Genome-Wide Prediction and Expression Characterization of the GATA Gene Family under Nitrogen and Phosphate Deficiency in Panax ginseng
Abstract
:1. Introduction
2. Methodology
2.1. Plant Materials, Cultivation and Stress Treatments
2.2. Identifying PgGATA genes in P. ginseng
2.3. Phylogenetic Analysis of PgGATAs
2.4. Chromosomal Scaffold and Gene Duplications
2.5. Gene Structures and Protein Motif Analyses
2.6. Cis-Elements in the Promoter Regions of PgGATA Genes
2.7. Gene Expression Analysis
3. Results
3.1. Identifying PgGATAs in P. ginseng
3.2. Gene Classification and Structural Analysis of PgGATAs
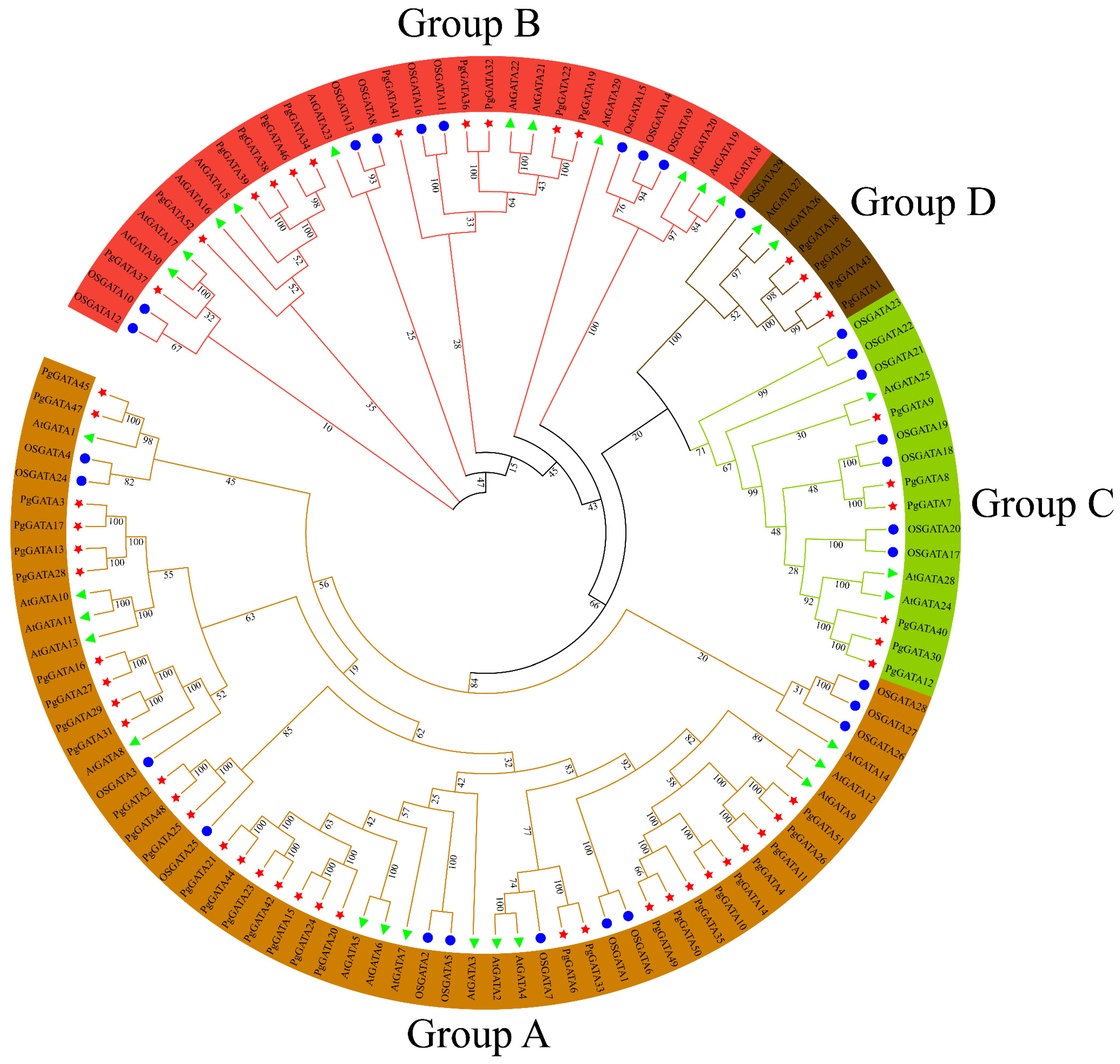
3.3. Phylogenetic Analysis of the PgGATA Proteins
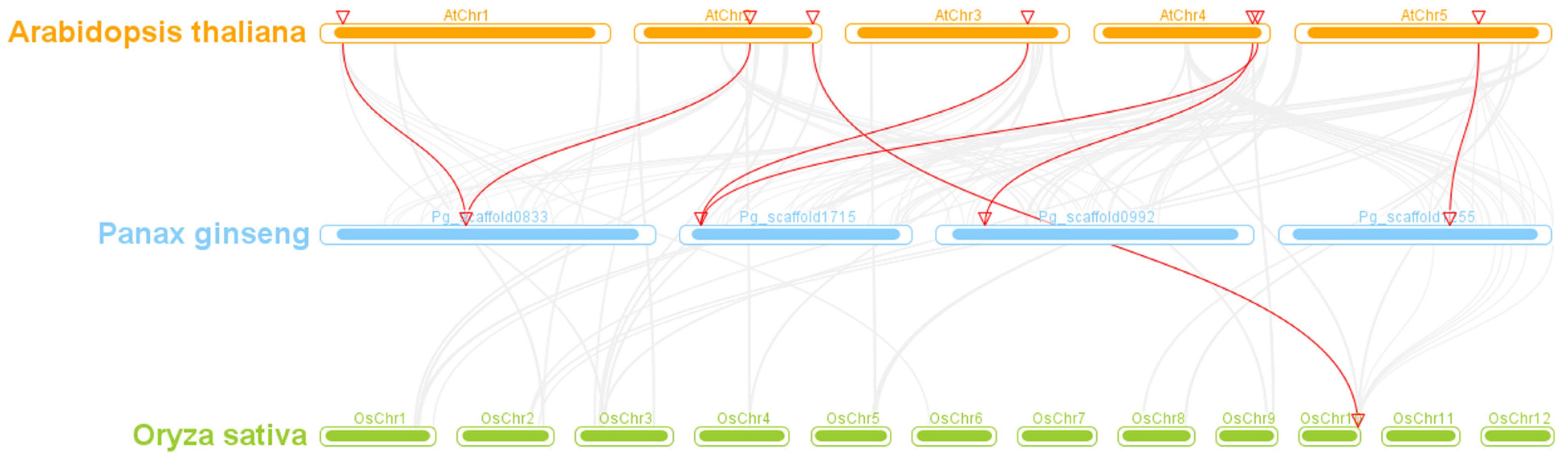
3.4. Collinearity and Ka/Ks Analyses of the PgGATA Family Members
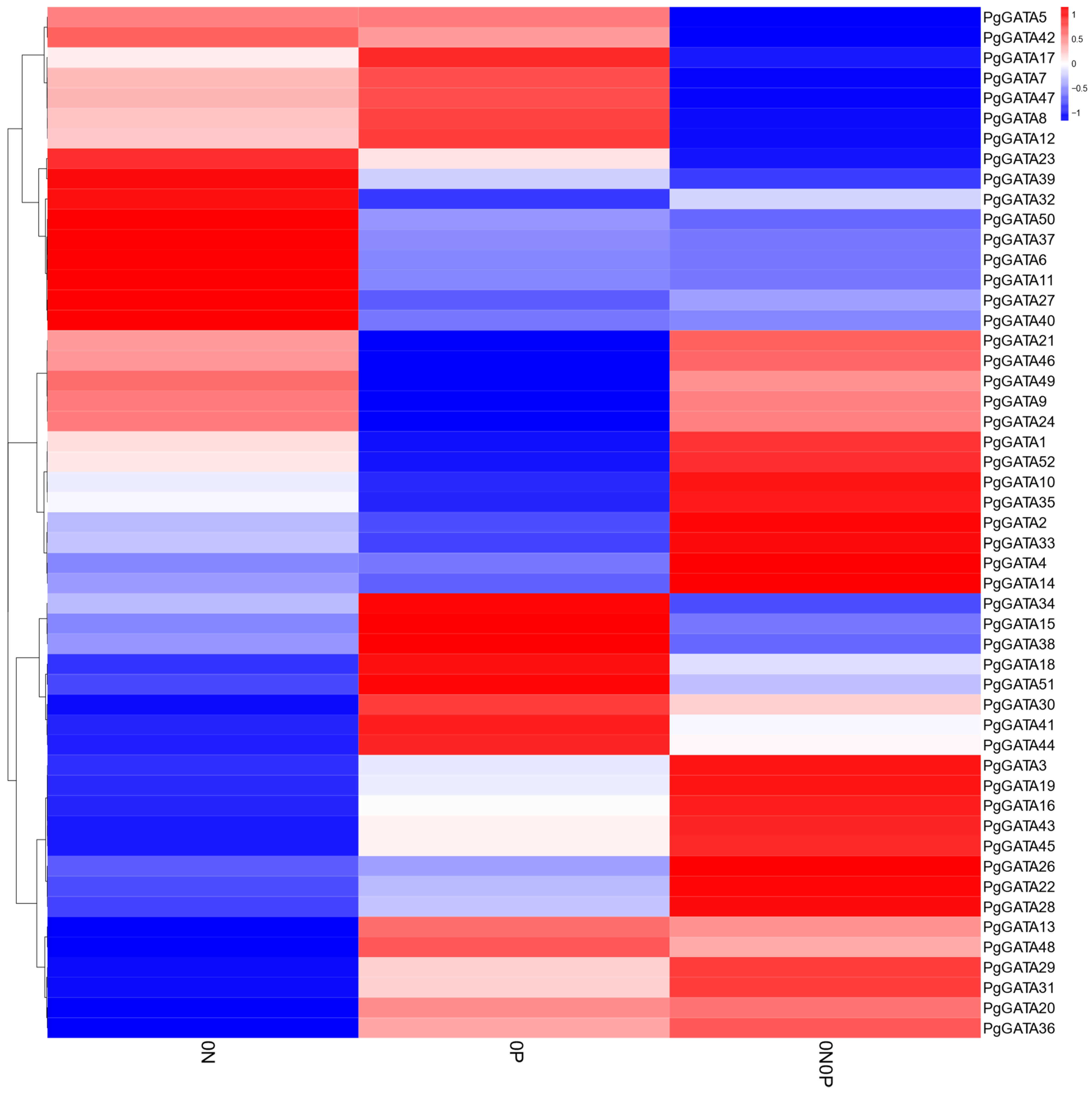
3.5. Gene Expression Analysis under N and P Deficiency
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jin, J.; He, K.; Tang, X.; Li, Z.; Lv, L. An Arabidopsis transcriptional regulatory map reveals distinct functional and evolutionary features of novel transcription factors. Mol. Biol. Evol. 2015, 32, 1767–1773. [Google Scholar] [CrossRef] [PubMed]
- Franco-Zorrilla, J.M.; López-Vidriero, I.; Carrasco, J.L.; Godoy, M.; Vera, P.; Solano, R. DNA-binding specifcities of plant transcription factors and their potential to defne target genes. Proc. Natl. Acad. Sci. USA 2014, 111, 2367–2372. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, B.; Erffelinck, M.-L.; Lacchini, E.; Ceulemans, E.; Colinas, M.; Williams, C.; Van Hamme, E.; De Clercq, R.; Perassolo, M.; Goossens, A. Interference between ER stress-related bZIP-type and jasmonate-inducible bHLH-type transcription factors in the regulation of triterpene saponin biosynthesis in Medicago truncatula. Front. Plant Sci. 2022, 13, 903793. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Sun, J.; Jiang, Z.; Ren, W.; Wang, Z.; Zhang, M.; Liu, X.; Wang, L.; Ma, W.; Xu, J. Identification and expression analysis of YABBY family genes in Platycodon grandiflorus. Plant Signal. Behav. 2023, 18, 1. [Google Scholar] [CrossRef]
- Long, G.; Zhao, C.; Zhao, P.; Zhou, C.; Zhou, Y. Transcriptomic response to cold of thermophilous medicinal plant Marsdenia tenacissima. Gene 2020, 742, 144602. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, X.; Zhang, X.; Bi, Y.; Gao, W. A bZIP transcription factor, PqbZIP1, is involved in the plant defense response of American ginseng. PeerJ 2022, 10, e12939. [Google Scholar] [CrossRef] [PubMed]
- Honghong, J.; Zhongyi, H.; Junhui, Z.; Jin, H.; Yuyang, Z.; Yingping, W.; Yuan, Y.; Luqi, H. Genome-wide analysis of Panax MADS-box genes reveals role of PgMADS41 and PgMADS44 in modulation of root development and ginsenoside synthesis. Int. J. Biol. Macromol. 2023, 233, 123648. [Google Scholar] [CrossRef]
- Reyes, J.C.; Muro-Pastor, M.I.; Florencio, F.J. The GATA family of transcription factors in Arabidopsis and rice. Plant Physiol. 2004, 134, 1718–1732. [Google Scholar] [CrossRef]
- Zhang, Z.; Ren, C.; Zou, L.; Wang, Y.; Li, S. Characterization of the GATA gene family in Vitis vinifera: Genome-wide analysis, expression profiles, and involvement in light and phytohormone response. Genome 2018, 61, 713–723. [Google Scholar] [CrossRef]
- Daniel-Vedele, F.; Caboche, M. A tobacco cDNA clone encoding a GATA-1 zinc finger protein homologous to regulators of nitrogen metabolism in fungi. Mol. Gen. Genet. 1993, 240, 365–373. Available online: https://hal.inrae.fr/hal-02703632 (accessed on 10 July 2023). [CrossRef]
- Tudzynski, B. Nitrogen regulation of fungal secondary metabolism in fungi. Front. Microbiol. 2014, 5, 656. [Google Scholar] [CrossRef]
- Zhao, Y.; Medrano, L.; Ohashi, K.; Fletcher, J.C.; Yu, H.; Sakai, H.; Meyerowitz, E.M. HANABA TARANU is a GATA transcription factor that regulates shoot apical meristem and flower development in Arabidopsis. Plant Cell 2004, 16, 2586–2600. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.P.; Koizuka, N.; Martin, R.C.; Nonogaki, H. The BME3 (Blue Micropylar End 3) GATA zinc finger transcription factor is a positive regulator of Arabidopsis seed germination. Plant J. 2005, 44, 960–971. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.M.; Zhang, Y.; Signorelli, T.; Zhao, R.; Zhu, T.; Rothstein, S. Genetic analysis of Arabidopsis GATA transcription factor gene family reveals a nitrate-inducible member important for chlorophyll synthesis and glucose sensitivity. Plant J. 2005, 44, 680–692. [Google Scholar] [CrossRef]
- Hudson, D.; Guevara, D.; Yaish, M.W.; Hannam, C.; Long, N.; Clarke, J.D.; Bi, Y.M.; Rothstein, S.J. GNC and CGA1 modulate chlorophyll biosynthesis and glutamate synthase (GLU1/Fd-GOGAT) expression in Arabidopsis. PLoS ONE 2011, 6, e26765. [Google Scholar] [CrossRef] [PubMed]
- Shikata, M.; Matsuda, Y.; Ando, K.; Nishii, A.; Takemura, M.; Yokota, A.; Kohchi, T. Characterization of Arabidopsis ZIM, a member of a novel plant-specific GATA factor gene family. J. Exp. Bot. 2004, 55, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Kiba, T.; Naitou, T.; Koizumi, N.; Yamashino, T.; Sakakibara, H.; Mizuno, T. Combinatorial microarray analysis revealing Arabidopsis genes implicated in cytokinin responses through the His->Asp Phosphorelay circuitry. Plant Cell Physiol. 2005, 46, 339–355. [Google Scholar] [CrossRef]
- Richter, R.; Behringer, C.; Zourelidou, M.; Schwechheimer, C. Convergence of auxin and gibberellin signaling on the regulation of the GATA transcription factors GNC and GNL in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2013, 110, 13192–13197. [Google Scholar] [CrossRef]
- Huang, X.Y.; Chao, D.Y.; Gao, J.P.; Zhu, M.Z.; Shi, M.; Lin, H.X. A previously unknown zinc finger protein, DST, regulates drought and salt tolerance in rice via stomatal aperture control. Genes Dev. 2009, 23, 1805–1817. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.M.; Lin, W.H.; Zhu, S.; Zhu, J.Y.; Sun, Y.; Fan, X.Y.; Cheng, M.; Hao, Y.; Oh, E.; Tian, M.; et al. Integration of light- and brassinosteroid-signaling pathways by a GATA transcription factor in Arabidopsis. Dev. Cell 2010, 19, 872–883. [Google Scholar] [CrossRef]
- Hudson, D.; Guevara, D.R.; Hand, A.J.; Xu, Z.; Hao, L.; Chen, X.; Zhu, T.; Bi, Y.M.; Rothstein, S.J. Rice cytokinin GATA transcription Factor1 regulates chloroplast development and plant architecture. Plant Physiol. 2013, 162, 132–144. [Google Scholar] [CrossRef]
- Wang, L.; Yin, H.; Qian, Q.; Yang, J.; Huang, C.; Hu, X.; Luo, D. NECK LEAF 1, a GATA type transcription factor, modulates organogenesis by regulating the expression of multiple regulatory genes during reproductive development in rice. Cell Res. 2009, 19, 598–611. [Google Scholar] [CrossRef] [PubMed]
- Jayakodi, M.; Choi, B.S.; Lee, S.C.; Kim, N.H.; Park, J.Y.; Jang, W.; Lakshmanan, M.; Mohan, S.V.G.; Lee, D.Y.; Yang, T.J. Ginseng Genome Database: An open-access platform for genomics of Panax ginseng. BMC Plant Biol. 2018, 18, 62. [Google Scholar] [CrossRef] [PubMed]
- Paek, K.Y.; Chakrabarty, D.; Hahn, E.J. Application of bioreactor systems for large scale production of horticultural and medicinal plants. Plant Cell Tiss. Organ. Cult. 2005, 81, 287–300. [Google Scholar] [CrossRef]
- Hayama, R.; Coupland, G. Shedding light on the circadian clock and the photoperiodic control of flowering. Curr.Opin. Plant Biol. 2003, 6, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Chiang, Y.H.; Zubo, Y.O.; Tapken, W.; Kim, H.J.; Lavanway, A.M.; Howard, L.; Pilon, M.; Kieber, J.J.; Schaller, G.E. Functional characterization of the GATA transcription factors GNC and CGA1 reveals their key role in chloroplast development, growth, and division in Arabidopsis. Plant Physiol. 2012, 160, 332–348. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The water culture method for growing plants without soil. Calif. Agric. Exp. Stn. Circ. 1950, 347, 1–32. [Google Scholar] [CrossRef]
- Qin, Y.; Li, X.; Wu, Y.; Wang, H.; Han, G.; Yan, Z. The Effect of Soil Enzymes and Polysaccharides Secreted by the Roots of Salvia miltiorrhiza Bunge under Drought, High Temperature, and Nitrogen and Phosphorus Deficits. Phyton-Int. J. Exp. Bot. 2024, 93, 119–135. [Google Scholar] [CrossRef]
- Kersey, P.J.; Allen, J.E.; Allot, A. Ensembl Genomes 2018: An integrated omics infrastructure for non-vertebrate species. Nucleic Acids Res. 2018, 46, D802–D808. [Google Scholar] [CrossRef]
- Finn, R.D.; Clements, J.; Eddy, S.R. HMMER web server: Interactive sequence similarity searching. Nucleic Acids Res. 2011, 39, W29–W37. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Finn, R.D.; Bateman, A.; Clements, J.; Coggill, P.; Eberhardt, R.Y.; Eddy, S.R.; Heger, A.; Hetherington, K.; Holm, L.; Mistry, J.; et al. Pfam: The protein families database. Nucleic Acids Res. 2014, 42, D222–D230. [Google Scholar] [CrossRef] [PubMed]
- Artimo, P.; Jonnalagedda, M.; Arnold, K.; Baratin, D.; Csardi, G.; Castro, E.; Duvaud, S.; Flegel, V.; Fortier, A.; Gasteiger, E.; et al. ExPASy: SIB bioinformatics resource portal. Nucleic Acids Res. 2012, 40, W597–W603. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Nei, M.; Dudley, J.; Tamura, K. MEGA: A biologist-centric software for evolutionary analysis of DNA and protein sequences. Brief. Bioinform. 2008, 9, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL) v4: Recent updates and new developments. Nucleic Acids Res. 2019, 47, W256–W259. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tang, H.; Debarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.H.; Jin, H.; Marler, B.; Guo, H.; et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhang, Y.; Zhang, Z.; Zhu, J.; Yu, J. KaKs_Calculator 2.0: A toolkit incorporating gamma-series methods and sliding window strategies. Genom. Proteom. Bioinform. 2010, 8, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Jin, J.; Guo, A.Y.; Zhang, H.; Luo, J.; Gao, G. GSDS 2.0: An upgraded gene feature visualization server. Bioinformatics 2015, 31, 1296–1307. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. MEME SUITE: Tools for motif discovery and searching. Nucleic Acids Res. 2009, 37, W202–W208. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant. 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Peer, Y.V.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Waterhouse, A.M.; Procter, J.B.; Martin, D.M.; Clamp, M.; Barton, G.J. Jalview Version 2—A multiple sequence alignment editor and analysis workbench. Bioinformatics 2009, 25, 1189–1191. [Google Scholar] [CrossRef]
- Mundy, J.; Yamaguchi-Shinozaki, K.; Chua, N.H. Nuclear proteins bind conserved elements in the abscisic acid-responsive promoter of a rice rab gene. Proc. Natl. Acad. Sci. USA 1990, 87, 1406–1410. [Google Scholar] [CrossRef] [PubMed]
- Dolferus, R.; Klok, E.J.; Ismond, K.; Delessert, C.; Wilson, S.; Good, A.; Peacock, J.; Dennis, L. Molecular basis of the anaerobic response in plants. IUBMB Life 2001, 51, 79–82. [Google Scholar] [CrossRef]
- Shin, R.; Burch, A.Y.; Huppert, K.A.; Tiwari, S.B.; Murphy, A.S.; Guilfoyle, T.J.; Schachtman, D.P. The Arabidopsis transcription factor MYB77 modulates auxin signal transduction. Plant Cell 2007, 19, 2440–2453. [Google Scholar] [CrossRef]
- Mizoguchi, T.; Wheatley, K.; Hanzawa, Y.; Wright, L.; Mizoguchi, M.; Song, H.R.; Carre, I.A.; Coupland, G. LHY and CCA1 are partially redundant genes required to maintain circadian rhythms in Arabidopsis. Dev. Cell. 2002, 2, 629–641. [Google Scholar] [CrossRef]
- Lopez-Maury, L.; Marguerat, S.; Bahler, J. Tuning gene expression to changing environments: From rapid responses to evolutionary adaptation. Nat. Rev. Genet. 2008, 9, 583–593. [Google Scholar] [CrossRef] [PubMed]
- Abe, H.; Urao, T.; Ito, T.; Seki, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Arabidopsis AtMYC2 (bHLH) and AtMYB2 (MYB) function as transcriptional activators in abscisic acid signaling. Plant Cell 2003, 15, 63–78. [Google Scholar] [CrossRef]
- Stracke, R.; Ishihara, H.; Hirofumi, H.; Gunnar, B.; Barsch, A.; Aiko, M.; Mehrtens, F.; Niehaus, K.; Weisshaar, B. Differential regulation of closely related R2R3-MYB transcription factors controls flavonol accumulation in different parts of the Arabidopsis thaliana seedling. Plant J. 2007, 50, 660–677. [Google Scholar] [CrossRef]
- Gilmartin, P.M.; Sarokin, L.; Memelink, J.; Chua, N.H. Molecular light switches for plant genes. Plant Cell 1990, 2, 369. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, L.; Liu, L.; Jia, B.; Ye, Z.; Tang, X.; Heng, W.; Liu, L. Functional Analysis of PbbZIP11 Transcription Factor in Response to Cold Stress in Arabidopsis and Pear. Plants 2023, 13, 24. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, K.; Yang, L.; Lv, X.; Wu, Y.; Liu, H.; Lu, Q.; Chen, D.; Qiu, D. Identification and characterization of MYC transcription factors in Taxus sp. Gene 2018, 675, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.K.; Geisler, M.; Springer, P.S. LATERAL ORGAN FUSION1 and LATERAL ORGAN FUSION2 function in lateral organ separation and axillary meristem formation in Arabidopsis. Development 2009, 136, 2423–2432. [Google Scholar] [CrossRef]
- Banerjee, J.; Sahoo, D.K.; Raha, S.; Sarkar, S.; Dey, N.; Maiti, I.B. A region containing an as-1 element of Dahlia Mosaic Virus (DaMV) subgenomic transcript promoter plays a key role in green tissue-and root-specific expression in plants. Plant Mol. Biol. Rep. 2015, 33, 532–556. [Google Scholar] [CrossRef]
- Ali, Q.; Mushtaq, N.; Amir, R.; Gul, A.; Tahir, M.; Munir, F. Genome-wide promoter analysis, homology modeling and protein interaction network of Dehydration Responsive Element Binding (DREB) gene family in Solanum tuberosum. PLoS ONE 2021, 16, e0261215. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, G.; Qu, L.J.; Gu, H. Characterization of Arabidopsis MYB transcription factor gene AtMYB17 and its possible regulation by LEAFY and AGL15. J. Genet. Genom. 2009, 36, 99–107. [Google Scholar] [CrossRef]
- Dikstein, R. The unexpected traits associated with core promoter elements. Transcription 2011, 2, 201–206. [Google Scholar] [CrossRef]
- Paz-Ares, J.; Ghosal, D.; Wienand, U.; Peterson, P.A.; Saedler, H. The regulatory c1 locus of Zea mays encodes a protein with homology to myb proto-oncogene products and with structural similarities to transcriptional activators. EMBO J. 1987, 6, 3553–3558. [Google Scholar] [CrossRef]
- Liu, T.; Luo, T.; Guo, X.; Zou, X.; Zhou, D.; Afrin, S.; Li, G.; Zhang, Y.; Zhang, R.; Luo, Z. PgMYB2, a MeJA-Responsive Transcription Factor, Positively Regulates the Dammarenediol Synthase Gene Expression in Panax Ginseng. Int. J. Mol. Sci. 2019, 20, 2219. [Google Scholar] [CrossRef]
- Jie, Z.; Juan, L.; Chao, J.; Tie-Gui, N.; Li-Ping, K.; Li, Z.; Yuan, Y.; Lu-Qi, H. Expression analysis of transcription factor ERF gene family of Panax ginseng. China J. Chin. Mater. Medica 2020, 45, 2515–2522. [Google Scholar] [CrossRef]
- Di, P.; Wang, P.; Yan, M.; Han, P.; Huang, X.; Yin, L.; Yan, Y.; Xu, Y.; Wang, Y. Genome-wide characterization and analysis of WRKY transcription factors in Panax ginseng. BMC Genom. 2021, 22, 834. [Google Scholar] [CrossRef]
- Chu, Y.; Xiao, S.; Su, H.; Liao, B.; Zhang, J.; Xu, J.; Chen, S. Genome-wide characterization and analysis of bHLH transcription factors in Panax ginseng. Acta Pharm. Sin. B 2018, 8, 666–677. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Xiaoming, Y.; Dianyuan, C.; Hu, F.; Jianming, L. Identification, Phylogenetic Evolution and Expression Analysis of GATA Transcription Factor Family in Maize (Zea mays). Int. J. Agric. Biol. 2020, 23, 637–643. [Google Scholar]
- Lai, D.; Yao, X.; Yan, J.; Gao, A.; Yang, H.; Xiang, D.; Ruan, J.; Fan, Y.; Cheng, J. Genome-wide identification, phylogenetic and expression pattern analysis of GATA family genes in foxtail millet (Setaria italica). BMC Genom. 2022, 23, 549. [Google Scholar] [CrossRef]
- Feng, X.; Yu, Q.; Zeng, J.; He, X.; Liu, W. Genome-wide identification and characterization of GATA family genes in wheat. BMC Plant Biol. 2022, 22, 372. [Google Scholar] [CrossRef]
- Li, X.; Deng, X.; Han, S.; Zhang, X.; Dai, T. Genome-Wide Identification and Expression Analysis of GATA Gene Family under Different Nitrogen Levels in Arachis hypogaea L. Agronomy 2023, 13, 215. [Google Scholar] [CrossRef]
- Yu, R.; Chang, Y.; Chen, H.; Feng, J.; Wang, H.; Tian, T.; Gao, G. Genome-wide identification of the GATA gene family in potato (Solanum tuberosum L.) and expression analysis. J. Plant Biochem. Biotechnol. 2022, 31, 37–48. [Google Scholar] [CrossRef]
- Panchy, N.; Lehti-Shiu, M.; Shiu, S.H. Evolution of Gene Duplication in Plants. Plant Physiol. 2016, 171, 2294–2316. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Nan, S.; Li, Y.; Yu, C.; Zhou, L.; Hu, J.; Jin, X.; Han, Y.; Wang, S. Comprehensive Analysis and Characterization of the GATA Gene Family, with Emphasis on the GATA6 Transcription Factor in Poplar. Int. J. Mol. Sci. 2023, 24, 14118. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Lai, D.; Zhou, M.; Ruan, J.; Ma, C.; Wu, W.; Weng, W.; Fan, Y.; Cheng, J. Genome-wide identification, evolution and expression pattern analysis of the GATA gene family in Sorghum bicolor. Front. Plant Sci. 2023, 14, 1163357. [Google Scholar] [CrossRef]
- Hu, Y.; Huang, J.; Yu, L.; Wang, C.; Zhang, X.; Cheng, X.; Yu, H.; Zhang, K. Identification, Characterization, and Expression Profiling of Maize GATA Gene Family in Response to Abiotic and Biotic Stresses. Agronomy 2023, 13, 1921. [Google Scholar] [CrossRef]
- Yao, X.; Zhou, M.; Ruan, J.; He, A.; Ma, C.; Wu, W.; Lai, D.; Fan, Y.; Gao, A.; Weng, W.; et al. Genome-Wide Identification, Evolution, and Expression Pattern Analysis of the GATA Gene Family in Tartary Buckwheat (Fagopyrum tataricum). Int. J. Mol. Sci. 2022, 23, 12434. [Google Scholar] [CrossRef] [PubMed]
- Terzaghi, W.B.; Cashmore, A.R. Light-regulated transcription. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1995, 46, 445–474. [Google Scholar] [CrossRef]
- Jeong, M.J.; Shih, M.C. Interaction of a GATA factor with cis-acting elements involved in light regulation of nuclear genes encoding chloroplast glyceraldehyde-3-phosphate dehydrogenase in Arabidopsis. Biochem. Biophys. Res. Commun. 2003, 300, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Jing, Z.; Zehua, S.; Dengke, S.; Shuqi, S.; Lingdan, L.; Liang, S.; Ang, R.; Hanshou, Y.; Mingwen, Z. Dual functions of AreA, a GATA transcription factor, on influencing ganoderic acid biosynthesis in Ganoderma lucidum. Environ. Microbiol. 2019, 21, 4166–4179. [Google Scholar] [CrossRef]
- Ostadi, A.; Javanmard, A.; Machiani, M.A.; Morshedloo, M.R.; Nouraein, M.; Rasouli, F.; Maggi, F. Effect of different fertilizer sources and harvesting time on the growth characteristics, nutrient uptakes, essential oil productivity and composition of Mentha x piperita L. Ind. Crops Prod. 2020, 148, 112290. [Google Scholar] [CrossRef]
- Sun, J.; Luo, H.; Yu, Q.; Kou, B.; Jiang, Y.; Weng, L.; Xiao, C. Optimal NPK Fertilizer Combination Increases Panax ginseng Yield and Quality and Affects Diversity and Structure of Rhizosphere Fungal Communities. Front. Microbiol. 2022, 13, 919434. [Google Scholar] [CrossRef]
- Guo, Q.; Yan, L.; Korpelainen, H.; Niinemets, Ü.; Li, C. Plant-plant interactions and n fertilization shape soil bacterial and fungal communities. Soil. Biol. Biochem. 2018, 128, 127–138. [Google Scholar] [CrossRef]
- Du, Y.; Wang, T.; Wang, C.; Anane, P.S.; Liu, S.; Paz-Ferreiro, J. Nitrogen fertilizer is a key factor affecting the soil chemical and microbial communities in a Mollisol. Can. J. Microbiol. 2019, 65, 510–521. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Han, X.; Tang, S.; Xia, X.; Yin, W. Erratum to: Poplar GATA transcription factor PdGNC is capable of regulating chloroplast ultrastructure, photosynthesis, and vegetative growth in Arabidopsis under varying nitrogen levels. Plant Cell 2014, 119, 329. [Google Scholar] [CrossRef]
- Lowry, J.A.; Atchley, W.R. Molecular evolution of the GATA family of transcription factors: Conservation within the DNA-binding domain. J. Mol. Evol. 2000, 50, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Devaiah, B.N.; Nagarajan, V.K.; Raghothama, K.G. Phosphate homeostasis and root development in Arabidopsis are synchronized by the zinc finger transcription factor ZAT6. Plant Physiol. 2007, 145, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.C.; Wang, Y.; Wang, E.T. Improving the utilization efficiency of nitrogen, phosphorus and potassium: Current situation and future perspectives. Sci. Sin. Vitae 2021, 51, 1415–1423. [Google Scholar] [CrossRef]
- Hu, B.; Jiang, Z.; Wang, W.; Qiu, Y.; Zhang, Z.; Liu, Y.; Li, A.; Gao, X.; Liu, L.; Qian, Y.; et al. Nitrate-NRT1.1B-SPX4 cascade integrates nitrogen and phosphorus signalling networks in plants. Nat. Plants 2019, 5, 401–413. [Google Scholar] [CrossRef]
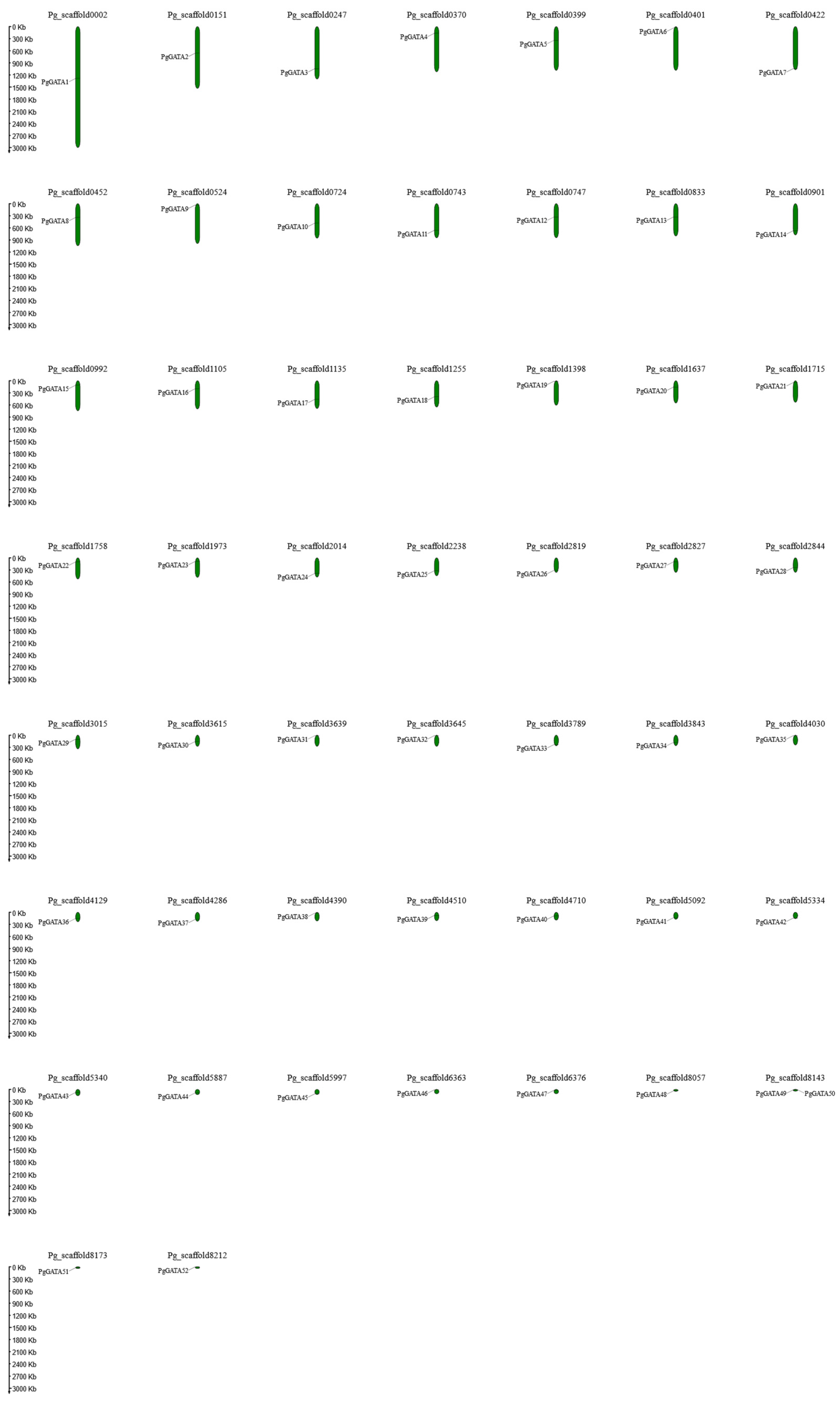
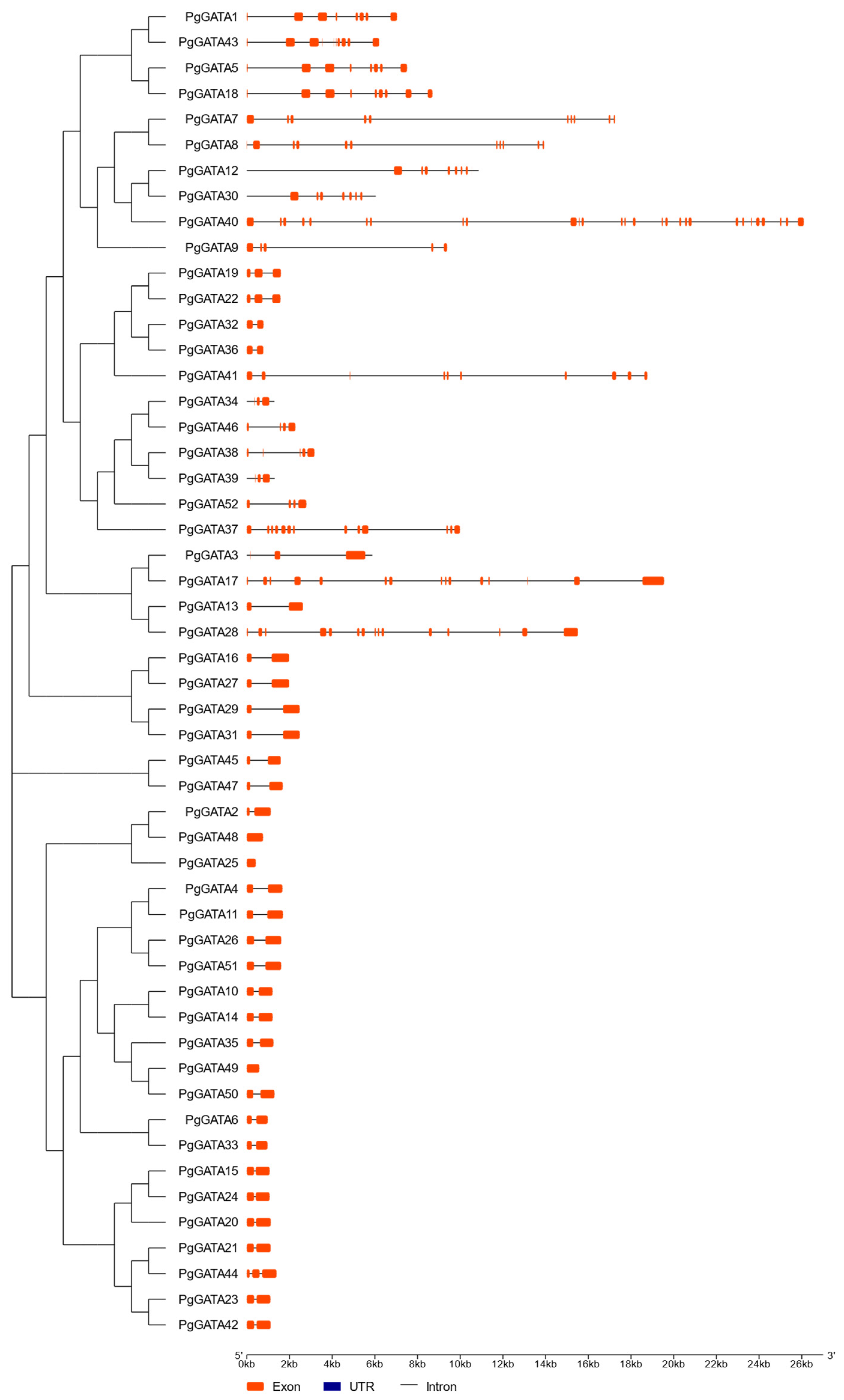
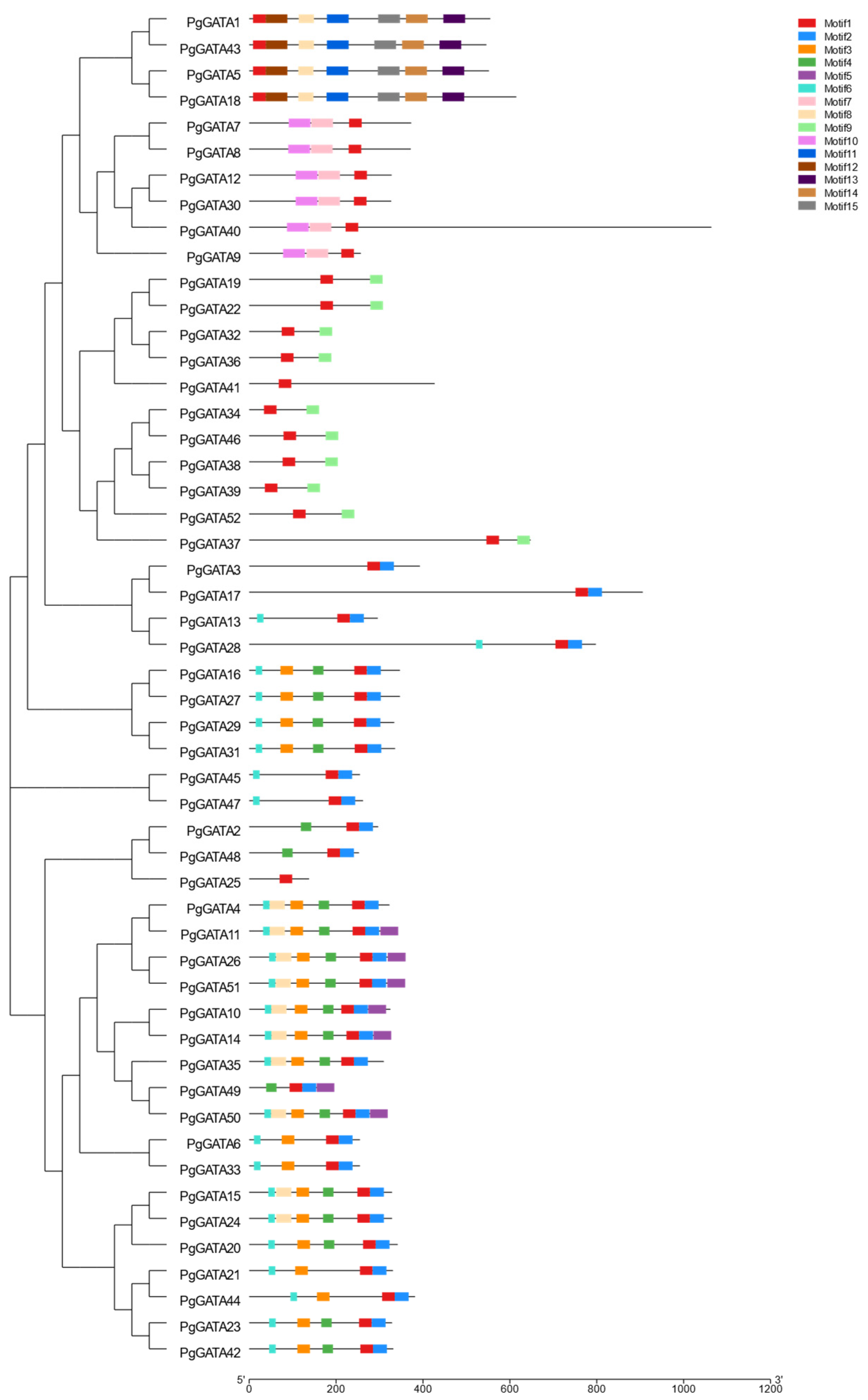
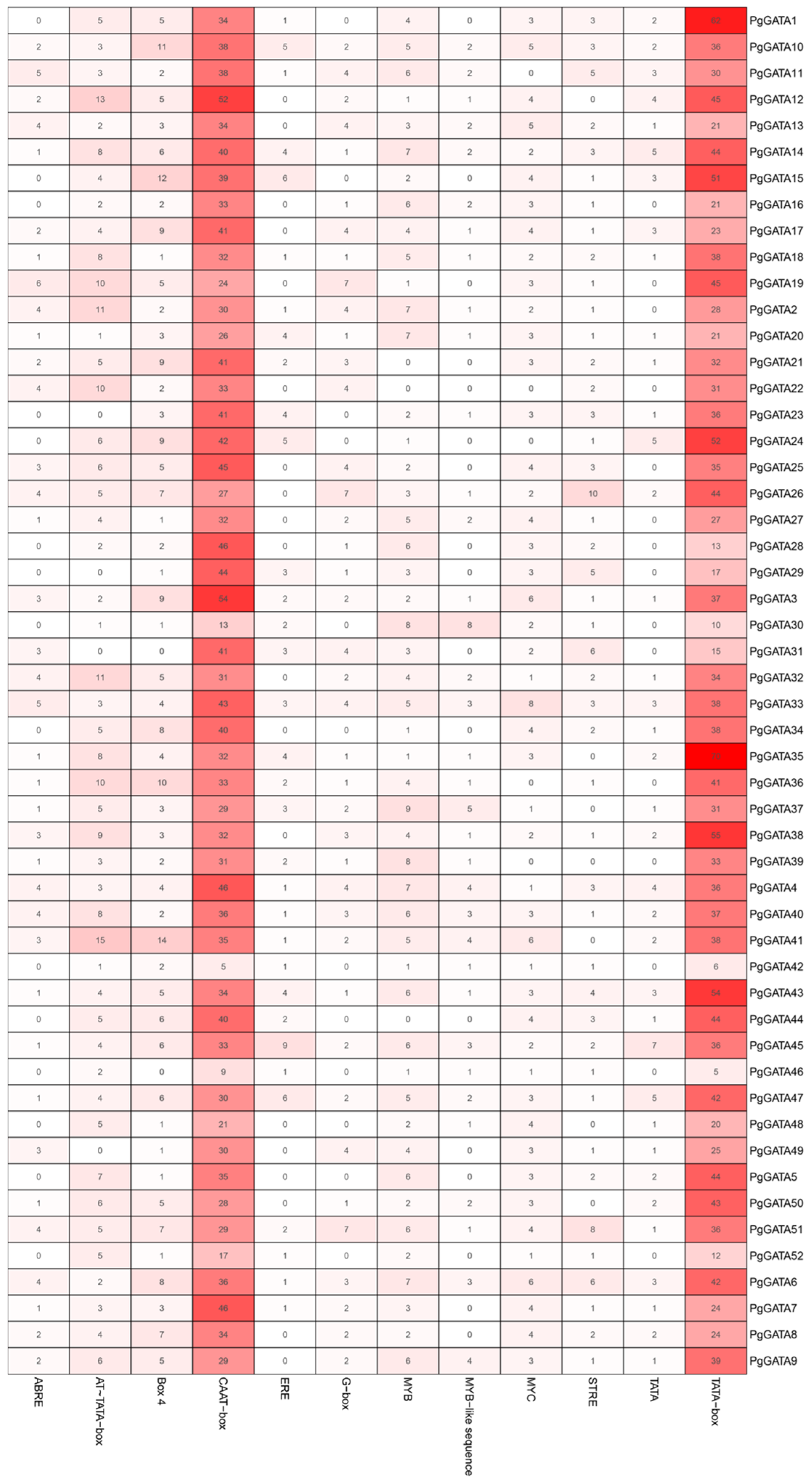

| Gene Name | Gene ID | Pfam ID | Chromosomes | Protein | Position of GATA Domain | |||
|---|---|---|---|---|---|---|---|---|
| Amino Acids | MW (kDa) | pI | From | To | ||||
| PgGATA1 | Pg_S0002.19 | PF00320.28 | Pg_scaffold0002:1288028-1295063 | 555 | 60.85929 | 7.86 | 1 | 35 |
| PgGATA2 | Pg_S0151.6 | PF00320.28 | Pg_scaffold0151:654526-655644 | 297 | 32.88809 | 8.61 | 1 | 35 |
| PgGATA3 | Pg_S0247.64 | PF00320.28 | Pg_scaffold0247:1040767-1046648 | 393 | 44.42883 | 8.93 | 1 | 35 |
| PgGATA4 | Pg_S0370.2 | PF00320.28 | Pg_scaffold0370:163245-164909 | 323 | 35.79844 | 5.95 | 1 | 34 |
| PgGATA5 | Pg_S0399.28 | PF00320.28 | Pg_scaffold0399:340107-347611 | 552 | 61.40093 | 7.91 | 1 | 35 |
| PgGATA6 | Pg_S0401.2 | PF00320.28 | Pg_scaffold0401:29144-30122 | 255 | 28.86266 | 6.45 | 1 | 34 |
| PgGATA7 | Pg_S0422.4 | PF00320.28 | Pg_scaffold0422:1034667-1051922 | 373 | 40.82508 | 5.67 | 1 | 35 |
| PgGATA8 | Pg_S0452.1 | PF00320.28 | Pg_scaffold0452:329642-343566 | 372 | 40.66498 | 5.52 | 1 | 35 |
| PgGATA9 | Pg_S0524.1 | PF00320.28 | Pg_scaffold0524:33560-42945 | 257 | 28.11685 | 6.17 | 1 | 33 |
| PgGATA10 | Pg_S0724.42 | PF00320.28 | Pg_scaffold0724:481073-482279 | 325 | 36.24711 | 6.01 | 1 | 34 |
| PgGATA11 | Pg_S0743.16 | PF00320.28 | Pg_scaffold0743:662369-664059 | 342 | 38.09921 | 5.9 | 1 | 34 |
| PgGATA12 | Pg_S0747.56 | PF00320.28 | Pg_scaffold0747:329359-340222 | 328 | 35.28915 | 5.46 | 1 | 35 |
| PgGATA13 | Pg_S0833.16 | PF00320.28 | Pg_scaffold0833:332798-335429 | 296 | 33.44801 | 8.9 | 1 | 35 |
| PgGATA14 | Pg_S0901.25 | PF00320.28 | Pg_scaffold0901:668023-669233 | 326 | 36.11471 | 5.35 | 1 | 34 |
| PgGATA15 | Pg_S0992.10 | PF00320.28 | Pg_scaffold0992:111377-112445 | 329 | 36.29037 | 5.58 | 1 | 34 |
| PgGATA16 | Pg_S1105.5 | PF00320.28 | Pg_scaffold1105:191951-193930 | 347 | 38.21527 | 6.46 | 1 | 34 |
| PgGATA17 | Pg_S1135.23 | PF00320.28 | Pg_scaffold1135:457378-476922 | 906 | 10.274274 | 8.83 | 1 | 35 |
| PgGATA18 | Pg_S1255.7 | PF00320.28 | Pg_scaffold1255:388046-396740 | 615 | 68.22211 | 8.62 | 1 | 35 |
| PgGATA19 | Pg_S1398.1 | PF00320.28 | Pg_scaffold1398:8780-10376 | 306 | 34.09219 | 9.35 | 1 | 34 |
| PgGATA20 | Pg_S1637.11 | PF00320.28 | Pg_scaffold1637:158676-159794 | 342 | 38.15677 | 5.42 | 1 | 34 |
| PgGATA21 | Pg_S1715.1 | PF00320.28 | Pg_scaffold1715:48429-49542 | 331 | 36.3334 | 6.72 | 1 | 35 |
| PgGATA22 | Pg_S1758.8 | PF00320.28 | Pg_scaffold1758:102416-103992 | 307 | 34.26337 | 9.45 | 1 | 34 |
| PgGATA23 | Pg_S1973.5 | PF00320.28 | Pg_scaffold1973:85000-86103 | 329 | 36.08194 | 5.13 | 1 | 35 |
| PgGATA24 | Pg_S2014.30 | PF00320.28 | Pg_scaffold2014:381887-382955 | 329 | 36.30244 | 5.74 | 1 | 34 |
| PgGATA25 | Pg_S2238.19 | PF00320.28 | Pg_scaffold2238:317522-317938 | 138 | 15.77091 | 9.25 | 1 | 35 |
| PgGATA26 | Pg_S2819.19 | PF00320.28 | Pg_scaffold2819:309794-311405 | 359 | 39.95244 | 6.08 | 1 | 34 |
| PgGATA27 | Pg_S2827.1 | PF00320.28 | Pg_scaffold2827:98960-100941 | 347 | 38.11916 | 6.71 | 1 | 34 |
| PgGATA28 | Pg_S2844.7 | PF00320.28 | Pg_scaffold2844:230542-246053 | 798 | 90.19806 | 8.66 | 1 | 35 |
| PgGATA29 | Pg_S3015.2 | PF00320.28 | Pg_scaffold3015:102177-104654 | 334 | 36.69519 | 6.56 | 1 | 34 |
| PgGATA30 | Pg_S3615.15 | PF00320.28 | Pg_scaffold3615:147233-153273 | 327 | 35.2272 | 5.38 | 1 | 35 |
| PgGATA31 | Pg_S3639.1 | PF00320.28 | Pg_scaffold3639:5840-8320 | 336 | 36.80338 | 6.71 | 1 | 34 |
| PgGATA32 | Pg_S3645.1 | PF00320.28 | Pg_scaffold3645:5740-6522 | 190 | 21.18405 | 9.27 | 1 | 34 |
| PgGATA33 | Pg_S3789.4 | PF00320.28 | Pg_scaffold3789:234760-235726 | 255 | 28.87458 | 6.5 | 1 | 34 |
| PgGATA34 | Pg_S3843.20 | PF00320.28 | Pg_scaffold3843:179679-180972 | 160 | 17.32369 | 9.86 | 1 | 35 |
| PgGATA35 | Pg_S4030.1 | PF00320.28 | Pg_scaffold4030:8653-9897 | 310 | 34.27078 | 5.33 | 1 | 34 |
| PgGATA36 | Pg_S4129.5 | PF00320.28 | Pg_scaffold4129:153794-154568 | 188 | 21.09301 | 9.45 | 1 | 34 |
| PgGATA37 | Pg_S4286.18 | PF00320.28 | Pg_scaffold4286:165564-175546 | 648 | 71.9843 | 6 | 1 | 35 |
| PgGATA38 | Pg_S4390.1 | PF00320.28 | Pg_scaffold4390:25504-28673 | 203 | 21.98218 | 9.41 | 1 | 35 |
| PgGATA39 | Pg_S4510.13 | PF00320.28 | Pg_scaffold4510:75064-76371 | 162 | 17.51885 | 9.71 | 1 | 35 |
| PgGATA40 | Pg_S4710.5 | PF00320.28 | Pg_scaffold4710:72488-98579 | 1064 | 117.47788 | 6.42 | 1 | 35 |
| PgGATA41 | Pg_S5092.5 | PF00320.28 | Pg_scaffold5092:121295-140056 | 427 | 47.29692 | 7.51 | 1 | 34 |
| PgGATA42 | Pg_S5334.7 | PF00320.28 | Pg_scaffold5334:138662-139775 | 332 | 36.51437 | 4.96 | 1 | 35 |
| PgGATA43 | Pg_S5340.2 | PF00320.28 | Pg_scaffold5340:76449-82649 | 546 | 60.08213 | 6.94 | 1 | 35 |
| PgGATA44 | Pg_S5887.5 | PF00320.28 | Pg_scaffold5887:88824-90214 | 382 | 41.96394 | 6.6 | 1 | 35 |
| PgGATA45 | Pg_S5997.3 | PF00320.28 | Pg_scaffold5997:103747-105336 | 255 | 28.66512 | 6.95 | 1 | 34 |
| PgGATA46 | Pg_S6363.2 | PF00320.28 | Pg_scaffold6363:10458-12732 | 204 | 22.31168 | 9.93 | 1 | 35 |
| PgGATA47 | Pg_S6376.1 | PF00320.28 | Pg_scaffold6376:16784-18465 | 262 | 29.36977 | 6.95 | 1 | 34 |
| PgGATA48 | Pg_S8057.2 | PF00320.28 | Pg_scaffold8057:31367-32128 | 253 | 28.25107 | 9.19 | 1 | 35 |
| PgGATA49 | Pg_S8143.1 | PF00320.28 | Pg_scaffold8143:10176-10763 | 195 | 21.86161 | 9.98 | 1 | 34 |
| PgGATA50 | Pg_S8143.2 | PF00320.28 | Pg_scaffold8143:14520-15815 | 318 | 35.28189 | 5.34 | 1 | 34 |
| PgGATA51 | Pg_S8173.3 | PF00320.28 | Pg_scaffold8173:21446-23055 | 358 | 39.67611 | 6.08 | 1 | 34 |
| PgGATA52 | Pg_S8212.2 | PF00320.28 | Pg_scaffold8212:3522-6309 | 241 | 26.38869 | 9.91 | 1 | 35 |
| Sequence | Method | Ka | Ks | Ka/Ks | p-Value (Fisher) | Length |
|---|---|---|---|---|---|---|
| PgGATA1-PgGATA11 | MA | 0.921489 | 1.275 | 1.18348 | 1.49 × 10−12 | 1026 |
| PgGATA1-PgGATA12 | MA | 0.89025 | 1.40254 | 1.07002 | 7.05 × 10−24 | 984 |
| PgGATA1-PgGATA13 | MA | 0.889125 | 1.48231 | 1.05362 | 4.50 × 10−27 | 888 |
| PgGATA1-PgGATA14 | MA | 0.903851 | 1.34656 | 1.02993 | 2.90 × 10−16 | 978 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, H.; Sun, H.; Shao, C.; Qian, J.; Lv, B.; Zhu, J.; Zhang, Y. Genome-Wide Prediction and Expression Characterization of the GATA Gene Family under Nitrogen and Phosphate Deficiency in Panax ginseng. Horticulturae 2024, 10, 282. https://doi.org/10.3390/horticulturae10030282
Liang H, Sun H, Shao C, Qian J, Lv B, Zhu J, Zhang Y. Genome-Wide Prediction and Expression Characterization of the GATA Gene Family under Nitrogen and Phosphate Deficiency in Panax ginseng. Horticulturae. 2024; 10(3):282. https://doi.org/10.3390/horticulturae10030282
Chicago/Turabian StyleLiang, Hao, Hai Sun, Cai Shao, Jiaqi Qian, Bochen Lv, Jiapeng Zhu, and Yayu Zhang. 2024. "Genome-Wide Prediction and Expression Characterization of the GATA Gene Family under Nitrogen and Phosphate Deficiency in Panax ginseng" Horticulturae 10, no. 3: 282. https://doi.org/10.3390/horticulturae10030282
APA StyleLiang, H., Sun, H., Shao, C., Qian, J., Lv, B., Zhu, J., & Zhang, Y. (2024). Genome-Wide Prediction and Expression Characterization of the GATA Gene Family under Nitrogen and Phosphate Deficiency in Panax ginseng. Horticulturae, 10(3), 282. https://doi.org/10.3390/horticulturae10030282






