Modeling the Effect of Temperature on the Severity of Blueberry Stem Blight and Dieback with a Focus on Neofusicoccum parvum and Cultivar Susceptibility
Abstract
1. Introduction
- (i)
- To compare the average in vitro growth rate of the four confirmed blueberry stem blight and dieback pathogens in Northwestern Italy at different temperatures (i.e., Neofusicoccum parvum, Diaporthe rudis, Cadophora luteo-olivacea and Peroneutypa scoparia);
- (ii)
- To evaluate the susceptibility of different northern highbush blueberry cvs. to these four pathogens;
- (iii)
- To model the in vitro effect of temperature on the mycelial growth rate of Neofusicoccum parvum;
- (iv)
- To assess and model the in planta effect of temperature on the severity of blueberry stem blight and dieback caused by Neofusicoccum parvum, the most virulent of the four pathogens studied here.
2. Materials and Methods
2.1. Fungal Species and Strain Selection
2.2. Plant Material
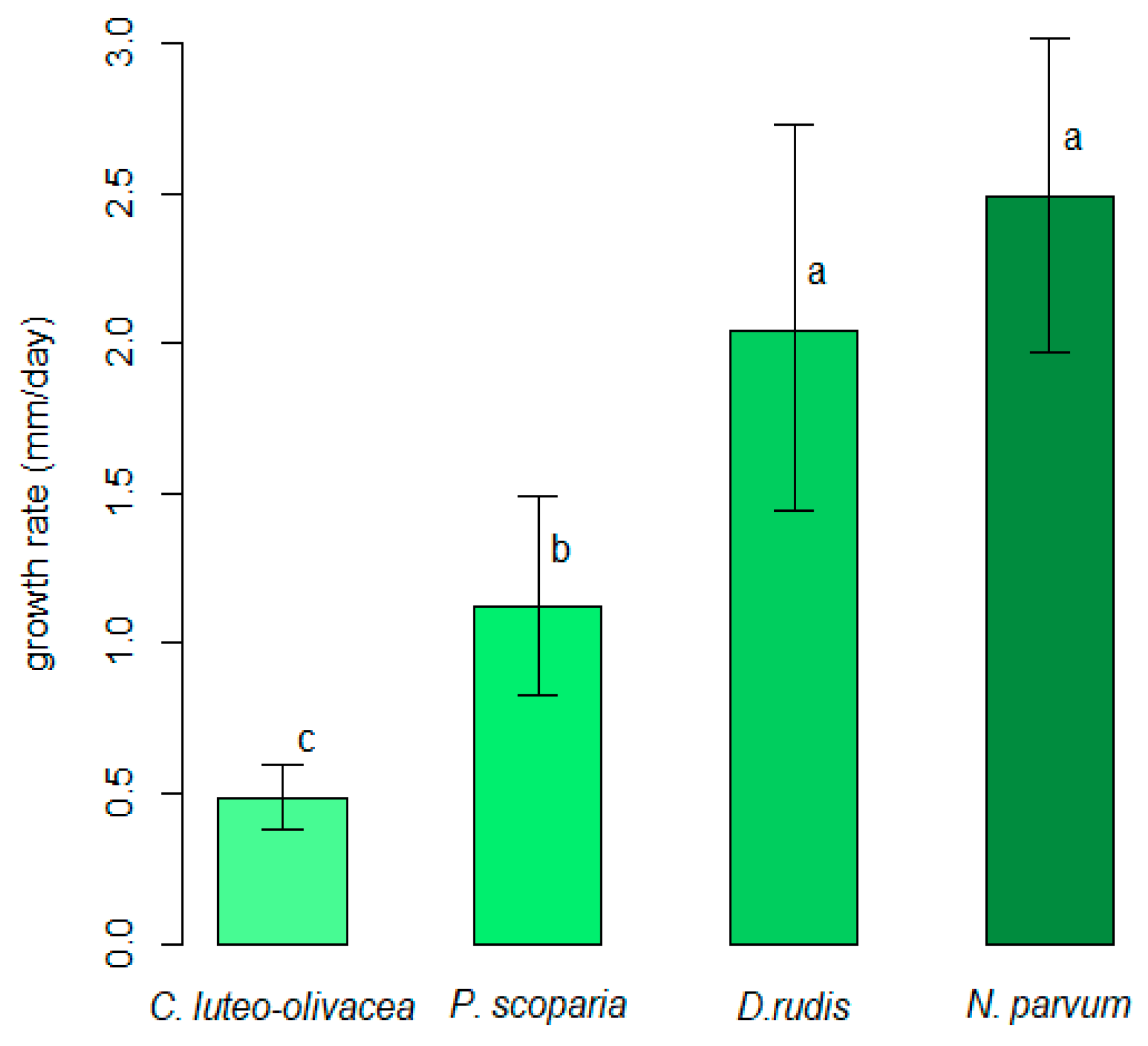
2.3. Comparison of In Vitro Growth Rates of Cadophora luteo-olivacea, Diaporthe rudis, Neofusicoccum parvum and Peroneutypa scoparia
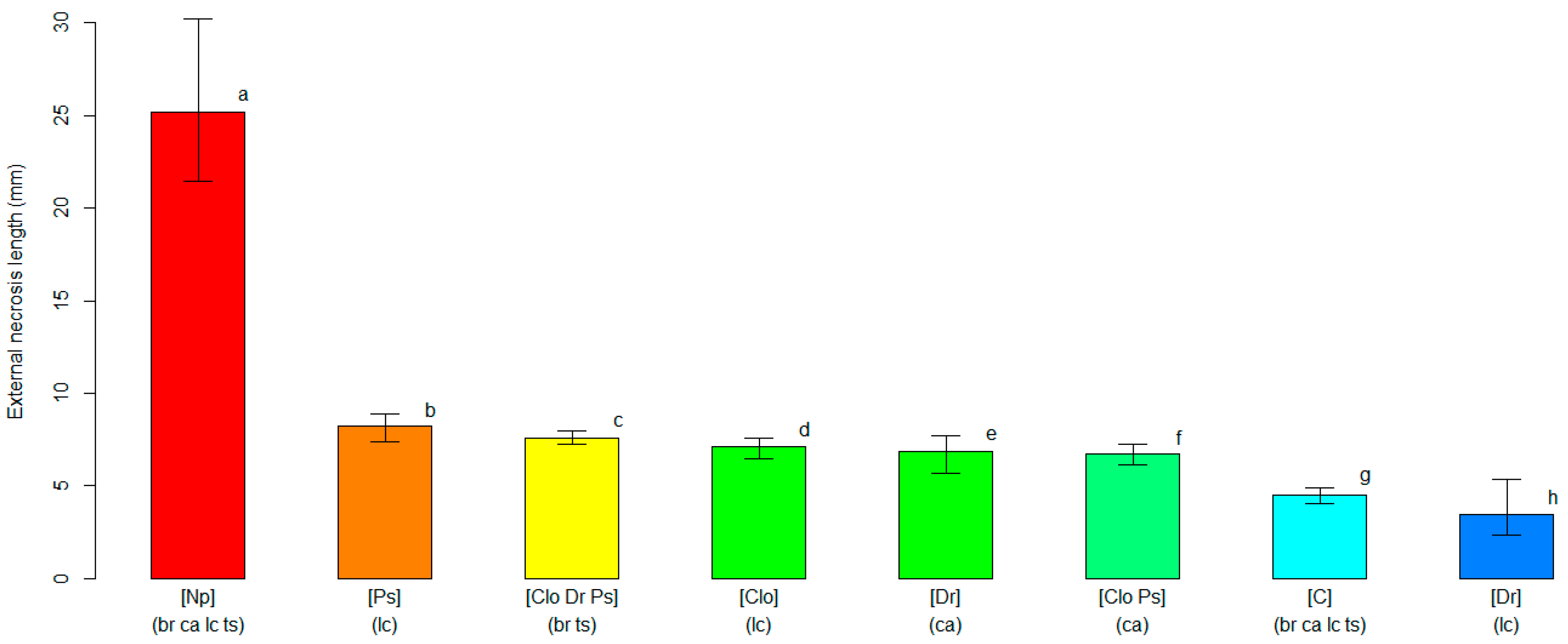
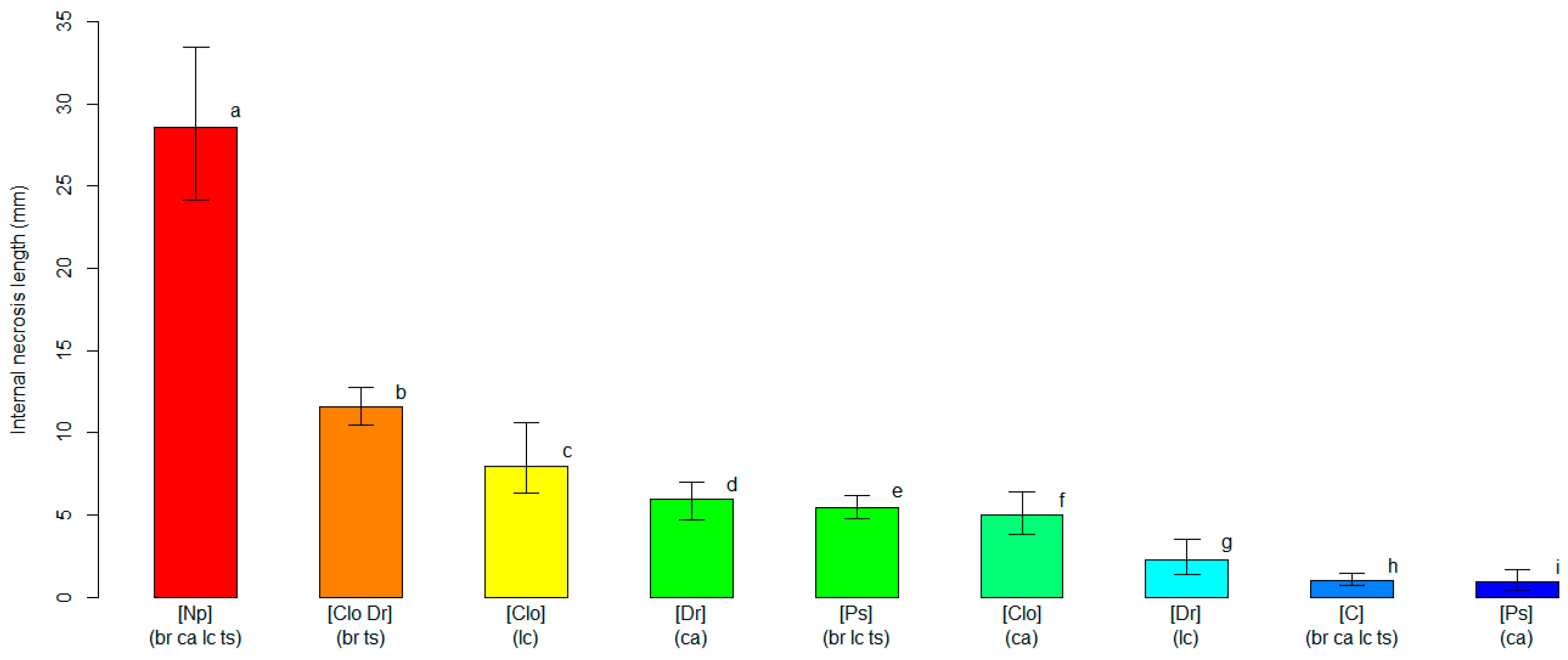
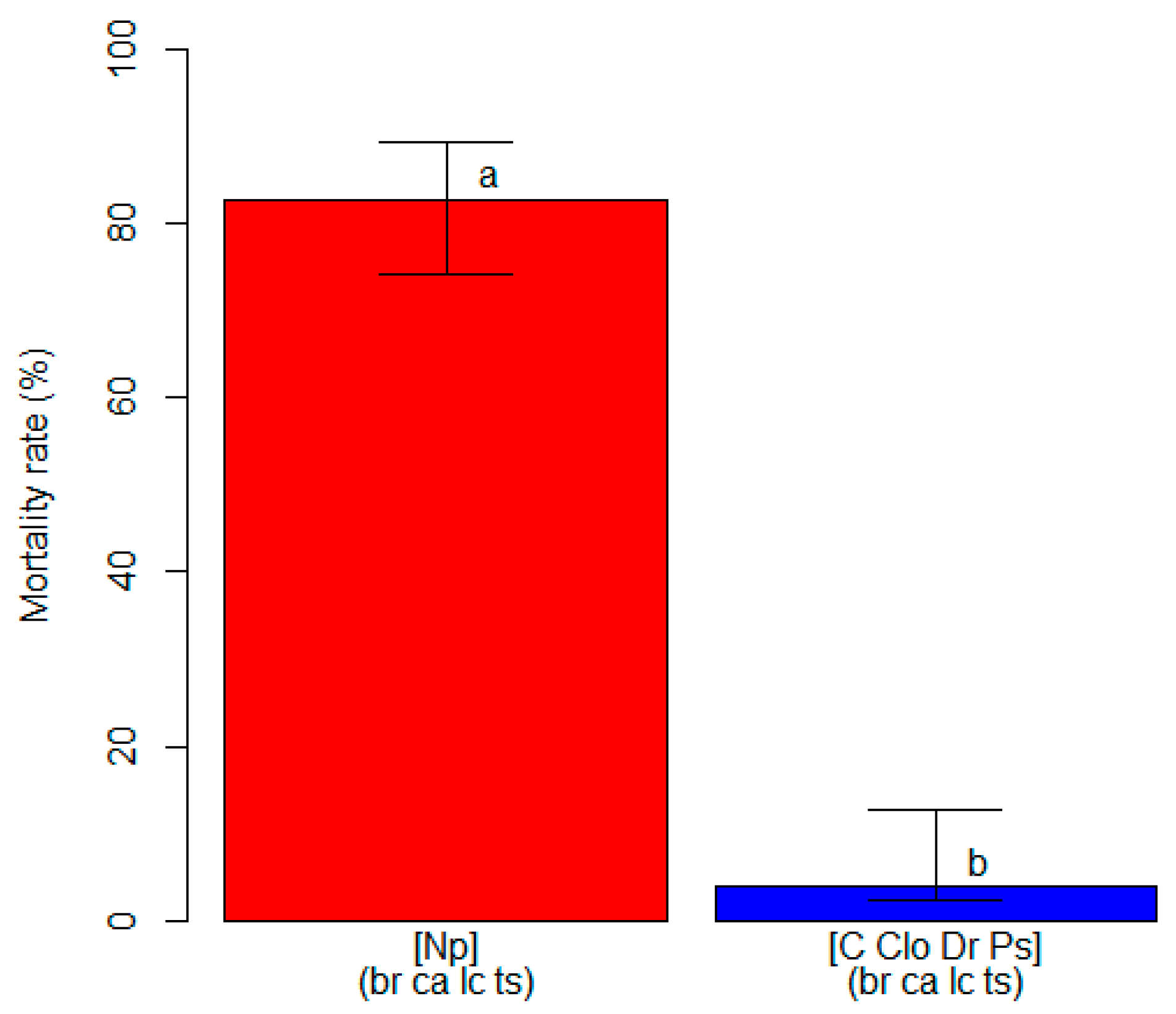
2.4. Varietal Susceptibility Test
- Inoculation treatment (2 levels: “plants inoculated with isolates of the target fungal pathogens” and “control plants mock-inoculated with plugs of sterile PDA-S”);
- Blueberry cultivar (4 levels: ‘Blue Ribbon’, ‘Cargo’, ‘Last Call’ and ‘Top Shelf’);
- Target pathogen species (5 levels: “C. luteo-olivacea”, “D. rudis”, “N. parvum”, “P. scoparia” and “none” for control plants).
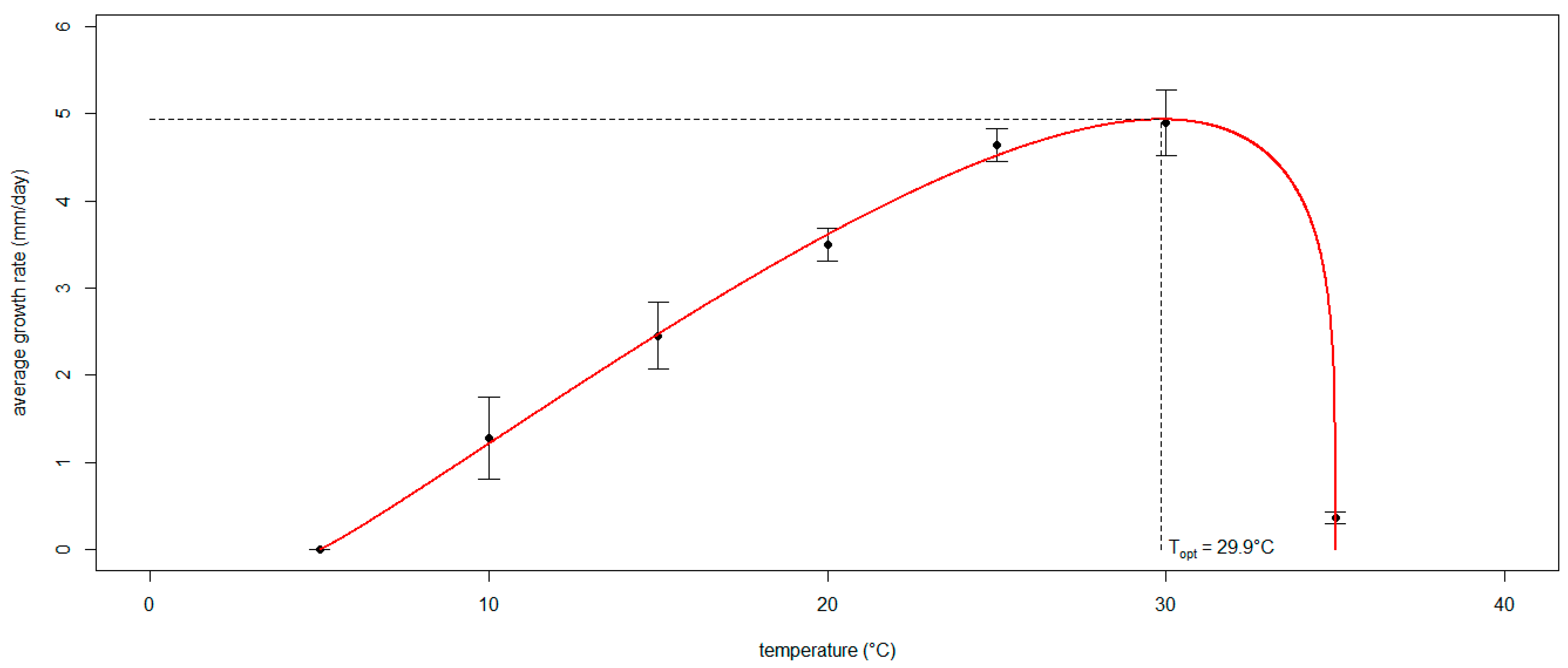
2.5. Modeling the Effect of Temperature on the Mycelial Growth Rate of Neofusicoccum parvum In Vitro
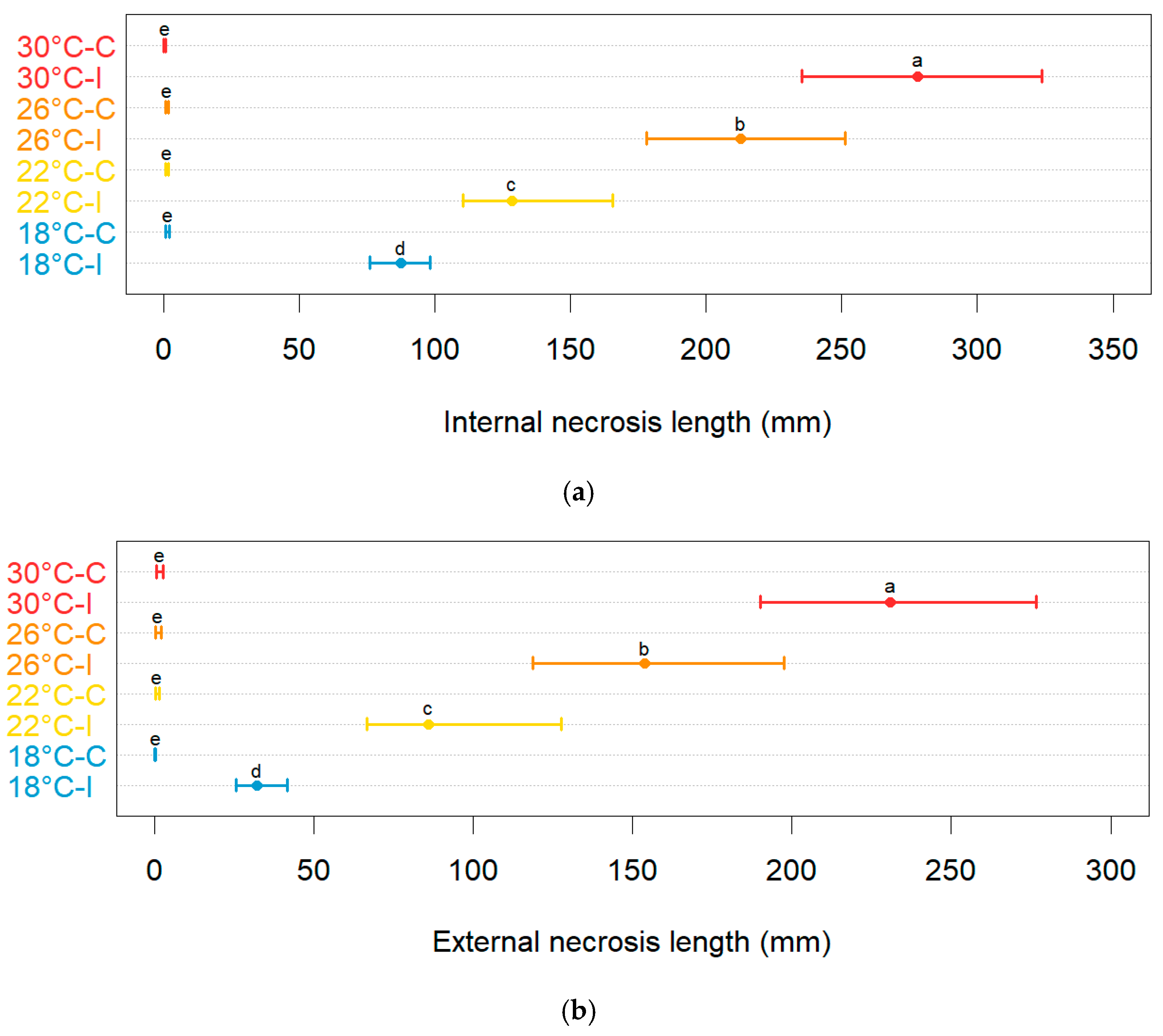
2.6. In Planta Effect of Temperature on Colonization Rate and Branch Mortality of N. parvum
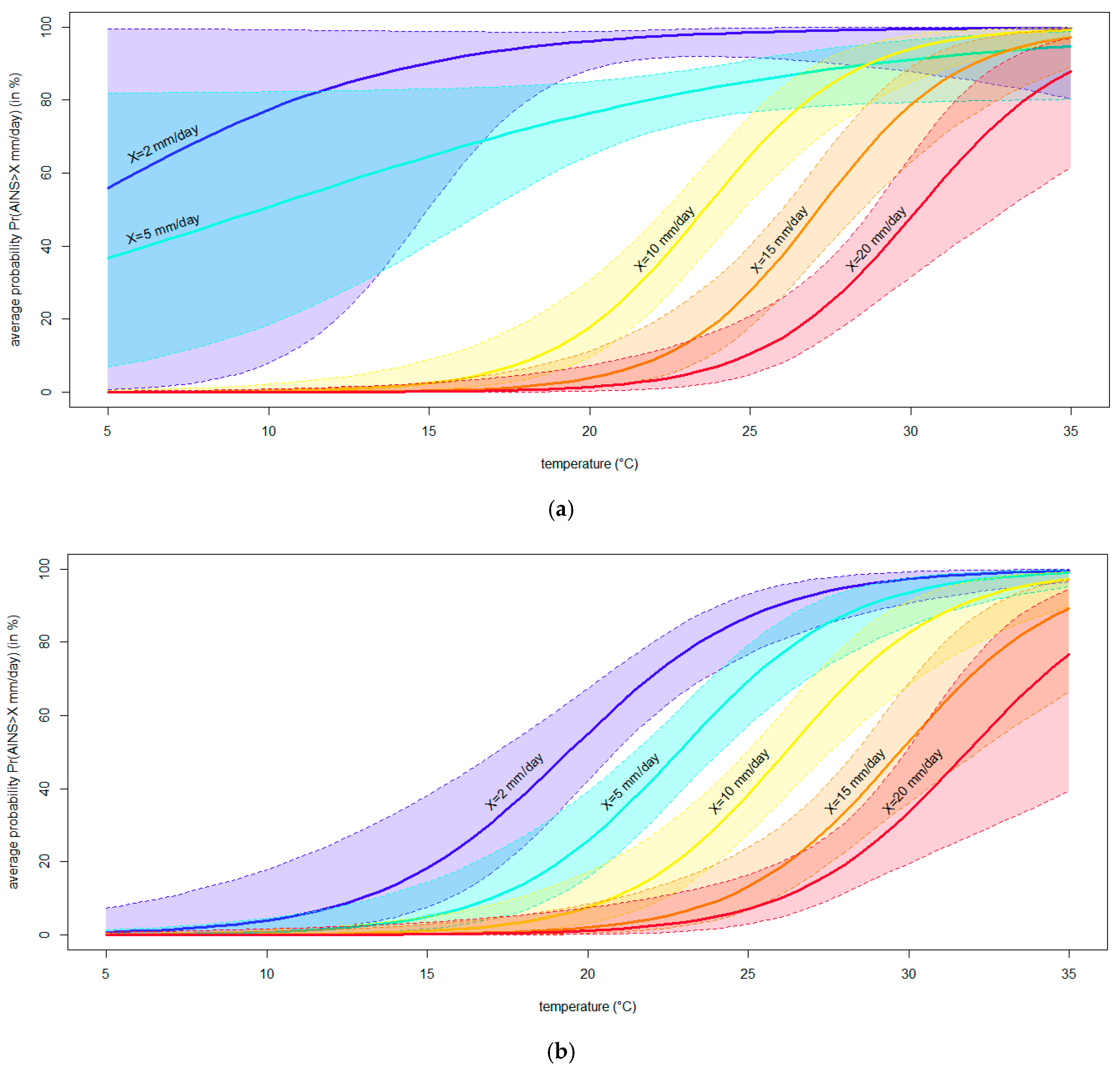
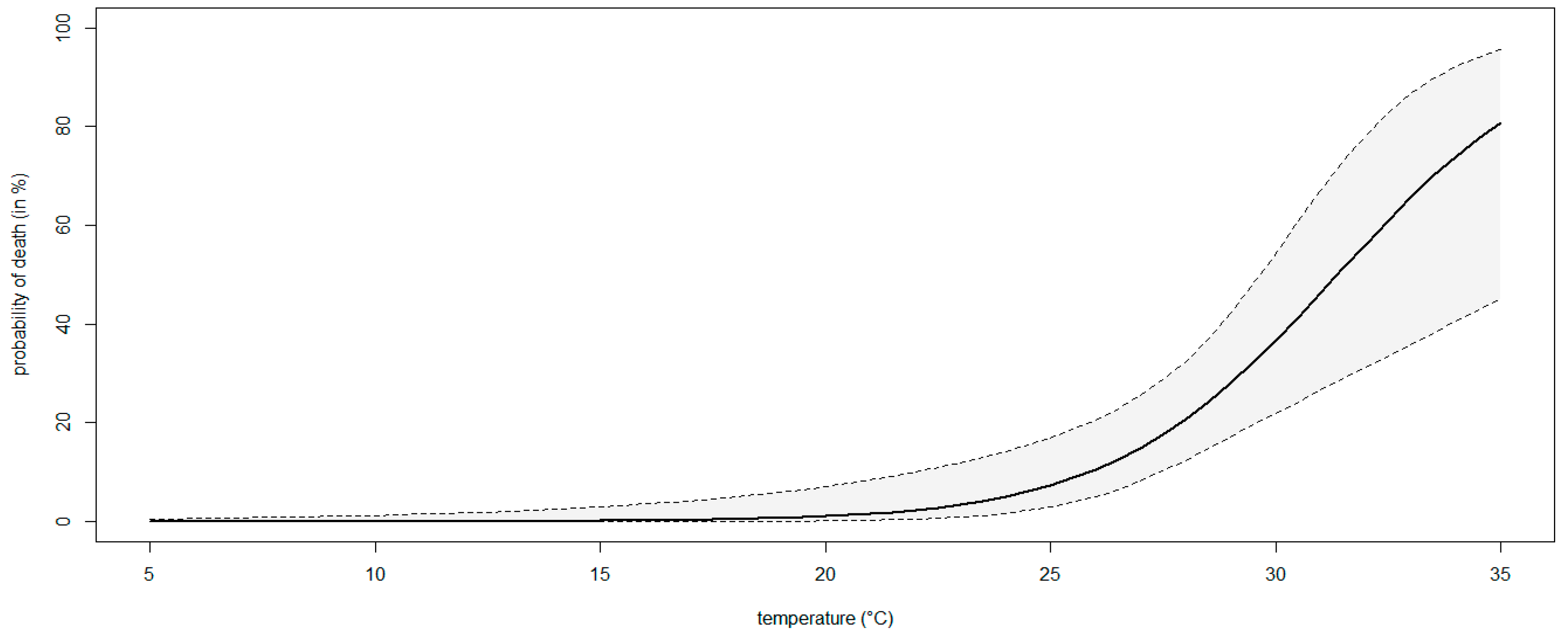
2.7. Modeling the Effect of Temperature on the Severity of Neofusicoccum parvum in Planta
- Inoculation treatment (2 levels: “plants inoculated with the selected isolate of N. parvum” and “control plants mock-inoculated with plugs of sterile PDA-S”);
- Temperature (4 levels: “18 °C”, “22 °C”, “27 °C” and “30 °C”).
3. Results
3.1. Comparison of In Vitro Growth Rates of Cadophora luteo-olivacea, Diaporthe rudis, Neofusicoccum parvum and Peroneutypa scoparia
3.2. Varietal Susceptibility Test
3.3. Modeling the Effect of Temperature on the Mycelial Growth Rate of Neofusicoccum parvum In Vitro
3.4. Modeling the Effect of Temperature on the Severity of Neofusicoccum parvum in Planta
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Retamales, J.B.; Hancock, J.F. Blueberries, 2nd ed.; CABI: Wallingford, UK, 2018. [Google Scholar]
- Guarnaccia, V.; Kraus, C.; Markakis, E.; Alves, A.; Armengol, J.; Eichmeier, A.; Compant, S.; Gramaje, D. Fungal Trunk Diseases of Fruit Trees in Europe: Pathogens, Spread and Future Directions. Phytopathol. Mediterr. 2022, 61, 563–599. [Google Scholar] [CrossRef]
- Espinoza, J.G.; Briceño, E.X.; Chávez, E.R.; Úrbez-Torres, J.R.; Latorre, B.A. Neofusicoccum spp. Associated with Stem Canker and Dieback of Blueberry in Chile. Plant Dis. 2009, 93, 1187–1194. [Google Scholar] [CrossRef]
- Rodríguez-Gálvez, E.; Hilário, S.; Lopes, A.; Alves, A. Diversity and Pathogenicity of Lasiodiplodia and Neopestalotiopsis Species Associated with Stem Blight and Dieback of Blueberry Plants in Peru. Eur. J. Plant Pathol. 2020, 157, 89–102. [Google Scholar] [CrossRef]
- Phillips, A.J.L.; Oudemans, P.V.; Correia, A. Characterisation and Epitypification of Botryosphaeria corticis, the Cause of Blueberry Cane Canker. Fungal Divers. 2006, 21, 141–155. [Google Scholar]
- Tennakoon, K.M.S.; Ridgway, H.J.; Jaspers, M.V.; Eirian Jones, E. Botryosphaeriaceae Species Associated with Blueberry Dieback and Sources of Primary Inoculum in Propagation Nurseries in New Zealand. Eur. J. Plant Pathol. 2018, 150, 363–374. [Google Scholar] [CrossRef]
- Scarlett, K.A.; Shuttleworth, L.A.; Collins, D.; Rothwell, C.T.; Guest, D.I.; Daniel, R. Botryosphaeriales Associated with Stem Blight and Dieback of Blueberry (Vaccinium spp.) in New South Wales and Western Australia. Australas. Plant Pathol. 2019, 48, 45–57. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, H.; Zhou, Z.; Hu, T.; Wang, S.; Wang, Y.; Cao, K. Identification and Distribution of Botryosphaeriaceae Species Associated with Blueberry Stem Blight in China. Eur. J. Plant Pathol. 2015, 143, 737–752. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Bhoyroo, V.; Rampadarath, S.; Jeewon, R. Multigene Phylogenetics and Morphology Reveal Five Novel Lasiodiplodia Species Associated with Blueberries. Life 2021, 11, 657. [Google Scholar] [CrossRef]
- Ivić, D.; Novak, A.; Pilipovi, P. Diaporthe eres Nitschke Is the Only Diaporthe Species Found on Blueberry in Croatia. Fragm. Phytomed. 2018, 32, 23–30. [Google Scholar]
- Lombard, L.; van Leeuwen, G.C.M.; Guarnaccia, V.; Polizzi, G.; van Rijswick, P.C.J.; Rosendahl, K.C.H.M.; Gabler, J.; Crous, P.W. Diaporthe Species Associated with Vaccinium, with Specific Reference to Europe. Phytopathol. Mediterr. 2014, 53, 23–30. [Google Scholar] [CrossRef]
- Castillo, S.; Borrero, C.; Castaño, R.; Rodríguez, A.; Avilés, M. First Report of Canker Disease Caused by Neofusicoccum parvum and N. australe on Blueberry Bushes in Spain. Plant Dis. 2013, 97, 1112. [Google Scholar] [CrossRef]
- Borrero, C.; Pérez, S.; Avilés, M. First Report of Canker Disease Caused by Lasiodiplodia theobromae on Blueberry Bushes in Spain. Plant Dis. 2019, 103, 2684. [Google Scholar] [CrossRef]
- Pečenka, J.; Tekielska, D.; Kocanová, M.; Peňázová, E.; Berraf-Tebbal, A.; Eichmeier, A. First Report of Lasiodiplodia theobromae Causing Decline of Blueberry (Vaccinium corymbosum) in the Czech Republic. Plant Dis. 2021, 105, 215. [Google Scholar] [CrossRef]
- Hilário, S.; Lopes, A.; Santos, L.; Alves, A. Botryosphaeriaceae Species Associated with Blueberry Stem Blight and Dieback in the Centre Region of Portugal. Eur. J. Plant Pathol. 2020, 156, 31–44. [Google Scholar] [CrossRef]
- Hilário, S.; Amaral, I.A.; Gonçalves, M.F.M.; Lopes, A.; Santos, L.; Alves, A. Diaporthe Species Associated with Twig Blight and Dieback of Vaccinium corymbosum in Portugal, with Description of Four New Species. Mycologia 2020, 112, 293–308. [Google Scholar] [CrossRef]
- ISTAT. 2023. Available online: https://www.istat.it/ (accessed on 27 November 2023).
- Guarnaccia, V.; Martino, I.; Tabone, G.; Brondino, L.; Gullino, M.L. Fungal Pathogens Associated with Stem Blight and Dieback of Blueberry in Northern Italy. Phytopathol. Mediterr. 2020, 59, 229–245. [Google Scholar] [CrossRef]
- ISTAT. 2023. Available online: http://dati.istat.it (accessed on 11 March 2024).
- Slippers, B.; Wingfield, M.J. Botryosphaeriaceae as Endophytes and Latent Pathogens of Woody Plants: Diversity, Ecology and Impact. Fungal Biol. Rev. 2007, 21, 90–106. [Google Scholar] [CrossRef]
- Sessa, L.; Abreo, E.; Lupo, S. Diversity of Fungal Latent Pathogens and True Endophytes Associated with Fruit Trees in Uruguay. J. Phytopathol. 2018, 166, 633–647. [Google Scholar] [CrossRef]
- Hilário, S.; Gonçalves, M.F.M. Mechanisms Underlying the Pathogenic and Endophytic Lifestyles in Diaporthe: An Omics-Based Approach. Horticulturae 2023, 9, 423. [Google Scholar] [CrossRef]
- Salvatore, M.M.; Andolfi, A.; Nicoletti, R. The Thin Line between Pathogenicity and Endophytism: The Case of Lasiodiplodia theobromae. Agriculture 2020, 10, 488. [Google Scholar] [CrossRef]
- Lione, G.; Giordano, L.; Sillo, F.; Gonthier, P. Testing and Modelling the Effects of Climate on the Incidence of the Emergent Nut Rot Agent of Chestnut Gnomoniopsis castanea. Plant Pathol. 2015, 64, 852–863. [Google Scholar] [CrossRef]
- Velásquez, A.C.; Castroverde, C.D.M.; He, S.Y. Plant–Pathogen Warfare under Changing Climate Conditions. Curr. Biol. 2018, 28, R619–R634. [Google Scholar] [CrossRef]
- Pour, F.; Ferreira, V.; Félix, C.; Serôdio, J.; Alves, A.; Duarte, A.S.; Esteves, A.C. Effect of Temperature on the Phytotoxicity and Cytotoxicity of Botryosphaeriaceae Fungi. Fungal Biol. 2020, 124, 571–578. [Google Scholar] [CrossRef]
- Hunjan, M.S.; Lore, J.S. Climate Change: Impact on Plant Pathogens, Diseases, and Their Management. In Crop Protection under Changing Climate; Jabran, K., Florentine, S., Chauhan, B.S., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 85–100. [Google Scholar] [CrossRef]
- Lung, T.; Dosio, A.; Becker, W.; Lavalle, C.; Bouwer, L.M. Assessing the Influence of Climate Model Uncertainty on EU-Wide Climate Change Impact Indicators. Clim. Change 2013, 120, 211–227. [Google Scholar] [CrossRef]
- Medda, S.; Fadda, A.; Mulas, M. Influence of Climate Change on Metabolism and Biological Characteristics in Perennial Woody Fruit Crops in the Mediterranean Environment. Horticulturae 2022, 8, 273. [Google Scholar] [CrossRef]
- Lead, C. Climate and Environmental Change in the Mediterranean Basin–Current Situation and Risks for the Future. In Union for the Mediterranean, Plan Bleu; UNEP/MAP: Marseille, France, 2020. [Google Scholar]
- Brondino, L.; Briano, R.; Massaglia, S.; Giuggioli, N.R. Influence of Harvest Method on the Quality and Storage of Highbush Blueberry. J. Agric. Food Res. 2022, 10, 100415. [Google Scholar] [CrossRef]
- DiCiccio, T.J.; Efron, B. Bootstrap Confidence Intervals. Stat. Sci. 1996, 11, 189–228. [Google Scholar] [CrossRef]
- Lione, G.; Giordano, L.; Sillo, F.; Brescia, F.; Gonthier, P. Temporal and Spatial Propagule Deposition Patterns of the Emerging Fungal Pathogen of Chestnut Gnomoniopsis castaneae in Orchards of North-western Italy. Plant Pathol. 2021, 70, 2016–2033. [Google Scholar] [CrossRef]
- Hothorn, T.; Zeileis, A. Partykit: A Modular Toolkit for Recursive Partytioning in R. J. Mach. Learn. Res. 2015, 16, 3905–3909. [Google Scholar]
- Hothorn, T.; Hornik, K.; Zeileis, A. Unbiased Recursive Partitioning: A Conditional Inference Framework. J. Comput. Graph. Stat. 2006, 15, 651–674. [Google Scholar] [CrossRef]
- Lione, G.; Giordano, L.; Turina, M.; Gonthier, P. Hail-Induced Infections of the Chestnut Blight Pathogen Cryphonectria Parasitica Depend on Wound Size and May Lead to Severe Diebacks. Phytopathology 2020, 110, 1280–1293. [Google Scholar] [CrossRef] [PubMed]
- Agresti, A. Categorical Data Analysis; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Zeileis, A.; Leisch, F.; Hornik, K.; Kleiber, C. Strucchange: An R Package for Testing for Structural Change in Linear Regression Models. J. Stat. Softw. 2002, 7, 1–38. [Google Scholar] [CrossRef]
- Lione, G.; Brescia, F.; Giordano, L.; Gonthier, P. Effects of Seasonality and Climate on the Propagule Deposition Patterns of the Chestnut Blight Pathogen Cryphonectria parasitica in Orchards of the Alpine District of North Western Italy. Agriculture 2022, 12, 644. [Google Scholar] [CrossRef]
- Kļaviņa, D.; Lione, G.; Kenigsvalde, K.; Pellicciaro, M.; Muižnieks, I.; Silbauma, L.; Jansons, J.; Gaitnieks, T.; Gonthier, P. Host-Associated Intraspecific Phenotypic Variation in the Saprobic Fungus Phlebiopsis gigantea. Microb. Ecol. 2023, 86, 1847–1855. [Google Scholar] [CrossRef] [PubMed]
- Venkatasubramaniam, A.; Wolfson, J.; Mitchell, N.; Barnes, T.; JaKa, M.; French, S. Decision Trees in Epidemiological Research. Emerg. Themes Epidemiol. 2017, 14, 11. [Google Scholar] [CrossRef] [PubMed]
- Blaker, H. Confidence Curves and Improved Exact Confidence Intervals for Discrete Distributions. Can. J. Stat. 2000, 28, 783–798. [Google Scholar] [CrossRef]
- López-Moral, A.; Raya-Ortega, M.C.; Agustí-Brisach, C.; Roca, L.F.; Lovera, M.; Luque, F.; Arquero, O.; Trapero, A. Morphological, Pathogenic, and Molecular Characterization of Colletotrichum acutatum Isolates Causing Almond Anthracnose in Spain. Plant Dis. 2017, 101, 2034–2045. [Google Scholar] [CrossRef] [PubMed]
- Moré, J.J. The Levenberg-Marquardt Algorithm: Implementation and Theory. In Numerical Analysis: Proceedings of the Biennial Conference Held at Dundee, UK, 28 June–1 July 1977; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Bates, D.M.; Watts, D.G. Nonlinear Regression Analysis and Its Applications. Sebastião Gazola 1988, 31, 95. [Google Scholar]
- Bates, D.M.; Chambers, J.M. Nonlinear Models. In Statistical Models; Wadsworth & Brooks: California, CA, USA, 1992; Chapter 10. [Google Scholar]
- Piñeiro, G.; Perelman, S.; Guerschman, J.P.; Paruelo, J.M. How to Evaluate Models: Observed vs. Predicted or Predicted vs. Observed? Ecol. Model. 2008, 216, 316–322. [Google Scholar] [CrossRef]
- Bliemel, F. Theil’s Forecast Accuracy Coefficient: A Clarification. J. Mark. Res. 1973, 10, 444–446. [Google Scholar] [CrossRef]
- Hosmer, D.W.; Lemeshow, S. Applied Logistic Regression; Johns Wiley & Sons: New York, NY, USA, 1989. [Google Scholar]
- Crawley, M.J. The R Book, 2nd ed.; John Wiley & Sons: Chichester, UK, 2013. [Google Scholar]
- Grueber, C.E.; Nakagawa, S.; Laws, R.J.; Jamieson, I.G. Multimodel Inference in Ecology and Evolution: Challenges and Solutions: Multimodel Inference. J. Evol. Biol. 2011, 24, 699–711. [Google Scholar] [CrossRef] [PubMed]
- Wagenmakers, E.-J.; Farrell, S. AIC Model Selection Using Akaike Weights. Psychon. Bull. Rev. 2004, 11, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Garbelotto, M.; Schmidt, D.; Swain, S.; Hayden, K.; Lione, G. The Ecology of Infection between a Transmissive and a Dead-end Host Provides Clues for the Treatment of a Plant Disease. Ecosphere 2017, 8, e01815. [Google Scholar] [CrossRef]
- Juroszek, P.; Racca, P.; Link, S.; Farhumand, J.; Kleinhenz, B. Overview on the Review Articles Published during the Past 30 Years Relating to the Potential Climate Change Effects on Plant Pathogens and Crop Disease Risks. Plant Pathol. 2020, 69, 179–193. [Google Scholar] [CrossRef]
- Ji, T.; Altieri, V.; Salotti, I.; Rossi, V. Effects of Temperature and Moisture Duration on Spore Germination of Four Fungi That Cause Grapevine Trunk Diseases. Plant Dis. 2023, 107, 1005–1008. [Google Scholar] [CrossRef]
- Batista, E.; Lopes, A.; Alves, A. What Do We Know about Botryosphaeriaceae? An Overview of a Worldwide Cured Dataset. Forests 2021, 12, 313. [Google Scholar] [CrossRef]
- Udayanga, D.; Castlebury, L.A.; Rossman, A.Y.; Hyde, K.D. Species Limits in Diaporthe: Molecular Re-Assessment of D. citri, D. cytosporella, D. foeniculina and D. rudis. Persoonia Mol. Phylogeny Evol. Fungi 2014, 32, 83–101. [Google Scholar] [CrossRef] [PubMed]
- Tennakoon, K.M.S.; Ridgway, H.J.; Jaspers, M.V.; Jones, E.E. Factors Affecting Neofuscicoccum Ribis Infection and Disease Progression in Blueberry. Eur. J. Plant Pathol. 2018, 151, 87–99. [Google Scholar] [CrossRef]
- Shafi, A.; Ridgway, H.J.; Jaspers, M.V.; Jones, E.E. Factors Influencing Virulence and Conidial Production of Neofusicoccum Species on Grapevine Shoots. Eur. J. Plant Pathol. 2019, 153, 1067–1081. [Google Scholar] [CrossRef]
- ARPA. 2023. Available online: https://www.arpa.piemonte.it/ (accessed on 14 October 2023).
- Pérez, R.; Laca, A.; Laca, A.; Díaz, M. Environmental Behaviour of Blueberry Production at Small-Scale in Northern Spain and Improvement Opportunities. J. Clean. Prod. 2022, 339, 130594. [Google Scholar] [CrossRef]
- Qiu, Y.; Steel, C.C.; Ash, G.J.; Savocchia, S. Effects of Temperature and Water Stress on the Virulence of Botryosphaeriaceae spp. Causing Dieback of Grapevines and Their Predicted Distribution Using CLIMEX in Australia. Acta Hortic. 2016, 1115, 171–182. [Google Scholar] [CrossRef]
- Van Dyk, M.; Spies, C.F.J.; Mostert, L.; Halleen, F. Survey of Trunk Pathogens in South African Olive Nurseries. Plant Dis. 2021, 105, 1630–1639. [Google Scholar] [CrossRef] [PubMed]
- Sakalidis, M.L.; Slippers, B.; Wingfield, B.D.; Hardy, G.E.S.J.; Burgess, T.I. The Challenge of Understanding the Origin, Pathways and Extent of Fungal Invasions: Global Populations of the Neofusicoccum parvum-N. ribis Species Complex. Divers. Distrib. 2013, 19, 873–883. [Google Scholar] [CrossRef]
- Martino, I.; Agustí-Brisach, C.; Nari, L.; Gullino, M.L.; Guarnaccia, V. Characterization and Pathogenicity of Fungal Species Associated with Dieback of Apple Trees in Northern Italy. Plant Dis. 2024, 108, 311–331. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martino, I.; Lione, G.; Garbelotto, M.; Gonthier, P.; Guarnaccia, V. Modeling the Effect of Temperature on the Severity of Blueberry Stem Blight and Dieback with a Focus on Neofusicoccum parvum and Cultivar Susceptibility. Horticulturae 2024, 10, 363. https://doi.org/10.3390/horticulturae10040363
Martino I, Lione G, Garbelotto M, Gonthier P, Guarnaccia V. Modeling the Effect of Temperature on the Severity of Blueberry Stem Blight and Dieback with a Focus on Neofusicoccum parvum and Cultivar Susceptibility. Horticulturae. 2024; 10(4):363. https://doi.org/10.3390/horticulturae10040363
Chicago/Turabian StyleMartino, Ilaria, Guglielmo Lione, Matteo Garbelotto, Paolo Gonthier, and Vladimiro Guarnaccia. 2024. "Modeling the Effect of Temperature on the Severity of Blueberry Stem Blight and Dieback with a Focus on Neofusicoccum parvum and Cultivar Susceptibility" Horticulturae 10, no. 4: 363. https://doi.org/10.3390/horticulturae10040363
APA StyleMartino, I., Lione, G., Garbelotto, M., Gonthier, P., & Guarnaccia, V. (2024). Modeling the Effect of Temperature on the Severity of Blueberry Stem Blight and Dieback with a Focus on Neofusicoccum parvum and Cultivar Susceptibility. Horticulturae, 10(4), 363. https://doi.org/10.3390/horticulturae10040363





