Abstract
To investigate the effect of calcium (Ca) application on post-harvest fruit quality in Nanfeng tangerines, this study sprayed three calcium fertilizers (Calbit, Larry 8 Calcium, and Suspended Calcium) before harvesting. The fruit quality was assessed over a storage period of 0–60 d at a controlled room temperature of 20 ± 5 °C. The evaluation methods included principal component analysis (PCA) and linearly weighted summation. Pre-harvest calcium treatments increased the fruit calcium content and maintained higher firmness and shear. Compared to the control group, fruits treated with Calbit exhibited decreased levels of decay, weight loss, and respiration rates. Additionally, they demonstrated higher values of L*, b*, C*, and H° and lower values of a* and CCI. Moreover, the Larry 8 Calcium and Calbit treatments increased the levels of soluble solids, total soluble sugars, titratable acids, and VC content in the fruit. They accelerated the decomposition of tartaric, oxalic, and citric acids in the pulp, promoting the accumulation of sucrose and glucose. PCA and comprehensive evaluation scores indicated that the comprehensive scores assessing the storage quality of Nanfeng tangerine fruits treated with pre-harvest Larry 8 Calcium and Calbit were higher than those of the control group. The highest composite quality scores for Calbit-treated fruits were observed at 40 and 60 d, and the highest scores for Larry 8 Calcium were recorded at other intervals. These findings suggest that pre-harvest calcium application improved the post-harvest fruit quality of Nanfeng tangerines, with Larry 8 Calcium and Calbit emerging as favorable options.
1. Introduction
The Nanfeng tangerine (Citrus reticulata Blanco cv. ‘Kinokuni’) is renowned as one of the best citrus varieties, with a cultivation legacy spanning over 1300 years in the Jiangxi Province, China [1]. Nanfeng tangerine fruit is important in the citrus fruit market because of its vibrant golden color, thin skin, tender flesh, and delightfully sweet flavor [2]. However, Nanfeng tangerine fruit is susceptible to fungal infections after harvest, leading to various physiological disorders, such as stem rot, green mold, and blue mold [3]. These infections significantly diminish the fruit quality and present challenges for transportation and storage. This leads to considerable financial losses and a reduction in commercial value [4]. Therefore, the primary considerations lie in enhancing the fruit quality and delaying the deterioration of the storage quality.
The maturity and development of fruits relies significantly on calcium (Ca), an essential mineral for fruit tree growth and development [5]. Furthermore, Ca regulates the physiological and metabolic functions of fruits as a counterion of organic and inorganic anions in vacuoles and a cytoplasmic second messenger associated with developmental or environmental events. This regulation is crucial for preserving the integrity and functionality of cell walls and membranes, thus enhancing the quality of the harvested fruits [6]. Fruits with higher Ca content may exhibit shorter shelf lives due to calcium binding to pectin in the cell wall, inhibiting enzymes such as polygalacturonase from breaking down the fruit [7]. High Ca levels can benefit fruit coloration, protein synthesis, membrane system integrity, and fruit cell structure stiffness [8], directly influencing the texture and flavor after harvest. Furthermore, numerous studies have established a connection between fruit storage-related physiological and pathological diseases and pericarp cell wall structure [9]. The stability of the cell wall structure is also associated with the synergistic interaction between calcium ions and the polygalacturonic acid chain. By impeding polygalacturonase-mediated pectin breakdown, various calcium treatments may affect pectin quantity in plant cell walls [10]. In addition to delaying fruit ripening and softening and avoiding physiological diseases and pests, this method facilitates the preservation of the cell membrane and cell wall structure, ensuring high fruit firmness and reducing rot [11]. Therefore, adjusting the Ca content effectively controls certain diseases [12].
Red soil utilized in citrus production typically exhibits acidic pH levels, leading to low Ca availability. Although Ca is the most common exchangeable cation in calcareous soils, citrus fruits often contain low levels of Ca [13]. This deficiency is primarily attributed to the restricted mobility of Ca in plants, resulting in inadequate transportation to organs with low transpiration rates, such as fruits, compared to shoots [14]. Hence, to augment fruit Ca content during cultivation, Ca cultivation can be commonly applied by spraying it onto fruit trees before harvesting. Although Ca treatment has been extensively researched in post-harvest fruit storage [11,12,13,14,15], there is a dearth of studies comparing the effects of various calcium preparations on Nanfeng tangerine post-harvest preservation. Therefore, this study aimed to evaluate the impact of pre-harvest calcium treatment on the post-harvest physiology, storability, and quality properties of Nanfeng tangerine fruit. Such investigations can establish foundational knowledge for future environment-friendly preservation technologies and fruit quality enhancement.
2. Materials and Methods
2.1. Plant Materials and Treatments
The experimental materials for this study comprised 18-year-old Nanfeng tangerine trees grafted onto trifoliate oranges. These plants were cultivated in the same plot at the Mingcheng Orchard, situated in Nanfeng County, Jiangxi Province (27°02′ N, 116°38′ E). The fruits were harvested on 31 October 2021, followed by immediate transfer to a ventilated storehouse for preservation at the Jiangxi Key Laboratory for Post-harvest Technology and Nondestructive Testing of Fruits and Vegetables, completed within 3 h.
This study employed three treatments: Larry 8 Calcium 1000× solution (Purchased from Wuhan city, China; Greencare Agricultural Science and Technology Co., Ltd., Ca + Mg ≥ 10.0%), Calbit 1000× solution (Purchased from Jiangmen City, China; Italy Valagro Co., Ltd., Ca ≥ 150 g/L), and Suspended Calcium 3000× solution (Purchased from Fuzhou City, China; Italy SCL group, CaO ≥ 600 g/L). Water spray (CK) served as the control. Six plants with similar yields, crown sizes, and tree vigor were selected. Each tree received two sprays, which were administered 60 and 30 d before harvesting.
2.2. Sample Collection and Post-Harvest Storage
After harvesting, fruits of identical maturity (based on the citrus color index and TSS content), uniform size, and free from mechanical damage were selected and stored for two days. Subsequently, 1000 fruits per treatment were placed in polyethylene plastic film bags (d = 0.03 mm) and stored at room temperature (20 ± 5 °C) with a relative humidity of 85–90%. Sample collection and data measurements were performed every 10 d during the storage period, which extended up to 60 d. At each time point, 20 fruits were sampled for each treatment. The fruits were then separated into peel and pulp, frozen in liquid nitrogen, and stored at −80 °C for further application. Before determination, frozen fruit flesh tissue samples were thoroughly ground into a powder using a refrigerated mixing mill (Retsch-MM 400, Retsch Inc.; Shanghai, China).
2.3. Fruit Quality Measurements
2.3.1. Measurement of Calcium Content
The Ca content was analyzed using inductively coupled plasma mass spectrometry (Agilent ICP OES, Santa Clara, CA, USA), with the specific mass-to-charge ratio (m/z) of the element utilized for qualitative analysis [16].
2.3.2. Measurement of Fruit Texture Index
Fruit firmness and shear force were assessed using a texture analyzer (FTC, TMS-Touch, Sterling, VA, USA). The flesh firmness was measured with a P/100 probe at a test speed of 120 mmmin−1, test and post-test speeds of 280 mmmin−1, a compression degree of 30%, and an initial force of 0.05 N. The flesh shear force was evaluated using the HDP/BS probe at a velocity of 90 mmmin−1 prior to the test and 280 mmmin−1 during and after the test, with a distance of 30 mm and an initial force of 0.5 N.
2.3.3. Measurement of Citrus Color Index, Decay Rate, Weight Loss Rate, and Respiration Rate
Twenty fruits were selected from each treatment group and organized into groups of five fruits each. After washing, colorimetric measurements, including L*, a*, b*, chroma (C*), hue angle (H°), and citrus color index (CCI), were conducted on the peels. Measurements were taken in two diagonal directions at the equator of the fruit epidermis using a colorimeter, and the average value was recorded. CCI was calculated as 1000 × a*/(L* × b*).
A total of 1200 fruits were allocated evenly across the four groups, with 300 fruits assigned to each treatment for decay rate assessment. Visible lesions on the fruit surface, approximately 1 mm in diameter, were indicative of rot. Subsequently, the decay rate (%) was calculated.
The weight loss rates of 33 randomly selected fruits were determined by calculating the percentage difference between the initial and final weights compared to the initial weight.
The fruit respiration rate was assessed using a fruit and vegetable respiration-measuring instrument (GXH-3051H, Junfanglihua Technology Research Institute, Beijing, China). Fifteen fruits were sampled from each group for each measurement, and the respiration rate was determined using the following equation:
where m is the mass of the Nanfeng tangerines (g), Vheadspace is the empty volume of the jar (mL), is the difference between the initial and final concentration of , and t is the recording time (min).
2.3.4. Measurement of Total Soluble Solids (TSS), Titratable Acidity (TA), Ascorbic Acid (VC) Content, and Solid–Acid Ratio (TSS/TA)
The nine fruits were divided into three groups. The pulp juice was combined, and the soluble solid content was measured using a handheld sugar meter (Atago Company, Tokyo, Japan, model PAL-1).
The titratable acidity content (%) in the fruits was determined through sodium hydroxide neutralization titration. The ascorbic acid content was assessed via 2,6-dichlorophenol indophenol titration, with each test conducted three times. The results were reported as mg/100 g [15]. The solid–acid ratio (%) was calculated as the ratio of the soluble solid content to the titratable acid content.
2.3.5. Measurement of Organic Acid and Sugar Component
Organic acids and sugars were extracted [17] with minor adjustments. The frozen fruit pulp samples were pulverized into powder using liquid nitrogen, and 4.0 g of the powdered sample was precisely weighed. Subsequently, the samples were incubated in a 35 °C water bath for 20 min, followed by centrifugation at 10,000 rpm for 15 min at room temperature (4000 rpm for 30 min in a 50 mL centrifuge tube). The resulting supernatant was collected in a 25 mL volumetric flask, combined, and diluted with 80% ethanol to a final volume of 25 mL. Finally, 1 mL of the extract was dried via rotary evaporation, followed by reconstitution with 1 mL of ultrapure water. The reconstituted extract was then filtered using a 13 mm aqueous filter head with a pore size of 0.44 μm for subsequent sample analysis.
The organic acid and sugar content of the fruit were analyzed using high-performance liquid chromatography (Shimadzu Malaysia Factory, Negeri Sembilan, Malaysia, Model LC-2030 Plus). For the determination of sugar components, chromatographic conditions were set as follows: Waters Spherisorb NH2 column (4.6 mm × 250 mm, 5.0 μm) maintained at a column temperature of 35 °C. The mobile phase consisted of an acetonitrile: ultrapure water ratio of 8.5:1.5, a flow rate of 0.8 mL/min, and an injection volume of 20 μL. The detection was performed using a refractive index detector (RID).
The chromatographic conditions for determining acid components were as follows: C18 column (4.6 mm × 250 mm) maintained at a column temperature of 30 °C. The mobile phase comprised 0.01 mol/L H2SO4 (pH 2.6), with a flow rate of 0.5 mL/min and an injection volume of 20 μL. The detection was conducted using a diode array detector (PDA) (Shimadzu, Kyoto, Japan, SPD-M20A).
2.4. Statistical Analysis
All data were statistically processed in Microsoft Excel and analyzed using SPSS 25.0. Graphs were generated using the Graphpad Prism 8.0.
3. Results
3.1. Effects of Pre-Harvest Calcium Treatment on Calcium Content
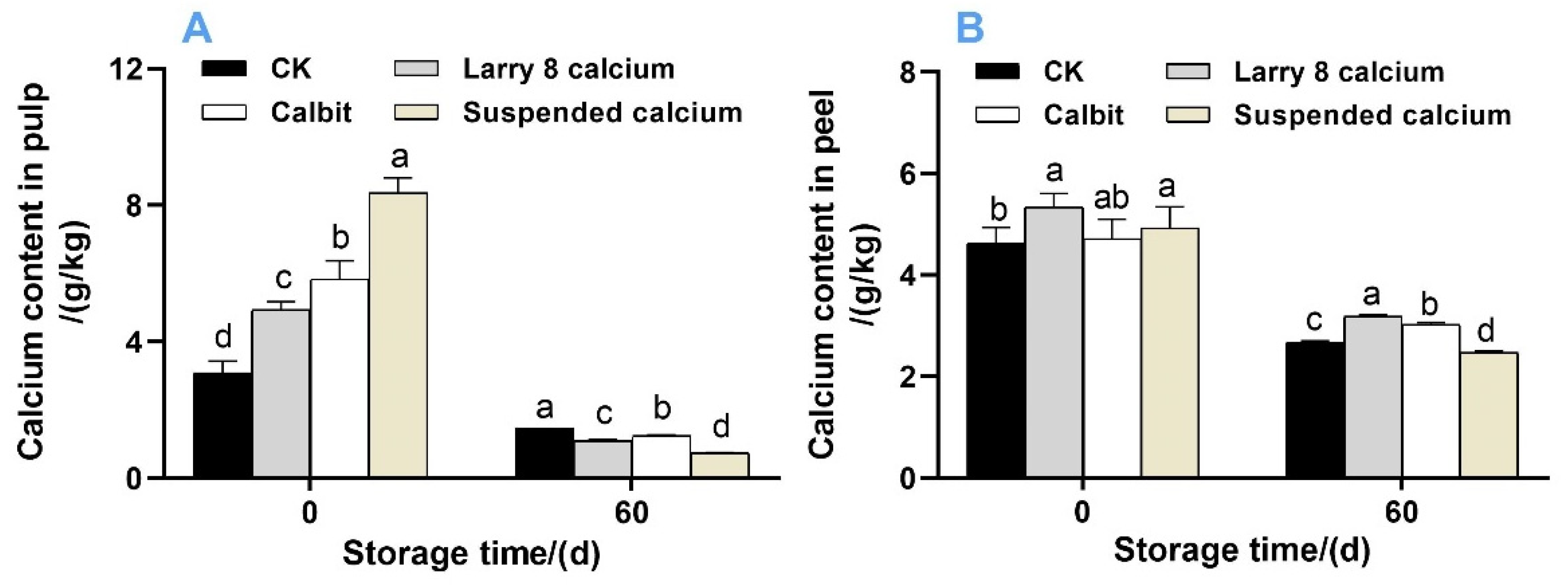
Pre-harvest Ca treatment significantly increased the Ca content in the pulp of Nanfeng tangerine fruit (Figure 1). The highest concentration was observed in fruits subjected to Suspended Ca treatment, followed by Calbit and Larry 8 Calcium. However, after 60 days of storage, the Ca content sharply declined, with the most substantial reduction observed in fruits treated with Suspended Ca, decreasing by 91.12% in the pulp (Figure 1A) and 49.80% in the peel (Figure 1B) compared to that on day 0.
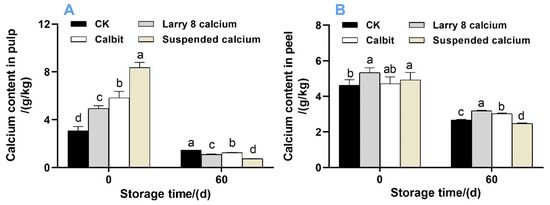
Figure 1.
Calcium content in the pulp (A) and peel (B) of post-harvest Nanfeng tangerines during storage. Vertical bars represent mean ± SE (n + 3). Different letters indicate significant differences according to t-test (p < 0.05) at the same storage stage; the same below.
In the fruit pulp, the Suspended Ca treatment exhibited the highest Ca content at 8.368 g/kg, and the control group showed the lowest Ca content at 3.084 g/kg. Regarding the peel, both the Larry 8 Calcium and Suspended calcium treatments demonstrated significantly higher Ca content than the CK. Among the treatments, the control group experienced the greatest reduction in Ca content in the pulp, whereas the Calbit treatment presented the lowest reduction in calcium content in the peel, decreasing by 52.89% and 35.76%, respectively, compared to day 0.
3.2. Effects of Pre-Harvest Calcium Treatment on Fruit Texture Quality
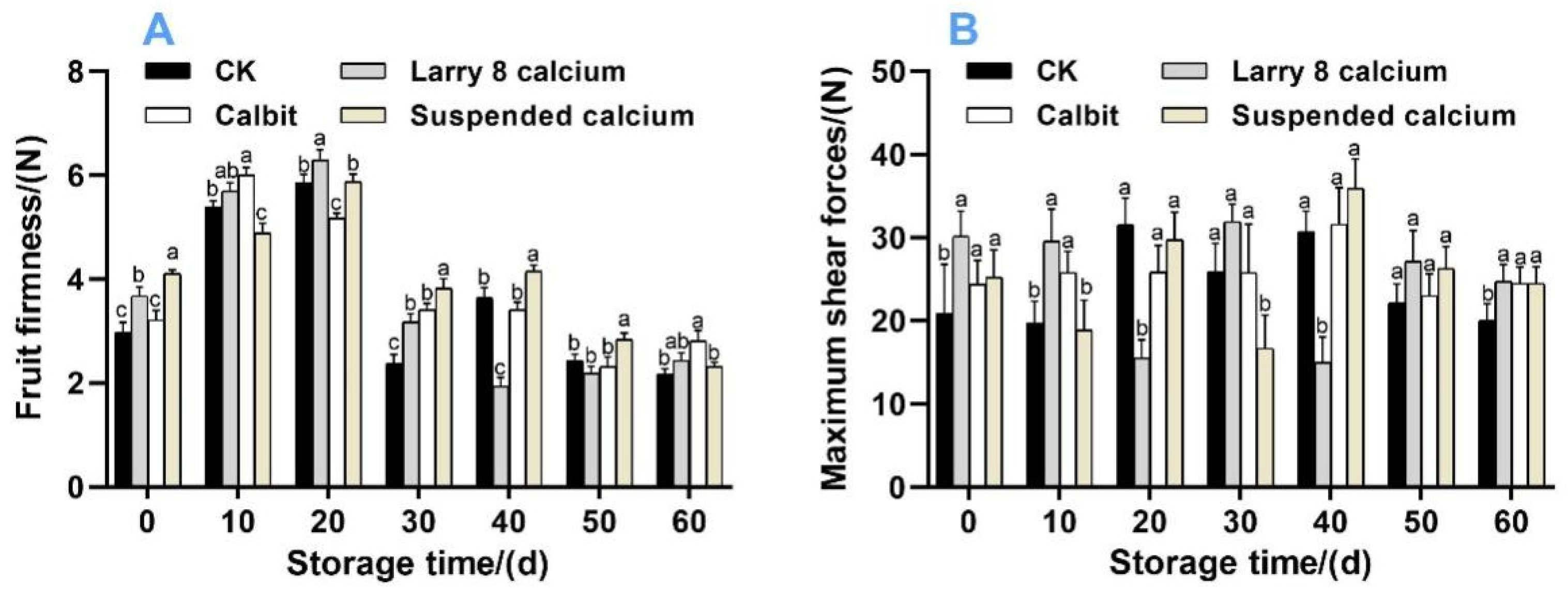
As illustrated in Figure 2A, the firmness of Nanfeng tangerines exhibited an initial increase, followed by a peak, subsequent decrease, and eventual stabilization with prolonged storage time. The fruit firmness peaked at day 10 for Calbit at 6.00 N, while Larry 8 Calcium, Suspended Calcium, and CK reached peaks of 6.30 N, 5.87 N, and 5.86 N, respectively, at day 20. Pre-harvest Ca treatment significantly mitigated the firmness decrease, resulting in 33.56%, 43.92%, and 61.18% higher firmness at day 30 than the CK. At the end of storage, Calbit fruit exhibited a firmness of 2.81 N, which was significantly higher than that of the other treatment groups and 28.90% greater than that of the CK.
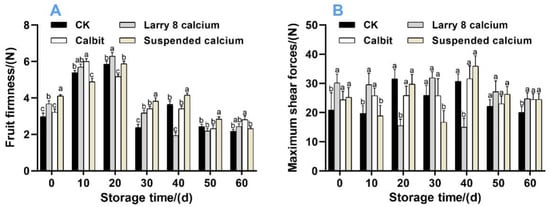
Figure 2.
Effects of different calcium treatments on firmness (A) and maximum shear force (B) of Nanfeng tangerine during storage. Different letters indicate significant differences according to t-test (p < 0.05) at the same storage stage.
The shear forces exhibited fluctuating changes (Figure 2B). At the end of the storage period, the maximum shear force of CK was 20.07 N, which was significantly lower than that of the other three treatment groups.
3.3. Effects of Pre-Harvest Calcium Treatment on Fruit Color Index
Throughout the storage period, the a* value generally exhibited an upward trend (Table 1). Calbit initially showed the lowest a* value of 5.72 on day 0. However, by day 60, CK displayed the smallest a* value of 31.87, which was significantly lower than those of the other three treatment groups. This suggested that pre-harvest Ca treatment enhanced the reddening of Nanfeng tangerine peels. The overall trend of peel b* values initially increased and then decreased. On day 60, the b* value of the Larry 8 Calcium treatment was 59.45, which was significantly lower than that of the Calbit and CK treatments. Calbit and Suspended Calcium increased, starting at 63.66 and 62.17 and decreasing to 60.65 and 60.10, respectively, by day 60, while CK decreased from 62.22 to 60.77 before storage.

Table 1.
Color difference index of Nanfeng tangerine fruit treated with different pre-harvest calcium during storage at room temperature.
The C* values of Nanfeng tangerines displayed a minor fluctuation pattern, initially increasing and then decreasing during storage. There were no significant differences in the C* values of the pericarp among the four treatment groups before or after storage. Larry 8 Calcium reached its maximum value of 68.41 after 30 days of storage. Calbit peaked at 69.73 after 50 days of storage, decreasing slightly to 69.44 by the end of storage; it remained higher than the other treatment groups. Suspended Calcium reached a peak of 70.64 after 50 d, whereas CK increased to 68.77 after 40 days of storage. The H° value exhibited a decreasing trend during storage, decreasing from 78.94, 85.05, 82.23, and 80.79 before storage to 61.00, 60.93, 60.86, and 62.34, respectively. After 60 days of storage, the CK color tone angle H° value was 62.34, which was significantly higher than that of other treatment groups. The L* values decreased with the storage time. Larry 8 Calcium exhibited the largest change, from 68.20 to 62.25; the smallest change occurred in CK, with the L* value decreasing from 66.19 to 63.08. On day 0, the peel color of Larry 8 Calcium was the highest, significantly higher than that of other three treatment groups, indicating the effectiveness of pre-harvest Larry 8 Calcium application in improving peel brightness.
The CCI values of the fruits exhibited an upward trend. At 0 and 50 d, the CCI values of Larry 8 Calcium were 2.56 and 9.07, respectively, significantly higher than those of other three treatment groups. During the initial 50 days of storage, the CCI value of Calbit remained consistently lower than that of the other treatments. These findings suggest that pre-harvest Calbit treatment had a suppressing effect on the increase in CCI value in Nanfeng tangerines stored at room temperature.
3.4. Effects of Pre-Harvest Calcium Treatment on Decay Rates, Weight Loss Rate, and Respiratory Rate
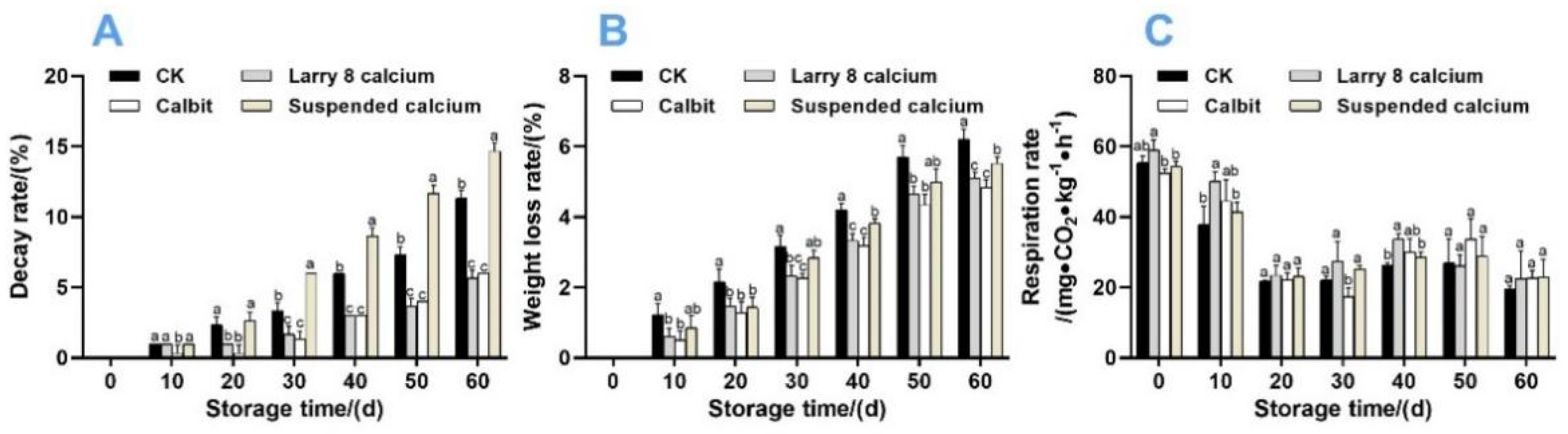
Both the Larry 8 Calcium and Calbit treatments notably reduced the decay rate of Nanfeng tangerine fruit, whereas the Suspended Calcium treatment increased it compared to the control. The highest decay rate (14.67%) was observed in fruits treated with Suspended Calcium, and the lowest decay rate (5.67%) occurred in those treated with Larry 8 Calcium after 60 days of storage.
Pre-harvest Ca treatments, particularly Larry 8 Ca and Calbit, reduced the weight loss rate of Nanfeng tangerine fruit during 60 days of storage (Figure 3B). After 60 d, the weight loss rate of CK was 27.08%, 21.17%, and 16.75% higher than that of the Calbit, Larry 8, and Suspended Calcium treatments, respectively.
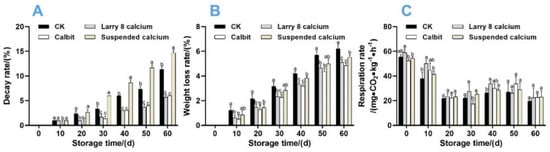
Figure 3.
Effects of different calcium treatments on decay rate (A), weight loss rate (B), and respiration rate (C) of Nanfeng tangerine during storage. Different letters indicate significant differences according to t-test (p < 0.05) at the same storage stage.
Figure 3C illustrates that the overall trend of respiration intensity during storage was similar across different Ca treatments, displaying an initial decrease, followed by an increase, and then a gradual decrease. On day 30, the Calbit treatment group reached its lowest respiratory rate, notably lower than those of other treatment groups, at 78.86% of CK. The respiration rates of Larry 8 Calcium, Suspended Calcium, and CK were minimized at 60 d and did not present significant differences.
3.5. Effects of Pre-Harvest Calcium Treatment on TSS, TA, VC Content, and TSS/TA
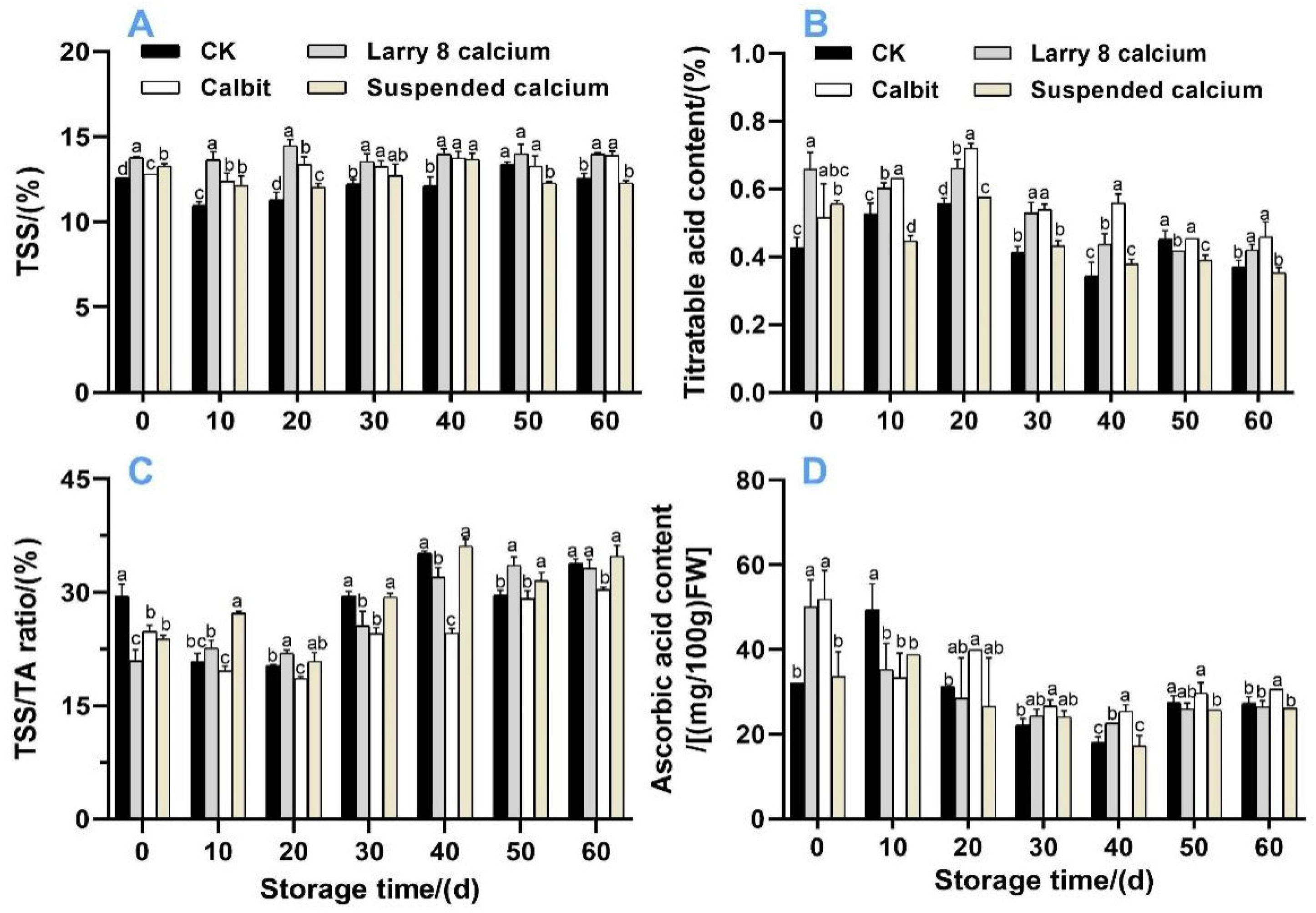
Figure 4 depicts the changes in the TSS, TA, VC, and TSS/TA ratios. The TSS content gradually increased during storage. Before 40-day storage, the pre-harvest Ca treatment significantly increased the TSS content (Figure 4A), and no differences were observed after 50 d. After 60 d, both the Calbit and Larry 8 Ca treatments showed significantly higher TSS contents than CK. The TA contents (Figure 4B) initially increased, and then decreased within 60 d. The Larry 8 Ca and Suspended Ca treatments exhibited significantly higher TA contents than CK in the initial phase of storage. Subsequently, from 20 d onwards, the TA content began to decline continuously, decreasing by 7.21%, 23.44%, 1.69%, and 12.22%, respectively. On day 60, the VC content in the Calbit and Larry 8 Ca treatments was significantly higher than that in the CK.
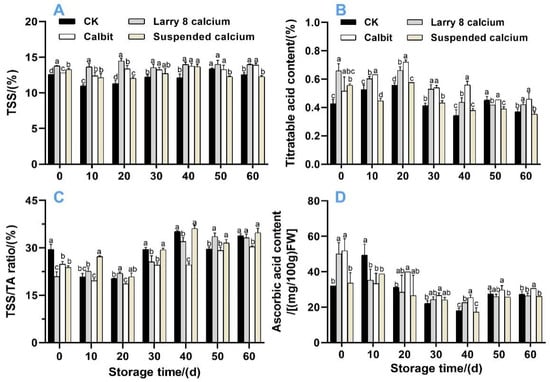
Figure 4.
Effects of different calcium treatments on soluble solids (A), titratable acid (B), TSS-TA ratio (C), and ascorbic acid (D) in Nanfeng tangerine during storage. Different letters indicate significant differences according to t-test (p < 0.05) at the same storage stage.
Pre-harvest Ca treatment delayed the increase in TSS/TA value, resulting in significantly lower values than in CK during the initial storage period, similar to the trend observed in TSS content. However, after 10 days of storage, the TSS/TA value for Suspended Calcium was 27.19%, which was significantly higher than that of the CK. The Larry 8 Ca treatment exhibited the highest TSS/TA value of 33.60% On day 50, it significantly surpassed CK and Calbit. These findings suggest that pre-harvest Ca treatment effectively hindered the reduction in the sugar and acid content, thereby influencing the flavor.
The VC content initially decreased and then gradually increased within 60 d, reaching its lowest value after 40 days of storage (Figure 4D). In the initial stage, the VC contents of Calbit and Larry 8 Calcium were 62.00% and 56.29% higher than those of CK, respectively. By the end of storage, the VC content in Calbit was 19.35% higher than that in the control.
This indicates that pre-harvest Ca treatment effectively enhanced the TSS, TA, and VC levels of Nanfeng tangerines, maintaining them at relatively high levels during storage. Consequently, it inhibited the decline in fruit quality and the occurrence of fruit diseases.
3.6. Effects of Pre-Harvest Calcium Treatment on Organic Acid and Sugar Components
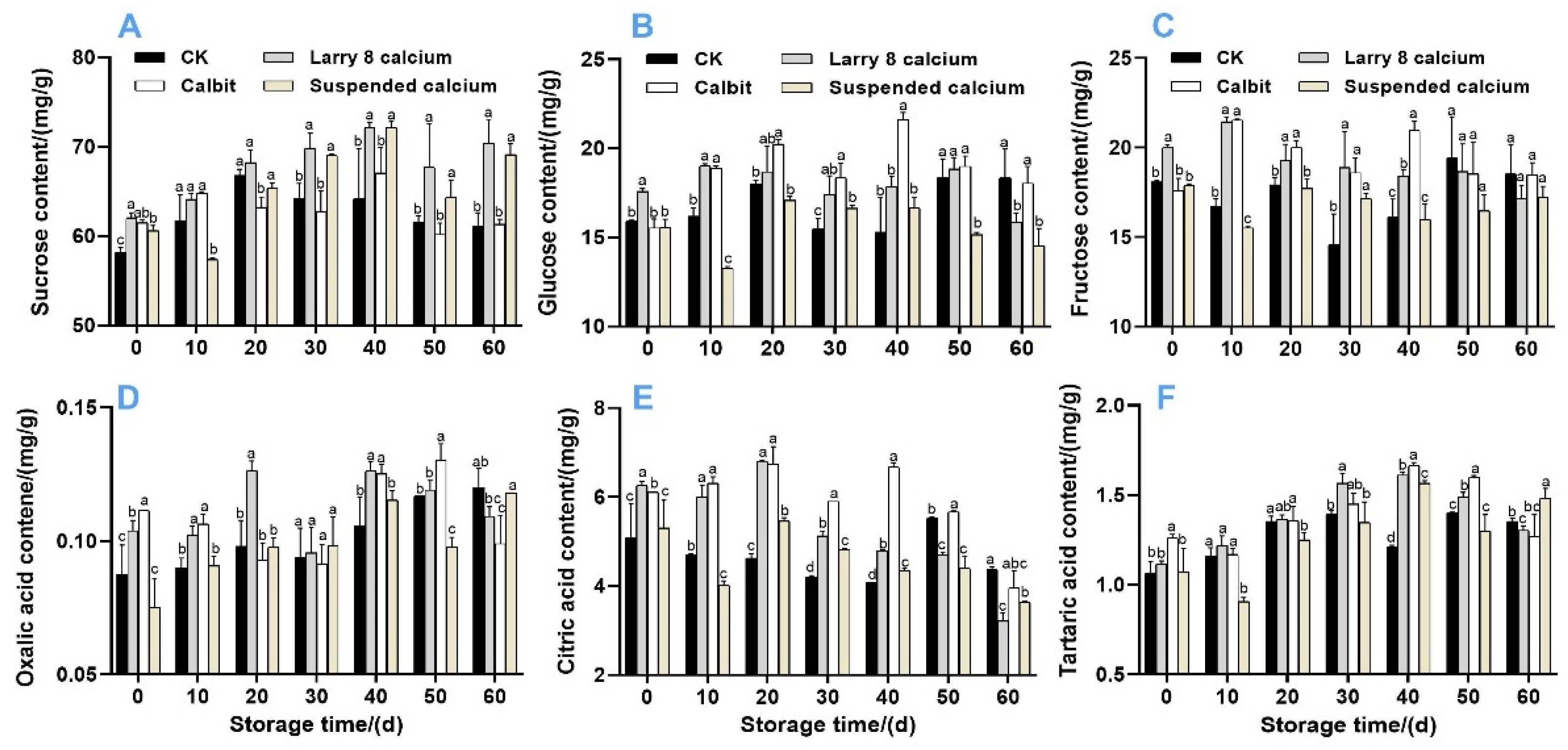
Further analysis of sucrose, fructose, and glucose revealed that the sucrose content was the highest, accounting for 64.68%, 62.25%, 66.88%, and 64.68% in Larry 8 Ca, Calbit, Suspended Ca, and CK, respectively. Throughout the storage period, the sucrose content in Nanfeng tangerine fruit exhibited an overall upward trend (Figure 5A). On day 0, the sucrose content of the pre-harvest Ca treatments was significantly higher than that of the control by 6.58%, 5.66%, and 4.11%, respectively. From 30 to 60 d of storage, there was no significant difference in sucrose content between Larry 8 Ca and Suspended Ca, but it was significantly higher than in other treatments. Moreover, the sucrose content of the Ca treatment groups did not significantly differ from that of the control group after 30 d. The glucose and fructose content initially increased and then decreased during storage (Figure 5B,C). On day 20, the fructose content of the Larry 8 Ca and Calbit treatments peaked at 21.43 mg/g and 21.53 mg/g, respectively, significantly higher than the CK and Suspended Ca treatments. The glucose content of Calbit reached its highest value of 21.61 mg/g on day 40, which was significantly higher than that of the other treatments. However, on day 60, the glucose content of the control group was significantly higher than that of Larry 8 Ca and Suspended Ca groups.
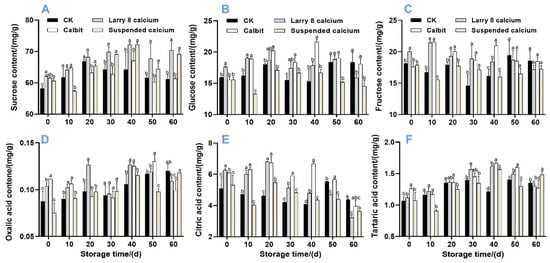
Figure 5.
Effects of different calcium treatments on sucrose (A), glucose (B), fructose (C), oxalic acid (D), citric acid (E), tartaric acid (F), and organic acid content of Nanfeng tangerine during storage. Different letters indicate significant differences according to t-test (p < 0.05) at the same storage stage.
As depicted in Figure 5D–F, the oxalic acid and tartaric acid contents exhibited a similar trend, initially increasing and then gradually decreasing. By 40 days of storage, the tartaric acid content of the pre-harvest Ca treatment group was significantly higher than that of the CK. The content of CK and Calbit peaked on day 50, with the Calbit treatment showing a significantly higher content than other three treatments (0.13 mg/g). The citric acid content demonstrated a general downward trend (Figure 5E). From days 0 to 40, the citric acid content of the Larry 8 Ca and Calbit treatments was significantly higher than that of the CK, averaging 27.87% and 40% higher than that of the CK, respectively. However, on day 60 of storage, the citric acid content of CK was significantly higher than that of Larry 8 Ca and Suspended Ca (4.37 mg/g).
3.7. Principal Component Analysis (PCA) of Storage Quality of Nanfeng Tangerine
Before the comprehensive evaluation of various Ca treatments, the inconsistencies in the quantitative profiles of each indicator were acknowledged. To mitigate differences in the quantitative profiles and magnitude of the raw data across the 16 indicators detected, the raw data from 28 groups (4 treatments × 7 storage periods) underwent initial preprocessing for homogenization using the Z-Score method in SPSS 25.0. The homogenization formula X = (raw value − mean value)/standard deviation was applied, resulting in a mean of zero and a standard deviation of 1 for each index.
To assess the suitability of the homogenized 16 sets of raw data for principal component analysis (PCA), KMO and Bartlett’s tests were initially conducted. The results, presented in Table 2, revealed a KMO value of 0.598, falling below the threshold of 0.600. To enhance the reliability of the data model, indicators with minimal statistical variance, including “citric acid”, “firmness”, and “shear force”, were excluded, and the remaining 13 indicators underwent a subsequent PCA step. The KMO and Bartlett’s test results for these 13 quality indicators are also displayed in Table 2, yielding a KMO value of 0.673 with a significance of 0.000, suggesting improved suitability for PCA. Utilizing PCA dimensionality reduction, the cumulative changes in quality across different treatment storage periods were divided into principal components based on eigenvalues exceeding 1. Table 3 illustrates three principal components with eigenvalues of 5.719, 3.454, and 1.070, explaining 43.990%, 70.555%, and 8.234% of the total variance in the information. This cumulative explanation accounted for 78.789% of the total information variance, effectively summarizing and reflecting most of the initial variables.

Table 2.
KMO and Bartlett tests.

Table 3.
Eigenvalues of the principal components and their contributions and cumulative contributions.
To visualize the advantages and drawbacks of various Ca treatments concerning the ambient storage quality of Nanfeng tangerine, the composite scores were calculated based on PCA-derived data. First, the eigenvectors for the 13 factors were derived from the loading coefficients and eigenvalues of 13 quality indices. Subsequently, the scores for the three principal components were constructed using these eigenvectors as weights:
Y1 = 0.340 X1 + 0.369 X2 − 0.337 X3 + 0.330 X4 − 0.320 X5 + 0.297 X6 + 0.287 X7 + 0.261 X8 + 0.255 X9 − 0.034 X10 + 0.108 X11 − 0.212 X12 + 0.132 X13
Y2 = 0.058 X1 − 0.157 X2 + 0.086 X3 + 0.171 X4 + 0.068 X5 − 0.255 X6 + 0.102 X7 + 0.327 X8 + 0.166 X9 + 0.479 X10 + 0.464 X11 + 0.399 X12 + 0.342 X13
Y3 = 0.03 X1 + 0.234 X2 + 0.306 X3 + 0.065 X4 + 0.208 X5 + 0.247 X6 − 0.509 X7 − 0.295 X8 + 0.544 X9 + 0.188 X10 + 0.14 X11 − 0.192 X12 + 0.087 X13
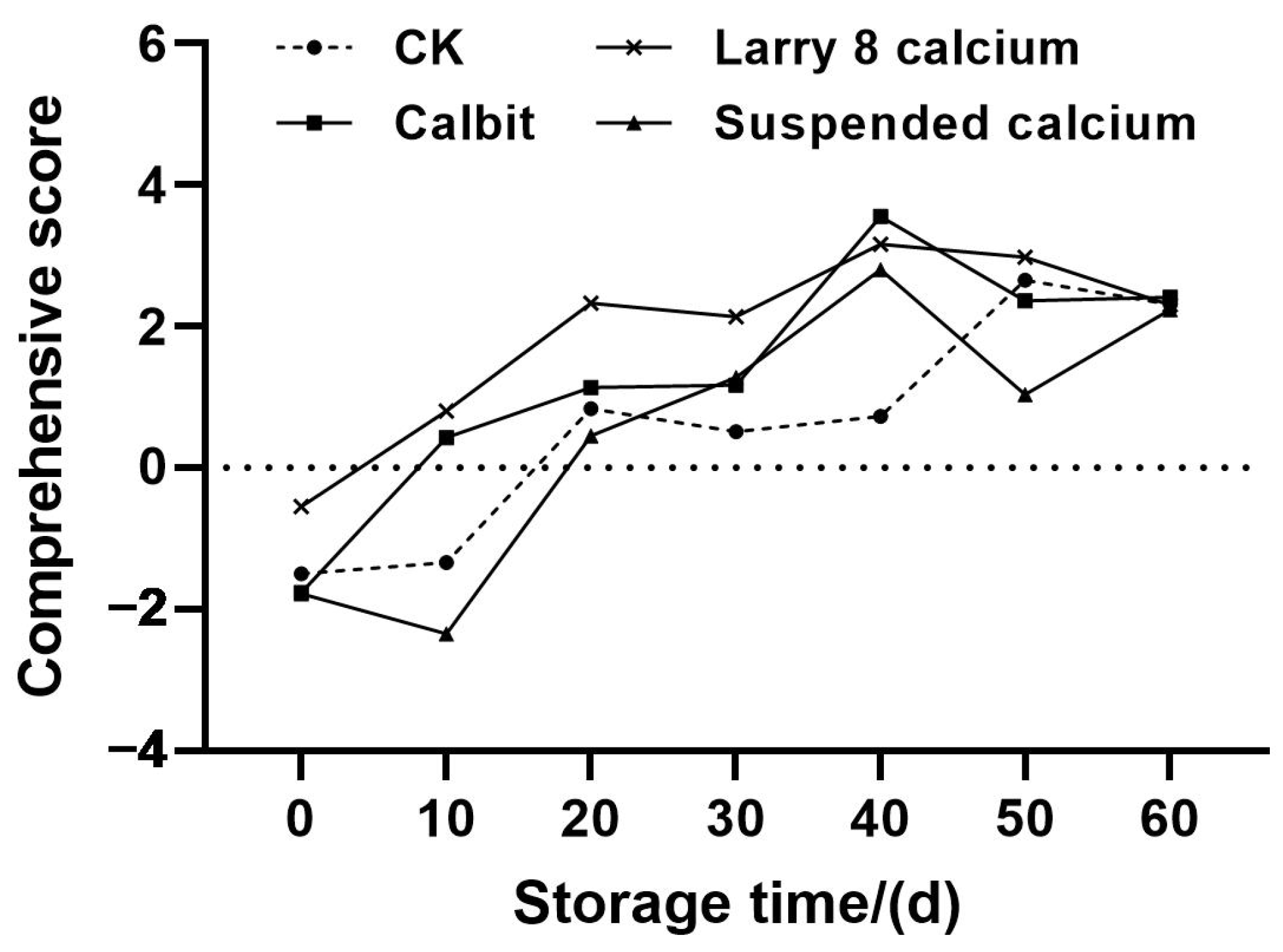
Utilizing the variance contribution rates corresponding to the three principal components as weights, a comprehensive evaluation model for the different Ca treatments was established: Y = 0.558 Y1 + 0.337 Y2 + 0.105 Y3. Subsequently, the measured values of ambient storage quality indices of Nanfeng tangerines with different Ca treatments were standardized and substituted into the above equations. The model was then employed to compute the comprehensive scores of various varieties and rankings, as depicted in Figure 6. The overall storage quality composite scores of Nanfeng tangerines with different Ca treatments exhibited an increasing trend, with the scores of the four treatment groups initially being negative on day 0. Larry Calcium and Calbit rose to positive values of 0.8 and 0.43, respectively, while Suspended Calcium and CK reached positive values on day 20, indicating a post-ripening process in Nanfeng tangerine after harvesting. The composite scores of Nanfeng tangerines treated with pre-harvest Ca peaked at 3.55, 3.16, and 2.8, respectively, on day 40. The CK reached its maximum value of 2.65 on day 50, suggesting that the quality of Nanfeng tangerine treated with pre-harvest Ca achieved optimal quality at approximately 40 to 50 d of storage. However, considering the increasing rate of rotting over extended storage periods, timely warehousing and sales after 50 days storage were advisable. Except for day 40 of storage, the scores of the Larry 8 Calcium treatment group were significantly higher than those of other three treatments from days 0 to 50. Furthermore, except for days 40 and 60 of storage, the scores of pre-harvest Larry 8 Calcium treatment surpassed those of other three treatments, indicating that the storage performance of Nanfeng tangerines treated with Larry 8 Calcium was generally superior.
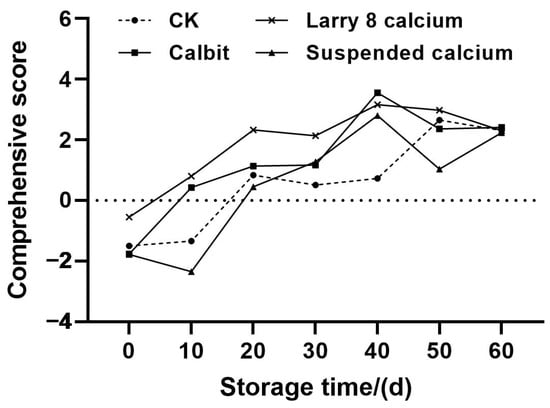
Figure 6.
Comprehensive score of Nanfeng tangerines treated with different calcium during storage.
4. Discussion and Conclusions
Ca plays a vital role in both plant growth and development and serves as a crucial component of the cell wall. Pre-harvest Ca treatment of fruits may effectively delay softening by preserving the tissue structure and cell wall constituents while also reducing the activities of cell-wall-degrading enzymes. Additionally, the foliar application of Ca fertilizer is an environmentally friendly method for preserving freshness, enhancing firmness, increasing Ca content in fruit [18], delaying senescence and ripening [19], reducing physiological and pathological diseases [20], improving fruit quality, and extending storage time [21]. Hence, pre-harvest Ca treatment is a prevalent and efficient agricultural practice [22]. This study aimed to assess the impact of various Ca fertilizers on the post-harvest quality and storage characteristics of Nanfeng tangerines. The findings revealed that pre-harvest Ca treatment significantly enhanced the Ca content, firmness, and maximum shear force of the fruits. It notably decelerated fruit softening and upheld high fruit hardness throughout storage, with Suspended Ca treatment yielding the most favorable results. Moreover, pre-harvest treatments with Calbit and Larry 8 Ca effectively mitigated dehydration and decay in Nanfeng tangerines while boosting sugar, acid, and VC levels in the fruit, maintaining them at relatively high levels during storage. Consequently, these treatments delayed fruit ripening and senescence, thus extending the fruit storage duration.
Fruit firmness and maximum shear force are crucial indicators of post-harvest fruit quality, reflecting fruit senescence and deterioration, and are often positively associated with Ca content [23]. Ca fertilizer application enhances fruit calcium content at harvest, improving flesh firmness and shear force, which is indicative of improved storage performance. Specifically, Ca2+ plays a role in maintaining or reinforcing cell membrane and cell wall integrity, inhibiting cell wall component degradation, reducing fruit respiration and ethylene synthesis, and regulating senescence-related enzyme activities and metabolism [24]. Additionally, Ca facilitates reducing fruit hydraulic conductivity and minimizing weight loss, primarily due to water loss during storage [25]. However, excessive cytoplasmic Ca2+ can damage membranes and elevate respiratory rates, leading to increased weight loss, as observed when CaCl2 concentrations exceeded 1.0%. For example, Guo et al. [26] demonstrated that post-harvest treatment of lychee with Ca chloride strengthened the cell wall structure, delaying enzymatic browning of the pericarp, pulp softening, and disease development.
Peel color significantly influences the overall quality of citrus fruits during post-harvest storage and marketing [27]. Parameters such as a*, b*, L*, C*, H°, and CCI values were utilized to characterize the surface color changes in Nanfeng tangerines post-harvest. The a* values represent red (positive) and green (negative), whereas the b* values represent yellow (positive) and blue (negative). C* indicates the color saturation degree and H° denotes the hue angle, where color transitions from yellow (90°) to fuchsia (0°). L* values indicate peel glossiness, and CCI values represent a comprehensive color difference index, with positive values indicating red and negative values indicating blue-green [28]. Zydlik et al. [29] demonstrated that pre-harvest Ca treatment significantly enhanced blueberry fruit color. Similarly, as observed in this study, pre-harvest Ca treatment effectively increased the peel brightness and color saturation indices during storage while delaying the increase in fruit CCI values. This effect may result from Ca treatment promoting carotenoid accumulation, thereby hastening fruit coloring [30].
The decay rate and weight loss are crucial indicators of fruit storage quality [31]. Ca treatment reduces free pectic acid content, increases Ca pectin content, and mitigates changes in fruit membrane permeability, enhancing resistance to decay and storage. Ultimately, this prolongs storage and shelf life and enhances fruit and vegetable quality [32]. Previous studies have demonstrated that pre-harvest spray treatment with 1% CaCl2 effectively increases fruit Ca2+ concentration and reduces enzyme activity, weight loss, and decay during storage [33]. Mostafa et al. [34] suggested that pre-harvest calcium treatment of loquat decreased post-harvest decay and improved storage quality, possibly due to Ca pectate hydrogels enhancing water retention and delaying dehydration [35]. Respiratory behavior significantly affects post-harvest fruit and vegetable metabolisms, with higher respiration intensities consuming more nutrients and energy and accelerating fruit senescence [36]. Therefore, lower respiration rates are favorable for maintaining the post-harvest fruit storage quality [37].
The degradation of nutrients in fruits during storage directly affects their flavor and quality. Hence, nutrients such as TSS, TA, VC, and TSS/TA serve as indicators of fruit aging and storage quality, with the taste of citrus fruits being primarily reliant on the sugar–acid ratio in the pulp [38]. Wang et al. [21] discovered that spraying Ca fertilizer during the young fruit, expansion, and coloring stages enhanced the individual fruit weight, firmness, TSS, and VC content in apples. Sabir et al. [39] demonstrated that post-harvest treatment of blackberries with 2% CaCl2 extended their storage life and delayed changes in TA, TSS, VC, and total phenol content. Similarly, Wang et al. [40] reported that applying different concentrations of Ca fertilizer to wine grapes resulted in an optimal concentration of 30 kg/ha, effectively enhancing the sugar–acid ratio, total phenol, reducing sugar, and soluble sugar content of the fruits, which was consistent with the findings of this study. These results highlight the varying effects of different calcium preparations and treatment durations on the intrinsic quality and flavor of fruits. Notably, organic acids generally decrease during ripening as they respire or are converted to sugars. Hence, the higher TSS content observed in the Calbit and Larry 8 Ca treatments at the end of the storage period may be attributed to the earlier conversion of TA during the initial storage period. VC, comprising AsA and dehydroascorbic acid, is among the most important nutritional constituents of fresh fruits and vegetables. In this study, pre-harvest Ca application was identified to inhibit the decline in VC in Nanfeng tangerines. Correspondingly, Erbaş et al. [41] documented that pre- and post-harvest treatments with Ca-Gluconate reduced the weight loss and decay rate while preserving the titratable acidity, fruit firmness, and sensory quality in sweet cherries.
Sugars and organic acids are vital components of citrus pulp and are commercially utilized as indicators of harvest ripeness. The composition, content, and ratio of soluble sugars and organic acids significantly influence fruit flavor development, thus affecting its inherent quality. Carbohydrates, which constitute the primary soluble fraction of citrus pulp (75–80%), predominantly determine the juice’s sweetness, with sucrose, glucose, and fructose typically in a 2:1:1 ratio. According to Goldenberg et al. [42], high polymeric sugars such as starch and pectin serve as substrates for respiratory metabolism, continuously converting into monosaccharides by enzymes and thereby augmenting fruit soluble sugar content [43]. The comparison of chemical composition and sensory profiles highlights a significant correlation between sweetness and the sugar-to-acid ratio, total organic acid concentration, and citric and shikimic acid levels [44]. Variations in organic acid composition among citrus varieties have been observed, with citric acid typically being predominant, constituting 70–90% of the total acid content [45]. Additionally, Ca influences organic acid accumulation because Ca2+ can form insoluble Ca salts with organic acids, thereby contributing to sugar accumulation in the pulp [46]. Li et al. [5] demonstrated that the application of 600 mg/L Prohexadione-Ca in grape berries increased the soluble sugar content by 11.28% and significantly elevated citric acid by 97.80% and malic acid by 68.86%. He et al. [47] indicated that calcium spraying one week before harvest reduced the decay rate of Olea europaea fruit during storage and notably enhanced the contents of sucrose, glucose, sorbitol, malic acid, succinic acid, tartaric acid, citric acid, and oxalic acid. Similarly, in this study, pre-harvest Larry 8 Ca and Calbit treatments augmented fructose, glucose, citric acid, and sucrose levels in Nanfeng tangerines at harvest, thereby promoting their synthesis throughout storage. We also noticed that the citric acid content of CK fruits increased rapidly after the 50 d of storage. It was higher than that of fruits treated with pre-harvest Larry 8 Calcium and Calbit. The reason for this remains to be studied further, but one possibility is that the weight loss was higher in the CK fruits during the late stage of storage.
PCA facilitates the transformation of numerous related indicators into a concise set with low correlations, enabling the computation of comprehensive scores for each treatment while mitigating information loss [48]. Yang et al. [49] employed citric acid content and gene expression in citric acid degradation pathways across control and treated samples at four intervals to explore sample classification and physiological parameters using PCA. They proposed that ACLα1/β in the acetyl-CoA pathway is a potential key gene for 2,4-D-induced citric acid degradation. Zhang et al. [50] utilized PCA and correlation analysis to reveal that short-term temperature treatments changed the metabolic flow, with GABA indicating positive correlations with sugars and organic acids. Peng et al. [51] conducted a comprehensive evaluation of 15 quality indices using PCA, determining that a 300 µmol/L MT treatment significantly enhanced disease resistance and preserved kiwifruit quality. The PCA results in this study demonstrated that the first three principal components collectively contributed to 78.789% of the variance. The efficacy of each pre-harvest Ca treatment in improving Nanfeng tangerine fruit quality was assessed based on score rankings, with Larry 8 Ca consistently exhibiting the highest comprehensive score during storage, thus indicating its superior effect in enhancing Nanfeng tangerine fruit quality.
5. Conclusions
Based on the PCA and the findings outlined above, pre-harvest Larry 8 Ca treatment demonstrated the most favorable effects during most of the storage period for Nanfeng tangerines, followed by Calbit and Suspended Ca. These treatments effectively extended the post-harvest room-temperature storage duration and elevated the Ca content, fruit firmness, and maximum shear forces. Moreover, they enhanced the fruit brightness and delayed the fruit respiration rate, decay, and weight loss while increasing the contents of TSS, TA, VC, organic acid, and soluble sugar in the flesh at harvest. This study can offer valuable insights into the selection of suitable calcium fertilizers for Nanfeng tangerine pre-harvest applications.
Author Contributions
Conceptualization, Q.G.; writing—review and editing, Q.W.; methodology, Q.M.; writing—original draft preparation and data curation, Z.C.; software, S.Z.; formal analysis, X.L.; investigation, D.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Natural Science Foundation of China (NSFC) (32060649, 32260751) and Key Project of Jiangxi Key R&D Plan (20171ACF60025).
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors would like to thank the Jiangxi agricultural university (JXAU) and Jiangxi Key Laboratory for Post-Harvest Technology and Nondestructive Testing of Fruits and Vegetables for their support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Chen, C.; Peng, X.; Zeng, R.; Chen, M.; Wan, C.; Chen, J. Ficus hirta fruits extract incorporated into an alginate-based edible coating for Nanfeng mandarin preservation. Sci. Hortic. 2016, 202, 41–48. [Google Scholar] [CrossRef]
- Chen, J.H.; Yang, C.H.; Wang, Y.S.; Lee, J.G.; Cheng, C.H.; Chou, C.C. Acrylamide-induced mitochondrial collapse and apoptosis in human astrocytoma cells. Food Chem. Toxicol. 2013, 51, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shen, Y.; Chen, C.; Gan, Z.; Chen, J.; Wan, C. Loquat leaf extract and alginate based green composite edible coating for preserving the postharvest quality of Nanfeng tangerines. Sustain. Chem. Pharm. 2022, 27, 100674. [Google Scholar] [CrossRef]
- Xiao, L.; Luo, Z.; Fu, Y.; Zeng, J.; Xiang, M.; Chen, J.; Chen, M. First Report of Postharvest Fruit Rot on Citrus reticulata Caused by Fusarium concentricum in China. Plant Dis. 2022, 107, 962. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Yang, J.; Dai, Z.; Chen, Y.; Shao, Z.; Wang, C.; Jin, X.; Wang, Y.; Feng, L. Prohexadione-calcium improves grape quality by regulating endogenous hormones, sugar and acid metabolism and related enzyme activities in grape berries. BMC Plant Biol. 2024, 24, 122. [Google Scholar] [CrossRef] [PubMed]
- Conway, W.S.; Sams, C.E.; Brown, G.A.; Kennedy, L.S.; Beavers, W.B.; Tobias, R.B. Pilot Test for the Commercial Use of Postharvest Pressure Infiltration of Calcium into Apples to Maintain Fruit Quality in Storage. HortTechnology 1994, 4, 239–243. [Google Scholar] [CrossRef]
- Guo, S. Soilless Cultivation; China Agriculture Press: Beijing, China, 2003. [Google Scholar]
- Jia, L.; Li, Y.; Liu, G.; He, J. Acidic electrolyzed water improves the postharvest quality of jujube fruit by regulating antioxidant activity and cell wall metabolism. Sci. Hortic. 2022, 304, 111253. [Google Scholar] [CrossRef]
- Wu, H.; Wang, F.; Yang, Q.; Tang, J.; Chen, L.; Shi, Z.; He, X.; Deng, J. Carboxymethyl chitosan different durations induces disease resistance of grapefruit by modulating ascorbate-glutathione cycle and cell wall metabolism. Postharvest Biol. Technol. 2024, 211, 112845. [Google Scholar] [CrossRef]
- Cabanne, C.; Bernard, D. Purification and characterization of two isozymes of polygalacturonase from botrytis cinerea. effect of calcium ions on polygalacturonase activity. Microbiol. Res. 2002, 157, 183–189. [Google Scholar] [CrossRef]
- Beiparysa, A.; Topno, E.S.; Joseph, V.A.; Bahadur, V.; Kerketta, A.; Kesharwani, L. Effect of Calcium Chloride (CaCl2) and Carbon Dioxide (CO2) onPost Harvest Quality of Apple Fruit (Malus domestica) cv. Gala. Int. J. Soil Sci. 2023, 35, 199–207. [Google Scholar]
- Jorunn, B.; Eivind, V.; Arne, S. Preharvest application with calcium and maturity at harvest affects postharvest fungal fruit decay of European plum. Eur. J. Plant Pathol. 2023, 166, 199–208. [Google Scholar]
- Cao, S.; Ouyang, M.; Zhou, W.; Cui, H.; Liu, P.; Tan, J.; Huang, L.; Liu, S. Multivariate analysis and simulation of mineral nutrients in fruit with soil nutrients and pH in Wenzhou mandarin fruit, Hunan, China. J. Fruit Sci. 2019, 36, 1029–1039. [Google Scholar]
- Lobos, T.E.; Retamales, J.B.; Hanson, E.J. Early preharvest calcium sprays improve postharvest fruit quality in ‘Liberty’ highbush blueberries. Sci. Hortic. 2021, 277, 109790. [Google Scholar] [CrossRef]
- Gao, J. Experimental Guidance for Plant Physiology; Higher Education Press: Beijing, China, 2006. [Google Scholar]
- GB 5009.268-2016; National Food Safety Standard—Determination of Calcium, Iron, Zinc, Sodium, Potassium, Magnesium, Copper and Manganese in Foods for Infants and Young Children, Milk and Milk Products. National Health and Family Planning Commission of the PRC: Beijing, China; State Food and Drug Administration: Beijing, China, 2016.
- Chen, M.; Jiang, Q.; Yin, X.R.; Lin, Q.; Chen, J.Y.; Allan, A.C.; Xu, C.J.; Chen, X.S. Effect of hot air treatment on organic acid and sugar metabolism in ponkan (Citrus reticulata) fruit. Sci. Hortic. 2012, 147, 118–125. [Google Scholar] [CrossRef]
- Sotiropoulos, T.; Voulgarakis, A.; Karaiskos, D.; Chatzistathis, T.; Manthos, I.; Dichala, O.; Mpountla, A. Foliar calcium fertilizers impact on several fruit quality characteristics and leaf and fruit nutritional status of the ‘Hayward’ kiwifruit cultivar. Agronomy 2021, 11, 235. [Google Scholar] [CrossRef]
- Hashmatt, M.; Morton, A.R.; Heyes, J.A.; Armour, D.; Lowe, T.; Black, M.; Kerckhoffs, L.H.J. Effect of preharvest foliar calcium application on fruit quality in Gold3 kiwifruit. Acta Hortic. 2019, 1253, 327–334. [Google Scholar] [CrossRef]
- Motlhalamme, T.; Mohamed, H.; Kaningini, A.G.; More, G.K.; Thema, F.T.; Maaza, M. Bio-synthesized calcium carbonate (CaCO3) nanoparticles: Their anti-fungal properties and application as nanofertilizer on Lycopersicon esculentum growth and gas exchange measurements. Plant Nano Biol. 2023, 6, 100050. [Google Scholar] [CrossRef]
- Wang, G.; Wang, J.; Han, X.; Chen, R.; Xue, X. Effects of spraying calcium fertilizer on photosynthesis, mineral content, sugar-acid metabolism and fruit quality of Fuji apples. Agronomy 2022, 12, 2563. [Google Scholar] [CrossRef]
- Nayak, S.; Tarai, R.K.; Swain, S.C.; Samal, S.; Pradhan, S.; Sethy, B.K.; Behera, S.K.; Vali, D.M.; Ranjith, P.; Mandal, P.; et al. Effect of Foliar Feeding with Nutrients and Bioregulators on Yield and Quality Attributes of Litchi cv. Bombai. Horticulturae 2024, 10, 188. [Google Scholar] [CrossRef]
- Ganai, A.N.; Rasool, K.; Ali, A.; Hassan, G.I.; Javeed, K.; Wani, A.H. Effect of Calcium Chloride on Fruit Quality and Shelf Life of RedVelox Apple. Int. J. Soil Sci. 2023, 35, 489–494. [Google Scholar]
- Jain, V.; Chawla, S.; Choudhary, P.; Jain, S. Post-harvest calcium chloride treatments influence fruit firmness, cell wall components and cell wall hydrolyzing enzymes of Ber (Ziziphus mauritiana Lamk.) fruits during storage. J. Food Sci. Technol. 2019, 56, 4535–4542. [Google Scholar] [CrossRef]
- Gao, Q.; Xiong, T.; Li, X.; Chen, W.; Zhu, X. Calcium and calcium sensors in fruit development and ripening. Sci. Hortic. 2019, 253, 412–421. [Google Scholar] [CrossRef]
- Guo, X.; Li, Q.; Luo, T.; Han, D.; Zhu, D.; Wu, Z. Postharvest calcium chloride treatment strengthens cell wall structure to maintain litchi fruit quality. Foods. 2023, 12, 2478. [Google Scholar] [CrossRef]
- Zhang, J. Establishment of a Method for Recognizing the Glossiness of Citrus Fruit Surface. Master’s Thesis, Huazhong Agricultural University, Wuhan, China, 2022. (In Chinese). [Google Scholar]
- Ma, Q.; Lin, X.; Wei, Q.; Yang, X.; Zhang, Y.; Chen, J. Melatonin treatment delays postharvest senescence and maintains the organoleptic quality of ‘Newhall’ navel orange (Citrus sinensis (L.) Osbeck) by inhibiting respiration and enhancing antioxidant capacity. Sci. Hortic. 2021, 286, 110236. [Google Scholar] [CrossRef]
- Zydlik, Z.; Zydlik, P.; Kafkas, N.E.; Yesil, B.; Cieśliński, S. Foliar Application of Some Macronutrients and Micronutrients Improves Yield and Fruit Quality of Highbush Blueberry (Vaccinium corymbosum L.). Horticulturae 2022, 8, 664. [Google Scholar] [CrossRef]
- Liu, Y. Effects of Harvest Time, Storage Method, and temperature on the Color of Navel Orange Peel during Storage of ‘Newhall’. Master’s Thesis, Jiangxi Agricultural University, Nanchang, China, 2015. (In Chinese). [Google Scholar]
- Zeng, J.; Chen, C.; Chen, M.; Chen, J. Comparative transcriptomic and metabolomic analyses reveal the delaying effect of naringin on postharvest decay in citrus fruit. Front. Plant Sci. 2022, 13, 1045857. [Google Scholar] [CrossRef] [PubMed]
- de Freitas, S.T.; Handa, A.K.; Wu, Q.; Park, S.; Mitcham, E.J. Role of pectin methylesterases in cellular calcium distribution and blossom-end rot development in tomato fruit. Plant J. 2012, 71, 824–835. [Google Scholar] [CrossRef]
- Souza, J.M.A.; Leonel, S.; Leonel, M.; Garcia, E.L.; Ribeiro, L.R.; Ferreira, R.B.; Martins, R.C.; de Souza Silva, M.; Monteiro, L.N.H.; Duarte, A.S. Calcium Nutrition in Fig Orchards Enhance Fruit Quality at Harvest and Storage. Horticulturae 2023, 9, 123. [Google Scholar] [CrossRef]
- Mostafa, Y.S.; Sultan, M.Z. Calcium chloride combined with antioxidants increases keeping quality and limits postharvest decay of loquat fruit. Acta Hortic. 2018, 1194, 157–164. [Google Scholar] [CrossRef]
- Turmanidze, T.; Gulua, L.; Jgenti, M.; Wicker, L. Effect of Calcium Chloride Treatments on Quality Characteristics of Blackberry Fruit During Storage. Int. J. Food Allied Sci. 2016, 2, 36. [Google Scholar] [CrossRef]
- Aghdam, M.S.; Luo, Z.; Li, L.; Jannatizadeh, A.; Fard, J.R.; Pirzad, F. Melatonin treatment maintains nutraceutical properties of pomegranate fruits during cold storage. Food Chem. 2020, 303, 125385. [Google Scholar] [CrossRef] [PubMed]
- Koyuncu, M.A.; Kuleaşan, H.; Erbaş, D.; Bodur, E. Using low dose fungicide by combining with intermittent ozone treatment to reduce fungicide residue, microbial load and quality losses in orange fruit during long term storage. Food Control 2023, 144, 109363. [Google Scholar] [CrossRef]
- Kahramanoğlu, İ.; Chen, C.; Chen, Y.; Chen, J.; Gan, Z.; Wan, C. Improving Storability of “Nanfeng” Mandarins by Treating with Postharvest Hot Water Dipping. J. Food Qual. 2020, 2020, 1–12. [Google Scholar] [CrossRef]
- Sabir, F.; Sabir, A.; Ozcelik, S.; Kucukbasmaci, A. Maintenance of postharvest quality of blackberry (Rubus fructicosus L.) fruits through salicylic acid and CaCl2 immersions. Acta Sci. Pol. Hortorum Cultus 2019, 18, 121–128. [Google Scholar] [CrossRef]
- Wang, R.; Qi, Y.; Wu, J.; Shukla, M.K.; Sun, Q. Influence of the application of irrigated water-soluble calcium fertilizer on wine grape properties. PLoS ONE 2019, 14, e0222104. [Google Scholar] [CrossRef]
- Erbaş, D.; Koyuncu, M.A. The Effect of Pre- and Postharvest Calcium Gluconate Treatments on Physicochemical Characteristics and Bioactive Compounds of Sweet Cherry during Cold Storage. Food Sci. Technol. Int. 2023, 29, 299–309. [Google Scholar] [CrossRef]
- Goldenberg, L.; Yaniv, Y.; Kaplunov, T.; Doron-Faigenboim, A.; Porat, R.; Carmi, N. Genetic diversity among mandarins in fruit-quality traits. J. Agric. Food Chem. 2014, 62, 4938–4946. [Google Scholar] [CrossRef] [PubMed]
- Hussain, P.R.; Dar, M.A.; Meena, R.S.; Wani, A.M.; Mir, M.A.; Shafi, F. Changes in quality of apple (Malus domestica) cultivars due to {gamma}-irradiation and storage conditions. J. Food Sci. Technol. 2008, 45, 44–49. [Google Scholar]
- Maletsika, P.; Liava, V.; Sarrou, E.; Titeli, V.S.; Nasiopoulou, E.; Martens, S.; Karagiannis, E.; Grigoriadou, K.; Molassiotis, A.; Nanos, G.D. Foliar Calcium Effects on Quality and Primary and Secondary Metabolites of White-Fleshed ‘Lemonato’ Peaches. Horticulturae 2023, 9, 299. [Google Scholar] [CrossRef]
- Lado, J.; Gambetta, G.; Zacarias, L. Key determinants of citrus fruit quality: Metabolites and main changes during maturation. Sci. Hortic. 2018, 233, 238–248. [Google Scholar] [CrossRef]
- Wang, B. Plant Physiology, 5th ed.; Science Press: Beijing, China, 2004. [Google Scholar]
- He, Y.; Li, W.; Ye, L.; Zhang, Z.S.; Wang, A.Q.; Hao, J.H.; Chang, L.J. Effects of preharvest calcium spraying on decay rate and sugar and acid content of European plum fruit during storage. Food Sci. 2016, 37, 247–252. [Google Scholar]
- Zhao, L.; Yan, S.; Wang, Y.; Xu, G.; Zhao, D. Evaluation of the Effect of Preharvest Melatonin Spraying on Fruit Quality of ‘Yuluxiang’ Pear Based on Principal Component Analysis. Foods 2023, 12, 3507. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Lin, X.; Wei, Q.; Chen, M. Understanding the influence of 2,4-dichlorophenoxyacetic acid and melatonin treatments on the sweet and acidic flavors and citric acid metabolism of ‘Olinda’ orange (Citrus sinensis (L.) Osbeck). Sci. Hortic. 2022, 304, 111287. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, R.; Sun, G.; Cheng, Y.; Wang, Z. The Effect of short-term temperature pretreatments on sugars, organic acids, and amino acids metabolism in valencia orange fruit. J. Food Qual. 2022, 2022, 8188000. [Google Scholar] [CrossRef]
- Peng, J.; Zhu, S.; Lin, X.; Wan, X.; Zhang, Q.; Njie, A.; Luo, D.; Long, Y.; Fan, R.; Dong, X. Evaluation of Preharvest Melatonin on Soft Rot and Quality of Kiwifruit Based on Principal Component Analysis. Foods 2023, 12, 1414. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).