Abstract
Dendrobium brymerianum Rchb. f. is a species of orchid with pharmacological interest for its potential to inhibit the growth of human lung cancer cells. The identification of the Dendrobium species is a notable problem due to morphological similarities and the limitations of universal DNA barcodes. To overcome these difficulties, this study employed complete chloroplast (cp) genome sequences as useful resources for the identification of D. brymerianum. Based on Illumina sequencing, the complete cp genomes of five D. brymerianum individuals were assembled. These genomes were in the quadripartite structure, diverse in length between 151,832 and 152,189 bp, and comprised 126 genes. Moreover, significant differences were found in the Small Single-Copy (SSC) and Large Single-Copy (LSC) regions in comparison to the Inverted Repeat (IR) regions. This study recognized hotspot regions and simple sequence repeat (SSR) loci, providing valuable insights into genetic markers. The phylogenetic relationship of Dendrobium species was discovered, highlighting the need for more precise differentiation practices. To address this, ARMS-specific primers, mainly AAob1/AAob2, confirmed strong specificity, permitting the accurate identification of D. brymerianum from other species through ARMS-qPCR. Overall, this study of D. brymerianum chloroplast genomes has generated valuable data about sequence variations, phylogenetics, and mutation dynamics. These perceptions will be valuable in future research on population genetics, taxonomy, and species identification within the Dendrobium genus.
1. Introduction
Orchidaceae, which is the second-largest family of flowering plants after Asteraceae, consists of about 30,000 identified species [1]. This family is important for biodiversity, conservation efforts, and its role in producing a variety of medicinal composites, nourishing foods, and decorative plants [2,3] The Dendrobium genus of orchids is mainly important as one of the largest genera of flowering plants, with a notable count of over 1450 species, mostly found in Asia and Oceania [4,5]. In China, about 90 Dendrobium species have been identified [6]. Besides attractiveness [7], Dendrobium species also have therapeutic and pharmaceutical importance [8]. These species have been employed in traditional Chinese medicine for centuries due to their healthcare benefits [9]. Xu [10] discussed the ancient use of several Dendrobium species, belonging to the Orchidaceae family, as tonics in traditional Chinese medicine. Among these species, Dendrobium brymerianum Rchb. f. is known to be dispersed in Thailand, Burma, Laos, and China [11,12]. It is worth noting that a methanol extract from the D. brymerianum plant exhibited substantial cytotoxic effects on human lung cancer H460 cells [13]. The commercial and ornamental significance of Dendrobium orchids has driven wide research, leading to abundant taxonomic studies, mainly in species identification [14,15,16]. The process of detecting Dendrobium species is particularly problematic because traditional methods rely on morphological features, which can be influenced by ecological aspects and pollinator selection pressures [17,18,19]. Furthermore, the process of intensive processing can make it even more challenging to differentiate between Dendrobium species [20]. Therefore, there is a persistent need to develop a simple and precise method for identifying D. brymerianum.
Chloroplasts are organelles that contain genetic material and are found in terrestrial plants, algae, and a few protozoa. Within a cell, chloroplast genomes can take on numerous forms, with the most common being a double-stranded circular structure that contains a Small Single-Copy region (SSC) and a Large Single-Copy region (LSC). The chloroplast genome in plants is composed of two Inverted Repeat regions, IRa and IRb, which together form a distinct four-part structure. The size of the chloroplast genome differs between 120 and 160 kb [21]. Unlike the mitochondrial or nuclear genomes, the chloroplast genome in plants shows a high degree of conservation in its structure, gene count, and gene composition. Its evolutionary rate falls within a moderate range, lying between the rates observed in the nuclear and mitochondrial genomes [22]. The lack of recombination, combined with the compact size of the genome and its high number of copies per cell [23,24], has resulted in the extensive application of complete cp genome sequences in phylogenetic analysis and species identification [25,26]. Studies have shown that the complete cp genome provides further information that increases the accuracy of phylogenetic analysis [27,28,29].
The study of chloroplast (cp) genome sequences through comparative analysis presents a valuable opportunity to expose sequence variations, identify mutation hotspots, and detect events of gene loss and duplication. Moreover, the mutation hotspot regions and single sequence repeats (SSRs) extracted from these chloroplast genome sequences are influential molecular markers for species identification and population genetics, as confirmed by studies on orchids [23,30,31,32]. For instance, the psbA-trnH and trnF-ndhJ regions are mainly useful for phylogenetic analysis within the Oncidium genus of orchids [33]. Correspondingly, non-coding loci such as rpl32-trnL, trnE-trnT, trnH-psbA, trnK-rps16, and trnT-trnL are effective for the identification of Cymbidium species [34]. Furthermore, DNA barcoding has been applied to detect Dendrobium species by employing various genetic loci or combinations, such as ITS [35], ITS + matK [16], and rbcL + matK [36]. However, many of these studies have produced unreliable results due to their requirement of a limited number of DNA sequences. Numerous genetic hotspots, such as rbcL, matK, and psbA-trnH, have shown potential in plant species identification and phylogenetic research [37]. Despite the consequences of complete cp genomes as highly stable genetic markers for low-level taxonomic studies of Dendrobium species, there have been limited complete investigations into these genomes. In a previous study, a molecular phylogenetic analysis that used a combination of partial chloroplast markers and ITS-rDNA sequences did not constantly align with the morphological sections, as observed in Xiang’s research [5]. As a result, complete cp genome sequences have become extensively used in plant identification and phylogenetic studies to overcome this limitation.
The need for a clear and accurate method for identifying D. brymerianum is demanding. Modern techniques for examining physical and chemical properties involve innovative analytical methods such as high-performance liquid chromatography, infrared spectroscopy, and gas chromatography [38]. The importance of these methods in identifying Dendrobium species cannot be overstated. However, their complexity and laborious nature encouraged us to explore ARMS-qPCR as a more effective method for identifying D. brymerianum. A single-nucleotide polymorphism (SNP) is a kind of genetic marker that is present across all chromosomes of numerous species. It denotes a single-base difference, including conversions, transversions, insertions, and deletions. One of the key features of SNPs is that they remain persistent and unaffected by deviations in the sample or the developing stage. Specific chloroplast SNPs might be useful to create primers for ARMS-qPCR, to reveal the genetic diversity of these hotspots. These markers have progressed rapidly and are presently being used in several fields such as ecology, agronomy, drug and medicines, and biological evolution [39]. The Amplification Refractory Mutation System (ARMS) comprises incorporating one or more mismatched nucleotides at the 3′ end of specific primers. Additionally, it can join together several SNP sequences to determine the genotype. Previous studies have shown the usefulness of ARMS technology in identifying single Dendrobium through PCR and electrophoresis [40]. Dong and Niu both used diverse methods to confirm Dendrobium plants. Dong used high-resolution melting curve technology to target the trn-L region, while Niu used SNP sites to distinguish D. huoshanense from other Dendrobium species, but both studies emphasized the efficiency of real-time PCR combined with the ARMS method for rapidly and accurately identifying D. huoshanense and its medicinal slices [41,42].
As a singular genome, the cp genome has become a widely accepted medium for providing visions into the evolution of plant species. We designed a research method to sequence, assemble, and annotate the complete chloroplast genome of D. brymerianum. Our main objectives are centered around four main goals: (1) comparing the complete cp genome of Dendrobium to expose the evolution of its genome structure, (2) detecting highly variable regions that can be used for species identification, (3) explaining the evolutionary relationships among different Dendrobium species using complete cp genome data, and (4) screening specific nucleotide sites that are unique to D. brymerianum and developing a precise technique for distinguishing it from other Dendrobium species using ARMS-qPCR technology.
2. Materials and Methods
2.1. Plant DNA Extraction
The DNA of D. brymerianum was extracted from freshly collected plant samples (Table S1). These plants were carefully cultured and preserved within the greenhouse facilities at Nanjing Normal University. Plants were maintained in the greenhouse at 22–24 °C, 70–80% relative humidity (RH), and with a 12 h photo-period for three months. The procedure of extracting genomic DNA used around 0.2 g of small leaf flakes per sample, by employing the CTAB method [43], with a few minor changes. This DNA, which was derived from fresh plant material, served as the template for conventional PCR and agarose gel electrophoresis and was used to assess the efficiency of the designed specific primers in amplifying the diagnostic fragment. The Invitrogen Qubit Fluorometer and the Thermo Scientific NanoDrop Spectrophotometer were used to measure the quantity and purity of DNA. Five high-quality and abundant DNA samples from individual D. brymerianum were chosen for library construction. To conduct ARMS-qPCR, high-quality D. brymerianum DNA samples were used with other Dendrobium species’ DNA samples.
2.2. Illumina Sequencing, Assembly, and Annotation
This study employed second-generation sequencing methods to sequence five individual D. brymerianum cp genomes on the Illumina HiSeq platform, resulting in full-length sequences. The TruSeq TM Nano DNA Library Prep Kit from Illumina (San Diego, CA, USA) was used to produce a DNA-seq library using 1 μg of DNA, beginning with DNA fragmentation using a fragmentation buffer. After that, following Illumina’s library construction protocol, end repair, phosphorylation, and the addition of ‘A’ bases were carried out on the fragmented DNA. The resulting paired-end DNA-sequencing library was assessed, and sequencing data were obtained using the Illumina Novaseq 6000 platform with a read length of 2 × 150 bp. Using the Illumina HiSeq 4000 platform, we obtained 150 base pair (bp) paired-end sequences on five samples, yielding 50 Gb pairs of reads for each sample after sequencing. Afterward, the raw reads were subjected to trimming to remove those with an error probability of <0.05. The Geneious Prime software 8.1 [44] (https://www.geneious.com/) accessed on 5 May 2023, was used to assemble the paired-end reads by using a combination of de novo assembly and reference-guided assembly approaches. To improve this method, the chloroplast genome of D. brymerianum, with accession number NC_035323, was utilized as a reference. By referencing the sequence, we compared the fragments obtained with the chloroplast genome sequence of D. brymerianum. Subsequently, the complete cp genomes were obtained for five individuals of D. brymerianum through assembly and gap filling.
The complete cp genome sequences were examined and annotated using Vector NTI software version 13.0 [45]. To confirm accuracy, the boundaries of each gene were manually verified by comparing them to similar genes in the reference genome of D. brymerianum (NC_035323). For genes with low similarity, the initiator (ATG, ATC, ATT), terminator (TAA, TAG, TGA), intron (primarily GT-AG, AT-AC at both ends), and exon information were recognized manually. The annotated genes were further refined through BLAST and multiple sequence alignment. Finally, a complete circular map of the chloroplast genome was created using the web-based tool GenomeVx [46].
2.3. Comparative Genomic Analysis
The mVISTA online software (http://genome.lbl.gov/vista/index.shtml) accessed on 5 May 2023 [47] was used to visually compare and analyze the complete cp genomes of 12 Dendrobium species including D. acinaciforme, D. brymerianum, D. chrysanthum, D. crepidatum, D. devonianum, D. exile, D. falconeri, D. fimbriatum, D. henryi, D. lohohense, D. salaccense and D. wilsonii (Figure S1). Among them, D. acinaciforme was chosen as the reference genome (NC_071778.1). Our study focused on gene location data and IR/SC junction evidence across the Dendrobium species, highlighting occasions of IR expansions and contractions.
2.4. Identification of Mutational Hotspots in Dendrobium Brymerianum Chloroplast Genomes
With the 11 previously sequenced Dendrobium cp genomes, we extracted sequences from non-coding regions, such as intergenic and intronic regions, from 5 newly sequenced individuals of D. brymerianum. We identified syntenic loci by comparing those flanked by the same genes/exons and removed those smaller than 100 bp. After gathering 89 non-coding syntenic loci, we achieved sequence alignments using the MAFFT v7.221 software [48]. The gaps were removed excluding gaps located at the 5′ and 3′ ends of alignments. The number of SNPs (single-nucleotide polymorphisms), indels (insertions/deletions), variable mutation sites, parsimony information sites, and total conserved DNA sequences was determined by using DnaSP v5 [49]. The Zhitao method was applied to calculate Sequence Variability [50]. The SV was calculated as (the number of nucleotide mutations + the number of InDel events)/(the number of conserved sites + the number of nucleotide mutations + the number of InDel events) × 100%. Lastly, we calculated the average SV and nucleotide diversity (pi) for each syntenic locus. Moreover, the DnaSP v5.10 software was used to compute the nucleotide diversity within the chloroplast genome by executing a sliding window analysis with a predefined length of 600 bp and a step size of 100 bp. The MISA tool, available at https://webblast.ipk-gatersleben.de/misa/, accessed on 5 May 2023, was used to find simple sequence repeats (SSRs) in the selected syntenic loci and the sixteen complete cp genomes. We set the detection standards as follows: ten repeat units for mononucleotide SSRs, five repeat units for dinucleotide SSRs, four repeat units for trinucleotide SSRs, and three repeat units for tetra-, penta-, and hexanucleotide SSRs.
2.5. Phylogenetic Analysis
Fifty-nine cp genome sequences were obtained from the GenBank database at NCBI for phylogenetic analysis. We used Bulbophyllum reptans and Pholidota imbricata as outgroups. The list of species, along with their corresponding complete cp genome and accession numbers, can be found in Table S2. The alignment of the complete cp genome sequences for these species was performed using MAFFT v7.221 [48]. The Python program version 3.11.4 was used to eliminate gaps, and the resulting data were used in the construction of maximum likelihood (ML) and Bayesian inference (BI) trees using RAxML 8.0.2 [51] and MrBayes 3.2 [52], respectively. To confirm the accuracy of the ML tree, 1000 bootstrap replicates were used to estimate robustness. In our Bayesian inference analysis, we utilized four Markov Chain Monte Carlo (MCMC) chains, with a sampling rate of one tree every 50,000 generations, resulting in a total of 5,000,000 generations. We wasted the first 25% of the sampled trees as burn-in, using the remaining trees to calculate the posterior probability.
2.6. ARMS-qPCR Analysis
Specific nucleotide sites related to D. brymerianum were identified by collecting the complete cp genome sequences of 40 Dendrobium species (Table S3). This study involved sequencing of the complete cp genome of D. brymerianum, while the remaining 39 sequences were obtained from prior publications by this research group and the GenBank public database. The alignment of the 40 complete cp genomes was made possible by using MAFFT v7 software [48], and MEGA 7 software [53] was employed to identify specific nucleotide sites in D. brymerianum’s complete cp genome sequence. Primers were designed using MEGA 7 and Vector NTI 10 software, based on specific nucleotide sites identified in D. brymerianum. To increase the specificity of the primers, one or two mismatched bases were added near the 3′ end of the primers. The optimized primers were then confirmed by conducting ordinary PCR reactions on DNA samples from D. brymerianum and other Dendrobium species. After PCR, the resulting products were subjected to electrophoresis by using 1.5% agarose gel electrophoresis and EB staining, then an ARMS-specific primer that can effectively distinguish the D. brymerianum among Dendrobium species was chosen based on the results of the PCR amplification. Additionally, the optimal annealing temperature for each primer pair was determined to ensure the best performance.
DNA samples from D. brymerianum and other Dendrobium samples (Table S1) were subjected to qPCR amplification using ARMS-specific primers that were identified through ordinary PCR and gel electrophoresis. The cycle threshold (Cq) values for each species were determined, which represent the number of cycles required to reach the fluorescence threshold [Cq = f (log10 initial concentration of the specific target)] [54]. The Cq values and their standard deviations (Cq ± SD) were calculated for each amplification result.
3. Results
3.1. Comparative Analysis of the Complete Chloroplast Genome
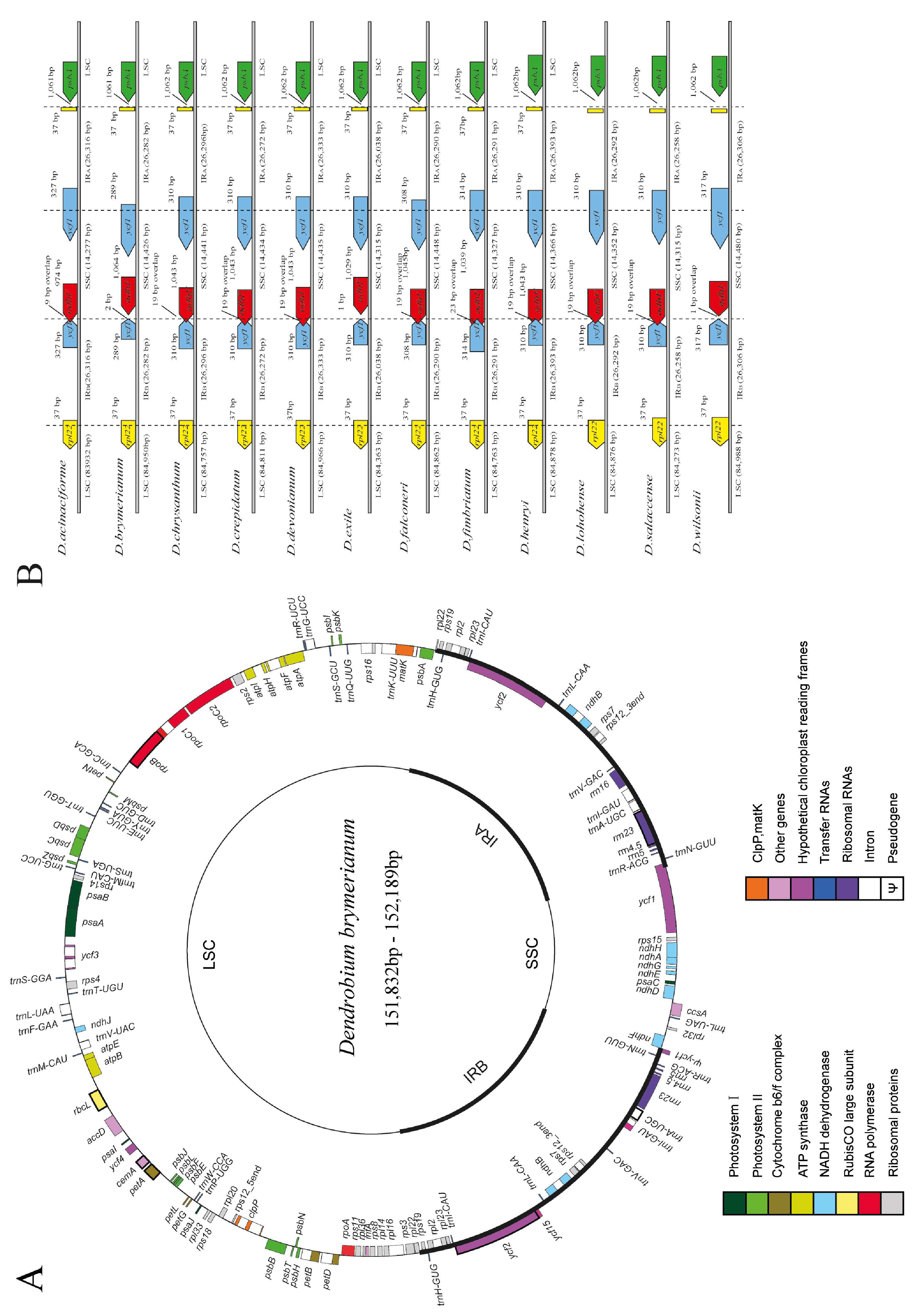
In this study, we sequenced the complete cp genomes of five individuals (LC794130–LC794134) of D. brymerianum. The newly sequenced genomes were compared to the cp genome of the reference cp genome to authenticate the accuracy of the complete cp genome sequences. Pairwise alignments exposed that the complete cp genomes were similar to D. brymerianum, as every region of the assembled genome confirmed 100% resemblance to the previously sequenced genome of the same species. The length of the complete cp genomes of five D. brymerianum individuals varied, ranging from 151,832 to 152,189 bp (Figure 1A). Our results showed that the complete cp genome of D. brymerianum has a circular, double-stranded, quadripartite structure, which comprises two Inverted Repeats (IRs), divided by the Large Single-Copy region (LSC) and the Small Single-Copy region (SSC). The detailed features of five individuals of D. brymerianum and other Dendrobium species can be observed in Table 1. The cp genomes of the five D. brymerianum individuals showed a substantial level of conservation concerning their gene composition and arrangement. The five genomes contain 126 unique genes, which comprise 84 protein-coding genes, 38 tRNA genes, and 4 rRNA genes. Moreover, nine pseudogenes were recognized, along with partial repetitions of genes (rpl22 and ycf1) at IR/SC borders, and remnants of ndh genes (ndhA, B, D, E, F, G, H, and J). These genes are characterized into 18 functional groups. The IR regions comprise seven protein-coding and seven tRNA genes, while the LSC and SSC regions comprise sixty-six and four protein-coding genes, separately, with twenty and one tRNA genes each. The IR regions also comprise duplicates of seven protein-coding genes, eight tRNA genes, and all four rRNA genes (Figure 1A, Table S4). Among the 17 genes with introns, 6 tRNA and 11 protein-coding genes were found (Table S5). Among these, 15 genes have a single intron, while ycf3 and clpP possess two each. The trnK-UUU gene has the largest intron of about 2788 bp. Additionally, the rps12 gene exhibits trans-splicing, with its 5′ end in the LSC region and 3′ end in the IR region.
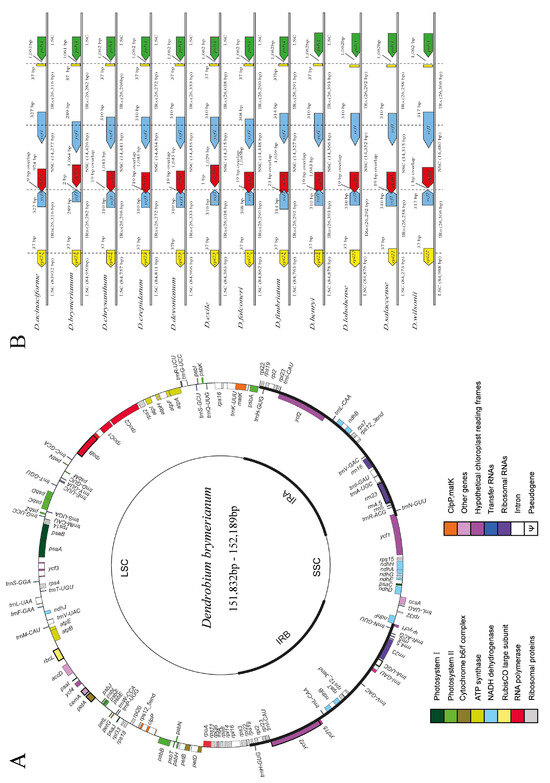
Figure 1.
(A) Visualization of D. brymerianum cp genome gene maps, with inner-circle genes transcribed clockwise and outer-circle genes transcribed counter-clockwise. Gene functions are indicated by color coding. (B) Comparative analysis of the boundaries between Large Single-Copy (LSC), Small Single-Copy (SSC), and Inverted Repeat (IR) regions across Dendrobium species.

Table 1.
The general feature of the complete cp genome of Dendrobium species.
The complete cp genomes of Dendrobium species (Table 1) were compared using mVista, with the cp genome of D. acinaciforme used as the reference. The coding regions are shown in blue and the non-coding regions are in red in the results (Figure S1). The results showed a considerable amount of resemblance in the sequencing of cp genomes, suggesting a strong conservation. In contrast to coding regions, the non-coding parts presented greater modifications. Moreover, the Large Single-Copy (LSC) and Small Single-Copy (SSC) regions were found to be more variable when compared to the Inverted Repeat (IR) region. The highest level of genetic divergence was detected in trnK-UUU, petN, trnL-UAA, trnT-UGU, ndhJ, psbT, rpI16, ndhF, ndhA, and ycf1.
Our study showed a comparison of the sequences flanking the Inverted Repeat/Single-Copy (IR/SC) junctions in D. brymerianum and other Dendrobium species (Figure 1B). While the boundaries of the Inverted Repeat/Large Single-Copy (IR/LSC) regions varied among these species, the degree of differences was not significant. Remarkably, the junctions of IRa/SSC were constantly detected at the 5′ end of ycf1, leading to the duplication of ycf1 in the IRb regions. The length of the ψycf1 gene remained constant at 310 base pairs in seven Dendrobium species: D. chrysanthum, D. crepidatum, D. devonianum, D. exile, D. henryi, D. lohohense, and D. salaccense. However, it exhibited slight variations in D. acinaciforme, D. brymerianum, D. falconeri, D. fimbriatum, and D. wilsonii, with lengths ranging from 289 to 327 base pairs. Moreover, the IRb/SSC junction in D. brymerianum was found to be located two base pairs upstream of the ψndhF gene, while in D. exile, it was located one base pair upstream. The overlapping region of ψycf1 and ψndhF is 19 base pairs long, and it occurs at the junctions of six Dendrobium species: D. chrysanthum, D. crepidatum, D. devonianum, D. falconeri, D. henryi, D. lohohense, and D. salaccense at the 3′ end of ψndhF. On the other hand, the junctions of D. acinaciforme, D. fimbriatum, and D. wilsonii all extended into the 3′ end of ψndhF, with an overlap of 9, 23, and 1 base pairs, respectively. This recommends that the extension/reduction of Inverted Repeats (IRs) is conserved among D. brymerianum and the other Dendrobium species.
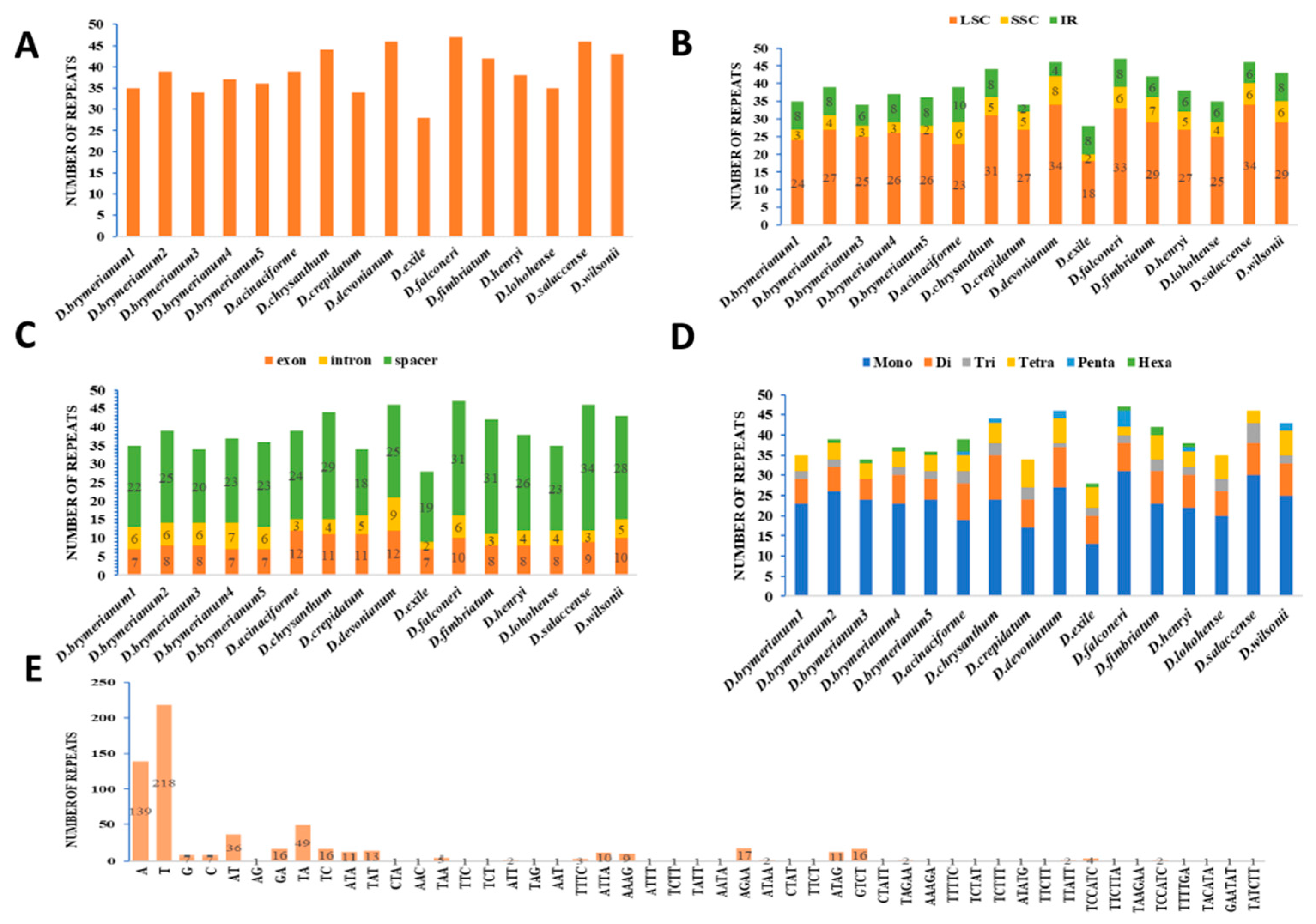
A total of 623 simple sequence repeat (SSR) (Table S6) loci were recognized in the 16 complete cp genomes of the Dendrobium species that were selected (Table 1, Figure 2). The number of SSRs varied from 28 in D. exile to 47 in D. falconeri (Figure 2A). These SSRs were dispersed randomly across the cp genome. Precisely, 438 SSRs were located in the Large Single-Copy (LSC) region, 75 in the Small Single-Copy (SSC) region, and 110 in the Inverted Repeat (IR) region (Figure 2B). At the same time, a total of 401 SSRs were identified within spacer regions, while 79 were found in introns and 143 in exons (Figure 2C). In terms of specific genomes, the majority of SSRs were identified in the Long Single-Copy (LSC) region, ranging from 18 in D. exile to 34 in D. devonianum (Figure 2B). Moreover, SSRs were abundant in spacer regions, with a range of 18 in D. crepidatum to 34 in D. salaccense (Figure 2C). Among 623 simple sequence repeats (SSRs), the different types of repeats were mononucleotide (371), dinucleotide (118), trinucleotide (37), tetranucleotide (74), pentanucleotide (11), and hexanucleotide (12) (Figure 2D,E).
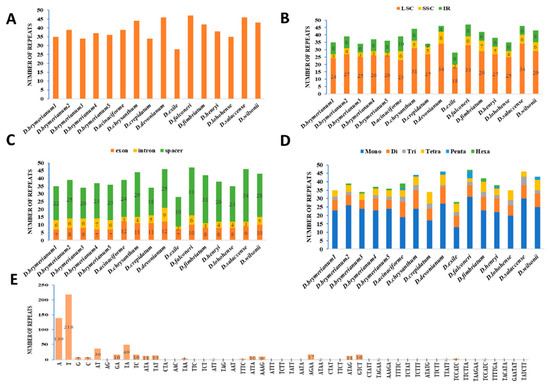
Figure 2.
Analysis of simple sequence repeats (SSRs) in sixteen Dendrobium chloroplast genomes: type and distribution. (A) Frequency of SSRs in Dendrobium species. (B) Frequency of SSRs in LSC, SSC, and IR regions. (C) Number of SSRs in exons, introns, and spacers. (D) Number of different SSR types. (E) Comparison of SSR motif frequencies across various repeat class types among 16 cp genomes.
3.2. Identification of Hotspots and Variability in Dendrobium Species
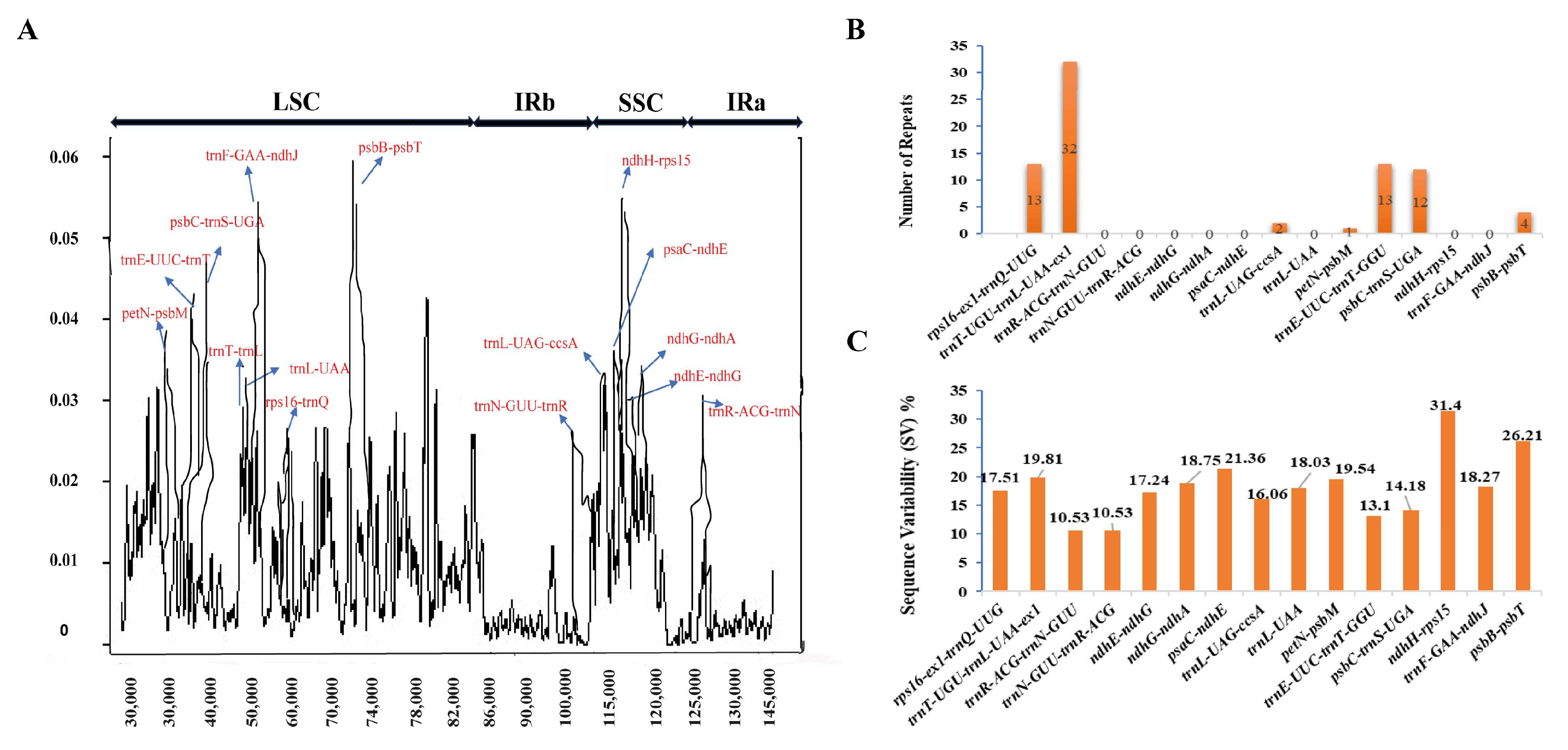
Eighty-nine syntenic non-coding regions were identified successfully that were consistent across all Dendrobium species (Table 1 and Table S7). To find regions of interest with higher variability, we compared the mean values of pairwise interspecific Sequence Variability (SV) and nucleotide diversity (Pi) of these regions. These 89 syntenic intergenic spacers and introns were then characterized into their particular regions based on their positions, including the LSC, SSC, and IR regions. The alignment of Dendrobium cp genomes formed a matrix with a total length of 146,540 bp, comprising 6650 variable sites (4.5%) and 1920 parsimony-informative sites (1.3%), and then calculated average nucleotide diversity, which was 0.00869. The values for detected SV and Pi ranged from 0.95% to 31.4% and from 0.00092 to 0.05911, respectively. Out of the 89 syntenic regions, 51 were located in the LSC, 9 in the SC, and 29 in the IR. Our in-depth analysis of the chosen cp genomes within the Dendrobium genus discovered that both the SSC and LSC regions showed significantly higher SV and Pi values related to the IR regions (Table S7). This result proposes that the IR regions are conserved. In particular, when comparing the regions evaluated, trnI-GAU in the IR region showed the lowest nucleotide diversity (Pi) value at 0.00092. On the other hand, the LSC region encompassing psbB-psbT showed the highest Pi value at 0.05911. Moreover, the IR region housing rps12 showed the lowest Sequence Variability (SV) value at 0.95%, while the SSC region comprising ndhH-rps15 showed the highest SV value at 31.4% (Table S7). To detect areas of significant sequence variation, nucleotide diversity (Pi) values were determined for a sliding window of 600 base pairs (Figure 3). The measured Pi values for the sliding windows ranged from 0 to 0.0052. Out of the 89 locations measured, only 15 were selected as hotspots, showing a nucleotide diversity of more than 0.026 (Table S8). Among them, eight markers were positioned in the LSC region, two were positioned in the IR region, and the remaining five were found in the SSC region (Figure 3A). The lengths of these 15 markers varied, with psbC-trnS-UGA being the shortest at 132 base pairs and rps16-ex1-trnQ-UUG being the longest at 1106 base pairs (Table S8). It is worth noting that rps16-ex1-trnQ-UUG had the highest number of variable sites among these markers. Of the fifteen regions with significant variability, SSRs were identified in only seven regions (Figure 3B, Table S9). The detected SV for these regions ranged from 10.53% (trnR-ACG-trnN-GUU, trnN-GUU-trnR-ACG) to 31.4% (ndhH-rps15) (Figure 3C).
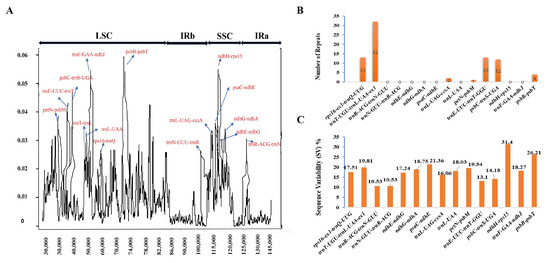
Figure 3.
Chloroplast genome analysis: identifying the top 15 syntenic intergenic and intronic loci with maximum genetic diversity across 12 studied Dendrobium species. (A) Sliding window analysis with a window length of 600 bp and a step size of 100 bp reveals nucleotide diversity (Pi) across the complete chloroplast genomes of Dendrobium species. (B) Number of SSRs in top hotspots. (C) Sequence variability among these top regions.
3.3. Phylogenetic Analysis
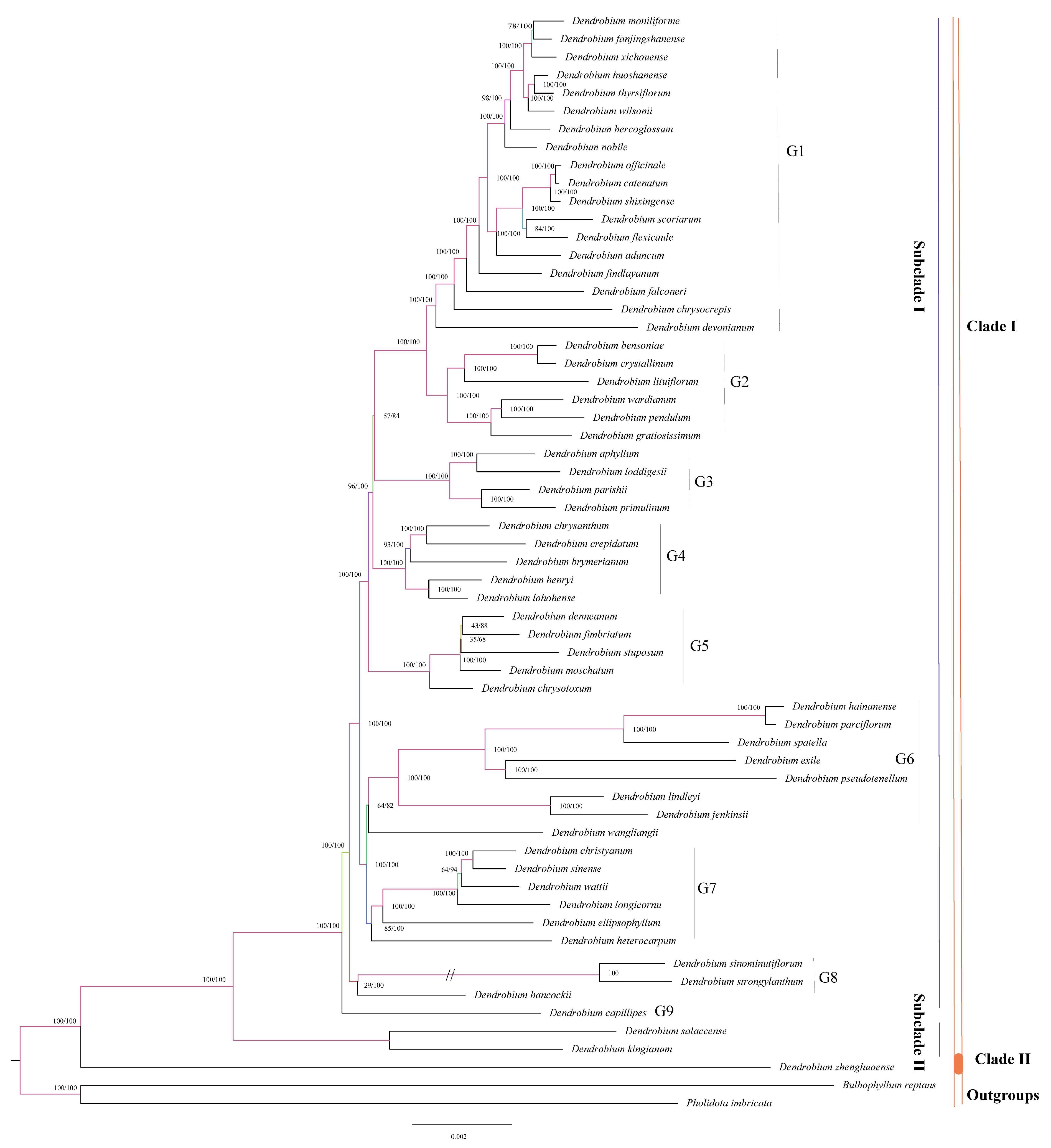
Utilizing complete cp genomes greatly improved the accuracy of phylogenetic relationships in our analysis. We used a total of 59 complete cp genome sequences obtained from the NCBI GenBank database (Table S2). Our study used Bulbophyllum reptans and Pholidota imbricata as an outgroup. The results of our research support the general structure of the trees produced by both the maximum likelihood (ML) and Bayesian inference (BI) methods, except for a few collapsed nodes. The Bayesian analysis yielded posterior probability (PP) values that were greater than the Bootstrap Percentage (BS) values. The major tree used to examine evolutionary connections is the ML topology derived from the comprehensive cp genome dataset (Figure 4). Our study concluded that D. zhenghuoense is closely related to other Dendrobium species with 100% support, forming a sister clade. However, the overall tree is paraphyletic. Comparing the sister clades, Clade I shows greater diversity than Clade II. Within the Dendrobium genus, two main subclades receive support. Among the sister clades, Clade I consist of 58 Dendrobium species (with 100% support in both parsimony and bootstrap analyses), while Clade II comprises only one species, D. Zhenghuoense (again with 100% support in both methods). Clade I is separated into two main subclades: Subclade I (PP = 100, BS = 100) and Subclade II (PP = 100, BS = 100). Subclade I comprises fifty-six species and is divided into nine well-supported groups: G1 (PP = 100, BS = 100) with eighteen species, G2 (PP = 100, BS = 100) comprising six species, G3 (PP = 100, BS = 100) with four species, G4 (PP = 100, BS = 100) including five species, G5 (PP = 100, BS = 100) with five species, G6 (PP = 82, BS = 64) having eight species, G7 (PP = 100, BS = 100) with six species, G8 (PP = 100, BS = 100) consisting of three species, and G9 (PP = 100, BS = 100) with only one species. Subclade II comprises just two species (PP = 100, BS = 100). Clade II (PP = 100, BS = 100) comprises only one species.
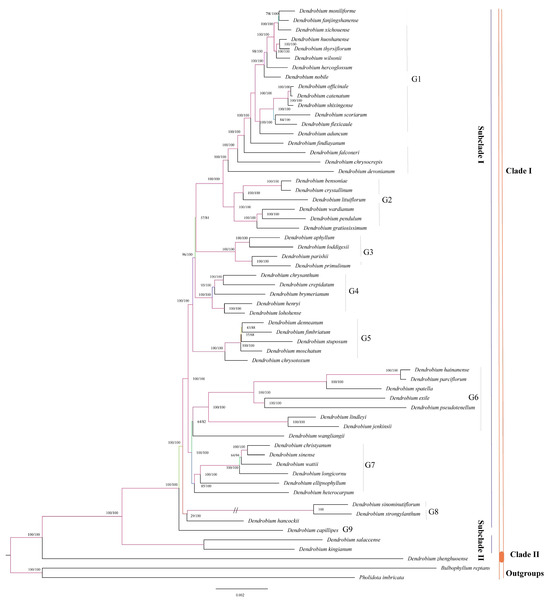
Figure 4.
Phylogenetic tree depicting the relationships among 59 Dendrobium species reconstructed through maximum likelihood and Bayesian inference methods using complete cp genome sequences. The branches are labeled with their respective support values, representing ML bootstrap support values and Bayesian posterior probabilities. Branches shortened by 50% are denoted by double slashes // on the tree.
3.4. Identification of Specific Nucleotide Sites of D. brymerianum and Design of ARMS-Specific Primers
After examining 40 cp genome sequences (Table S3) of Dendrobium species, 12 specific nucleotide sites (Table S10) from D. brymerianum were identified and selected to design a specific primer. Specifically, the Aoa1 and Aoa2 sites were situated in the matK gene region, while the Aoc1 and Aoc2 sites were situated in the ycf1 gene region. The Aod1 and Aod2 sites were situated in the trnN-GUU gene region, and the Aof1 and Aof2 sites were also situated in the trnN-GUU gene region. To design the specific primers for D. brymerianum, the combination of the above sites was used to design the Aoa1/Aoa2, Aoc1/Aoc2, Aod1/Aod2, and Aof1/Aof2 primers. These specific sites on D. brymerianum were different from other species; thus, they were selected based on the conditions essential for DNA amplification length. At the sites of Aoa1, Aoa2, Aoc1, Aoc2, Aod1, Aod2, Aof1, and Aof2, all species were identical except for D. brymerianum. The Aob1 site, situated in the rbcL region, appears to be a comparatively specific site for D. brymerianum. At this site, both D. brymerianum and D. salaccense were the same. To create specific primers for D. brymerianum, it was essential to identify a site that differ between these two species. Accordingly, we screened a suitable Aob2 site and then combined both Aob1 and Aob2 to design an Aob1/Aob2-specific primer. At the Aoe1 site situated within the ycf1 gene region, D. brymerianum and D. acinaciforme share the same genetic sequence. To create a specific primer for D. brymerianum, we selected different Aoe2 sites near both species and applied the above-mentioned principle. After screening 12 nucleotide sites, we designed a total of six pairs of specific primers for D. brymerianum (Table S11). To improve the specificity of the primers, we modified selected specific primers based on the ARMS primer mismatch principle. The mismatched bases were added to the primers, with the positions of the mismatched bases mostly located at the third-last nucleotide of the 3′ end of the primer. The number of mismatched bases was set between 1 and 3, and the types of mismatches included T/C, A/G, C/G, G/A, and C/A (Table S11).
3.5. Analysis of ARMS-qPCR
The DNA samples from the Dendrobium species (Table S1) were amplified using ordinary PCR utilizing six pairs of ARMS-specific primers (Table S11). Following the amplification results, one pair of ARMS-specific primers with effective discrimination was initially selected, namely Aob1/Aob2. Based on the results of gel electrophoresis, the Aob1/Aob2 primer showed strong specificity and good identification ability compared to other primers. To obtain more accurate and authentic results, the mismatched bases were modified and the PCR was run using the AAob1/AAob2 primer (Table S11). After adjusting their annealing temperatures, the optimal annealing temperature was determined to be 55 °C (Table 2). The gel electrophoresis results indicated that the band corresponds to D. brymerianum, as evidenced by the clear and bright band and amplified fragment size of approximately 250 bp. This is consistent with the size of the target band (Table 2). The AAob1/AAob2 primer exhibited high specificity, allowing for the clear distinction of the amplified band corresponding to D. brymerianum from other species in the gel electrophoresis results. General PCR was employed as a preliminary screening method for the specific primers. To assess the efficacy and sensitivity of this selected primer in identifying D. brymerianum, qPCR technology was subsequently utilized for further detection and analysis.

Table 2.
ARMS-specific primers of D. brymerianum screened by conventional PCR amplification.
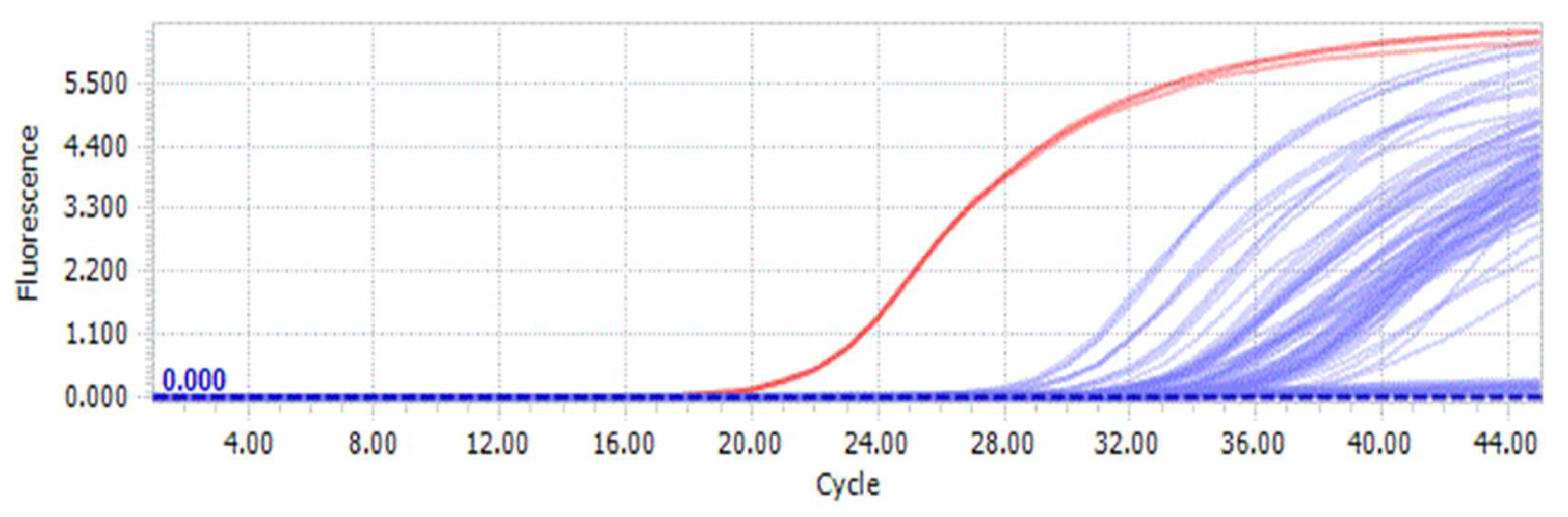
Using the previously used DNA samples for ordinary PCR of each species (Table S1), fluorescence quantitative PCR was carried out using the corresponding ARMS-specific primer pair (AAob1/AAob2). The qPCR amplification results (Figure 5) show a red curve for D. brymerianum and blue curves for other Dendrobium species. The result of amplifying a specific pair of primers determined that the red curve emerged initially. For D. brymerianum, the red curve emerged after approximately 20 cycles, while for the other species, it appeared after 28 cycles. Upon examining the Cq values, it was observed that the average Cq value with primer AAob1/AAob2 was 20.62 for D. brymerianum (Figure 5, Table 3). The average Cq values for the other species were considerably higher than those of D. brymerianum. A smaller Cq ± SD value signifies a greater degree of DNA amplification. Our findings suggest that the primer screened in this study can effectively distinguish D. brymerianum from the other species.
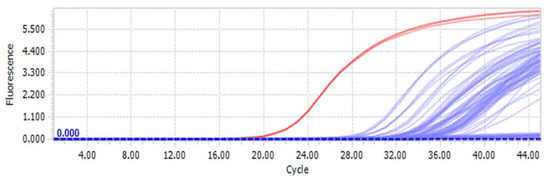
Figure 5.
Fluorescence amplification curve and Cq values of D. brymerianum and other Dendrobium species based on a pair of primers, AAob1/AAob2. Red lines show D. brymerianum and blue lines show other Dendrobium species. All species were examined in triplicate.

Table 3.
Cq values of D. brymerianum and other Dendrobium species from ARMS-qPCR amplification.
4. Discussion
4.1. The Evolution of the Chloroplast Genome of D. brymerianum
Chloroplast genomes have become valuable tools for studying genetic modifications between closely related species [55]. However, there are numerous genetic events, such as the gaining or losing of genes and reshuffling in several land plant genomes [56]. In our research, the cp genomes of D. brymerianum showed a reliable quadripartite structure, comprising two Inverted Repeats (IRs) and two Single Copies (SCs) (Figure 1A). The genomes showed a high degree of constancy in both their structure and size with no significant divergence, which was observed in the genomes of other angiosperm chloroplasts [57,58]. Our findings exhibited that the IR regions had the highest GC content, which is likely attributed to the occurrence of ribosomal RNA (rRNA) in these areas. The IR region is typically the most conserved in the cp genome [59].
The distribution of exon and intron lengths in plant cp genomes provides significant information. The gene rps12 exhibits trans-splicing, which occurs when its 5′ end is situated in the LSC region and its 3′ end is located in the IR region [60]. Numerous studies have emphasized the importance of ycf1 as a pseudogene, affecting cp genome variation and contributing to the encoding of Tic214 in plants [61,62]. The capacity to influence gene expression levels in various temporal and spatial settings has been widely documented [63,64]. Though there have been no studies to date that have delved into the exact methods leading to intron regulation in Dendrobium, it is necessary to identify that further research into introns present in cp genomes can yield appreciated knowledge for the identification of medicinal plants.
Evolution commonly results in expansions and contractions within the IR, LSC, and SSC regions, which significantly influence the cp genome sizes. Remarkably, variations in the size of boundary regions are a main factor that contributes to the detected differences among various chloroplast sections [21]. Variations in cp genome size can be primarily credited to three primary factors. The first factor involves variations, such as shrinkage, expansion, or loss, within the IR region [65]. The size of several cp genomes typically changes due to changes in the IR region and the border that separates the LSC and SSC regions. Goulding proposed that there was a slight increase in the number of genes at the boundary of the IR region, followed by the combination of the LSC boundary [66]. This amplification of genes at the IR region’s boundary is considered essential for conserving the stability of the IR region [67]. The second factor that affects SSC regions is the gain or loss of genes within them. The third reason involves a decrease in the length of introns or intergenic regions between genes. Our study observed slight variations in the IR region and its neighboring boundaries between the IR and the LSC or SSC regions across Dendrobium species’ cp genomes (Figure 1B). Studies propose that genes in numerous species can be found at the boundary, across the boundary, or located completely within the IR region. The junctions that join the IRa and SSC regions are sited at the 5′ end of the ycf1 gene, which results in a duplicated occurrence of ycf1 within the IRb regions. Our results highlighted the regularity of extension or reduction within IRs across Dendrobium species. The modification of sequence arrangements, which leads to variations in the structure of the cp genome in similar species, may suggest a connection to plant genetic diversity information. Accordingly, these findings offer promising potential for application in molecular identification and evolutionary studies.
4.2. Identification of D. brymerianum Based on Complete Chloroplast Genome Sequence
SSRs (simple sequence repeats) are frequently found in the organelle genomes of eukaryotes, providing a rich source of polymorphism, high reproducibility, strong reliability, and co-dominant inheritance [68]. Naturally, microsatellites are composed of 1–6 nucleotide repeat units and are generally distributed throughout the genome, significantly influencing genome recombination and rearrangement [69]. In our study, we successfully recognized a total of 623 SSRs in the complete cp genome of Dendrobium species. The majority of SSRs were noticed in the LSC and spacer regions on an individual genome basis (Figure 2). A significant number of SSRs have been recognized in various plant species, including Forsythia suspense [70], D. nobile, D. officinale, and others [30].
The existence of mutation events within the cp genome is not accidental; rather, they tend to be combined in specific regions, which are referred to as “hotspot” regions [23]. The use of cp genome mutation hotspots serves as a practical and efficient means for creating DNA barcodes, a technique that has been effectively applied in the research of orchids [71,72]. Our research found hypervariable regions in the complete cp genome of Dedndrobium species, mainly trnK-UUU, petN, trnL-UAA, trnT-UGU, ndhJ, psbT, rpl16, ndhF, ndhA, and ycf1 (Figure S1). The IR region and the coding regions showed significantly less variability than the other regions. Our results align with previous research outcomes of cp genomes in other higher plants, such as Hernandia nymphaeifolia [73] and Saposhnikovia divaricata [74]. This trend is also supported by a previous study on the Dendrobium genus [26,75], which proposes that non-coding regions evolve faster than coding regions in Dendrobium species. Hence, when creating DNA barcodes for Dendrobium species, it is important to focus on non-coding regions. Our research has documented that the SSC and LSC loci have higher Sequence Variability (SV) and nucleotide diversity (Pi) values compared to the IR loci. Accordingly, the IR regions are supposed to be conserved, suggesting that regions other than the IR have the potential to be used for the development of molecular markers. This result aligns with previous studies conducted on Paraphalaenopsis species [76]. Out of the 89 loci we assessed, we found 15 loci to be significant hotspots, with nucleotide diversity values exceeding 0.026 (Table S8). Previous research has described the occurrence of several hotspot regions which we identified [34,77]. Out of these fifteen regions, SSRs were only noticed in seven regions (Figure 3B, Table S9). The diverse range of SSR loci showing variations within the cp genomes of various species makes them highly valuable as molecular markers for precisely identifying species [28,78]. Though we have discovered numerous potential barcoding regions, further research is needed to determine whether these highly variable markers can be successfully utilized for identification and phylogenetic analyses of Dendrobium species.
The use of complete cp genome sequences, which contain a substantial number of variable sites, has been confirmed to be a useful method for confirming species and determining phylogenetic relationships in taxonomically complicated groups. This method has been successfully applied in various studies, such as those on Orychophragmus [79]. Xiang discovered the potential of four plastid DNA markers and nrDNA ITS data in determining the complex phylogenetic relationships among Dendrobium species [14]. However, Xiang highlighted that phylogenies based on morphological sections with incomplete chloroplast markers and ITS-rDNA data often exhibited inconsistencies [5]. By using complete cp genomes, our research offers valuable molecular insights into the evolutionary relationships and conservation of Dendrobium species. Our study suggests that, overall, Dendrobium species are paraphyletic (Figure 4). Studies by Wongsawad and Clements have previously suggested that the section Dendrobium is not monophyletic and may be polyphyletic, respectively [80,81]. However, Xiang found that the section Dendrobium is paraphyletic, a conclusion that aligns with our findings [14]. Our research showed different relationships among Dendrobium species compared to Xiang’s study [5]. Though D. lituiflorum was grouped with D. wardianum, D. pendulum, and D. gratiosissimum in Xiang’s study, it was grouped with D. bensoniae and D. crystallinum in our study. These differences between our findings and those of Xiang are likely due to the scarcity of data available on the complete cp genome of Dendrobium species. Xiang studied the evolutionary history of Dendrobium species using partial chloroplast markers and ITS-rDNA from a diverse group of species, while our study utilized complete cp genomes from species within the same genus. Thus, incorporating supplementary individuals with complete cp genomes from various Dendrobium species can offer further insights into their evolutionary relationships.
4.3. ARMS-qPCR Technology Can Be Used to Identify the Medicinal Products of D. brymerianum
The accurate identification of Dendrobium species based solely on their morphological features is challenging due to their wide distribution, significant intra-species variations, and overlapping traits with similar species. Many researchers have struggled to use physicochemical identification methods, such as high-pressure liquid chromatography (HPLC) [82] and capillary electrophoresis (CE) [83], for the identification of Dendrobium species. There is a significant gap in the accessibility of a reliable method for herbal identification. Quantitative PCR (qPCR), an advanced system of conventional PCR, is a sensitive method for quantifying nucleic acids [84]. Developing an ARMS-qPCR identification system demands the design of ARMS-specific primers that are tailored to ARMS technology and incorporate fluorescence qPCR. Before designing ARMS-specific primers for D. brymerianum, it was significant to determine the specific nucleotide sites specific to D. brymerianum. The vast amount of cp genome data served as a valuable resource, establishing a solid foundation for the identification of specific nucleotide sites of D. brymerianum. Identifying specific nucleotide sites specific to the D. brymerianum was a challenging task. To identify the targeted species, which has limited specific identification sites, this study closely examined variations between D. brymerianum and other species using the primer design principles [85]. Following these principles, specific primers for D. brymerianum were successfully designed (Table S11). To increase the specificity of the primers, the ARMS primer mismatch principle was applied, which involved intentionally introducing mismatched bases near the 3′ end of the primers. Our research primarily focused on five mismatch types, including T/C, A/G, GA, and CA, which represented pyrimidine/pyrimidine and purine/purine mismatches. Furthermore, the C/G pyrimidine/purine mismatch was included to evaluate specificity. Previous research has suggested that it is more effective to position mismatched bases at the second, third, and fourth positions from the 3′ end of the primer [42]. In our study, AAob1/AAob2 primer pair (Table 2) showed strong specificity, allowing easy distinction of the amplified band of D. brymerianum from other species in the gel electrophoresis results. The Aob1 and Aob2 sites are located in the rbcL gene region, which has been regularly utilized for plant identification [86]. Previous research used ARMS technology for the identification of D. officinale and found that incorporating mismatched bases at various positions from the 3′ end of the primer affected the range of annealing temperature of the primer [87]. By introducing mismatched bases at the third reciprocal position of the 3′ end, this study established a method for the stable identification of D. brymerianum across an annealing temperature of 55 °C. Utilizing ARMS primer design principles and an understanding of mismatches, the development of the AAob1/AAob2 primer pair effectively enabled the consistent identification of D. brymerianum among other Dendrobium species (Table S1).
During ARMS-qPCR amplification with the AAob1/AAob2 primer set, the fluorescence amplification curve permitted for clear differentiation of D. brymerianum from other Dendrobium species. Considerable differences in Cq values between D. brymerianum and other species were detected, further confirming the sensitivity and efficacy of qPCR techniques in identifying Dendrobium species. André and co-authors established an identification method for D. nobile using ARMS-qPCR technology, which effectively distinguished it from other closely related species [88]. In our study, we combined ARMS and qPCR to create an ARMS-qPCR identification system. This system boasts high specificity, sensitivity, and ease of use, making it a promising tool for identifying D. brymerianum and other species. Our research involved gathering raw Cq values for several Dendrobium species (Figure 5, Table 3). These values signify the number of cycles necessary to achieve the fluorescence threshold level detectable in ARMS-qPCR reactions. The Cq values across all species varied from 20.62 to 36.67, and from these values, we can infer the relative expression abundance of DNA in each species. Lower Cq values indicate higher DNA expression abundance, so D. brymerianum had the highest expression abundance among all species. In recent years, this technique has found application in identifying various other Dendrobium species, such as D. Huoshanensis [42] and D. officinale [38]. The successful utilization of this method for different Dendrobium species emphasizes its effectiveness and feasibility in species identification. The identification of Dendrobium species has always been challenging, particularly when it comes to obtaining DNA. However, the method established in this study for D. brymerianum has proven to be effective, accurate, and efficient. This approach can be easily implemented for quality control purposes during the production and processing of D. brymerianum. The key advantage of this method is that it ensures the quality and safety of D. brymerianum, making it a valuable tool for the industry.
5. Conclusions
This study has provided a valuable genomic resource for the Dendrobium genus and revealed that there is a significant conservation of gene composition and arrangement in D. brymerianum, while there is a great deal of variability in the non-coding regions among the compared Dendrobium species. The analysis also revealed highly variable regions and emphasized the usefulness of complete cp genomes as a super-barcode for authenticating Dendrobium species and their products. Additionally, the phylogenetic analysis revealed that Dendrobium species are paraphyletic, which means that they do not form a single, natural group. Therefore, it is necessary to include more genomic data to gain a comprehensive understanding of their evolutionary relationships. The ARMS-qPCR method that was developed exhibited remarkable specificity in differentiating D. brymerianum from other species, providing an accurate identification approach. Although there were obstacles to overcome, the use of complete cp genomes shows potential for widely authenticating plants, which could enhance taxonomic accuracy in complicated plant groups.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/horticulturae10030260/s1, Figure S1: Illustration of chloroplast genome alignment among twelve Dendrobium species using D. acinaciforme as the reference through mVISTA analysis; Table S1: Information of species with their names for DNA extraction; Table S2: The list of species and their corresponding complete cp genomes and accession numbers for phylogenetic study; Table S3: Species information to obtain complete cp genomes to obtain SNP sites; Table S4: List of genes encoded by D. brymerianum complete cp genome; Table S5: Genes with introns in the complete cp genome of D. brymerianum; Table S6: A total of 623 simple sequence repeat (SSR) loci, recognized in the 16 complete cp genomes of Dendrobium species; Table S7: Eighty-nine syntenic non-coding regions and their Sequence Variability (SV) and nucleotide diversity (Pi); Table S8: Variability patterns of hypervariable markers among Dendrobium species; Table S9: Simple sequence repeats (SSRs) in seven syntenic loci; Table S10: Information of 12 nucleotide sites for designing specific primers of D. brymerianum; Table S11: Sequences of ARMS-specific primers designed for D. brymerianum.
Author Contributions
Conceptualization, X.D., Z.N. and Q.X.; methodology, Z.N., Q.X. and A.K.; software, J.Y., Z.H., C.L. and A.K.; validation, J.Y., Z.H., M.W., C.L. and A.K.; formal analysis, J.Y., Z.H., C.L. and A.K.; data curation, Z.N., Q.X. and A.K.; writing—original draft preparation, A.K.; writing—review and editing, Z.N., Q.X. and A.K.; supervision, X.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially funded by NSFC (Natural Science Foundation of China), grant numbers 32070353 and 31670330.
Data Availability Statement
The assembled complete chloroplast genome sequences of five individual of D. brymerianum were submitted to the DDBJ with accession numbers LC794131, LC794132, LC794133, and LC794134.
Acknowledgments
We would like to thank the College of Life Sciences, Nanjing Normal University for supporting this work. I am personally grateful to Ding Xiaoyu, Niu Zhitao, and Xue Qingyun for the helpful comments on the manuscript of this paper and the support on technical data analysis.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Govaerts, R.; Bernet, P.; Kratochvil, K.; Gerlach, G.; Carr, G.; Alrich, P.; Wood, J.J. World Checklist of Orchidaceae, 2nd ed.; Facilitated by the Royal Botanic Gardens: London, UK, 2017; pp. 97–100. [Google Scholar]
- Willis, K. State of the World’s Plants 2017, 2nd ed.; Royal Botanics Gardens: London, UK, 2017; pp. 1–100. [Google Scholar]
- Hinsley, A.; De Boer, H.J.; Fay, M.F.; Gale, S.W.; Gardiner, L.M.; Gunasekara, R.S.; Phelps, J. A review of the trade in orchids and its implications for conservation. Bot. J. Linn. Soc. 2018, 186, 435–455. [Google Scholar] [CrossRef]
- Cribb, P.; Govaerts, R. Just how many orchids are there? In Proceedings of the 18th World Orchid Conference, Dijon, France, 11–20 March 2005; Naturalia Publications: Dijion, France, 2005. [Google Scholar]
- Xiang, X.G.; Mi, X.C.; Zhou, H.L.; Li, J.W.; Chung, S.W.; Li, D.Z.; Jin, X.H. Biogeographical diversification of mainland Asian Dendrobium (Orchidaceae) and its implications for the historical dynamics of evergreen broad-leaved forests. J. Biogeogr. 2016, 43, 1310–1323. [Google Scholar] [CrossRef]
- Zhang, F.P.; Yin, Z.L.; He, H.P. Characterization of the complete chloroplast genome of Dendrobium christyanum and its phylogenetic analysis. Mitochondrial DNA Part B 2021, 6, 2605–2606. [Google Scholar] [CrossRef] [PubMed]
- da Silva, J.A.T.; Jin, X.; Dobránszki, J.; Lu, J.; Wang, H.; Zotz, G.; Zeng, S. Advances in Dendrobium molecular research: Applications in genetic variation, identification and breeding. Mol. Phylogenet. Evol. 2016, 95, 196–216. [Google Scholar] [CrossRef] [PubMed]
- Teixeira da Silva, J.A.; Tsavkelova, E.A.; Ng, T.B.; Parthibhan, S.; Dobránszki, J.; Cardoso, J.C.; Zeng, S. Asymbiotic in vitro seed propagation of Dendrobium. Plant Cell Rep. 2015, 34, 1685–1706. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.Z.; Dan, Y.; Liu, Y.Z.; Peng, Y.J.C.H.M. Pharmacopeia of the People’s Republic of China (2010 edition): A Milestone in Development of China’s Healthcare. Chin. Herb. Med. CHM 2010, 2, 157–159. [Google Scholar]
- Xu, J.; Han, Q.B.; Li, S.L.; Chen, X.J.; Wang, X.N.; Zhao, Z.Z.; Chen, H.B. Chemistry, bioactivity and quality control of Dendrobium, a commonly used tonic herb in traditional Chinese medicine. Phytochem. Rev. 2013, 12, 341–367. [Google Scholar] [CrossRef]
- Seidenfaden, G. Orchid genera in Thailand XII, Dendrobium Sw. Opera Bot. 1985, 83, 5–295. [Google Scholar]
- Chen, Y.; Yu, H.; Liu, Y. Chemical constituents from Dendrobium brymerianum Rchb. f. B Biochem. Syst. Ecol. 2014, 57, 175–177. [Google Scholar] [CrossRef]
- Klongkumnuankarn, P.; Busaranon, K.; Chanvorachote, P.; Sritularak, B.; Jongbunprasert, V.; Likhitwitayawuid, K. Cytotoxic and anti-migratory activities of phenolic compounds from Dendrobium brymerianum. Evid.-Based Complement. Alternat. Med. 2015, 2015, 350410. [Google Scholar] [CrossRef]
- Xiang, X.G.; Schuiteman, A.; Li, D.Z.; Huang, W.C.; Chung, S.W.; Li, J.W.; Jin, X.H. Molecular systematics of Dendrobium (Orchidaceae, Dendrobieae) from mainland Asia based on plastid and nuclear sequences. Mol. Phylogenet. Evol. 2013, 69, 950–960. [Google Scholar] [CrossRef]
- Feng, S.; Jiang, Y.; Wang, S.; Jiang, M.; Chen, Z.; Ying, Q.; Wang, H. Molecular identification of Dendrobium species (Orchidaceae) based on the DNA barcode ITS2 region and its application for phylogenetic study. Int. J. Mol. Sci. 2015, 16, 21975–21988. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Li, D.; Li, J.; Xiang, X.; Jin, W.; Huang, W.; Huang, L. Evaluation of the DNA barcodes in Dendrobium (Orchidaceae) from mainland Asia. PLoS ONE 2015, 10, e0115168. [Google Scholar] [CrossRef] [PubMed]
- Adams, P.B. Systematics of Dendrobiinae (Orchidaceae), with special reference to Australian taxa. Bot. J. Linn. Soc. 2011, 166, 105–126. [Google Scholar] [CrossRef]
- Morris, M.W.; Stern, W.L.; Judd, W.S. Vegetative anatomy and systematics of subtribe Dendrobiinae (Orchidaceae). Bot. J. Linn. Soc. 1996, 120, 89–144. [Google Scholar] [CrossRef]
- Yukawa, T.; Uehara, K. Vegetative diversification and radiation in subtribe Dendrobiinae (Orchidaceae): Evidence from chloroplast DNA phylogeny and anatomical characters. Plant Syst. Evol. 1996, 201, 1–14. [Google Scholar] [CrossRef]
- Xu, H.; Hou, B.; Zhang, J.; Tian, M.; Yuan, Y.; Niu, Z.; Ding, X. Detecting adulteration of Dendrobium officinale by real-time PCR coupled with ARMS. Int. J. Food Sci. Technol. 2012, 47, 1695–1700. [Google Scholar] [CrossRef]
- Cheng, H.; Li, J.; Zhang, H.; Cai, B.; Gao, Z.; Qiao, Y.; Mi, L. The complete chloroplast genome sequence of strawberry (Fragaria × ananassa Duch.) and comparison with related species of Rosaceae. PeerJ 2017, 5, e3919. [Google Scholar] [CrossRef]
- Dong, W.; Xu, C.; Cheng, T.; Lin, K.; Zhou, S. Sequencing angiosperm plastid genomes made easy: A complete set of universal primers and a case study on the phylogeny of Saxifragales. Genome Biol. Evol. 2013, 5, 989–997. [Google Scholar] [CrossRef]
- Dong, W.; Liu, J.; Yu, J.; Wang, L.; Zhou, S. Highly variable chloroplast markers for evaluating plant phylogeny at low taxonomic levels and for DNA barcoding. PLoS ONE 2012, 7, e35071. [Google Scholar] [CrossRef]
- Twyford, A.D.; Ness, R.W. Strategies for complete plastid genome sequencing. Mol. Ecol. Resour. 2017, 17, 858–868. [Google Scholar] [CrossRef]
- Dong, W.; Xu, C.; Wu, P.; Cheng, T.; Yu, J.; Zhou, S.; Hong, D.Y. Resolving the systematic positions of enigmatic taxa: Manipulating the chloroplast genome data of Saxifragales. Mol. Phylogenet. Evol. 2018, 126, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, Y.; Yang, Y.; Xie, X.; Lu, Y.; Yang, Z.; Suo, Z. Interspecific chloroplast genome sequence diversity and genomic resources in Diospyros. BMC Plant Biol. 2018, 18, 210. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Xu, C.; Li, W.; Xie, X.; Lu, Y.; Liu, Y.; Suo, Z. Phylogenetic resolution in Juglans based on complete chloroplast genomes and nuclear DNA sequences. Front. Plant Sci. 2017, 8, 1148. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Su, Z.; Yu, S.; Liu, J.; Yin, X.; Zhang, G.; Li, B. Genome comparison reveals mutation hotspots in the chloroplast genome and phylogenetic relationships of Ormosia species. BioMed Res. Int. 2019, 2019, 7265030. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Li, X.; Li, H.; Yang, J.; Wang, H.; He, J. Comparative analysis of the complete chloroplast genomes of four Aconitum medicinal species. Molecules 2018, 23, 1015. [Google Scholar] [CrossRef]
- Kang, J.; Lu, J.; Qiu, S.; Chen, Z.; Liu, J.; Wang, H. Dendrobium SSR markers play a good role in genetic diversity and phylogenetic analysis of Orchidaceae species. Sci. Hortic. 2015, 183, 160–166. [Google Scholar] [CrossRef]
- Ahmed, I.; Matthews, P.J.; Biggs, P.J.; Naeem, M.; McLenachan, P.A.; Lockhart, P.J. Identification of chloroplast genome loci suitable for high-resolution phylogeographic studies of Colocasia esculenta (L.) Schott (Araceae) and closely related taxa. Mol. Ecol. Resour. 2013, 13, 929–937. [Google Scholar] [CrossRef]
- Luo, J.; Hou, B.W.; Niu, Z.T.; Liu, W.; Xue, Q.Y.; Ding, X.Y. Comparative chloroplast genomes of photosynthetic orchids: Insights into the evolution of the Orchidaceae and development of molecular markers for phylogenetic applications. PLoS ONE 2014, 9, e99016. [Google Scholar] [CrossRef]
- Pan, I.C.; Liao, D.C.; Wu, F.H.; Daniell, H.; Singh, N.D.; Chang, C.; Lin, C.S. Complete chloroplast genome sequence of an orchid model plant candidate: Erycina pusilla apply in tropical Oncidium breeding. PLoS ONE 2012, 7, e34738. [Google Scholar] [CrossRef]
- Yang, J.B.; Tang, M.; Li, H.T.; Zhang, Z.R.; Li, D.Z. Complete chloroplast genome of the genus Cymbidium: Lights into the species identification, phylogenetic implications, and population genetic analyses. BMC Evol. Biol. 2013, 13, 84. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wang, Z.; Ding, X.; Zhou, K.; Xu, L. Differentiation of Dendrobium species used as “Huangcao Shihu” by rDNA ITS sequence analysis. Planta Med. 2006, 72, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Asahina, H.; Shinozaki, J.; Masuda, K.; Morimitsu, Y.; Satake, M. Identification of medicinal Dendrobium species by phylogenetic analyses using matK and rbcL sequences. J. Nat. Med. 2010, 64, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Kress, W.J.; Erickson, D.L. A two-locus global DNA barcode for land plants: The coding rbcL gene complements the non-coding trnH-psbA spacer region. PLoS ONE 2007, 2, e508. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Chen, X.; Xu, J.; Cai, J.; Wang, X. Identification of Dendrobium officinale using DNA barcoding method combined with HRM and qPCR technology. Food Anal. Methods 2022, 15, 1310–1320. [Google Scholar] [CrossRef]
- Lan, Q.; Zhang, G.; Wang, Y.; Zhao, X.; Zhu, Z.; Cui, X.; Cheng, Y. SNP-based molecular assay for cucumber hybrid seed purity identification by pyrosequencing. China Veg. 2012, 6, 58–63. [Google Scholar]
- Lu, S.; Ding, X.; Ma, Y.; Han, L.; Zhang, W.; Qian, L.; Zhang, F. Confirming the genetic identity of Dendrobium fimbriatum using an amplification refractory mutation system (ARMS). Plant Mol. Biol. Report. 2010, 28, 712–716. [Google Scholar] [CrossRef]
- Dong, X.; Jiang, C.; Yuan, Y.; Peng, D.; Luo, Y.; Zhao, Y.; Huang, L. Application of high-resolution melting analysis for authenticity testing of valuable Dendrobium commercial products. J. Sci. Food Agric. 2018, 98, 549–558. [Google Scholar] [CrossRef]
- Niu, Z.; Pan, J.; Xue, Q.; Zhu, S.; Liu, W.; Ding, X. Plastome-wide comparison reveals new SNV resources for the authentication of Dendrobium huoshanense and its corresponding medicinal slice (Huoshan Fengdou). Acta Pharm. Sin. B 2018, 8, 466–477. [Google Scholar] [CrossRef]
- Doyle, J.J. Isolation of plant DNA from fresh tissue. Focus 1990, 12, 13–15. [Google Scholar]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Drummond, A. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Moriyama, E.N. Vector NTI, a balanced all-in-one sequence analysis suite. Brief. Bioinform. 2004, 5, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Conant, G.C.; Wolfe, K.H. GenomeVx: Simple web-based creation of editable circular chromosome maps. Bioinformatics 2008, 24, 861–862. [Google Scholar] [CrossRef] [PubMed]
- Frazer, K.A.; Pachter, L.; Poliakov, A.; Rubin, E.M.; Dubchak, I. VISTA: Computational tools for comparative genomics. Nucleic Acids Res. 2004, 32, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Librado, P.; Rozas, J. DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics 2009, 25, 1451–1452. [Google Scholar] [CrossRef] [PubMed]
- Zhitao, N.; Shuying, Z.; Jiajia, P.; Ludan, L.; Jing, S.; Xiaoyu, D. Comparative analysis of Dendrobium plastomes and utility of plastomic mutational hotspots. Sci. Rep. 2017, 7, 2073. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; Van Der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, J.; Lv, Y.; Zhang, X.; Xia, C.; Zhao, H.; Wen, C. Genetic diversity analysis and variety identification using SSR and SNP markers in melon. BMC Plant Biol. 2023, 23, 39. [Google Scholar] [CrossRef]
- Yuan, J.S.; Reed, A.; Chen, F.; Stewart, C.N. Statistical analysis of real-time PCR data. BMC Bioinform. 2006, 7, 85. [Google Scholar] [CrossRef]
- Xie, D.F.; Yu, Y.; Deng, Y.Q.; Li, J.; Liu, H.Y.; Zhou, S.D.; He, X.J. Comparative analysis of the chloroplast genomes of the Chinese endemic genus Urophysa and their contribution to chloroplast phylogeny and adaptive evolution. Int. J. Mol. Sci. 2018, 19, 1847. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Liu, H.; Hu, J.; Liang, Y.; Liang, J.; Wuyun, T.; Tan, X. Five complete chloroplast genome sequences from Diospyros: Genome organization and comparative analysis. PLoS ONE 2016, 11, e0159566. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Zhang, X.; Liu, G.; Yin, Y.; Chen, K.; Yun, Q.; Yu, J. The complete chloroplast genome sequence of date palm (Phoenix dactylifera L.). PLoS ONE 2010, 5, e12762. [Google Scholar] [CrossRef] [PubMed]
- Asaf, S.; Khan, A.L.; Khan, M.A.; Waqas, M.; Kang, S.M.; Yun, B.W.; Lee, I.J. Chloroplast genomes of Arabidopsis halleri ssp. gemmifera and Arabidopsis lyrata ssp. petraea: Structures and comparative analysis. Sci. Rep. 2017, 7, 7556. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Ma, P.F.; Wen, J.; Yi, T.S. Complete sequencing of five Araliaceae chloroplast genomes and the phylogenetic implications. PLoS ONE 2013, 8, e78568. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Guo, L.; Zhao, W.; Xu, J.; Li, Y.; Zhang, X.; Hou, X. Complete chloroplast genome sequence and phylogenetic analysis of Paeonia ostii. Molecules 2018, 23, 246. [Google Scholar] [CrossRef] [PubMed]
- de Cambiaire, J.C.; Otis, C.; Lemieux, C.; Turmel, M. The complete chloroplast genome sequence of the chlorophycean green alga Scenedesmus obliquus reveals a compact gene organization and a biased distribution of genes on the two DNA strands. BMC Evol. Biol. 2006, 6, 37. [Google Scholar] [CrossRef]
- Nakai, M.J. The TIC complex uncovered: The alternative view on the molecular mechanism of protein translocation across the inner envelope membrane of chloroplasts. Biochim. Biophys. Acta 2015, 1847, 957–967. [Google Scholar] [CrossRef]
- Le Hir, H.; Nott, A.; Moore, M.J. How introns influence and enhance eukaryotic gene expression. Trends Biochem. Sci. 2003, 28, 215–220. [Google Scholar] [CrossRef]
- Niu, D.K.; Yang, Y.F. Why eukaryotic cells use introns to enhance gene expression: Splicing reduces transcription-associated mutagenesis by inhibiting topoisomerase I cutting activity. Biol. Direct 2011, 6, 24. [Google Scholar] [CrossRef] [PubMed]
- Bock, R.; Knoop, V. Plastid Genomes of Seed Plants. In Genomics of Chloroplasts and Mitochondria, 1st ed.; Springer Science & Business Media: Bonn, Germany, 2012; Volume 35, pp. 103–126. [Google Scholar]
- Goulding, S.E.; Wolfe, K.H.; Olmstead, R.G.; Morden, C.W. Ebb and flow of the chloroplast inverted repeat. Mol. Gen. Genet. 1996, 252, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Ravi, V.; Khurana, J.P.; Tyagi, A.K.; Khurana, P.J.P.S. An update on chloroplast genomes. Plant Syst. Evol. 2008, 271, 101–122. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.; Cao, X.; Wu, D.; Hui, M.; Han, X.; Wang, H. Screening and validation of SSR molecular markers for identification of downy mildew resistance in intraspecific hybrid F1 progeny (V. vinifera). Horticulturae 2022, 8, 706. [Google Scholar] [CrossRef]
- Ni, L.; Zhao, Z.; Xu, H.; Chen, S.; Dorje, G. Chloroplast genome structures in Gentiana (Gentianaceae), based on three medicinal alpine plants used in Tibetan herbal medicine. Curr. Genet. 2017, 63, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yu, H.; Wang, J.; Lei, W.; Gao, J.; Qiu, X.; Wang, J. The complete chloroplast genome sequences of the medicinal plant Forsythia suspensa (Oleaceae). Int. J. Mol. Sci. 2017, 18, 2288. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.K.; Tu, X.D.; Zhao, Z.; Zeng, M.Y.; Zhang, S.; Ma, L.; Chen, S.P. Plastid phylogenomic data yield new and robust insights into the phylogeny of Cleisostoma–Gastrochilus clades (Orchidaceae, Aeridinae). Mol. Phylogenet. Evol. 2020, 145, 106729. [Google Scholar] [CrossRef]
- Kim, Y.K.; Jo, S.; Cheon, S.H.; Kwak, M.; Kim, Y.D.; Kim, K.J. Plastome evolution and phylogeny of subtribe Aeridinae (Vandeae, Orchidaceae). Mol. Phylogenet. Evol. 2020, 144, 106721. [Google Scholar] [CrossRef]
- Li, J.; Liu, Q.; Zhang, J.; Yang, Y.; Zhang, S.; Zhang, Y. Chloroplast genome of endangered mangrove plants Hernandia nymphaeifolia and its phylogenetic evolution. J. Northwest Forest. Univ. 2020, 35, 54–61. [Google Scholar]
- Yi, S.; Lu, H.; Wang, W.; Wang, G.; Xu, T.; Li, M.; Liu, D. The Chloroplast Genome of Wild Saposhnikovia divaricata: Genomic Features, Comparative Analysis, and Phylogenetic Relationships. Genes 2022, 13, 931. [Google Scholar] [CrossRef]
- Zong, D.; Gan, P.; Zhou, A.; Zhang, Y.; Zou, X.; Duan, A.; He, C. Plastome sequences help to resolve deep-level relationships of Populus in the family Salicaceae. Front. Plant Sci. 2019, 10, 5. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, F.; Zhao, Z.; Li, M.; Liu, Z.; Peng, D. Complete Chloroplast Genomes and Comparative Analyses of Three Paraphalaenopsis (Aeridinae, Orchidaceae) Species. Int. J. Mol. Sci. 2023, 24, 11167. [Google Scholar] [CrossRef] [PubMed]
- Shaw, J.; Shafer, H.L.; Leonard, O.R.; Kovach, M.J.; Schorr, M.; Morris, A.B. Chloroplast DNA sequence utility for the lowest phylogenetic and phylogeographic inferences in angiosperms: The tortoise and the hare IV. Am. J. Bot. 2014, 101, 1987–2004. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Tang, J.; Zeng, S.; Han, F.; Yuan, J.; Yu, J. Comparative plastid genomics of four Pilea (Urticaceae) species: Insight into interspecific plastid genome diversity in Pilea. BMC Plant Biol. 2021, 21, 25. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Hu, Q.; Al-Shehbaz, I.A.; Luo, X.; Zeng, T.; Guo, X.; Liu, J. Species delimitation and interspecific relationships of the genus Orychophragmus (Brassicaceae) inferred from whole chloroplast genomes. Front. Plant Sci. 2016, 7, 1826. [Google Scholar] [CrossRef] [PubMed]
- Wongsawad, P.; Handa, T.; Yukawa, T. Molecular phylogeny of Dendrobium Callista-Dendrobium complex. In Proceedings of the 17th World Orchid Conference, Shah Alam, Malaysia, 24–30 April 2002; Natural History Publications (Borneo) Sdn Bhd: Kota Kinabalu, Malaysia, 2005; pp. 131–133. [Google Scholar]
- Clements, M.A. Molecular Phylogenetics Systematics in Dendrobieae (Orchidaceae). Aliso J. Syst. Florist. Bot. 2006, 22, 465–480. [Google Scholar]
- Yang, L.; Wang, Z.; Xu, L. Simultaneous determination of phenols (bibenzyl, phenanthrene, and fluorenone) in Dendrobium species by high-performance liquid chromatography with diode array detection. J. Chromatogr. A 2006, 1104, 230–237. [Google Scholar] [CrossRef]
- Zha, X.Q.; Luo, J.P.; Wei, P. Identification and classification of Dendrobium candidum species by fingerprint technology with capillary electrophoresis. S. Afr. J. 2009, 75, 276–282. [Google Scholar] [CrossRef]
- Heid, C.A.; Stevens, J.; Livak, K.J.; Williams, P.M. Real-time quantitative PCR. Genome Res. 1996, 6, 986–994. [Google Scholar] [CrossRef]
- Liu, J.; Huang, S.; Sun, M.; Liu, S.; Liu, Y.; Wang, W.; Hua, W. An improved allele-specific PCR primer design method for SNP marker analysis and its application. Plant Methods 2012, 8, 34. [Google Scholar] [CrossRef]
- Lu, D.; Huang, Y.; Wen, H. Application of DNA barcoding in the authentication of a food product. Food Sci. 2015, 36, 248–253. [Google Scholar]
- Qian, L.; Ding, G.; Zhou, Q.; Feng, Z.; Ding, X.; Gu, S.; Chu, B. Molecular authentication of Dendrobium loddigesii Rolfe by amplification refractory mutation system (ARMS). Planta Med. 2008, 74, 470–473. [Google Scholar] [CrossRef]
- Hoerning, A.; Kalkavan, H.; Rehme, C.; Menke, J.; Worm, K.; Garritsen, H.S.; Hoyer, P.F. Quantitative real-time ARMS-qPCR for mitochondrial DNA enables accurate detection of microchimerism in renal transplant recipients. Pediatr. Transplant. 2011, 15, 809–818. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).