underscoring the significance of comprehending the soil environment in controlling diseases like Fusarium wilt. Moreover, investigations carried out in Colombia and Venezuela [64,65,66] have delved into soil predisposing elements and susceptibility mapping across different regions, offering valuable insights into the spatial dynamics of Fusarium oxysporum f. sp. cubense Tropical Race 4 (TR4). These findings highlight the multifaceted nature of banana cultivation systems and emphasize the necessity for a more comprehensive approach to disease management strategies.
Additionally, the study implicating putative effectors of the SIX genes in Foc’s pathogenicity aligns with the broader scientific discourse on microbial effectors’ role in plant–pathogen interactions. Olivares et al. [58] utilized supervised methods to pinpoint soil characteristics associated with banana wilt incidence, shedding light on potential factors influencing pathogen virulence. Similarly, the work of Rodriguez-Yzquierdo et al. [65] on soil predisposing elements in La Guajira, Colombia, aids in understanding regional variations in disease prevalence. These studies collectively underscore the importance of a multidisciplinary approach, integrating genetic, biochemical, and ecological viewpoints, to unravel the complexities of Fusarium wilt and guide banana genetic breeding programs. The potential correlation, as identified in the present study, of SIX effector proteins with avirulence in specific banana cultivars resonates with the broader trend in microbial pathogenicity research emphasizing the crucial role of effectors in host–pathogen interactions.
5. Conclusions
Our study allowed us to characterize a set of isolates in terms of the phenotypic characteristics of aggressiveness and virulence and to correlate them with the presence of putative pathogenicity effector genes (SIX genes). We verified that two homologs of the SIX8 gene, SIX8a and SIX8b, were detected by PCR in isolates of Foc from Brazil and that there is a correlation of the SIX8b gene with avirulence in the cultivar ‘Grand Naine’, belonging to the Cavendish subgroup. In addition, isolates 218A and 229A will be used to replace the standard isolate 0801 (race 1) in EMBRAPA’s banana breeding program due to their potential to better discriminate resistance/susceptible genotypes considering their aggressiveness. In view of the potential correlation of SIX effector proteins with avirulence in banana-specific cultures, our results bring new perspectives on the characterization of SIX genes and their role in Foc pathogenicity and align with the broader trend in microbial pathogenicity research, highlighting the key role of effectors in host–pathogen interactions. In the future, further research may be carried out aiming at a more complete identification of these isolates, as well as a more precise definition of the races and the presence of pathogenicity and avirulence genes; for example, via sequencing of the SIX regions or even sequencing of the genome of these isolates.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae10030228/s1, Figure S1: Identification of SIX1, SIX7, and SIX8 genes and homologs SIX8a and SIX8b and primer sequences, Foc-1/Foc-2 (FOC) and TR4 in Foc (Fusarium oxysporum f. sp. cubense). Gel analysis of DNA amplified from isolates in Table S1. The SIX 2, SIX 3, SIX 4, SIX 5, and SIX 6 genes do not appear because they do not have amplified bands. Pb: base pairs; Table S1: Description of isolates of Fusarium oxysporum f. sp. cubense from Brazil used in the study to assess the presence of genes secreted in xylem (SIX) and levels of aggressiveness and virulence. Embrapa, Brazil, 2020. Table S2: Analysis of variance for internal indexes of Fusarium wilt symptoms (ID) in the cultivars Prata-anã and Grand Naine based on the rhizome vascular discoloration, 85 days after inoculation in greenhouse with 52 isolates of Fusarium oxysporum f. sp. cubense. Table S3: Classification of aggressiveness and virulence of 52 isolates of Fusarium oxysporum f. sp. cubense from Brazil in the cultivars “Prata-Anã” and “Grand Naine” based on the internal disease index.
Author Contributions
Conceptualization, K.V.C.V., A.d.J.R. and E.P.A.; methodology, A.d.J.R., K.V.C.V. and M.d.S.F.; software, K.V.C.V., A.d.J.R. and M.d.S.F.; validation, K.V.C.V., A.d.J.R., M.d.S.F., K.N.P., V.B.O.A., S.A.S.d.O., C.F.F., F.H. and E.P.A.; formal analysis, A.d.J.R.; investigation, K.V.C.V., A.d.J.R., F.H., C.F.F., S.A.S.d.O. and E.P.A.; resources, E.P.A.; data curation, K.V.C.V., A.d.J.R., M.d.S.F. and V.B.O.A.; writing—original draft preparation, K.V.C.V., A.d.J.R. and E.P.A.; writing—review and editing, K.V.C.V., A.d.J.R., F.H., C.F.F., S.A.S.d.O. and E.P.A.; visualization, K.V.C.V., A.d.J.R., M.d.S.F., V.B.O.A., K.N.P., V.B.O.A., C.F.F., F.H., S.A.S.d.O. and E.P.A.; supervision, E.P.A.; project administration, E.P.A.; funding acquisition, E.P.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by IITA/The Bill and Melinda Gates Foundation—Accelerated Breeding of Better Bananas, ID OPP1093845.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Acknowledgments
The authors thank the State of Bahia Research Foundation (Fapesb), The Brazilian National Council for Scientific and Technological Development (CNPq), for partially financing the research.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- FAO. The Global Programme on Banana Fusarium Wilt Disease. Available online: https://www.fao.org/3/i7956e/i7956e.pdf (accessed on 22 September 2022).
- Ploetz, R.C. Diseases and pests: A review of their importance and management. InfoMusa 2004, 13, 11–16. [Google Scholar]
- Ploetz, R.C. Fusarium wilt of Banana. Phytopathology 2015, 105, 1512–1521. [Google Scholar] [CrossRef]
- Staver, C.; Pemsl, D.E.; Scheerer, L.; Perez Vicente, L.; Dita, M. Ex ante assessment of returns on research investments to address the impact of Fusarium wilt tropical race 4 on global banana production. Front. Plant Sci. 2020, 11, 844. [Google Scholar] [CrossRef]
- Gonçalves, Z.S.; Da Invenção, D.R.S.; Ledo, C.A.D.S.; Ferreira, C.F.; Amorim, E.P. Research Article Agronomic Performance of Plantain Genotypes and Genetic Variability Using Ward-MLM Algorithm. Genet. Mol. Res. 2018, 17, gmr16039882. [Google Scholar] [CrossRef]
- Waite, B.H.; Stover, R.H. Studies on Fusarium wilt of bananas: Vi. Variability and the cultivar concept in Fusarium oxysporum f. sp. cubense. Can. J. Bot. 1960, 38, 985–994. [Google Scholar] [CrossRef]
- Pérez-Vicente, L.; Dita, M.A. Fusarium Wilt of Banana or Panama Disease by Fusarium oxysporum f. sp. cubense: A Review on History, Symptoms, Biology, Epidemiology and Management. In Manual Prevention and Diagnostic of Fusarium Wilt (Panama Disease) of Banana Caused by Fusarium oxysporum f. sp. cubense Tropical Race 4 (TR4); Pérez-Vicente, L., Dita, M.A., de la Parte, M., Eds.; FAO: Rome, Italy, 2018; pp. 5–30. [Google Scholar]
- Dita, M.; Barquero, M.; Heck, D.; Mizubuti, E.S.G.; Staver, C.P. Fusarium Wilt of Banana: Current Knowledge on Epidemiology and Research Needs Toward Sustainable Disease Management. Front. Plant Sci. 2018, 9, 1468. [Google Scholar] [CrossRef] [PubMed]
- Ploetz, R.C. Fusarium Wilt of Banana Is Caused by Several Pathogens Referred to as Fusarium oxysporum f. sp. cubense. Phytopathol. 2006, 96, 653–656. [Google Scholar] [CrossRef] [PubMed]
- Molina, A.; Fabregar, E.; Sinohin, V.G.; Herradura, L.; Fourie, G.; Viljoen, A. Confirmation of Tropical Race 4 of Fusarium oxysporum f. sp. cubense Infecting Cavendish Bananas in the Philippines. In Proceedings of the Centennial Meeting of the American Phytopathological Society, Minneapolis, MN, USA, 26–28 July 2008; Available online: https://cgspace.cgiar.org/handle/10568/674 (accessed on 2 August 2016).
- Buddenhagen, I. Understanding strain diversity in Fusarium oxysporum f. sp. cubense and history of introduction of “Tropical Race 4” to better manage banana production. Acta Hortic. 2009, 828, 193–204. [Google Scholar] [CrossRef]
- Ordoñez, N.; García-Bastidas, F.; Laghari, H.B.; Akkary, M.Y.; Harfouche, E.N.; Al Awar, B.N.; Kema, G.H.J. First Report of Fusarium oxysporum f. sp. cubense Tropical Race 4 Causing Panama Disease in Cavendish Bananas in Pakistan and Lebanon. Plant Dis. 2016, 100, 209. [Google Scholar] [CrossRef]
- Özarslandan, M.; Akgül, D.S. First Report of Fusarium oxysporum f. sp. cubense Race 4 Causing Fusarium Wilt Disease of Banana in Turkey. Plant Dis. 2020, 104, 974. [Google Scholar] [CrossRef]
- Thangavelu, R.; Mostert, D.; Gopi, M.; Devi, P.G.; Padmanaban, B.; Molina, A.B.; Viljoen, A. First Detection of Fusarium oxysporum f. sp. cubense Tropical Race 4 (TR4) on Cavendish Banana in India. Eur. J. Plant Pathol. 2019, 154, 777–786. [Google Scholar] [CrossRef]
- Maryani, N.; Lombard, L.; Poerba, Y.S.; Subandiyah, S.; Crous, P.W.; Kema, G.H.J. Phylogeny and genetic diversity of the banana Fusarium wilt pathogen Fusarium oxysporum f. sp. cubense in the Indonesian centre of origin. Stud. Myco. 2019, 92, 155–194. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, W.T.; Henderson, J.; Pattemore, J.A.; O’Dwyer, C.; Perry, S.; Beasley, D.R.; Tan, Y.P.; Smyth, A.L.; Goosem, C.H.; Thomson, K.M.; et al. Detection of Fusarium oxysporum f. Sp. cubense Tropical Race 4 Strain in Northern Queensland. Australas. Plant Dis. Notes 2016, 11, 33. [Google Scholar] [CrossRef]
- García-Bastidas, F.A.; Quintero-Vargas, J.C.; Ayala-Vasquez, M.; Schermer, T.; Seidl, M.F.; Santos-Paiva, M.; Noguera, A.M.; Aguilera-Galvez, C.; Wittenberg, A.; Hofstede, R.; et al. First Report of Fusarium Wilt Tropical Race 4 in Cavendish Bananas Caused by Fusarium odoratissimum in Colombia. Plant Dis. 2020, 104, 994. [Google Scholar] [CrossRef]
- ICA. Instituto Colombiano Agropecuario. Available online: https://www.ica.gov.co/noticias/ica-amplia-y-refuerza-las-medidas-que-ya-venia-im. (accessed on 29 August 2019).
- Acuña, R.; Rouard, M.; Leiva, A.M.; Marques, C.; Olortegui, A.; Ureta, C.; Cabrera-Pintado, R.M.; Rojas, J.C.; López, D.; Cenci, A.; et al. First report of Fusarium oxysporum f. sp. cubense Tropical Race 4, causing Fusarium wilt in Cavendish bananas in Peru. Plant Dis. 2021, 106, 2268. [Google Scholar]
- Martínez, G.; Olivares, B.O.; Rey, J.C.; Rojas, J.; Cardenas, J.; Muentes, C.; Dawson, C. The Advance of Fusarium Wilt Tropical Race 4 in Musaceae of Latin America and the Caribbean: Current Situation. Pathogens 2023, 12, 277. [Google Scholar] [CrossRef] [PubMed]
- Takken, F.; Rep, M. The Arms Race between Tomato and Fusarium Oxysporum. Mol. Plant Pathol. 2010, 11, 309–314. [Google Scholar] [CrossRef]
- Meldrum, R.A.; Fraser-Smith, S.; Tran-Nguyen, L.T.T.; Daly, A.M.; Aitken, E.A.B. Presence of putative pathogenicity genes in isolates of Fusarium oxysporum f. sp. cubense from Australia. Australas. Plant Pathol. 2012, 41, 551–557. [Google Scholar] [CrossRef]
- Fraser-Smith, S.; Czislowski, E.; Meldrum, R.A.; Zander, M.; O’Neill, W.; Balali, G.R.; Aitken, E.A.B. Sequence Variation in the Putative Effector GeneSIX 8 Facilitates Molecular Differentiation ofF Usarium Oxysporum f. sp. cubense. Plant Pathol. 2014, 63, 1044–1052. [Google Scholar] [CrossRef]
- Czislowski, E.; Fraser-Smith, S.; Zander, M.; O’Neill, W.T.; Meldrum, R.A.; Tran-Nguyen, L.T.T.; Batley, J.; Aitken, E.A.B. Investigation of the Diversity of Effector Genes in the Banana Pathogen, Fusarium oxysporum f. sp. cubense, Reveals Evidence of Horizontal Gene Transfer. Mol. Plant Pathol. 2018, 19, 1155–1171. [Google Scholar] [CrossRef]
- Carvalhais, L.C.; Henderson, J.; Rincon-Florez, V.A.; O’Dwyer, C.; Czislowski, E.; Aitken, E.A.B.; Drenth, A. Molecular Diagnostics of Banana Fusarium Wilt Targeting Secreted-in-Xylem Genes. Front. Plant Sci. 2019, 10, 547. [Google Scholar] [CrossRef] [PubMed]
- Costa, S.N.; Bragança, C.A.D.; Ribeiro, L.R.; Amorim, E.P.; Oliveira, S.A.S.; Dita, M.A.; Laranjeira, F.F.; Haddad, F. Genetic Structure of Fusarium oxysporum f. sp. cubense in Different Regions from Brazil. Plant Pathol. 2015, 64, 137–146. [Google Scholar] [CrossRef]
- Batista, I.C.; Heck, D.W.; Santos, A.; Alves, G.; Ferro, C.G.; Dita, M.; Mizubuti, E.S. The Brazilian population of Fusarium oxysporum f. sp. cubense is not structured by VCG or by geographic origin. bioRxiv 2022, 112, 2416–2425. [Google Scholar]
- Ribeiro, L.R.; Oliveira, S.A.S.D.; Amorim, E.P.; Serejo, J.A.S.; Haddad, F. Sources of resistance to Fusarium oxysporum f. sp. cubense in banana germplasm. Rev. Bras. Frutic. 2018, 40, 1–8. [Google Scholar]
- Rebouças, T.A.; Haddad, F.; Ferreira, C.F.; De Oliveira, S.A.S.; Da Silva Ledo, C.A.; Amorim, E.P. Identification of Banana Genotypes Resistant to Fusarium Wilt Race 1 under Field and Greenhouse Conditions. Sci. Hortic. 2018, 239, 308–313. [Google Scholar] [CrossRef]
- Rocha, A.D.J.; Ferreira, M.D.S.; Rocha, L.D.S.; Oliveira, S.A.; Amorim, E.P.; Mizubuti, E.S.; Haddad, F. Interaction between Fusarium oxysporum f. sp. cubense and Radopholus similis can lead to changes in the resistance of banana cultivars to Fusarium wilt. Eur. J. Plant Pathol. 2020, 158, 403–417. [Google Scholar]
- Dita, M.A.; Garming, H.; Van den Bergh, I.; Staver, C.; Lescot, T. Banana in Latin America and the Caribbean: Current state, challenges and perspectives. Acta Hortic. 2011, 986, 365–380. [Google Scholar] [CrossRef]
- Dita, M.A.; Pérez Vicente, L.; Martínez, E. Inoculation of Fusarium oxysporum f. sp. cubense causal agent of fusarium wilt in banana. In Technical Manual: Prevention and Diagnostic of Fusarium Wilt of Banana Caused by Fusarium Oxysporum f. sp. cubense Tropical Race 4 (TR4); Pérez Vicente, L., Dita, M.A., de la Parte, E.M., Eds.; Food and Agriculture Organization of the United Nations: Rome, Italy, 2014; pp. 55–58. [Google Scholar]
- McKinney, H.H. Influence of Soil Temperature and Moisture on Infection of Wheat Seedlings by Helminthosporium sativum. J. Agric. Res. 1923, 26, 195. [Google Scholar]
- Phillips, J.M.; Hayman, D.S. Improved Procedures for Clearing Roots and Staining Parasitic and Vesicular-Arbuscular Mycorrhizal Fungi for Rapid Assessment of Infection. Trans. Br. Mycol. Soc. 1970, 55, 158-IN18. [Google Scholar] [CrossRef]
- Brundrett, M.; Bougher, N.; Dell, B.; Grove, T.; Malajczuk, N. (Eds.) Working with Mycorrhizas in Forestry and Agriculture; ACIAR Monograph 32: Canberra, Australia, 2006; p. 374. [Google Scholar]
- Doyle, J.J.; Doyle, J.L. Isolation of plant DNA from fresh tissue. Focus 1990, 12, 13–15. [Google Scholar]
- Dita, M.A.; Waalwijk, C.; Buddenhagen, I.W.; Souza, J.T.; Kema, G.H.J. A molecular diagnostic for tropical race 4 of the banana Fusarium wilt pathogen. Plant. Pathol. 2010, 59, 348–357. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Chang, J.-Y.; Liu, E.-T.; Chao, C.-P.; Huang, J.-W.; Chang, P.-F.L. Development of a Molecular Marker for Specific Detection of Fusarium Oxysporum f. Sp. Cubense Race 4. Eur. J. Plant Pathol. 2009, 123, 353–365. [Google Scholar] [CrossRef]
- O’Donnell, K.; Kistler, H.C.; Cigelnik, E.; Ploetz, R.C. Multiple Evolutionary Origins of the Fungus Causing Panama Disease of Banana: Concordant Evidence from Nuclear and Mitochondrial Gene Genealogies. Proc. Natl. Acad. Sci. USA 1998, 95, 2044–2049. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; The R Foundation for Statistical Computing: Vienna, Austria, 2020; Volume 1. [Google Scholar]
- Vale, F.X.R.D.; Parlevliet, J.E.; Zambolim, L. Concepts in plant disease resistance. Fitopatol. Bras. 2001, 26, 577–589. [Google Scholar] [CrossRef]
- Ma, L.-J.; Van Der Does, H.C.; Borkovich, K.A.; Coleman, J.J.; Daboussi, M.-J.; Di Pietro, A.; Dufresne, M.; Freitag, M.; Grabherr, M.; Henrissat, B.; et al. Comparative genomics reveals mobile pathogenicity chromosomes in Fusarium. Nature 2010, 464, 367–373. [Google Scholar] [CrossRef]
- Rocha, A.D.J.; Soares, J.M.D.S.; Nascimento, F.D.S.; Rocha, A.D.S.; Amorim, V.B.O.D.; Ramos, A.P.D.S.; Ferreira, C.F.; Haddad, F.; Amorim, E.P. Molecular, Histological and Histochemical Responses of Banana Cultivars Challenged with Fusarium Oxysporum f. Sp. Cubense with Different Levels of Virulence. Plants 2022, 11, 2339. [Google Scholar] [CrossRef] [PubMed]
- Fourie, G.; Steenkamp, E.T.; Ploetz, R.C.; Gordon, T.R.; Viljoen, A. Current Status of the Taxonomic Position of Fusarium Oxysporum Formae Specialis Cubense within the Fusarium Oxysporum Complex. Infect. Genet. Evol. 2011, 11, 533–542. [Google Scholar] [CrossRef]
- Czislowski, E.; Fraser-Smith, S.; Zander, M.; Aitken, E.A.B. Identifying Pathogenicity Genes in Fusarium oxysporum f. Sp. cubense. Acta Hortic. 2016, 1114, 101–106. [Google Scholar] [CrossRef]
- Maldonado, B.L.D.; Villarruel, O.J.L.; Calderón, O.M.A.; Sánchez, E.A.C. Secreted in Xylem (Six) genes in Fusarium oxysporum f. sp. cubense and their potential acquisition by horizontal transfer. Adv. Biotech. Micro. 2018, 10, 555779. [Google Scholar]
- Widinugraheni, S.; Niño-Sánchez, J.; Van Der Does, H.C.; Van Dam, P.; García-Bastidas, F.A.; Subandiyah, S.; Meijer, H.J.G.; Kistler, H.C.; Kema, G.H.J.; Rep, M. A SIX1 Homolog in Fusarium Oxysporum f.Sp. Cubense Tropical Race 4 Contributes to Virulence towards Cavendish Banana. PLoS ONE 2018, 13, e0205896. [Google Scholar] [CrossRef]
- An, B.; Hou, X.; Guo, Y.; Zhao, S.; Luo, H.; He, C.; Wang, Q. The effector SIX8 is required for virulence of Fusarium oxysporum f. sp. cubense tropical race 4 to Cavendish banana. Fungal. Biol. 2019, 123, 423–430. [Google Scholar]
- Stover, R.H. Fusarial Wilt (Panama Disease) of Bananas and Other Musa Species; Commonwealth Mycological Institute: Kew, UK, 1962; p. 117. [Google Scholar]
- Hall, C.; Heath, R.; Guest, D. The Infection Process of Fusarium Oxysporum f.Sp. Vasinfectum in Australian Cotton. Australas Plant Pathol. 2013, 42, 1–8. [Google Scholar] [CrossRef]
- Li, C.; Chen, S.; Zuo, C.; Sun, Q.; Ye, Q.; Yi, G.; Huang, B. The Use of GFP-Transformed Isolates to Study Infection of Banana with Fusarium oxysporum f. sp. cubense Race 4. Eur. J. Plant Pathol. 2011, 131, 327–340. [Google Scholar] [CrossRef]
- Li, C.; Yang, J.; Li, W.; Sun, J.; Peng, M. Direct Root Penetration and Rhizome Vascular Colonization by Fusarium oxysporum f. Sp. cubense Are the Key Steps in the Successful Infection of Brazil Cavendish. Plant Dis. 2017, 101, 2073–2078. [Google Scholar] [CrossRef]
- Carlier, J.; Hayden, H.; Rivas, G.; Zapater, M.F. Genetic differentiation in Mycosphaerella leaf spot pathogens. In Mycosphaerella Leaf Spot Diseases of Bananas: Present Status and Outlook. Proceedings of the Workshop on Mycosphaerella Leaf Spot Diseases; José, S., Rica, C., Jacome, L., Lepoivre, P., Marin, D., Ortiz, R., Eds.; The International Network for the Improvement of Banana and Plantain: Montpellier, France, 2002; pp. 123–129. [Google Scholar]
- Groenewald, S.; Van Den Berg, N.; Marasas, W.F.O.; Viljoen, A. The application of high-throughput AFLP’s in assessing genetic diversity of Fusarium oxysporum f. sp. cubense. Mycol. Res. 2006, 110, 297–305. [Google Scholar] [CrossRef]
- Truman, W.; Bennett, M.H.; Kubigsteltig, I.; Turnbull, C.; Grant, M. Arabidopsis Systemic Immunity Uses Conserved Defense Signaling Pathways and Is Mediated by Jasmonates. Proc. Natl. Acad. Sci. USA 2007, 104, 1075–1080. [Google Scholar] [CrossRef]
- Karangwa, P.; Mostert, D.; Ndayihanzamaso, P.; Dubois, T.; Niere, B.; Zum Felde, A.; Schouten, A.; Blomme, G.; Beed, F.; Viljoen, A. Genetic diversity of Fusarium oxysporum f. sp. cubense in east and central Africa. Plant Dis. 2018, 102, 552–560. [Google Scholar] [CrossRef]
- Cunha, C.M.S.; Hinz, R.H.; Pereira, A.; Tcacenco, F.A.; Stadnik, M.J. Aggressiveness and genetic diversity of Fusarium oxysporum f. sp. cubense from Santa Catarina, southern Brazil. Trop. Plant Pathol. 2015, 40, 326–334. [Google Scholar]
- Olivares, B.O.; Vega, A.; Calderón, M.A.R.; Rey, J.C.; Lobo, D.; Gómez, J.A.; Landa, B.B. Identification of Soil Properties Associated with the Incidence of Banana Wilt Using Supervised Methods. Plants 2022, 11, 2070. [Google Scholar] [CrossRef]
- Olivares Campos, B.O. Evaluation of the Incidence of Banana Wilt and Its Relationship with Soil Properties. In Banana Production in Venezuela; The Latin American Studies Book Series; Springer Nature: Cham, Switzerland, 2023; pp. 95–117. [Google Scholar] [CrossRef]
- Olivares, B.O.; Rey, J.C.; Perichi, G.; Lobo, D. Relationship of Microbial Activity with Soil Properties in Banana Plantations in Venezuela. Sustainability 2022, 14, 13531. [Google Scholar] [CrossRef]
- Olivares, B.O.; Rey, J.C.; Lobo, D.; Navas-Cortés, J.A.; Gómez, J.A.; Landa, B.B. Fusarium Wilt of Bananas: A Review of Agro-Environmental Factors in the Venezuelan Production System Affecting Its Development. Agronomy 2021, 11, 986. [Google Scholar] [CrossRef]
- Olivares, B.O.; Araya-Alman, M.; Acevedo-Opazo, C.; Rey, J.C.; Cañete-Salinas, P.; Kurina, F.G.; Balzarini, M.; Lobo, D.; Navas-Cortés, J.A.; Landa, B.B. Relationship Between Soil Properties and Banana Productivity in the Two Main Cultivation Areas in Venezuela. J. Soil. Sci. Plant Nutr. 2020, 20, 2512–2524. [Google Scholar] [CrossRef]
- Olivares, B.O.; Calero, J.; Rey, J.C.; Lobo, D.; Landa, B.B.; Gómez, J.A. Correlation of banana productivity levels and soil morphological properties using regularized optimal scaling regression. Catena 2022, 208, 105718. [Google Scholar] [CrossRef]
- Rodríguez-Yzquierdo, G.; Olivares, B.O.; Silva-Escobar, O.; González-Ulloa, A.; Soto-Suarez, M.; Betancourt-Vásquez, M. Mapping of the Susceptibility of Colombian Musaceae Lands to a Deadly Disease: Fusarium oxysporum f. sp. cubense Tropical Race 4. Horticulturae 2023, 9, 757. [Google Scholar] [CrossRef]
- Rodríguez-Yzquierdo, G.; Olivares, B.O.; González-Ulloa, A.; León-Pacheco, R.; Gómez-Correa, J.C.; Yacomelo-Hernández, M.; Carrascal-Pérez, F.; Florez-Cordero, E.; Soto-Suárez, M.; Dita, M.; et al. Soil Predisposing Factors to Fusarium oxysporum f. sp. cubense Tropical Race 4 on Banana Crops of La Guajira, Colombia. Agronomy 2023, 13, 2588. [Google Scholar] [CrossRef]
- Olivares, B. Machine learning and the new sustainable agriculture: Applications in banana production systems of Venezuela. Agric. Res. Updates 2022, 42, 133–157. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).