Genetic and Hormonal Regulation of Sweet Cherry (Prunus avium L.) Maturity across Altitudinal Gradients
Abstract
:1. Introduction
2. Materials and Methods
2.1. RNA Extraction and Quantitative Real-Time PCR Analysis
2.2. Detection and Quantitation of Phytohormones Using UPLC/MS
2.3. Statistical Analysis
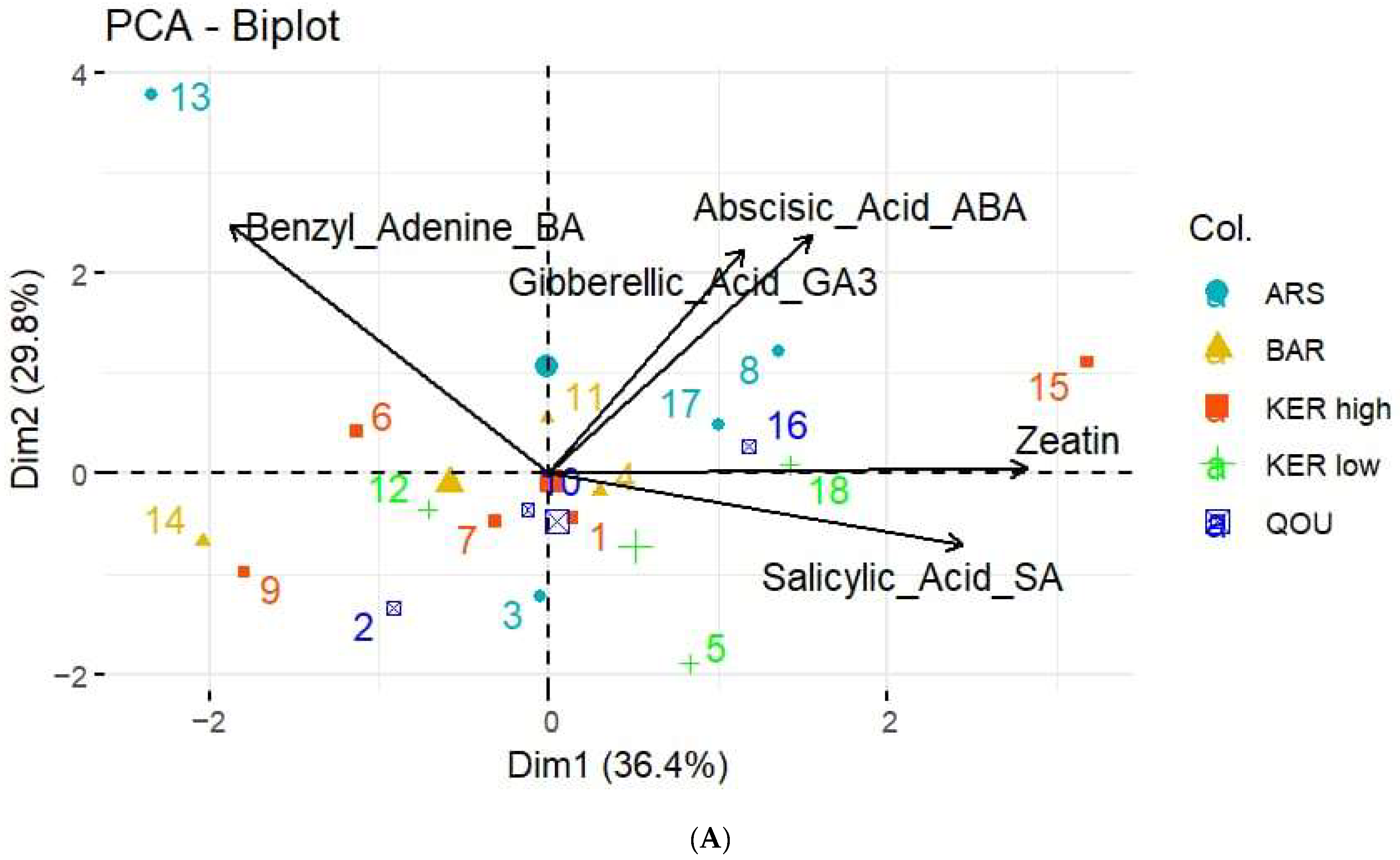
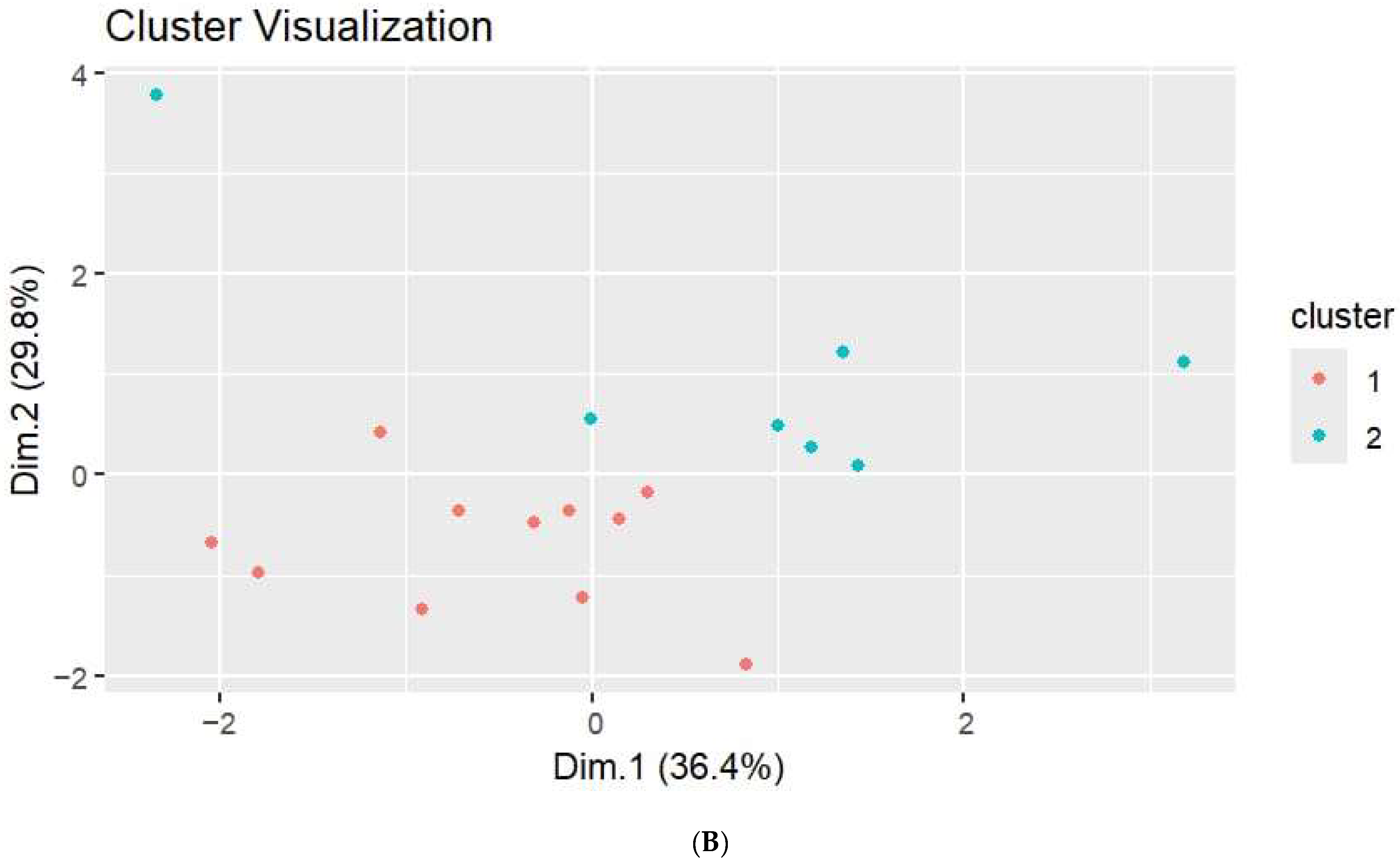
3. Results
3.1. Gene Expression
3.2. Hormone Quantification
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nacouzi, D.; Masry, R.; El Kayal, W. Quality and Phytochemical Composition of Sweet Cherry Cultivars Can Be Influenced by Altitude. Plants 2023, 12, 2254. [Google Scholar] [CrossRef]
- Salameh, M.; Nacouzi, D.; Lahoud, G.; Riachy, I.; El Kayal, W. Evaluation of Postharvest Maturity Indices of Commercial Avocado Varieties Grown at Various Elevations Along Lebanon’s Coast. Front. Plant Sci. 2022, 13, 895964. [Google Scholar] [CrossRef]
- Hardner, C.M.; Hayes, B.J.; Kumar, S.; Vanderzande, S.; Cai, L.; Piaskowski, J.; Quero-Garcia, J.; Campoy, J.A.; Barreneche, T.; Giovannini, D.; et al. Prediction of genetic value for sweet cherry fruit maturity among environments using a 6K SNP array. Hortic. Res. 2019, 6, 6. [Google Scholar] [CrossRef]
- Soto, A.; Ruiz, K.B.; Ravaglia, D.; Costa, G.; Torrigiani, P. ABA may promote or delay peach fruit ripening through modulation of ripening- and hormone-related gene expression depending on the developmental stage. Plant Physiol. Biochem. 2013, 64, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Götz, K.-P.; Chmielewski, F.-M.; Tarkowská, D.; Pěnčík, A.; Novák, O. Phytohormones in Sweet Cherry Buds During Winter Rest and Bud Development. J. Plant Growth Regul. 2023, 42, 2519–2529. [Google Scholar] [CrossRef]
- Teribia, N.; Tijero, V.; Munné-Bosch, S. Linking hormonal profiles with variations in sugar and anthocyanin contents during the natural development and ripening of sweet cherries. New Biotechnol. 2016, 33, 824–833. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, P.; Sun, L.; Li, Q.; Dai, S.; Sun, Y.; Kai, W.; Zhang, Y.; Liang, B.; Leng, P. Transcriptional regulation of PaPYLs, PaPP2Cs and PaSnRK2s during sweet cherry fruit development and in response to abscisic acid and auxin at onset of fruit ripening. Plant Growth Regul. 2015, 75, 455–464. [Google Scholar] [CrossRef]
- Kuhn, N.; Ponce, C.; Arellano, M.; Time, A.; Multari, S.; Martens, S.; Carrera, E.; Sagredo, B.; Donoso, J.M.; Meisel, L.A. ABA influences color initiation timing in P. avium L. fruits by sequentially modulating the transcript levels of ABA and anthocyanin-related genes. Tree Genet. Genomes 2021, 17, 20. [Google Scholar] [CrossRef]
- Liu, N. Effects of IAA and ABA on the Immature Peach Fruit Development Process. Hortic. Plant J. 2019, 5, 145–154. [Google Scholar] [CrossRef]
- Canli, F.A.; Pektas, M.; Ercisli, S. Benzyladenine and Gibberellin Applications Improve Fruit Weight and Delay Maturity of Sweet Cherry. Erwerbs-Obstbau 2015, 57, 71–75. [Google Scholar] [CrossRef]
- Zalabák, D.; Pospíšilová, H.; Šmehilová, M.; Mrízová, K.; Frébort, I.; Galuszka, P. Genetic engineering of cytokinin metabolism: Prospective way to improve agricultural traits of crop plants. Biotechnol. Adv. 2013, 31, 97–117. [Google Scholar] [CrossRef] [PubMed]
- Giménez, M.J.; Valverde, J.M.; Valero, D.; Guillén, F.; Martínez-Romero, D.; Serrano, M.; Castillo, S. Quality and antioxidant properties on sweet cherries as affected by preharvest salicylic and acetylsalicylic acids treatments. Food Chem. 2014, 160, 226–232. [Google Scholar] [CrossRef] [PubMed]
- El Kayal, W.; El-Sharkawy, I.; Dowling, C.; Paliyath, G.; Sullivan, J.A.; Subramanian, J. Effect of Pre-Harvest Application of Hexanal and Growth Regulators in Enhancing Shelf Life and Regulation of Membrane Associated Genes in Strawberry. Can. J. Plant Sci. 2017, 97, 1109–1120. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Setha, S.; Kondo, S.; Hirai, N.; Ohigashi, H. Quantification of ABA and its metabolites in sweet cherries using deuterium-labeled internal standards. Plant Growth Regul. 2005, 45, 183–188. [Google Scholar] [CrossRef]
- Tijero, V.; Teribia, N.; Muñoz, P.; Munné-Bosch, S. Implication of Abscisic Acid on Ripening and Quality in Sweet Cherries: Differential Effects during Pre- and Post-harvest. Front. Plant Sci. 2016, 7, 602. [Google Scholar] [CrossRef] [PubMed]
- Hamie, N.; Nacouzi, D.; Choker, M.; Salameh, M.; Darwiche, L.; El Kayal, W. Maturity Assessment of Different Table Grape Cultivars Grown at Six Different Altitudes in Lebanon. Plants 2023, 12, 3237. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Niu, L.; Zhang, Q.; Pan, B.-Z.; He, H.; Xu, Z.-F. Benzyladenine treatment promotes floral feminization and fruiting in a promising oilseed crop Plukenetia volubilis. Ind. Crops Prod. 2014, 59, 295–298. [Google Scholar] [CrossRef]
- Akinci-Yildirim, F.; Kepenek, G.; Şan, B.; Yildirim, A.N.; Kaçal, E. Effects of Ba+Ga4+7 Treatments on Fruit Quality in ‘Fuji’ Apple Variety. Turk. J. Agric. Nat. Sci. 2014, 1, 1387–1390. [Google Scholar]
- Blažek, J.; Zelený, L.; Suran, P. Sweet cherry research world overview 2015–2017. Hortic. Sci. 2022, 49, 121–146. [Google Scholar] [CrossRef]
- Aremu, A.O.; Fawole, O.A.; Makunga, N.P.; Masondo, N.A.; Moyo, M.; Buthelezi, N.M.D.; Amoo, S.O.; Spíchal, L.; Doležal, K. Applications of Cytokinins in Horticultural Fruit Crops: Trends and Future Prospects. Biomolecules 2020, 10, 1222. [Google Scholar] [CrossRef] [PubMed]
- Giménez, M.J.; Serrano, M.; Valverde, J.M.; Martínez-Romero, D.; Castillo, S.; Valero, D.; Guillén, F. Preharvest salicylic acid and acetylsalicylic acid treatments preserve quality and enhance antioxidant systems during postharvest storage of sweet cherry cultivars: Preharvest salicylic acid and acetylsalicylic acid treatments. J. Sci. Food Agric. 2017, 97, 1220–1228. [Google Scholar] [CrossRef] [PubMed]
- Valero, D.; Díaz-Mula, H.M.; Zapata, P.J.; Castillo, S.; Guillén, F.; Martínez-Romero, D.; Serrano, M. Postharvest Treatments with Salicylic Acid, Acetylsalicylic Acid or Oxalic Acid Delayed Ripening and Enhanced Bioactive Compounds and Antioxidant Capacity in Sweet Cherry. J. Agric. Food Chem. 2011, 59, 5483–5489. [Google Scholar] [CrossRef] [PubMed]
- Bal, E.; Torçuk, A.İ. Effect of Ultrasound Treatment Combined with Salicylic Acid Treatment Reduces Decay and Improves Storage life in Sweet Cherry Fruit. Erwerbs-Obstbau 2020, 62, 327–333. [Google Scholar] [CrossRef]
- Dobrev, P.I.; Vankova, R. Quantification of Abscisic Acid, Cytokinin, and Auxin Content in Salt-Stressed Plant Tissues. In Plant Salt Tolerance Methods in Molecular Biology; Shabala, S., Cuin, T.A., Eds.; Humana Press: Totowa, NJ, USA, 2012; pp. 251–261. [Google Scholar] [CrossRef]
- Karagiannis, E.; Michailidis, M.; Tanou, G.; Scossa, F.; Sarrou, E.; Stamatakis, G.; Samiotaki, M.; Martens, S.; Fernie, A.R.; Molassiotis, A. Decoding altitude-activated regulatory mechanisms occurring during apple peel ripening. Hortic. Res. 2020, 7, 120. [Google Scholar] [CrossRef]
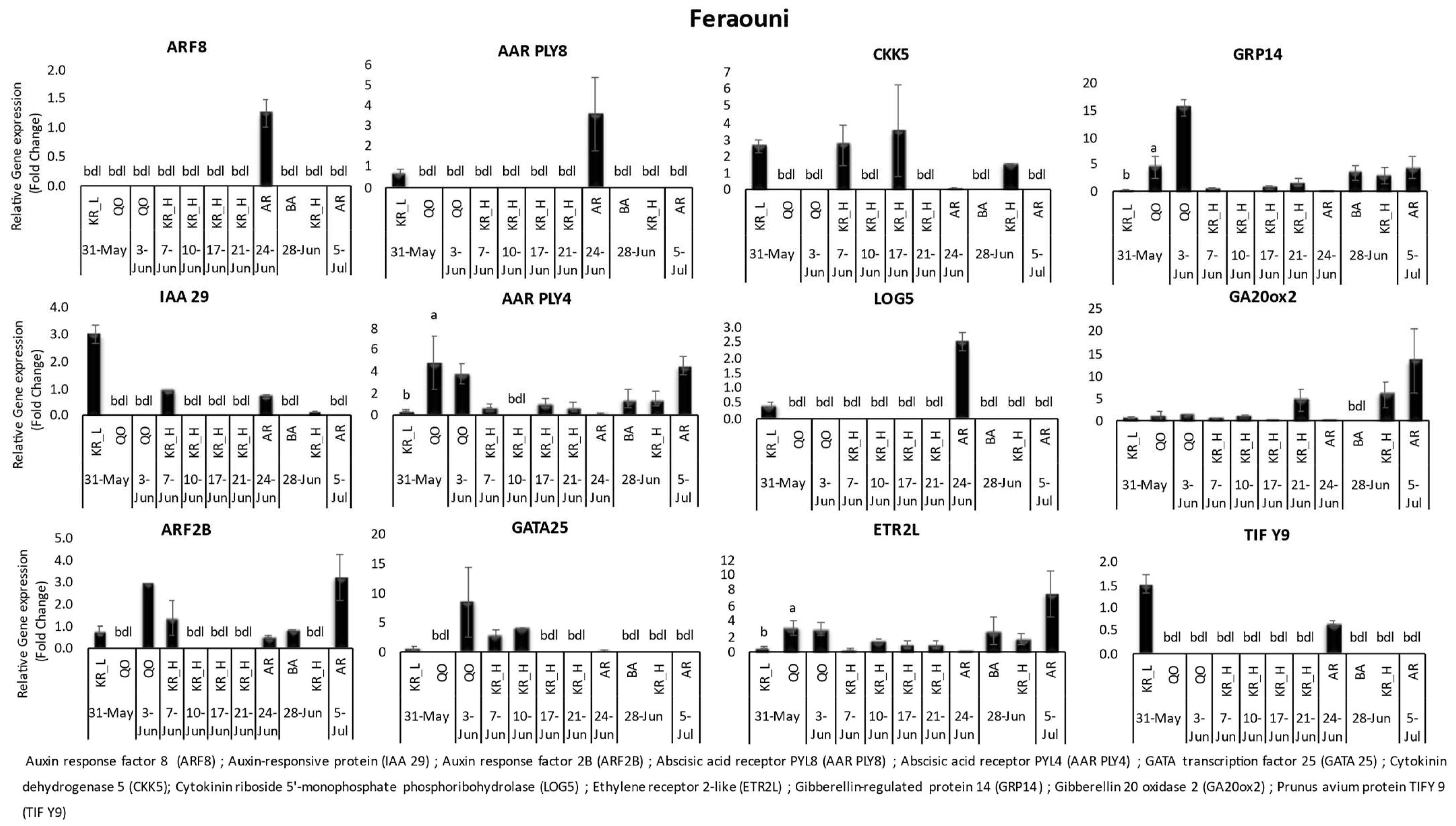
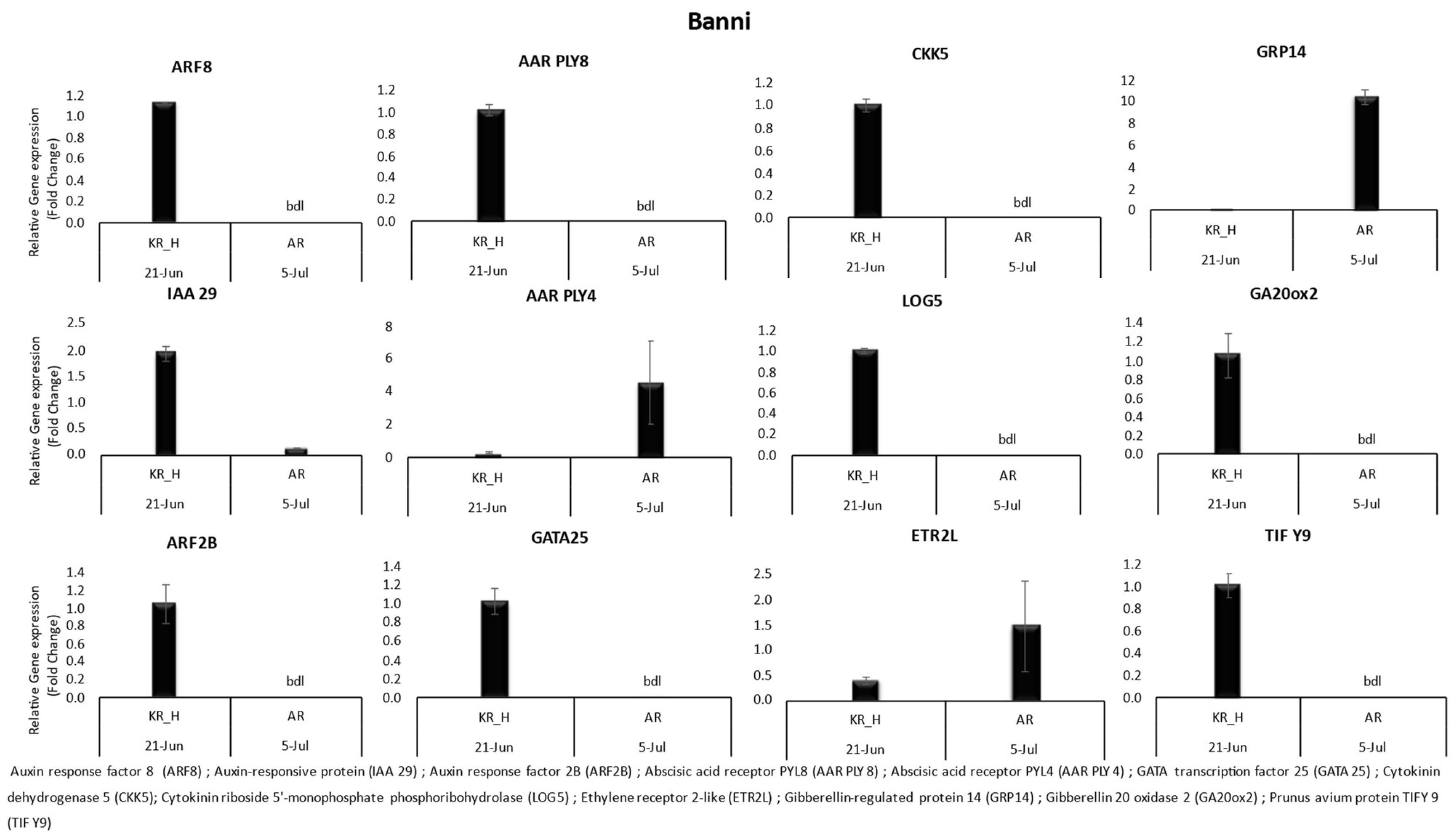

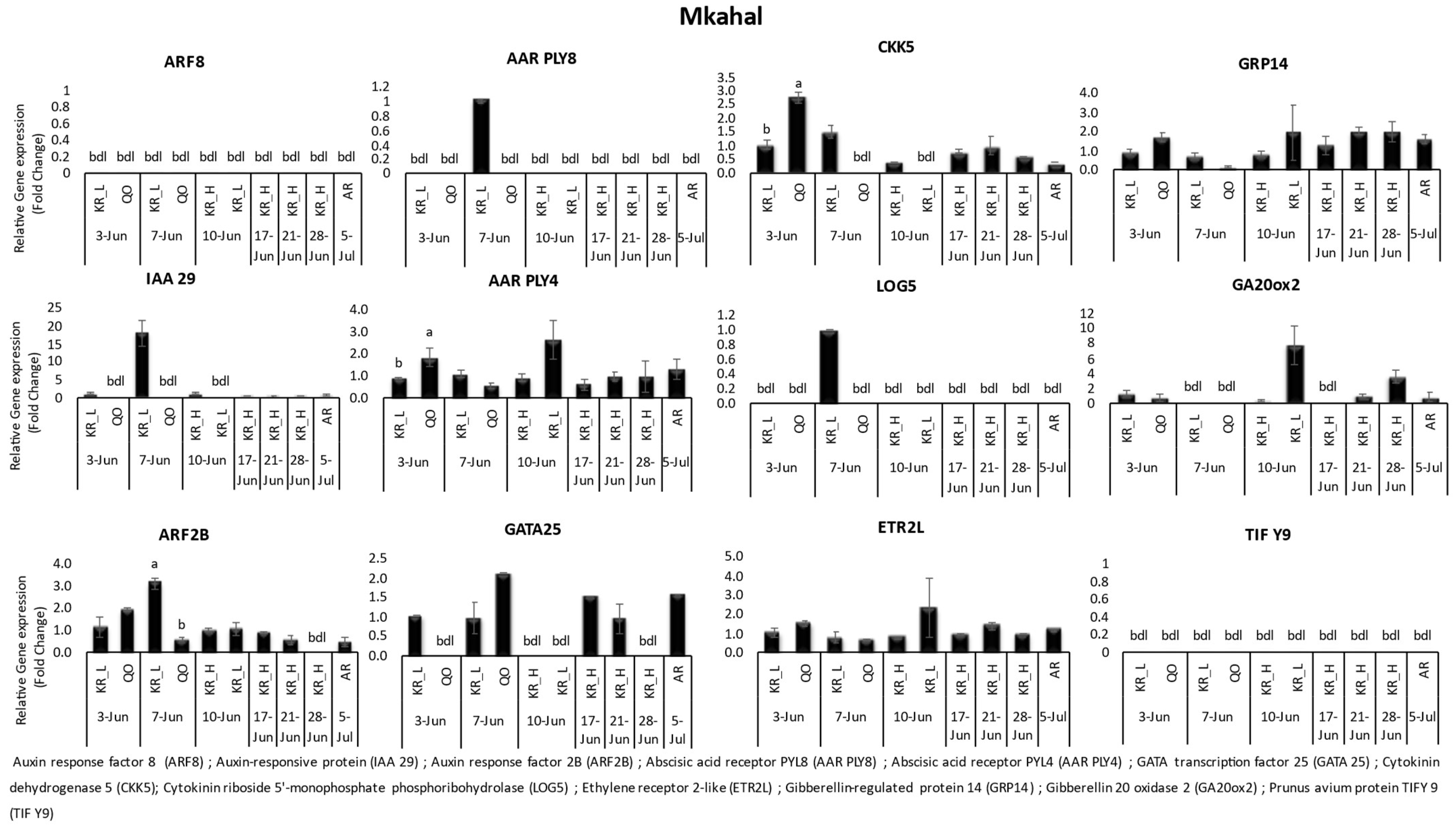
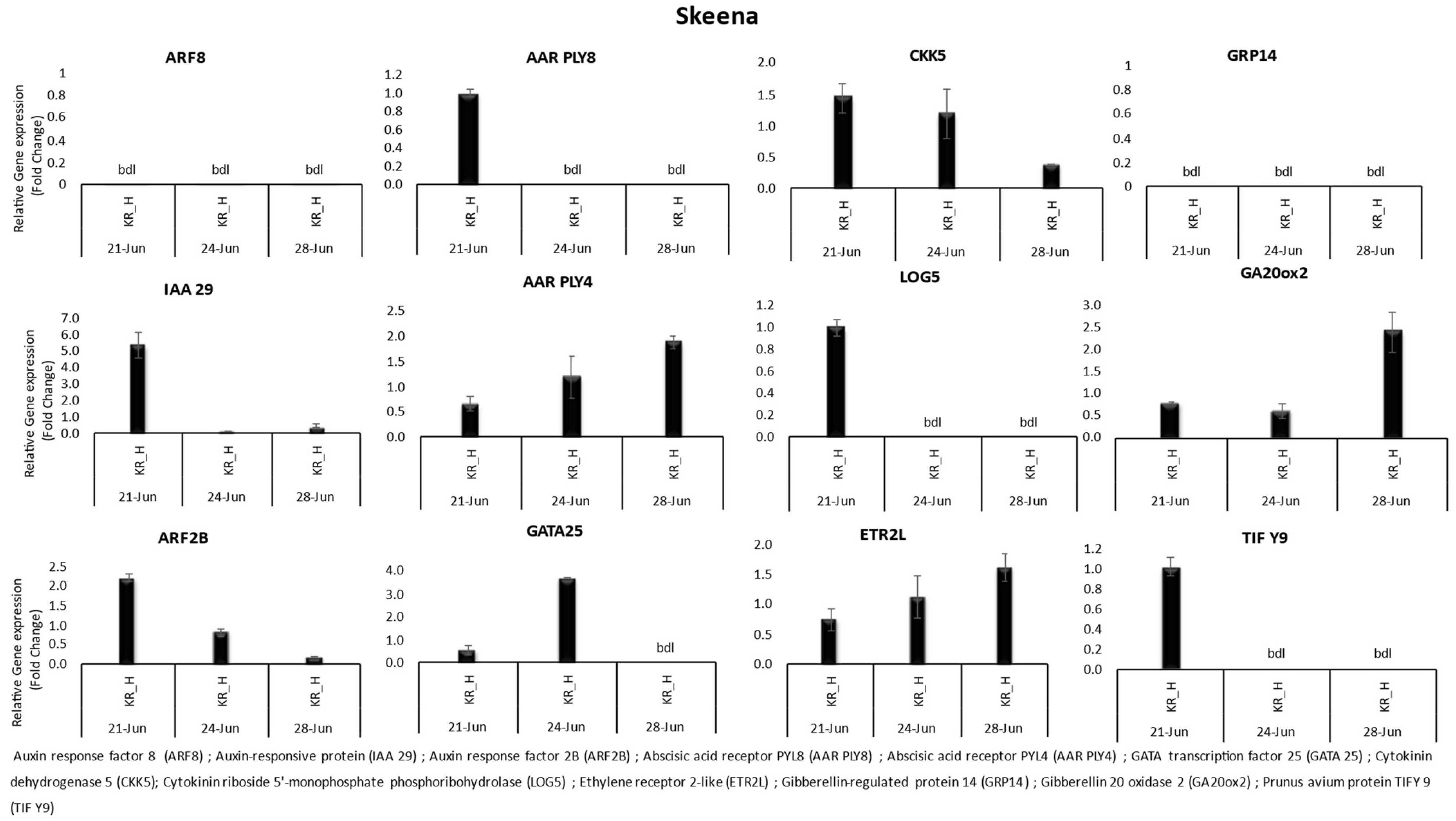

| Variety | Location | Date of Harvest | Benzyl Adenine | Zeatin | Salicylic Acid | Gibberellic Acid | Abscisic Acid | |
|---|---|---|---|---|---|---|---|---|
| BA (ng/g DW) | (ng/g DW) | SA (ng/g DW) | GA3 (ng/g DW) | ABA (ng/g DW) | ||||
| Banni (BA) | Arsal | AR | 5 July | 0.06 ± 0.02 | 4.11 ± 0.07 | 5.88 ± 0.18 | 2.24 ± 0.07 | 18.11 ± 0.24 |
| Kaa El Reem (high altitude) | KR_H | 21 June | 0.04 ± 0 | 1.85 ± 0.02 | 6.39 ± 0.35 | 0.91 ± 0.18 | 17.78 ± 0.33 | |
| Feraouni (FE) | Arsal | AR | 24 June | 0.08 ± 0 | 4.58 ± 0.08 a | 9.27 ± 0.28 a | 1.73 ± 0.17 a | 9.01 ± 0.27 a |
| 5 July | 0.06 ± 0 | 2.07 ± 0 b | 5.43 ± 0.55 b | 0.79 ± 0.05 b | 1.85 ± 0.12 b | |||
| Barqa | BA | 28 June | 0.1 ± 0 | 2.34 ± 0.09 | 9.36 ± 0.03 | 2.18 ± 0.13 | 5.52 ± 0.08 | |
| Kaa El Reem (high altitude) | KR_H | 7 June | ND | 3.98 ± 0.06 a | 19.01 ± 0.32 a | 1.84 ± 0.16 ab | 18.13 ± 0.25 a | |
| 10 June | ND | 3.74 ± 0.03 a | 7.45 ± 0.16 b | 1.78 ± 0.04 ab | 18.93 ± 0.11 a | |||
| 17 June | 0.06 ± 0.01 | 1.3 ± 0.12 c | 5.52 ± 0.51 c | 0.85 ± 0.05 b | 2.91 ± 0.14 b | |||
| 21 June | 0.11 ± 0 | 2.1 ± 0.07 b | 5.4 ± 0.26 c | 2.14 ± 0.06 a | 4.12 ± 0.41 b | |||
| 28 June | 0.18 ± 0.02 | 1.72 ± 0.02 b | 6.12 ± 0.08 c | 1.23 ± 0.46 ab | 2.63 ± 0.29 b | |||
| Kaa El Reem (low altitude) | KR_L | 31 May | 0.07 ± 0.01 | 3.54 ± 0.12 | 13.42 ± 0.92 | 1.12 ± 0.02 | 3.52 ± 0.04 | |
| Qoussaya | QO | 31 May | 0.19 ± 0.03 | 3.19 ± 0.51 | 3.92 ± 0.62 | 0.98 ± 0.08 | 5.98 ± 1.25 | |
| 3 June | 0.08 ± 0.02 | 3.59 ± 0.03 | 3.48 ± 0.15 | 0.82 ± 0.05 | 4.35 ± 0.06 | |||
| Irani (IR) | Barqa | BA | 17 June | 0.26 ± 0.02 | 1.9 ± 0.04 a | 3.78 ± 0.05 b | 1.73 ± 0.05 | 25.88 ± 0.16 a |
| 21 June | 0.21 ± 0 | 2.31 ± 0.13 a | 11.19 ± 0.02 a | 1.14 ± 0.04 | 9.94 ± 0.14 b | |||
| 28 June | ND | 1.42 ± 0.04 b | 10.99 ± 1.04 a | 2.85 ± 0.5 | 1.87 ± 0.15 c | |||
| Kaa El Reem (high altitude) | KR_H | 10 June | 0.06 ± 0 | 1.26 ± 0.03 b | 1.5 ± 0.15 b | 0.71 ± 0.03 | 2.89 ± 0.04 b | |
| 14 June | 0.15 ± 0 | 2.42 ± 0.09 a | 3.52 ± 0.04 a | 1.2 ± 0.26 | 11.57 ± 0.16 a | |||
| 28 June | 0.16 ± 0.02 | 1.05 ± 0.01 b | 2.37 ± 0.34 ab | 1.57 ± 0.02 | 1.16 ± 0.04 c | |||
| Kaa El Reem (low altitude) | KR_L | 31 May | 0.24 ± 0 a | 2.3 ± 0.01 c | 1.85 ± 0.81 | 1.33 ± 0.11 | 5.48 ± 0.35 b | |
| 3 June | 0.27 ± 0.02 a | 4.78b± 0.1 a | 1.61 ± 0.28 | 1.09 ± 0.08 | 6.8 ± 0.17 b | |||
| 7 June | 0.15 ± 0.01 b | 4.05 ± 0.15 b | 4.32 ± 0.82 | 1.32 ± 0.04 | 12.9 ± 0.13 a | |||
| Qoussaya | QO | 31 May | 0.19 ± 0.01 | 5.59 ± 0.07 a | 3.1 ± 0.33 | 1.55 ± 0.05 | 14.17 ± 0.1 a | |
| 3 June | 0.02 ± 0 | 2.73 ± 0.09 b | 3.86 ± 0.03 | 1.27 ± 0.07 | 3.35 ± 0.21 b | |||
| Mkahal (MK) | Arsal | AR | 5 July | 0.07 ± 0.01 | 4.49 ± 0.01 | 4.97 ± 0.14 | 1.63 ± 0.15 | 17.5 ± 0.02 |
| Kaa El Reem (high altitude) | KR_H | 10 June | 0.11 ± 0 | 7.44 ± 0.15 a | 17.28 ± 1.14 a | 2.01 ± 0.33 | 51.14 ± 0.25 a | |
| 17 June | 0.15 ± 0 | 7.71 ± 0.03 a | 7.87 ± 0.06 b | 2.16 ± 0.58 | 27.04 ± 0.04 b | |||
| 21 June | ND | 5.74 ± 0.02 b | 15.6 ± 0.81 a | 1.7 ± 0.17 | 7.62 ± 0.37 c | |||
| 28 June | ND | 3.78 ± 0.09 c | 6.32 ± 0.49 b | 1.75 ± 0.03 | 3.71 ± 0.04 d | |||
| Kaa El Reem (low altitude) | KR_L | 3 June | 0.05 ± 0.02 | 4.59 ± 0.06 a | 10.47 ± 0.16 | 1.92 ± 0.11 | 19.69 ± 0.86 a | |
| 7 June | ND | 5.03 ± 0.13 a | 8.05 ± 0.24 | 1.44 ± 0.34 | 15.39 ± 0.22 b | |||
| 10 June | ND | 3.21 ± 0.01 b | 8.28 ± 1 | 2.14 ± 0.2 | 4.36 ± 0.02 c | |||
| Qoussaya | QO | 3 June | 0.13 ± 0.04 | 5.1 ± 0 | 4.59 ± 0.13 | 1.21 ± 0.07 | 12.24 ± 0.44 | |
| 7 June | ND | 5.96 ± 0.68 | 5.49 ± 0.72 | 1.82 ± 0.18 | 17.62 ± 2.16 | |||
| Skeena (SK) | Kaa El Reem (high altitude) | KR_H | 21 June | 0.2 ± 0.02 | 2.82 ± 0.04 a | 2.77 ± 0.24 a | 2.15 ± 0.21 | 3.46 ± 0.29 ab |
| 24 June | 0.27± 0 | 2.49 ± 0.05 b | 3.01 ± 0.17 a | 2.61 ± 0.15 | 3.66 ± 0.06 a | |||
| 28 June | 0.3 ± 0.05 | 2.05 ± 0.04 c | 1.2 ± 0.12 b | 2.01 ± 0.14 | 2.06 ± 0.34 b | |||
| Teliani (TE) | Arsal | AR | 10 June | 1.17 ± 0.01 | 1.48 ± 0.01 | 2.31 ± 0.46 | 1.94 ± 0.01 | 18.96 ± 0.14 |
| Barqa | BA | 31 May | 0.34 ± 0.02 | 1.72 ± 0.03 a | 3.32 ± 0.31 | 1.47 ± 0.08 a | 8.97 ± 0.2 a | |
| 3 June | 0.28 ± 0.01 | 1.3 ± 0.03 b | 2.73 ± 0.24 | 0.31 ± 0.05 b | 6.03 ± 0.24 b | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nacouzi, D.; El Kayal, W. Genetic and Hormonal Regulation of Sweet Cherry (Prunus avium L.) Maturity across Altitudinal Gradients. Horticulturae 2024, 10, 408. https://doi.org/10.3390/horticulturae10040408
Nacouzi D, El Kayal W. Genetic and Hormonal Regulation of Sweet Cherry (Prunus avium L.) Maturity across Altitudinal Gradients. Horticulturae. 2024; 10(4):408. https://doi.org/10.3390/horticulturae10040408
Chicago/Turabian StyleNacouzi, Diana, and Walid El Kayal. 2024. "Genetic and Hormonal Regulation of Sweet Cherry (Prunus avium L.) Maturity across Altitudinal Gradients" Horticulturae 10, no. 4: 408. https://doi.org/10.3390/horticulturae10040408
APA StyleNacouzi, D., & El Kayal, W. (2024). Genetic and Hormonal Regulation of Sweet Cherry (Prunus avium L.) Maturity across Altitudinal Gradients. Horticulturae, 10(4), 408. https://doi.org/10.3390/horticulturae10040408






