BcBZR1 Regulates Leaf Inclination Angle in Non-Heading Chinese Cabbage (Brassica campestris ssp. chinensis Makino)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
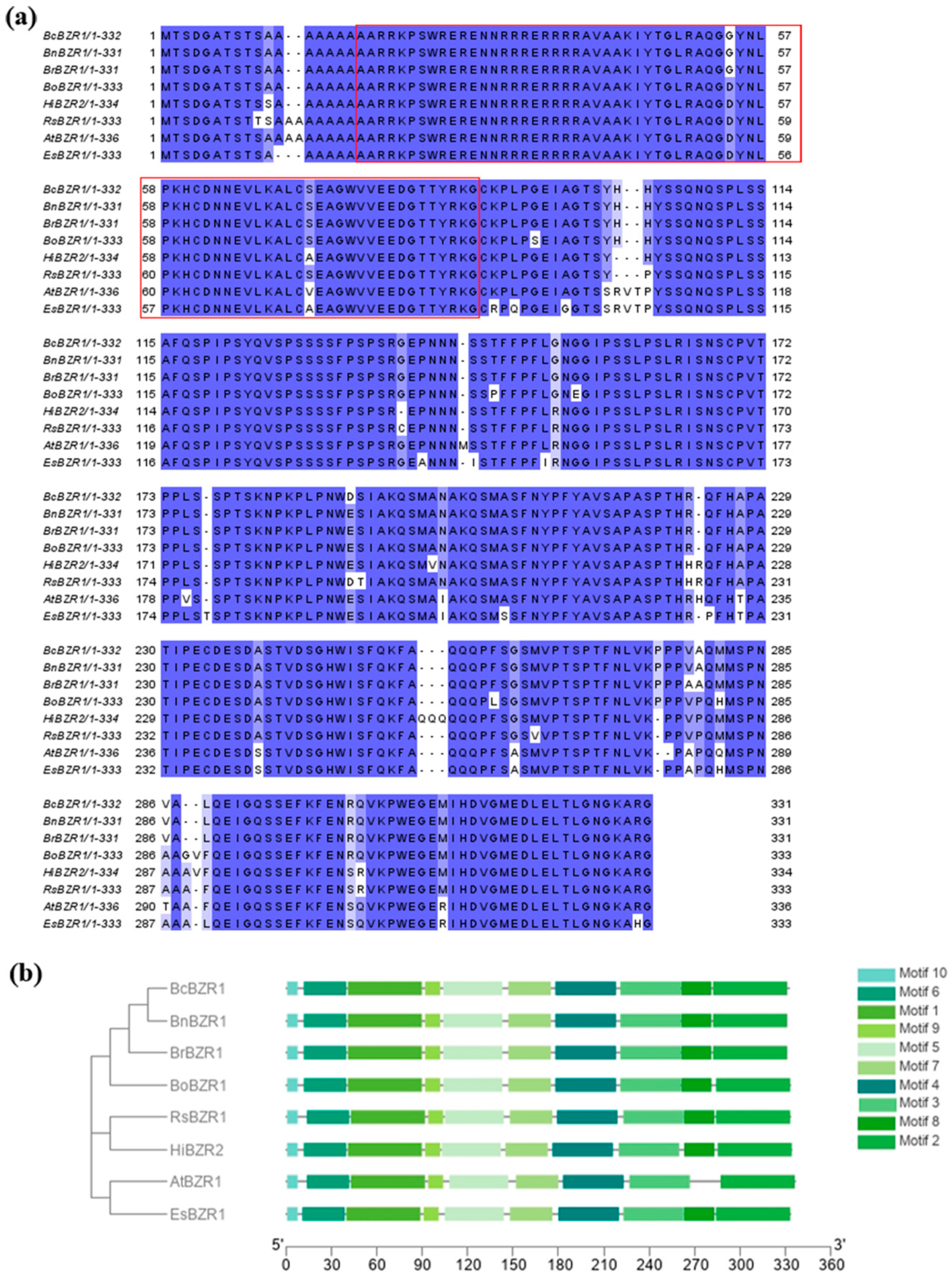
2.2. Sequence Analysis of BcBZR1
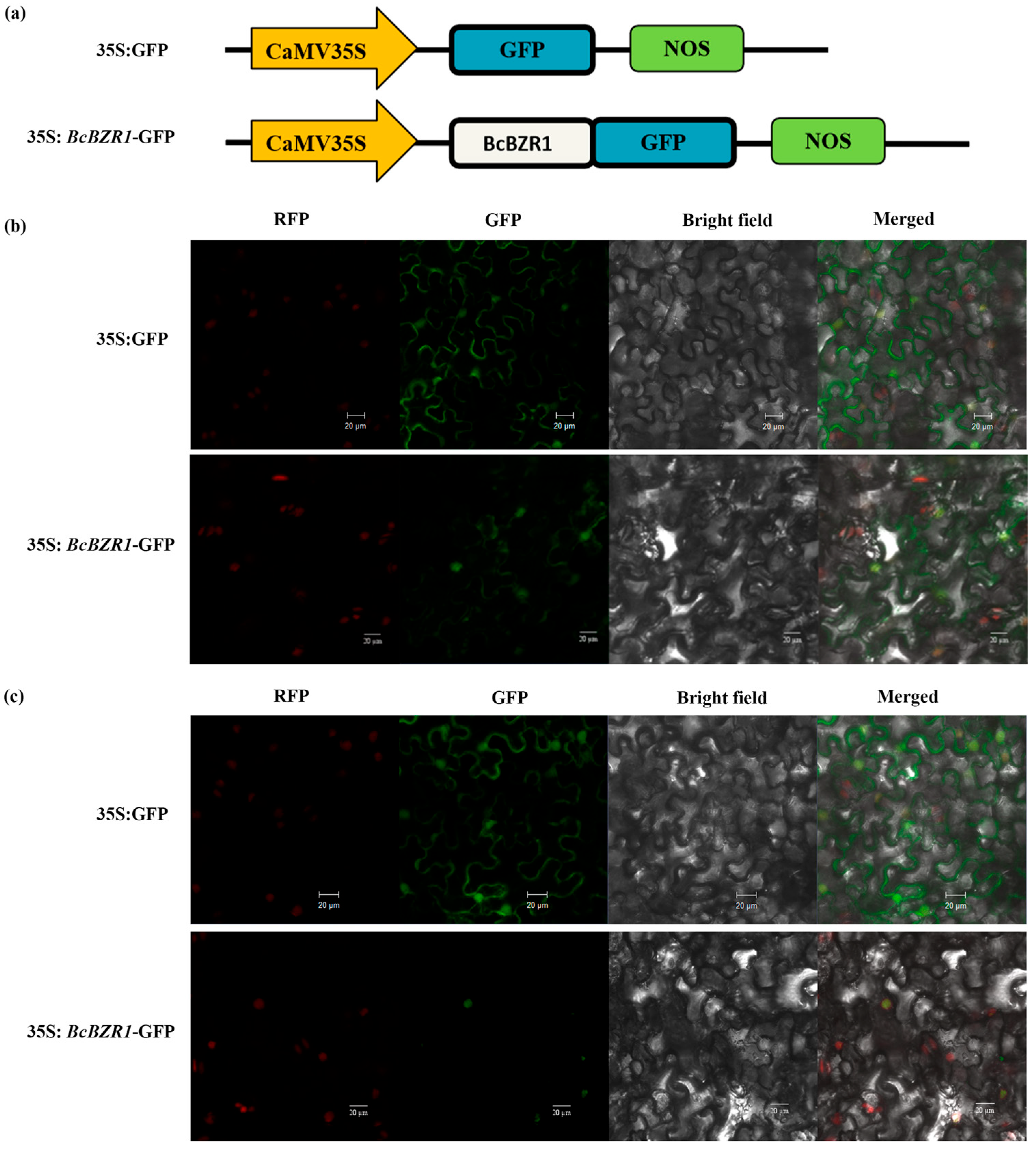
2.3. Subcellular Localization
2.4. Agrobacterium-Mediated Transformation
2.5. RNA Extraction and qRT-PCR Analysis
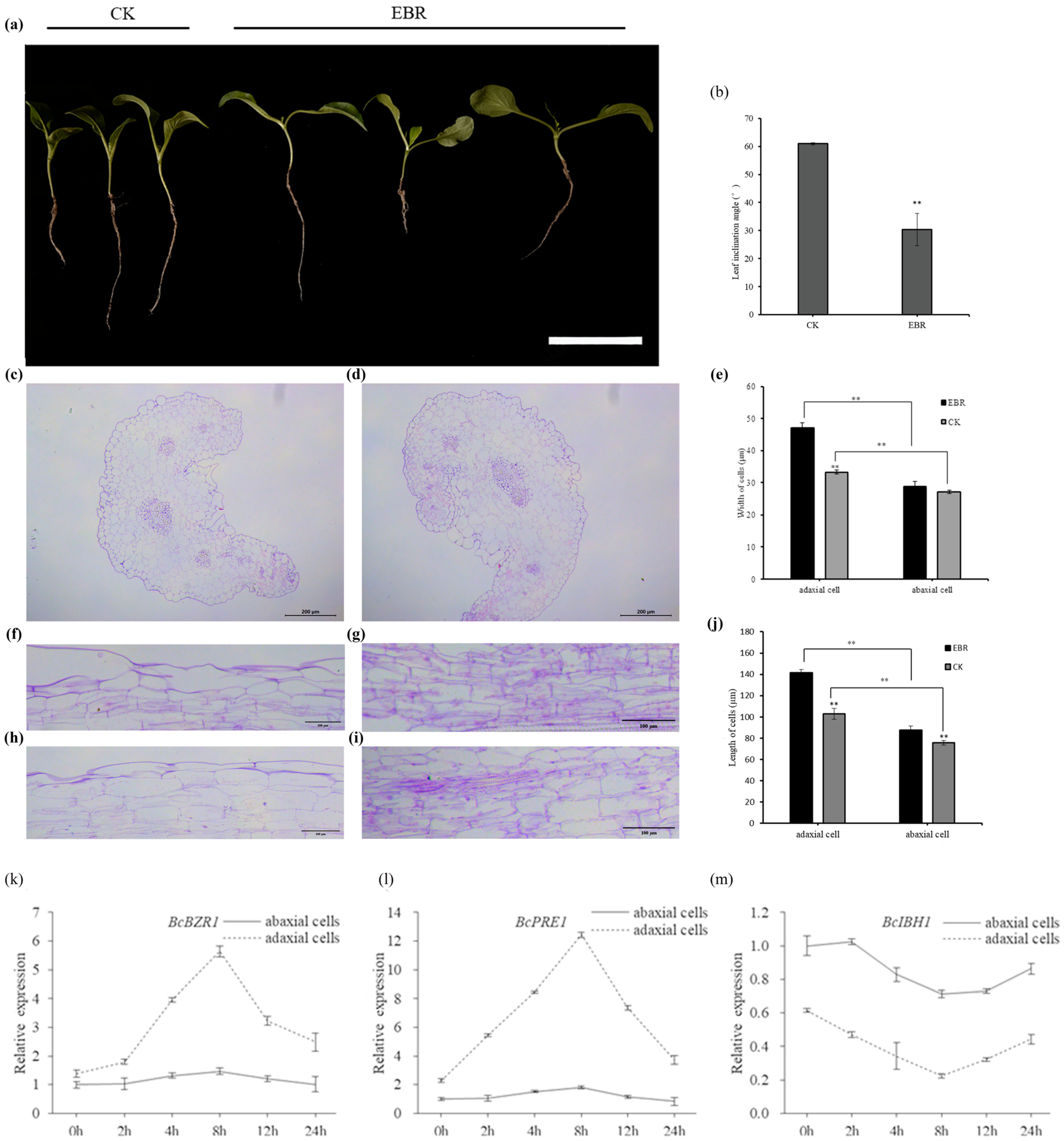
2.6. EBR Treatment
2.7. Low-Temperature Stress Treatment
2.8. Bimolecular Fluorescence Complementation
3. Results
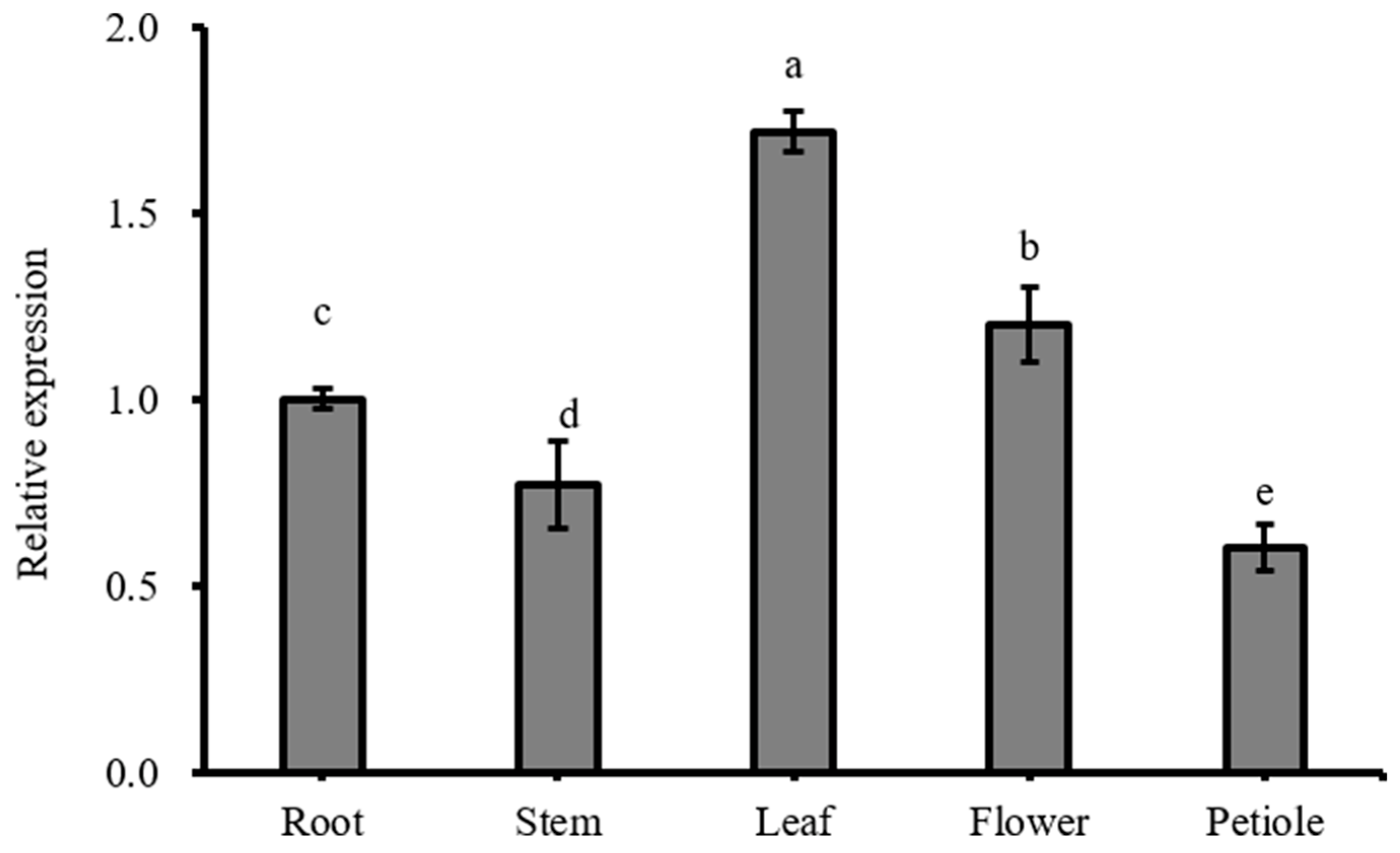
3.1. Sequence and Expression Analysis of BcBZR1
3.2. Subcellular Localization of BcBZR1 Protein
3.3. Overexpression of BcBZR1 in Arabidopsis Causes Leaf Inclination Angle Decrease
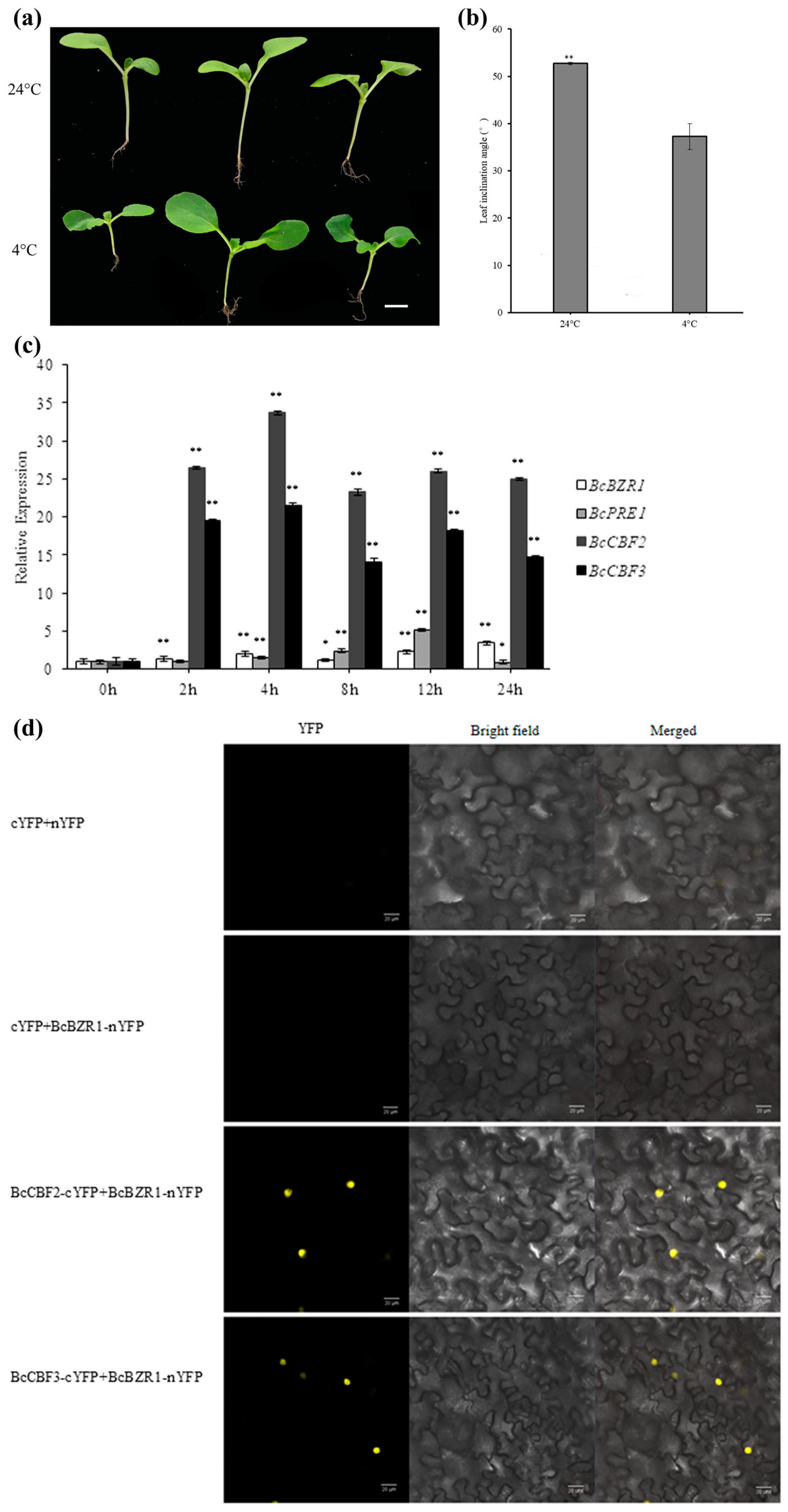
3.4. EBR Treatment Leads to Reduced Leaf Inclination Angle in Non-Heading Chinese Cabbage
3.5. Interaction between BcBZR1 and BsCBF2/3 In Vivo
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jin, L.B.; Zhang, J.W.; Li, B.; Cui, H.Y.; Dong, S.T.; Liu, P.; Zhao, B. Canopy Structure and Photosynthetic Characteristics of High Yield and High Nitrogen Efficiency Summer Maize. Sci. Agric. Sin. 2013, 46, 2430–2439. [Google Scholar] [CrossRef]
- Li, X.P.; Wang, S.; Huang, Y.C.; Jia, B.Y.; Wang, Y.; Zeng, Q.Y. Effects of spacing on the yields and canopy structure of japonica rice at full heading stage. Chin. J. Appl. Ecol. 2015, 26, 3329–3336. [Google Scholar] [CrossRef]
- Luo, X.Y.; Zheng, J.S.; Huang, R.Y.; Huang, Y.M.; Wang, H.C.; Jiang, L.R.; Fang, X.J. Phytohormones signaling and crosstalk regulating leaf angle in rice. Plant Cell Rep. 2016, 3512, 2423–2433. [Google Scholar] [CrossRef] [PubMed]
- Sreeramulu, S.; Mostizky, Y.; Sunitha, S.; Shani, E.; Nahum, H.; Salomon, D.; Ben Hayun, L.; Gruetter, C.; Rauh, D.; Ori, N.; et al. BSKs are partially redundant positive regulators of brassinosteroid signaling in Arabidopsis. Plant J. 2013, 74, 905–919. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.N.; Wang, S.K.; Xu, Y.X.; Yu, C.L.; Shen, C.J.; Qian, Q.; Geisler, M.; Jiang, D.A.; Qi, Y.H. The auxin response factor, OsARF19, controls rice leaf angles through positively regulating OsGH3-5 ang OsBRI1. Plant Cell Environ. 2015, 38, 638–654. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.Q.; Xiang, J.J.; Xue, H.W. Studies on the Rice LEAF INCLINATION1 (LC1), an IAA-amido Synthetase, Reveal the Effects of Auxin in Leaf Inclination Control. Mol. Plant 2013, 6, 174–187. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.; Lin, L.B.; Xue, H.W. Rice miR394 suppresses leaf inclination through targeting an F-box gene, LEAF INCLINATION 4. J. Integr. Plant Biol. 2019, 61, 406–416. [Google Scholar] [CrossRef]
- Xia, C. The Regulation and Mechanism of Gibberellin on Rice Leaf Angle; Huazhong Agriculture University: Wuhan, China, 2018. [Google Scholar]
- Dong, Z.Y.; Li, D.L.; Hu, X.X.; Liang, L.J.; Wu, G.K.; Zeng, S.Y.; Liu, E.B.; Wu, Y.; Wang, H.; Bhanbhro, L.; et al. Mining of favorable marker alleles for flag leaf inclination in some rice (Oryza sativa L.) accessions by association mapping. Euphytica 2018, 214, 117. [Google Scholar] [CrossRef]
- Ferrero-Serrano, A.; Assmann, S.M. The a-subunit of the rice heterotrimeric G protein, RGA1, regulates drought tolerance during the vegetative phase in the dwarf rice mutant d1. Exp. Bot. 2016, 67, 3433–3443. [Google Scholar] [CrossRef]
- Ueguchi-Tanaka, M.; Fujisawa, Y.; Ashikari, M.; Ashikari, M.; Iwasaki, Y.; Kitano, H.; Matsuoka, M. Rice dwarf mutant d1, which is defective in the alpha subunit of the heterotrimeric G protein, affects gibberellin signal transduction. Proc. Natl. Acad. Sci. USA 2000, 97, 11638–11643. [Google Scholar] [CrossRef]
- Jia, C.G.; Zhao, S.K.; Bao, T.T.; Zhao, P.Q.; Peng, K.; Guo, Q.X.; Gao, X.; Qin, J.C. Tomato BZR/BES transcription factor SlBZR1 positively regulates BR signaling and salt stress tolerance in tomato and Arabidopsis. Plant Sci. 2021, 302, 110719. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.L.; Gao, J.; Zhang, H.; Li, S.H.; Xing, J.H.; Wang, F.R.; Dong, J.N. The Regulation of Brassinosteroid (BR) on Elongation and Division of Rice (Oryza sativa) Cells. J. Agric. Biotechnol. 2015, 23, 71–79. [Google Scholar] [CrossRef]
- Sun, S.Y.; Chen, D.H.; Li, X.M.; Qiao, S.L.; Shi, C.N.; Li, C.X.; Shen, H.Y.; Wang, X.L. Brassinosteroid signaling regulates leaf erectness in Oryza sativa via the control of a specific U-type cyclin and cell proliferation. Dev. Cell 2015, 34, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, M.; Fujiwara, S.; Mitsuda, N.; Ohme-Takagi, M. A Triantagonistic Basic Helix-Loop-Helix System Regulates Cell Elongation in Arabidopsis. Plant Cell 2012, 24, 4483–4497. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, M.; Mitsuda, N.; Ohme-Takagi, M. ATBS1 INTERACTING FACTORs negatively regulate Arabidopsis cell elongation in the triantagonistic bHLH system. Plant Signal. Behav. 2013, 8, e23448. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.Y.; Bai, M.Y.; Wu, G.X.; Zhu, J.Y.; Wang, H.; Zhang, Z.G.; Wang, W.F. Antagonistic HLH/bHLH Transcription Factors Mediate Brassinosteroid Regulation of Cell Elongation and Plant Development in Rice and Arabidopsis. Plant Cell 2009, 21, 3767–3780. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Nakano, T.; Gendron, J.; He, J.; Chen, M.; Vafeados, D.; Yang, Y.; Fujioka, S.; Yoshida, S.; Asami, T.; et al. Nuclear-localized BZR1 mediates brassinosteroid-induced growth and feedback suppression of brassinosteroid biosynthesis. Dev. Cell 2002, 2, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Wang, Z.Y.; Mora-Garcia, S.; Li, J.; Yoshida, S.; Asami, T.; Chory, J. BES1 accumulates in the nucleus in response to brassinosteroids to regulate gene expression and promote stem elongation. Cell 2002, 109, 181–191. [Google Scholar] [CrossRef]
- Zhang, C.; Xu, Y.Y.; Guo, S.Y.; Zhu, J.Y.; Huan, G.; Liu, H.H.; Wang, L.; Luo, G.Z.; Wang, X.J.; Chong, K. Dynamics of Brassinosteroid Response Modulated by Negative Regulator LIC in Rice. PLoS Genet. 2012, 8, 651–664. [Google Scholar] [CrossRef]
- Bai, M.Y.; Shang, J.X.; Oh, E. Brassinosteroid, gibberellin and phytochrome impinge on a common transcription module in Arabidopsis. Nat. Cell Biol. 2012, 14, 810–817. [Google Scholar] [CrossRef]
- Li, Q.F.; He, J.X. BZR1 interacts with HY5 to mediate brassinosteroid- and lightregulated cotyledon opening in Arabidopsis in darkness. Mol. Plant 2016, 9, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ye, K.; Shi, Y.; Cheng, J.K.; Zhang, X.Y.; Yang, S.H. BZR1 Positively Regulates Freezing Tolerance via CBF-Dependent and CBF-Independent Pathways in Arabidopsis. Mol. Plant 2017, 10, 545–559. [Google Scholar] [CrossRef] [PubMed]
- Chen, J. Regulation and Biological Function of BZR1 Transcription Factor at Low Temperature; Lanzhou University: Lanzhou, China, 2018. [Google Scholar]
- Clough, S.J.; Bent, A.F.J. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 2010, 16, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Kenneth, J.K.; Schmitted, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Zhang, X.C.; Lv, C.G.; Hu, N.; Yao, K.M.; Zhang, Q.J.; Dai, Q.G. Simulation of Leaf Inclination Angle Distribution for Rice with Different Plant Types. Chin. J. Rice Sci. 2012, 26, 205–210. [Google Scholar] [CrossRef]
- Chen, T.K.; Yang, H.T.; Fang, S.C.; Lien, Y.C.; Yang, T.T.; Ko, S.S. Hybrid-Cut: An Improved Sectioning Method for Recalcitrant Plant Tissue Samples. JoVE-J. Vis. Exp. 2016, 117, e54754. [Google Scholar] [CrossRef]
- Li, Y.; Liu, G.F.; Ma, L.M.; Liu, T.K.; Zhang, C.W.; Xiao, D.; Zheng, H.K.; Chen, F.; Hou, X.L. A chromosome-level reference genome of non-heading Chinese cabbage Brassica campestris (syn. Brassica rapa) ssp. Chinensis. Hortic. Res. 2020, 7, 212. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.S.; Zhu, W.; Li, L. Brassinosteroids control male fertility by regulating the expression of key genes involved in Arabidopsis anther and pollen development. Proc. Natl. Acad. Sci. USA 2010, 107, 6100–6105. [Google Scholar] [CrossRef]
- Western, T.L.; Haughn, G.W. BELL1 and AGAMOUS genes promote ovule identity in Arabidopsis thaliana. Plant J. 1999, 18, 329–336. [Google Scholar] [CrossRef]
- Eremina, M.; Unterholzner, S.J.; Rathnayake, A.I.; Castellanos, M.; Khan, M.; Kugler, K.G.; May, S.T.; Mayer, K.F.X.; Rozhon, W.; Poppenberger, B. Brassinosteroids participate in the control of basal and acquired freezing tolerance of plants. Proc. Natl. Acad. Sci. USA 2016, 113, 5982–5991. [Google Scholar] [CrossRef]
- Raabe, K.; Pisek, J.; Sonnentag, O.; Annuk, K. Variations of leaf inclination angle distribution with height over the growing season and light exposure for eight broadleaf tree species. Agric. For. Meteorol. 2015, 214, 2–11. [Google Scholar] [CrossRef]
- Chen, S.H.; Zhou, L.J.; Xu, P.; Xue, H.W. SPOC domain-containing protein Leaf inclination3 interacts with LIP1 to regulate rice leaf inclination through auxin signaling. PLoS Genet. 2018, 14, e1007829. [Google Scholar] [CrossRef] [PubMed]
- Li, W.Y.; Yang, Z.X.; Yao, J.Y.; Li, J.S.; Song, W.B.; Yang, X.H. Cellulose synthase-like D1 controls organ size in maize. BMC Plant Biol. 2018, 18, 239. [Google Scholar] [CrossRef]
- Wang, N.; Cao, D.; Gong, F.P.; Ku, L.X.; Chen, Y.H.; Wang, W. Differences in properties and proteomes of the midribs contribute to the size of the leaf angle in two near-isogenic maize lines. J. Proteom. 2015, 128, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, S.; Yang, K.Y.; Kim, Y.M.; Park, S.Y.; Kim, S.Y.; Soh, M.S. Overexpression of PRE1 and its homologous genes activates gibberellin-dependent responses in Arabidopsis thaliana. Plant Cell Physiol. 2006, 47, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Zhiponova, M.K.; Morohashi, K.; Vanhoutte, I.; Machemer-Noonan, K.; Revalska, M.; Van Montagu, M.; Grotewold, E.; Russinova, E. Helix-loop-helix/basic helix-loop-helix transcription factor network represses cell elongation in Arabidopsis through an apparent incoherent feed-forward loop. Proc. Natl. Acad. Sci. USA 2014, 111, 2824–2829. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.M.; Tian, Y.C.; Han, C.; Zhou, C.N.; Bai, M.Y.; Fan, M. Phospho-Mutant Activity Assays Provide Evidence for the Negative Regulation of Transcriptional Regulator PRE1 by Phosphorylation. Int. J. Mol. Sci. 2020, 21, 9183. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ku, L.X.; Han, Z.P.; Guo, S.L.; Liu, H.J.; Zhang, Z.Z.; Chen, Y.H. The ZmCLA4 gene in the qLA4-1 QTL controls leaf angle in maize (Zea mays L.). J. Exp. Bot. 2014, 65, 5063–5076. [Google Scholar] [CrossRef]
- Ohmetakagi, M.; Shinshi, H. Ethylene-Inducible DNA-Binding Proteins That Interact with an Ethylene-Responsive Element. Plant Cell 1995, 7, 173–182. [Google Scholar] [CrossRef]
- Okamuro, J.K.; Caster, B.; Villarroel, R.; VanMontagu, M.; Jofuku, K.D. The AP2 domain of APETALA2 defines a large new family of DNA binding proteins in Arabidopsis. Proc. Natl. Acad. Sci. USA 1997, 94, 7076–7081. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, W.; Li, Y.; He, Y.; Wu, Y.; Hou, X. BcBZR1 Regulates Leaf Inclination Angle in Non-Heading Chinese Cabbage (Brassica campestris ssp. chinensis Makino). Horticulturae 2024, 10, 324. https://doi.org/10.3390/horticulturae10040324
Lin W, Li Y, He Y, Wu Y, Hou X. BcBZR1 Regulates Leaf Inclination Angle in Non-Heading Chinese Cabbage (Brassica campestris ssp. chinensis Makino). Horticulturae. 2024; 10(4):324. https://doi.org/10.3390/horticulturae10040324
Chicago/Turabian StyleLin, Wenyuan, Yiran Li, Ying He, Ying Wu, and Xilin Hou. 2024. "BcBZR1 Regulates Leaf Inclination Angle in Non-Heading Chinese Cabbage (Brassica campestris ssp. chinensis Makino)" Horticulturae 10, no. 4: 324. https://doi.org/10.3390/horticulturae10040324
APA StyleLin, W., Li, Y., He, Y., Wu, Y., & Hou, X. (2024). BcBZR1 Regulates Leaf Inclination Angle in Non-Heading Chinese Cabbage (Brassica campestris ssp. chinensis Makino). Horticulturae, 10(4), 324. https://doi.org/10.3390/horticulturae10040324







