Identification of Laccase Genes in Grapevine and Their Roles in Response to Botrytis cinerea
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant and Fungus Materials
2.2. Identification of the VvLAC Gene Family
2.3. Chromosomal Location, Phylogenetic Analysis and Collinearity Analysis
2.4. Gene Structures, Conserved Motifs and Cis-Elements
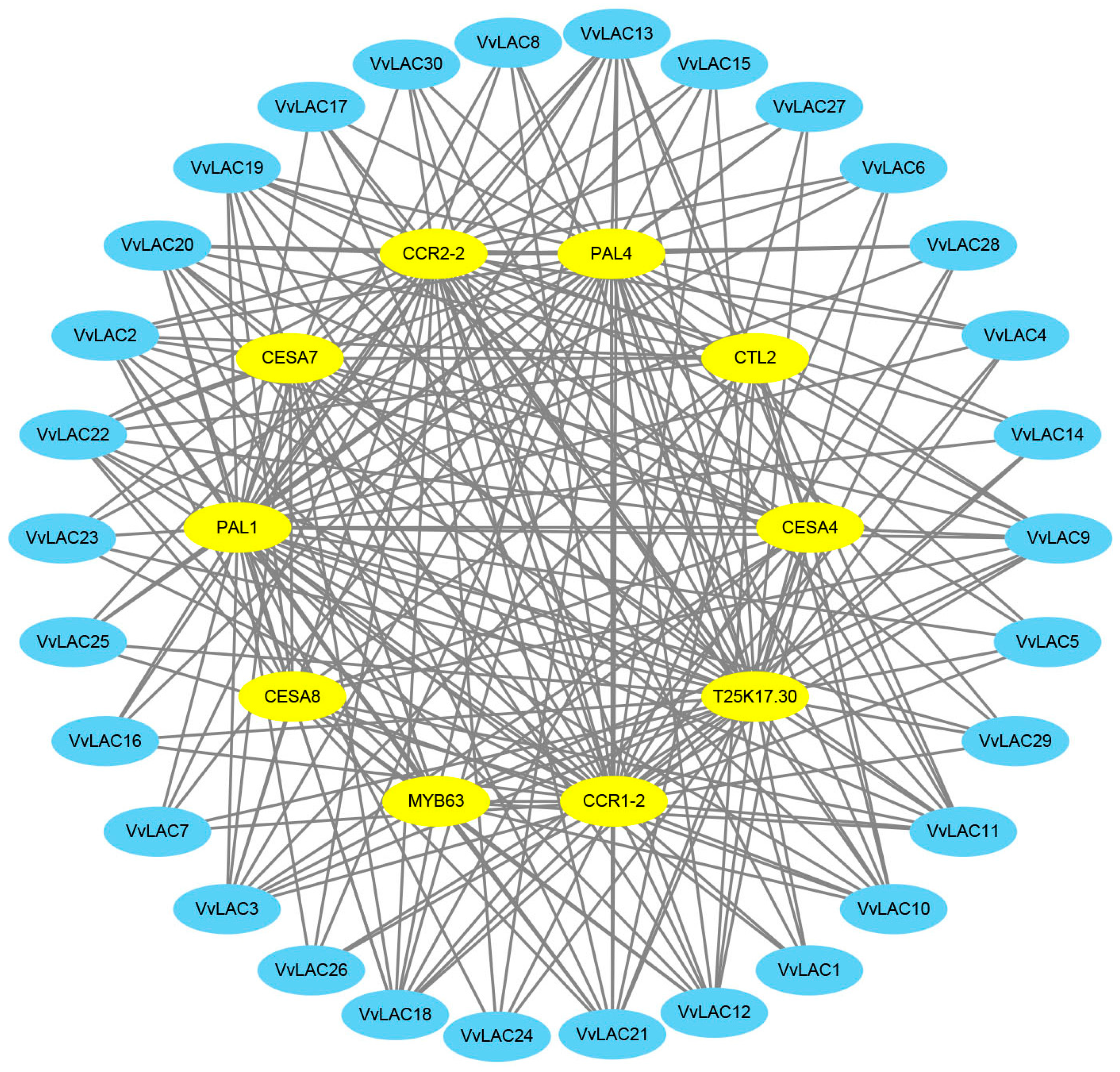
2.5. Protein Interaction Network Analysis
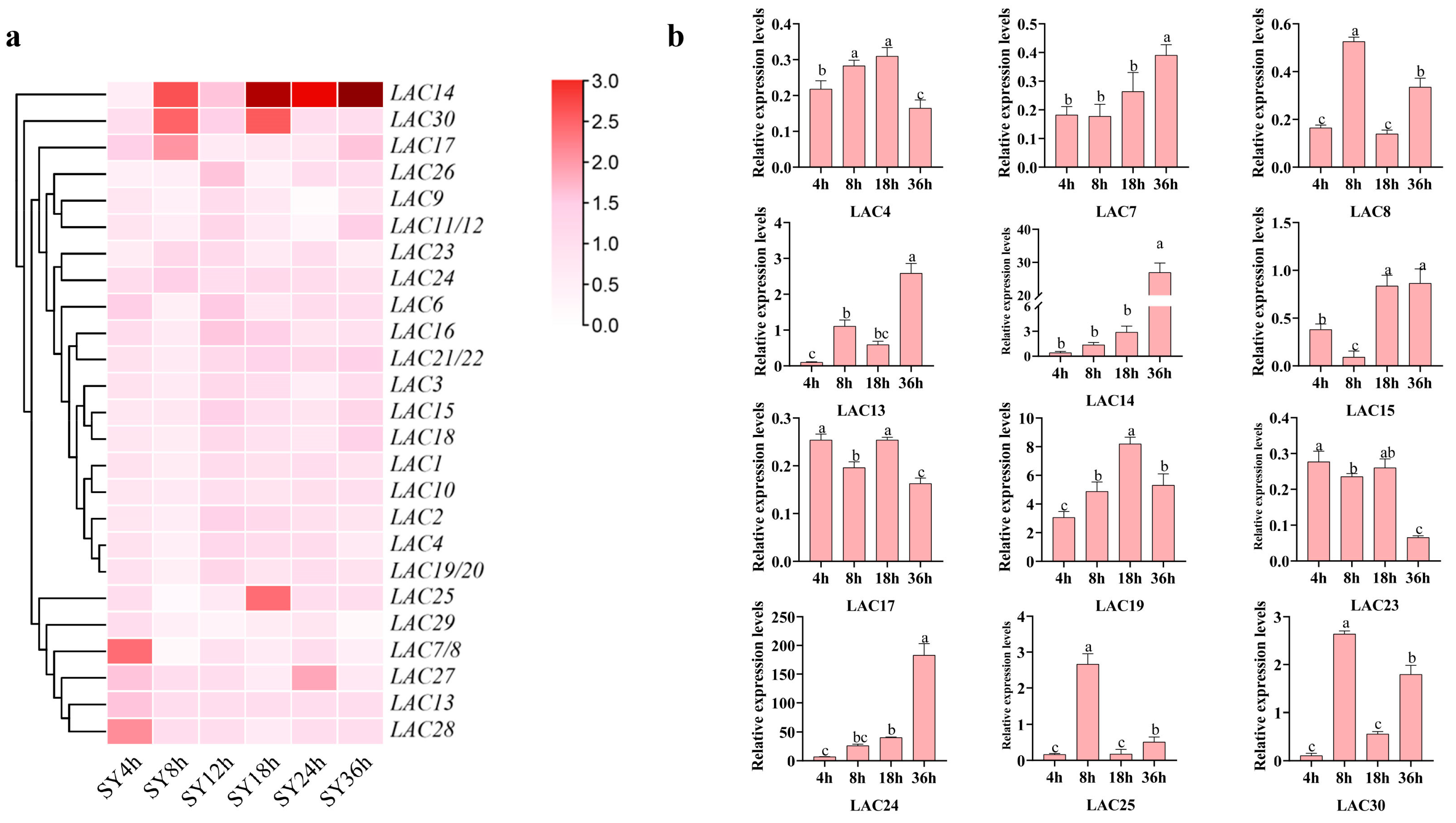
2.6. Expression Profile of Grape LAC Genes in SY Leaves against B. cinerea
2.7. RNA Extraction and qRT-PCR Analysis
2.8. Determination of Laccase Activity and Lignin Content
2.9. Data Analysis
3. Results
3.1. Laccase Activity and Lignin Content in SY Leaves in Response to B. cinerea
3.2. Identification of VvLAC Genes in Grapevine
3.3. Chromosomal Locations and Phylogenetic Analysis
3.4. Gene Duplication and Syntenic Analysis
3.5. Motif Composition and Gene Structure
3.6. Cis-Elements in Promoters of VvLAC Genes
3.7. Protein–Protein Interaction Network
3.8. Expression Profiles of LAC Genes in SY Leaves in Response against Botrytis cinerea
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shen, F.; Wu, W.; Han, X.; Wang, J.; Li, Y.; Liu, D. Study on the occurrence law and green control of grape gray mold from the perspective of ecological balance. Bioengineered 2021, 12, 779–790. [Google Scholar] [CrossRef] [PubMed]
- De Simone, N.; Pace, B.; Grieco, F.; Chimienti, M.; Tyibilika, V.; Santoro, V.; Capozzi, V.; Colelli, G.; Spano, G.; Russo, P. Botrytis cinerea and table grapes: A review of the main physical, chemical, and bio-based control treatments in post-harvest. Foods 2020, 9, 1138. [Google Scholar] [CrossRef] [PubMed]
- Wan, R.; Hou, X.; Wang, X.; Qu, J.; Singer, S.D.; Wang, Y.; Wang, X. Resistance evaluation of Chinese wild Vitis genotypes against Botrytis cinerea and different responses of resistant and susceptible hosts to the infection. Front. Plant Sci. 2015, 6, 160149. [Google Scholar] [CrossRef] [PubMed]
- Harper, L.A.; Paton, S.; Hall, B.; McKay, S.; Oliver, R.P.; Lopez-Ruiz, F.J. Fungicide resistance characterized across seven modes of action in Botrytis cinerea isolated from Australian vineyards. Pest Manag. Sci. 2022, 78, 1326–1340. [Google Scholar] [CrossRef] [PubMed]
- Bi, K.; Liang, Y.; Mengiste, T.; Sharon, A. Killing softly: A roadmap of Botrytis cinerea pathogenicity. Trends Plant Sci. 2023, 28, 211–222. [Google Scholar] [CrossRef]
- Blanco-Ulate, B.; Morales-Cruz, A.; Amrine, K.C.H.; Labavitch, J.M.; Powell, A.L.T.; Cantu, D. Genome-wide transcriptional profiling of Botrytis cinerea genes targeting plant cell walls during infections of different hosts. Front. Plant Sci. 2014, 5, 98301. [Google Scholar] [CrossRef] [PubMed]
- Veloso, J.; van Kan, J.A.L. Many shades of grey in Botrytis-host plant interactions. Trends Plant Sci. 2018, 23, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Goetz, G.; Fkyerat, A.; Métais, N.; Kunz, M.; Tabacchi, R.; Pezet, R.; Pont, V. Resistance factors to grey mould in grape berries: Identification of some phenolics inhibitors of Botrytis cinerea stilbene oxidase. Phytochemistry 1999, 52, 759–767. [Google Scholar] [CrossRef]
- Liu, Q.; Luo, L.; Zheng, L. Lignins: Biosynthesis and biological functions in plants. Int. J. Mol. Sci. 2018, 19, 335. [Google Scholar] [CrossRef] [PubMed]
- Lazniewska, J.; Macioszek, V.K.; Kononowicz, A.K. Plant-fungus interface: The role of surface structures in plant resistance and susceptibility to pathogenic fungi. Physiol. Mol. Plant Pathol. 2012, 78, 24–30. [Google Scholar] [CrossRef]
- Lee, M.-H.; Jeon, H.S.; Kim, S.H.; Chung, J.H.; Roppolo, D.; Lee, H.-J.; Cho, H.J.; Tobimatsu, Y.; Ralph, J.; Park, O.K. Lignin-based barrier restricts pathogens to the infection site and confers resistance in plants. Embo J. 2019, 38, e101948. [Google Scholar] [CrossRef] [PubMed]
- Asselbergh, B.; Curvers, K.; Franca, S.C.; Audenaert, K.; Vuylsteke, M.; Van Breusegem, F.; Hoefte, M. Resistance to Botrytis cinerea in sitiens, an abscisic acid-deficient tomato mutant, involves timely production of hydrogen peroxide and cell wall modifications in the epidermis. Plant Physiol. 2007, 144, 1863–1877. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, A.J.; Allwood, J.W.; Winder, C.L.; Dunn, W.B.; Heald, J.K.; Cristescu, S.M.; Sivakumaran, A.; Harren, F.J.M.; Mulema, J.; Denby, K.; et al. Metabolomic approaches reveal that cell wall modifications play a major role in ethylene-mediated resistance against Botrytis cinerea. Plant J. 2011, 67, 852–868. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Yi, H. Induction of disease resistance providing new insight into sulfur dioxide preservation in Vitis vinifera L. Sci. Hortic. 2017, 225, 567–573. [Google Scholar] [CrossRef]
- Hamiduzzaman, M.M.; Jakab, G.; Barnavon, L.; Neuhaus, J.M.; Mauch-Mani, B. β-Aminobutyric acid-induced resistance against downy mildew in grapevine acts through the potentiation of callose formation and jasmonic acid signaling. Mol. Plant-Microbe Interact. 2005, 18, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Wallis, C.M.; Chen, J. Grapevine phenolic compounds in xylem sap and tissues are significantly altered during infection by Xylella Fastidiosa. Phytopathology 2012, 102, 816–826. [Google Scholar] [CrossRef] [PubMed]
- Tziros, G.T.; Ainalidou, A.; Samaras, A.; Kollaros, M.; Karamanoli, K.; Menkissoglu-Spiroudi, U.; Karaoglanidis, G.S. Differences in defence-related gene expression and metabolite accumulation reveal insights into the resistance of Greek grape wine cultivars to Botrytis bunch rot. Oeno One 2022, 56, 111–123. [Google Scholar] [CrossRef]
- Perkins, M.L.; Schuetz, M.; Unda, F.; Chen, K.T.; Bally, M.B.; Kulkarni, J.A.; Yan, Y.; Pico, J.; Castellarin, S.D.; Mansfield, S.D.; et al. Monolignol export by diffusion down a polymerization-induced concentration gradient. Plant Cell 2022, 34, 2080–2095. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y. LACCASE2 negatively regulates lignin deposition of Arabidopsis Roots. Plant Physiol. 2020, 182, 1190–1191. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Nakashima, J.; Chen, F.; Yin, Y.; Fu, C.; Yun, J.; Shao, H.; Wang, X.; Wang, Z.-Y.; Dixon, R.A. LACCASE is necessary and nonredundant with PEROXIDASE for lignin polymerization during vascular development in Arabidopsis. Plant Cell 2013, 25, 3976–3987. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Davis, E.; Gardner, D.; Cai, X.; Wu, Y. Involvement of AtLAC15 in lignin synthesis in seeds and in root elongation of Arabidopsis. Planta 2006, 224, 1185–1196. [Google Scholar] [CrossRef] [PubMed]
- Berthet, S.; Demont-Caulet, N.; Pollet, B.; Bidzinski, P.; Cezard, L.; Le Bris, P.; Borrega, N.; Herve, J.; Blondet, E.; Balzergue, S.; et al. Disruption of LACCASE4 and 17 results in tissue-specific alterations to lignification of Arabidopsis thaliana stems. Plant Cell 2011, 23, 1124–1137. [Google Scholar] [CrossRef] [PubMed]
- Perkins, M.L.; Schuetz, M.; Unda, F.; Smith, R.A.; Sibout, R.; Hoffmann, N.J.; Wong, D.C.J.; Castellarin, S.D.; Mansfield, S.D.; Samuels, L. Dwarfism of high-monolignol Arabidopsis plants is rescued by ectopic LACCASE overexpression. Plant Direct 2020, 4, e00265. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Machemer-Noonan, K.; Golfier, P.; Unda, F.; Dechert, J.; Zhang, W.; Hoffmann, N.; Samuels, L.; Mansfield, S.D.; Rausch, T.; et al. The in vivo impact of MsLAC1, a Miscanthus laccase isoform, on lignification and lignin composition contrasts with its in vitro substrate preference. BMC Plant Biol. 2019, 19, 552. [Google Scholar] [CrossRef]
- Xue, C.; Yao, J.-L.; Qin, M.-F.; Zhang, M.-Y.; Allan, A.C.; Wang, D.-F.; Wu, J. PbrmiR397a regulates lignification during stone cell development in pear fruit. Plant Biotechnol. J. 2019, 17, 103–117. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Fan, C.; Li, X.; Li, Y.; Hu, J.; Li, C.; Luo, K. LACCASE14 is required for the deposition of guaiacyl lignin and affects cell wall digestibility in poplar. Biotechnol. Biofuels 2020, 13, 197. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhuo, C.; Xiao, X.; Wang, X.; Docampo-Palacios, M.; Chen, F.; Dixon, R.A. Substrate specificity of LACCASE8 facilitates polymerization of caffeyl alcohol for C-lignin biosynthesis in the seed coat of Cleome hassleriana. Plant Cell 2020, 32, 3825–3845. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, V.K.; Rai, K.M.; Thu, S.W.; Hii, M.M.; Mendu, V. Genome-wide identification of multifunctional laccase gene family in cotton (Gossypium spp.); expression and biochemical analysis during fiber development. Sci. Rep. 2016, 6, 34309. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Tang, Y.; Jia, P.; Zeng, Y.; Wang, B.; Wu, P.; Quan, Y.; Chen, A.; Li, Y.; Wu, J. A Cotton lignin biosynthesis gene, GhLAC4, fine-tuned by Ghr-miR397 modulates plant resistance against Verticillium dahliae. Front. Plant Sci. 2021, 12, 743795. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, L.; Wang, X.; Chen, B.; Zhao, J.; Cui, J.; Li, Z.; Yang, J.; Wu, L.; Wu, J.; et al. The cotton laccase gene GhLAC15 enhances Verticillium wilt resistance via an increase in defence-induced lignification and lignin components in the cell walls of plants. Mol. Plant Pathol. 2019, 20, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Min, L.; Yang, X.; Jin, S.; Zhang, L.; Li, Y.; Ma, Y.; Qi, X.; Li, D.; Liu, H.; et al. Laccase GhLac1 modulates broad-spectrum biotic stress tolerance via manipulating phenylpropanoid pathway and jasmonic acid synthesis. Plant Physiol. 2018, 176, 1808–1823. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Gong, H.; Cao, L.; Hou, Y.; Qu, S. MicroRNA397b negatively regulates resistance of Malus hupehensis to Botryosphaeria dothidea by modulating MhLAC7 involved in lignin biosynthesis. Plant Sci. 2020, 292, 110390. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Yu, X.; Chen, W.; Zhuang, W.; Wang, S.; Sun, C.; Cao, L.; Zhou, T.; Qu, S. MdWRKY75e enhances resistance to Alternaria alternata in Malus domestica. Hortic. Res. 2021, 8, 225. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Li, G.; Singh, J.; Khan, A.; Fazio, G.; Saltzgiver, M.; Xia, R. Laccase directed lignification is one of the major processes associated with the defense response against Pythium ultimum infection in apple roots. Front. Plant Sci. 2021, 12, 629776. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liu, Y.; Dong, X.; Liu, J.-J.; Zhao, D.-G. Identification of a novel laccase gene EuLAC1 and its potential resistance against Botrytis cinerea. Transgenic Res. 2022, 31, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Wan, R.; Guo, C.; Hou, X.; Zhu, Y.; Gao, M.; Hu, X.; Zhang, S.; Jiao, C.; Guo, R.; Li, Z.; et al. Comparative transcriptomic analysis highlights contrasting levels of resistance of Vitis vinifera and Vitis amurensis to Botrytis cinerea. Hortic. Res. 2021, 8, 103. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. Interactive tree of life (iTOL) v3: An online tool for the display and annotation of phylogenetic and other trees. Nucleic Acids Res. 2016, 44, W242–W245. [Google Scholar] [CrossRef] [PubMed]
- McCaig, B.C.; Meagher, R.B.; Dean, J.F.D. Gene structure and molecular analysis of the laccase-like multicopper oxidase (LMCO) gene family in Arabidopsis thaliana. Planta 2005, 221, 619–636. [Google Scholar] [CrossRef] [PubMed]
- Liao, B.; Wang, C.; Li, X.; Man, Y.; Ruan, H.; Zhao, Y. Genome-wide analysis of the Populus trichocarpa laccase gene family and functional identification of PtrLAC23. Front. Plant Sci. 2023, 13, 1063813. [Google Scholar] [CrossRef]
- Xu, X.; Zhou, Y.; Wang, B.; Ding, L.; Wang, Y.; Luo, L.; Zhang, Y.; Kong, W. Genome-wide identification and characterization of laccase gene family in Citrus sinensis. Gene 2019, 689, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Yao, J.; Tong, R.; Wang, S.; Li, M.; Song, C.; Wan, R.; Jiao, J.; Zheng, X. Genome-wide identification of laccase gene family from Punica granatum and functional analysis towards potential involvement in lignin biosynthesis. Horticulturae 2023, 9, 918. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, H.; Huang, K.; Guo, R.; Zhao, J.; Xie, H.; Zhu, J.; Gu, H.; Chen, H.; Li, G.; et al. Comprehensive analysis of the laccase gene family in tea plant highlights its roles in development and stress responses. BMC Plant Biol. 2023, 23, 129. [Google Scholar] [CrossRef] [PubMed]
- Wan, R.; Song, J.; Lv, Z.; Qi, X.; Han, X.; Guo, Q.; Wang, S.; Shi, J.; Jian, Z.; Hu, Q.; et al. Genome-wide identification and comprehensive analysis of the AP2/ERF gene family in pomegranate fruit development and postharvest preservation. Genes 2022, 13, 895. [Google Scholar] [CrossRef]
- Mayolo-Deloisa, K.; Gonzalez-Gonzalez, M.; Rito-Palomares, M. Laccases in food industry: Bioprocessing, potential industrial and biotechnological applications. Front. Bioeng. Biotechnol. 2020, 8, 222. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Davis, E.J.; Ballif, J.; Liang, M.; Bushman, E.; Haroldsen, V.; Torabinejad, J.; Wu, Y. Mutant identification and characterization of the laccase gene family in Arabidopsis. J. Exp. Bot. 2006, 57, 2563–2569. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Luo, L.; Wang, X.; Shen, Z.; Zheng, L. Comprehensive Analysis of Rice Laccase Gene (OsLAC) Family and ectopic expression of OsLAC10 enhances tolerance to copper stress in Arabidopsis. Int. J. Mol. Sci. 2017, 18, 209. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Liu, Y.; Yang, S.; Yang, Y.; Wang, C. Genome-wide characterization of laccase gene family from turnip and chinese cabbage and the role in xylem lignification in hypocotyls. Horticulturae 2022, 8, 522. [Google Scholar] [CrossRef]
- Sun, Z.; Zhou, Y.; Hu, Y.; Jiang, N.; Hu, S.; Li, L.; Li, T. Identification of wheat LACCASEs in response to Fusarium graminearum as potential deoxynivalenol trappers. Front. Plant Sci. 2022, 13, 832800. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, V.; Garcia-Andrade, J.; Vera, P. Enhanced disease resistance to Botrytis cinerea in myb46 Arabidopsis plants is associated to an early down-regulation of CesA genes. Plant Signal. Behav. 2011, 6, 911–913. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xin, H.; Fan, P.; Zhang, J.; Liu, Y.; Dong, Y.; Wang, Z.; Yang, Y.; Zhang, Q.; Ming, Y.; et al. The genome of Shanputao (Vitis amurensis) provides a new insight into cold tolerance of grapevine. Plant Fournal 2021, 105, 1495–1506. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhang, X.; Zhang, Q.; Chai, S.; Yin, W.; Gao, M.; Li, Z.; Wang, X. The transcription factors VaERF16 and VaMYB306 interact to enhance resistance of grapevine to Botrytis cinerea infection. Mol. Plant Pathol. 2022, 23, 1415–1432. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhu, Y.; Han, R.; Yin, W.; Guo, C.; Li, Z.; Wang, X. Expression of Vitis amurensis VaERF20 in Arabidopsis thaliana improves resistance to Botrytis cinerea and Pseudomonas syringae pv. tomato DC3000. Int. J. Mol. Sci. 2018, 19, 696. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Lee, C.; Zhong, R.; Ye, Z. MYB58 and MYB63 are transcriptional activators of the lignin biosynthetic pathway during secondary cell wall formation in Arabidopsis. Plant Cell 2009, 21, 248–266. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Huang, S.; Qian, J.; Qing, H.; Wan, Z.; Cheng, H.; Zhang, C. Genome-Wide identification of Petunia HSF genes and potential function of PhHSF19 in benzenoid/phenylpropanoid biosynthesis. Int. J. Mol. Sci. 2022, 23, 2974. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, A.P.; McEntee, K. Msn2p, a zinc finger DNA-binding protein, is the transcriptional activator of the multistress response in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 1996, 93, 5777–5782. [Google Scholar] [CrossRef] [PubMed]
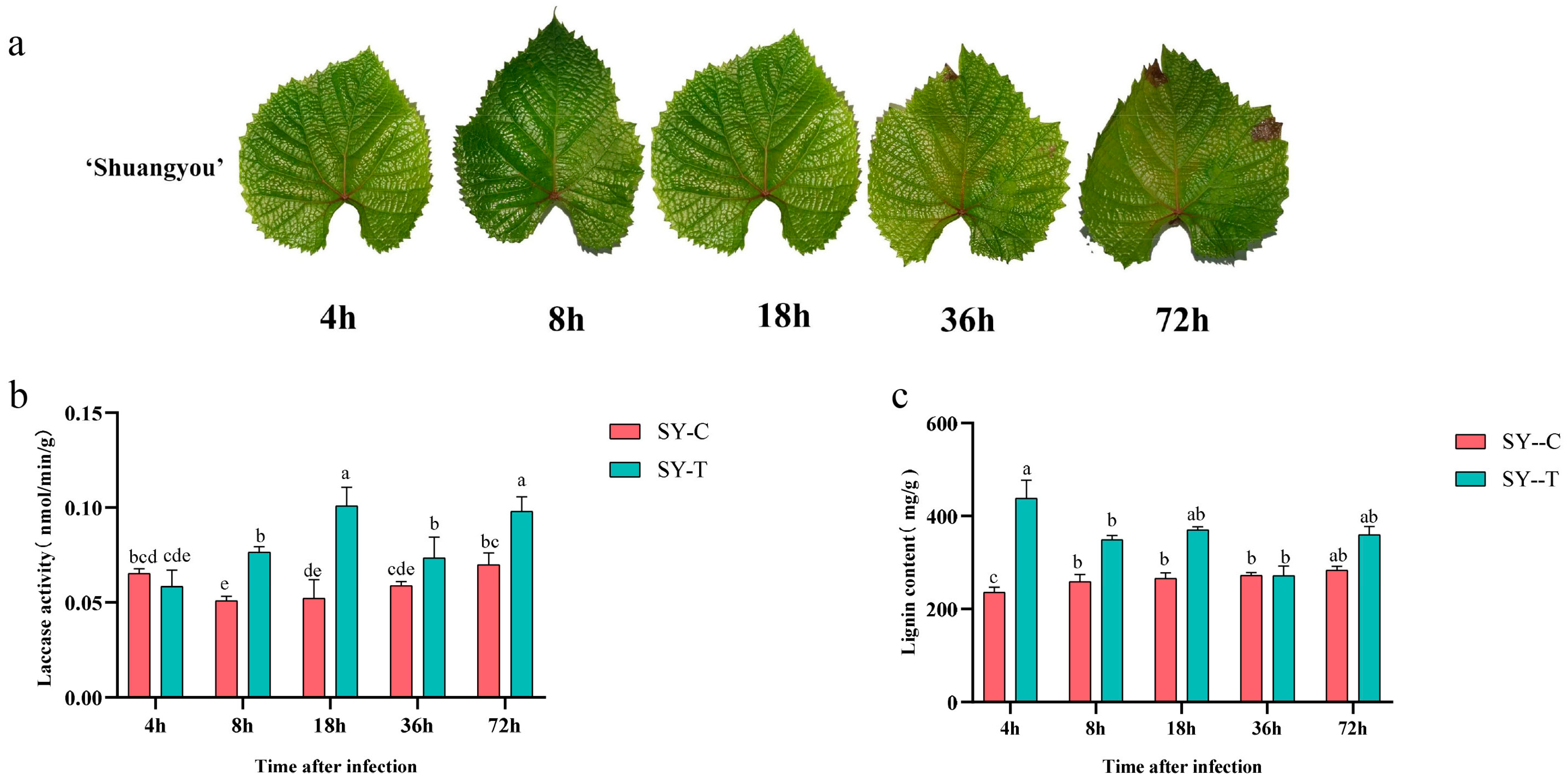
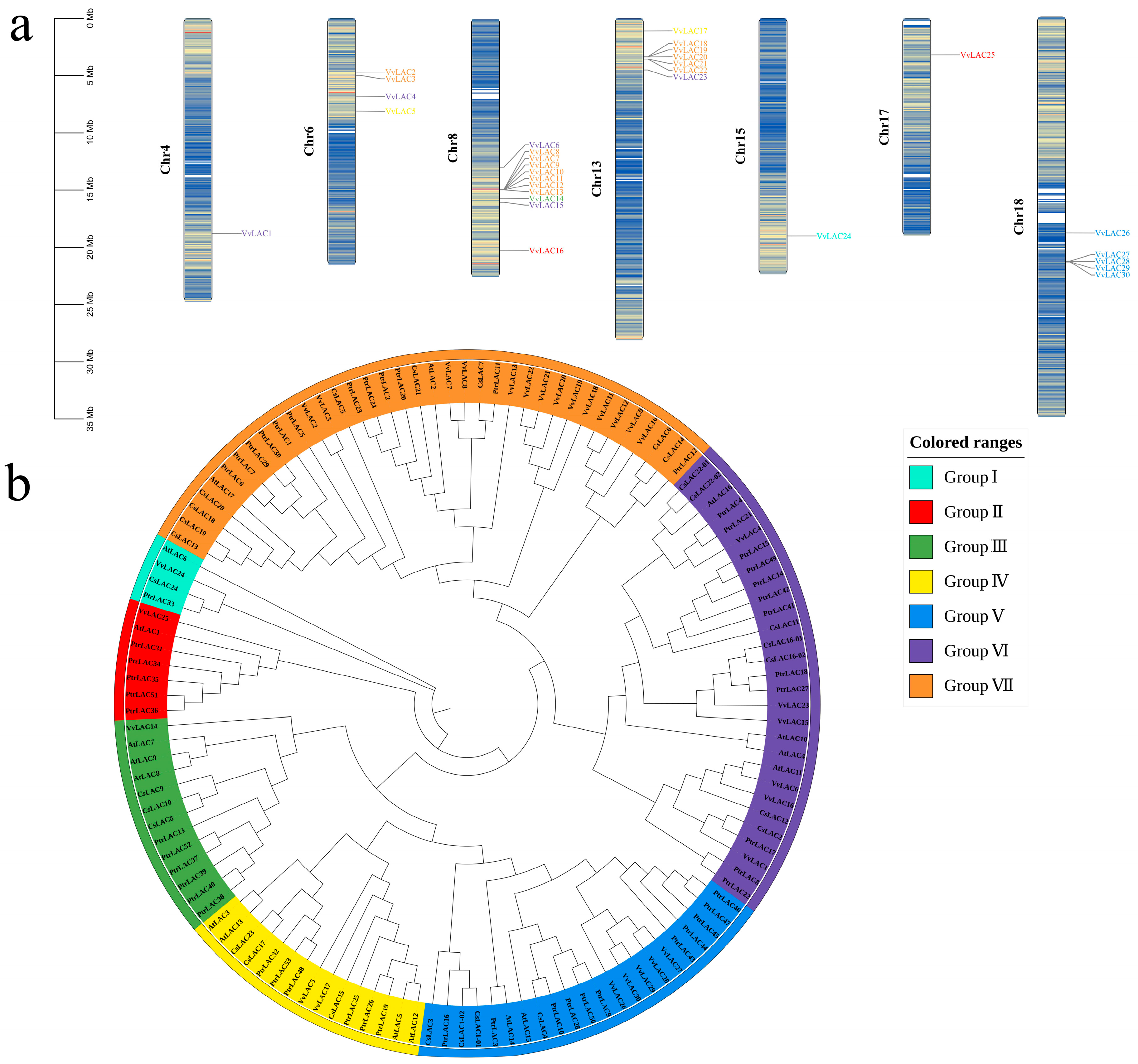

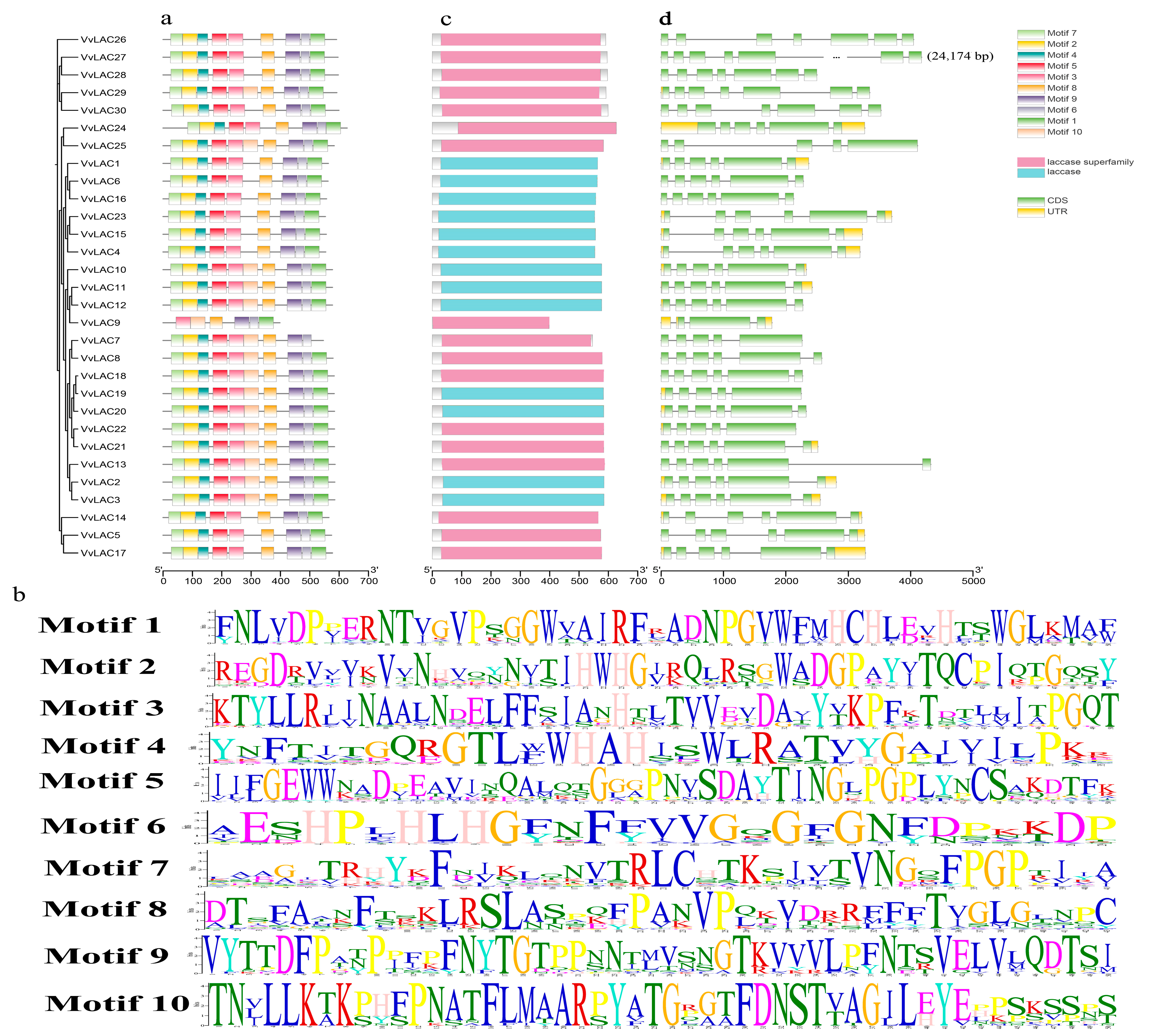

| Name | Gene ID | Chromosome | Gene Length (bp) | pI | MW (Kda) | Amino Acid (aa) | Subcellular Localization |
|---|---|---|---|---|---|---|---|
| VvLAC1 | Vitvi04g01317_t001 | Chr4 | 1692 | 8.49 | 62.23 | 563 | vacu |
| VvLAC2 | Vitvi06g00378_t001 | Chr6 | 1758 | 9.02 | 64.39 | 585 | chlo |
| VvLAC3 | Vitvi06g00405_t001 | Chr6 | 1758 | 8.71 | 64.30 | 585 | extr |
| VvLAC4 | Vitvi06g00591_t001 | Chr6 | 1665 | 9.26 | 60.70 | 554 | chlo |
| VvLAC5 | Vitvi06g00728_t001 | Chr6 | 1725 | 8.59 | 63.59 | 574 | vacu |
| VvLAC6 | Vitvi08g01031_t001 | Chr8 | 1689 | 8.37 | 62.49 | 562 | extr |
| VvLAC7 | Vitvi08g01223_t002 | Chr8 | 1641 | 9.32 | 59.90 | 546 | chlo |
| VvLAC8 | Vitvi08g01223_t001 | Chr8 | 1740 | 9.24 | 63.69 | 579 | chlo |
| VvLAC9 | Vitvi08g04233_t001 | Chr8 | 1197 | 9.74 | 43.53 | 398 | chlo |
| VvLAC10 | Vitvi08g02201_t001 | Chr8 | 1734 | 9.77 | 64.04 | 577 | chlo |
| VvLAC11 | Vitvi08g01228_t001 | Chr8 | 1734 | 9.79 | 64.04 | 577 | chlo |
| VvLAC12 | Vitvi08g04234_t001 | Chr8 | 1734 | 9.78 | 64.08 | 577 | chlo |
| VvLAC13 | Vitvi08g01229_t001 | Chr8 | 1761 | 9.22 | 64.96 | 586 | chlo |
| VvLAC14 | Vitvi08g01299_t001 | Chr8 | 1698 | 6.79 | 62.00 | 565 | vacu |
| VvLAC15 | Vitvi08g01335_t001 | Chr8 | 1671 | 9.12 | 60.48 | 556 | chlo |
| VvLAC16 | Vitvi08g01735_t001 | Chr8 | 1674 | 8.04 | 61.69 | 557 | extr |
| VvLAC17 | Vitvi13g00117_t001 | Chr13 | 1734 | 7.63 | 63.18 | 577 | cyto |
| VvLAC18 | Vitvi13g00321_t001 | Chr13 | 1752 | 9.44 | 64.30 | 583 | chlo |
| VvLAC19 | Vitvi13g00322_t001 | Chr13 | 1752 | 9.53 | 64.39 | 583 | chlo |
| VvLAC20 | Vitvi13g00323_t001 | Chr13 | 1755 | 9.35 | 64.61 | 584 | vacu |
| VvLAC21 | Vitvi13g00341_t001 | Chr13 | 1755 | 9.4 | 64.50 | 584 | chlo |
| VvLAC22 | Vitvi13g00342_t001 | Chr13 | 1755 | 9.22 | 64.45 | 584 | chlo |
| VvLAC23 | Vitvi13g00509_t001 | Chr13 | 1662 | 8.67 | 60.40 | 553 | chlo |
| VvLAC24 | Vitvi15g00941_t001 | Chr15 | 1884 | 8.19 | 69.76 | 627 | chlo |
| VvLAC25 | Vitvi17g00227_t001 | Chr17 | 1752 | 8.5 | 65.26 | 583 | chlo |
| VvLAC26 | Vitvi18g01488_t001 | Chr18 | 1776 | 5.3 | 66.34 | 591 | extr |
| VvLAC27 | Vitvi18g02924_t001 | Chr18 | 1791 | 4.89 | 66.31 | 596 | cyto |
| VvLAC28 | Vitvi18g02922_t001 | Chr18 | 1794 | 4.79 | 66.25 | 597 | pero |
| VvLAC29 | Vitvi18g02927_t001 | Chr18 | 1779 | 5.52 | 66.58 | 592 | plas |
| VvLAC30 | Vitvi18g02928_t001 | Chr18 | 1800 | 4.92 | 67.64 | 599 | cyto |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, R.; Yang, Z.; Liu, J.; Zhang, M.; Jiao, J.; Wang, M.; Zhang, K.; Hao, P.; Liu, Y.; Bai, T.; et al. Identification of Laccase Genes in Grapevine and Their Roles in Response to Botrytis cinerea. Horticulturae 2024, 10, 376. https://doi.org/10.3390/horticulturae10040376
Wan R, Yang Z, Liu J, Zhang M, Jiao J, Wang M, Zhang K, Hao P, Liu Y, Bai T, et al. Identification of Laccase Genes in Grapevine and Their Roles in Response to Botrytis cinerea. Horticulturae. 2024; 10(4):376. https://doi.org/10.3390/horticulturae10040376
Chicago/Turabian StyleWan, Ran, Zhenfeng Yang, Jun Liu, Mengxi Zhang, Jian Jiao, Miaomiao Wang, Kunxi Zhang, Pengbo Hao, Yu Liu, Tuanhui Bai, and et al. 2024. "Identification of Laccase Genes in Grapevine and Their Roles in Response to Botrytis cinerea" Horticulturae 10, no. 4: 376. https://doi.org/10.3390/horticulturae10040376
APA StyleWan, R., Yang, Z., Liu, J., Zhang, M., Jiao, J., Wang, M., Zhang, K., Hao, P., Liu, Y., Bai, T., Song, C., Song, S., Shi, J., & Zheng, X. (2024). Identification of Laccase Genes in Grapevine and Their Roles in Response to Botrytis cinerea. Horticulturae, 10(4), 376. https://doi.org/10.3390/horticulturae10040376





