Variability in Morphological, Biochemical, and Proximate Yield Composition among Predominant Amaranthus hybridus Cultivars in South-West Nigeria
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Field Evaluation
2.2. Percentage Germination
2.3. Agronomic Parameters
2.4. Proximate Composition Analysis
2.5. Phenolic Contents
2.6. Total Antioxidants
2.7. Data Analyses
3. Results
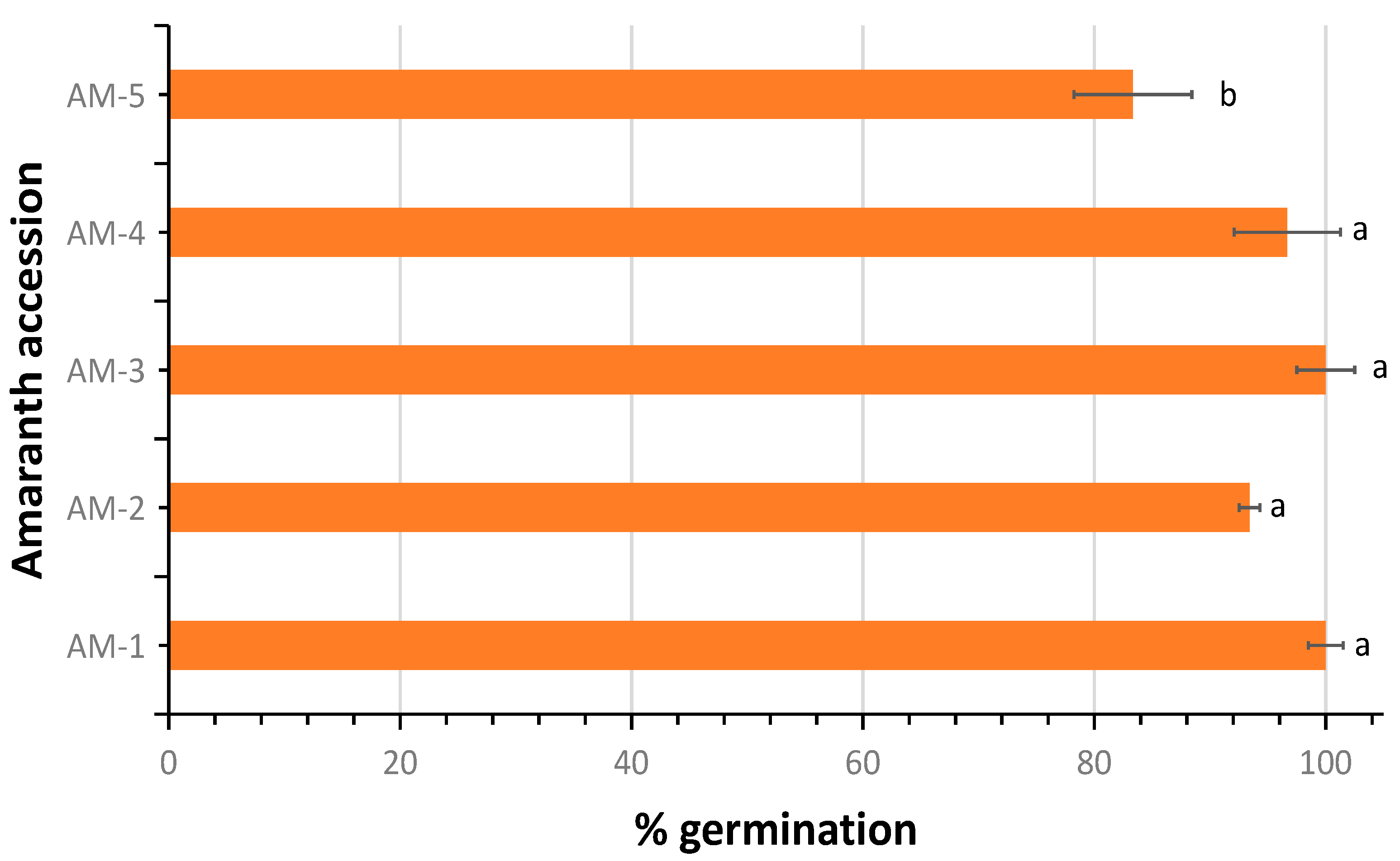
3.1. Germination Percentage
3.2. Quantitative and Morphological Characters
3.3. Qualitative and Yield-Related Parameters
3.4. Seed Proximate Composition
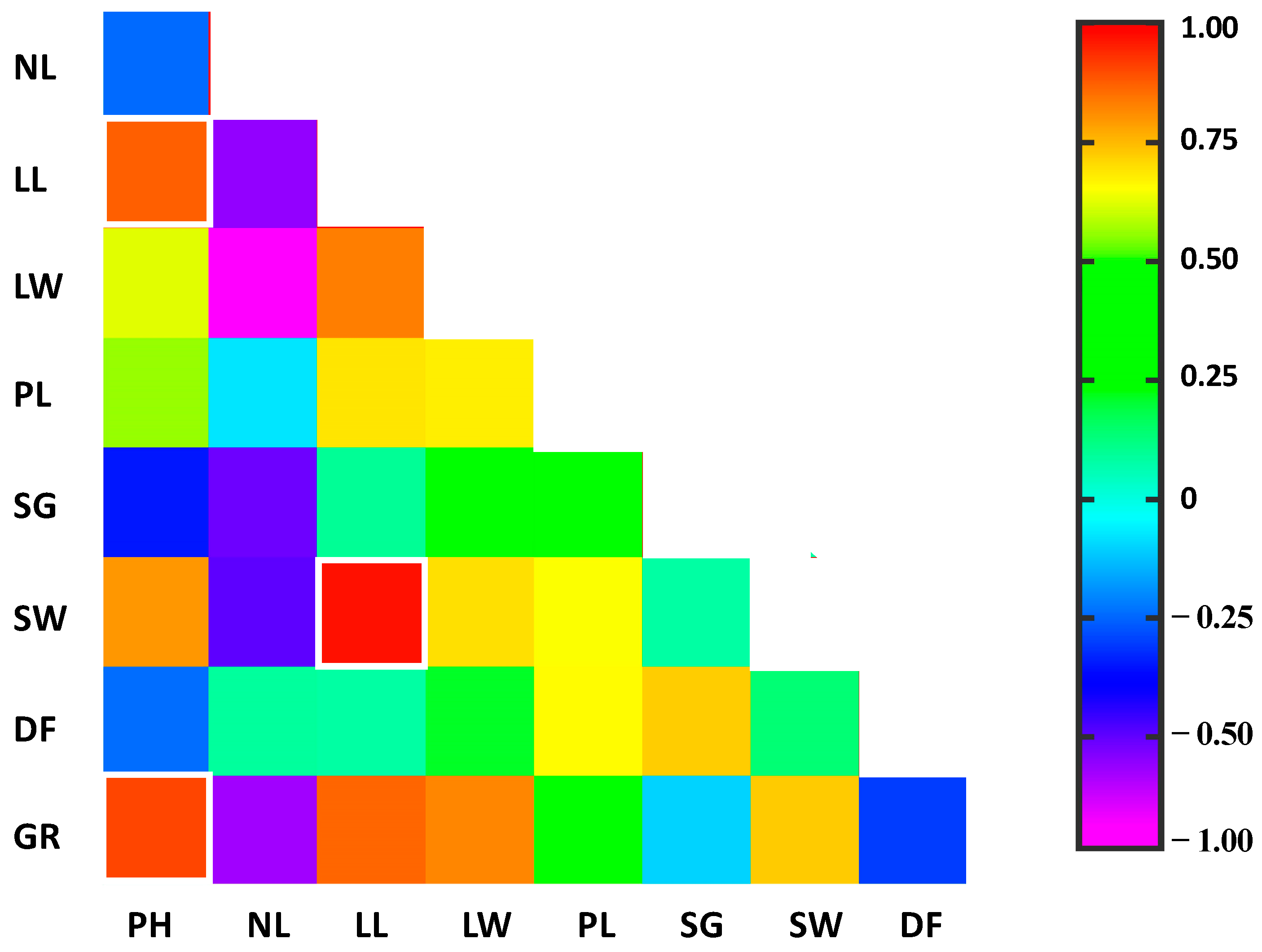
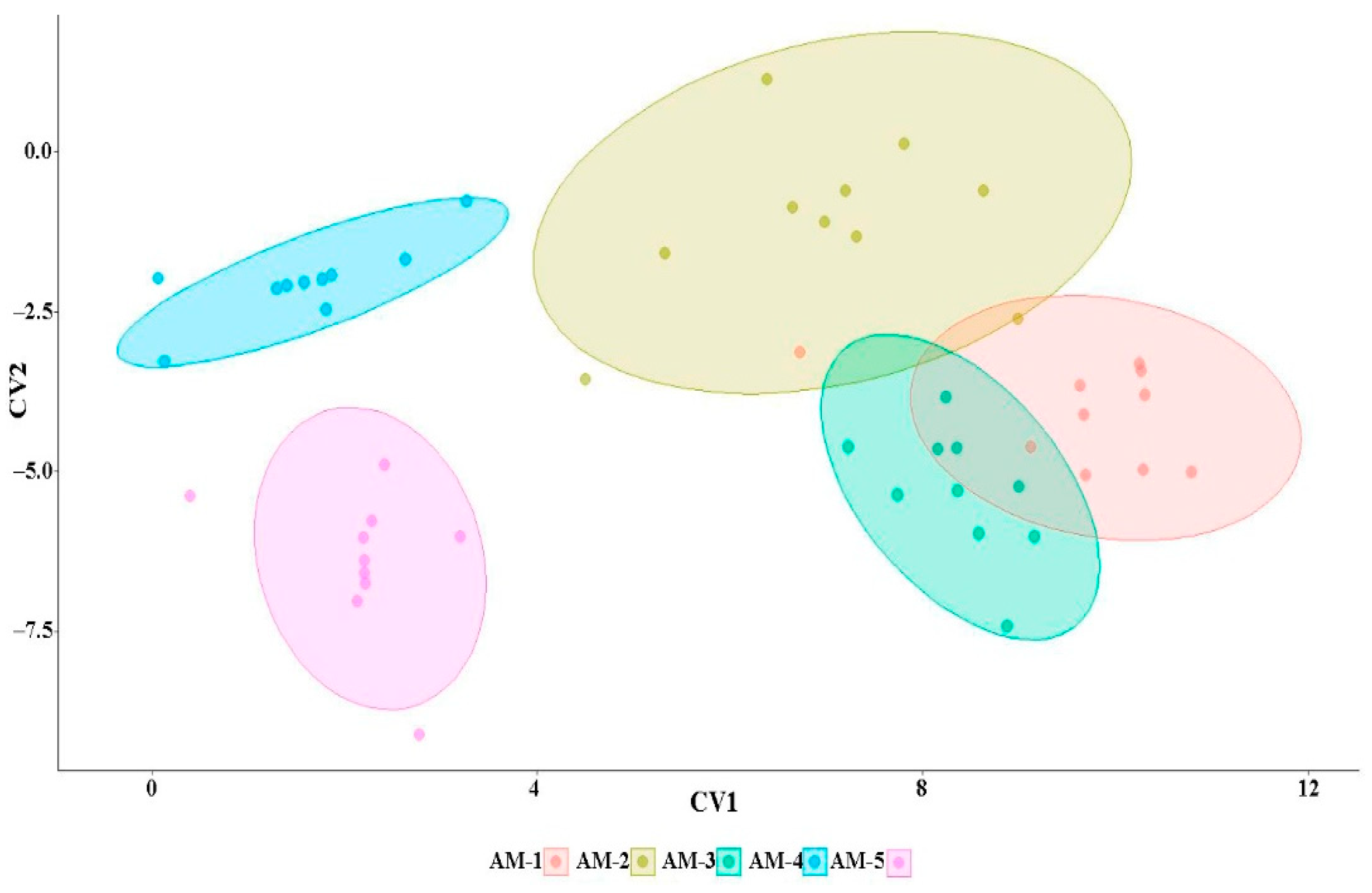
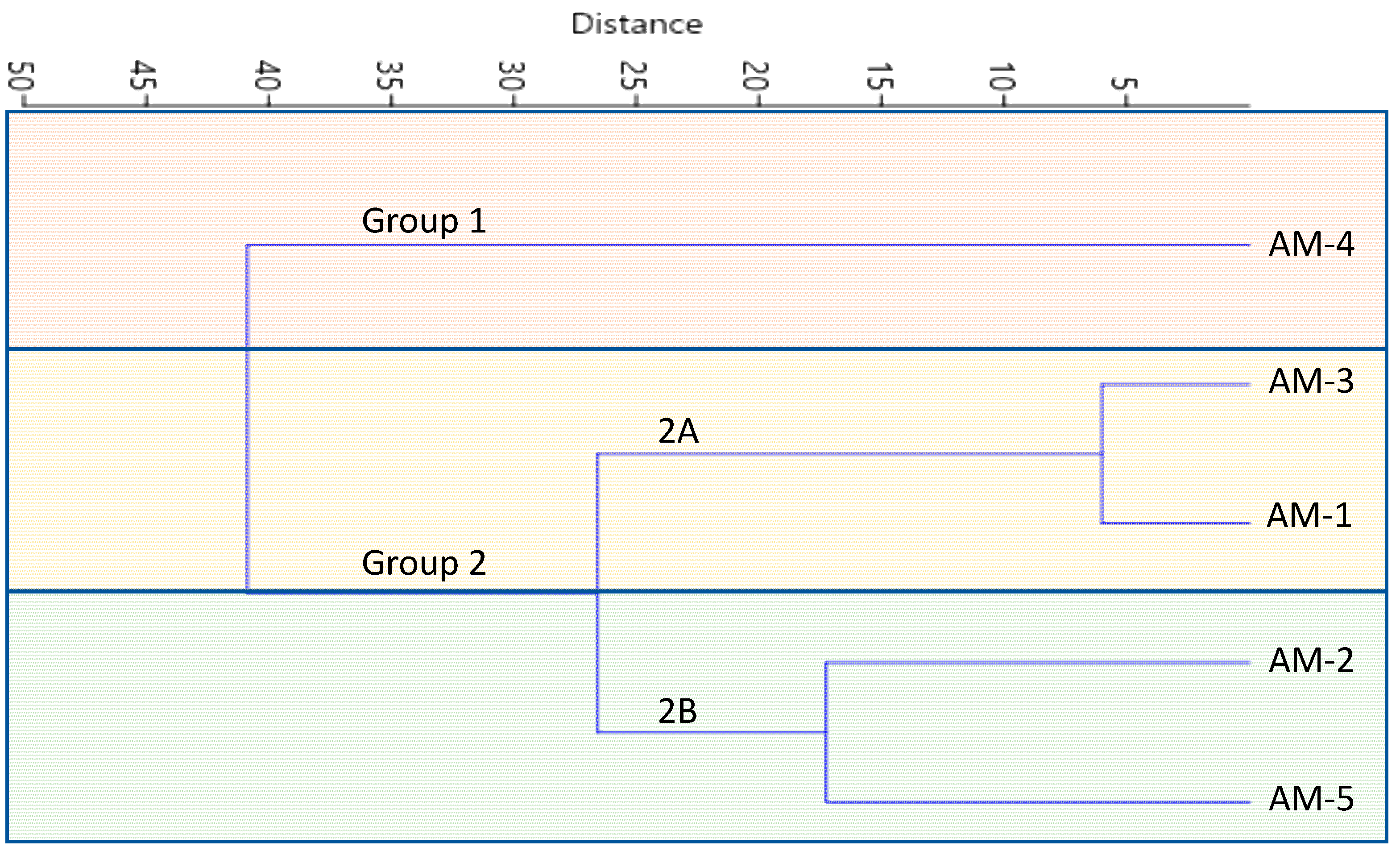
3.5. Multivariate Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Abolaji, G.T.; Olooto, F.M.; Ogundele, D.T.; Williams, F.E. Nutritional Characterization of Grain Amaranth Grown in Nigeria for Food Security and Healthy Living. Agrosearch 2016, 17, 1. [Google Scholar] [CrossRef]
- Nyonje, W.A.; Yang, R.-Y.; Kejo, D.; Makokha, A.O.; Owino, W.O.; Abukutsa-Onyango, M.O. Exploring the Status of Preference, Utilization Practices, and Challenges to Consumption of Amaranth in Kenya and Tanzania. J. Nutr. Metab. 2022, 2022, 2240724. [Google Scholar] [CrossRef] [PubMed]
- Nyonje, W.A.; Makokha, A.O.; Abukutsa-Onyango, M.O. Anti-Nutrient, Phytochemical and Antiradical Evaluation of 10 Amaranth (Amaranthus spp.) Varieties Before and After Flowering. J. Agric. Sci. 2014, 6, 68. [Google Scholar] [CrossRef]
- Christenhusz, M.J.M.; Byng, J.W. The Number of Known Plants Species in the World and Its Annual Increase. Phytotaxa 2016, 261, 201. [Google Scholar] [CrossRef]
- Waselkov, K.E.; Boleda, A.S.; Olsen, K.M. A Phylogeny of the Genus Amaranthus (Amaranthaceae) Based on Several Low-Copy Nuclear Loci and Chloroplast Regions. Syst. Bot. 2018, 43, 439–458. [Google Scholar] [CrossRef]
- Akaneme, F.; Ani, G. Morphological Assessment of Genetic Variability among Accessions of Amaranthus hybridus. World Appl. Sci. J. 2013, 28, 568–577. [Google Scholar] [CrossRef]
- Sarangi, D.; Jhala, A.J.; Govindasamy, P.; Brusa, A. Amaranthus spp. In Biology and Management of Problematic Crop Weed Species; Chauhan, B.S., Ed.; Elsevier, Inc.: Philadelphia, PA, USA, 2021; pp. 21–42. [Google Scholar] [CrossRef]
- Assad, R.; Reshi, Z.A.; Jan, S.; Rashid, I. Biology of Amaranths. Bot. Rev. 2017, 83, 382–436. [Google Scholar] [CrossRef]
- Langyan, S.; Bhardwaj, R.; Kumari, J.; Jacob, S.R.; Bisht, I.S.; Pandravada, S.R.; Singh, A.; Singh, P.B.; Dar, Z.A.; Kumar, A.; et al. Nutritional Diversity in Native Germplasm of Maize Collected from Three Different Fragile Ecosystems of India. Front. Nutr. 2022, 9, 812599. [Google Scholar] [CrossRef] [PubMed]
- Juliano, B.O. Rice: Role in Diet. In Encyclopedia of Food and Health; Caballero, B., Finglas, P., Toldrá, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 641–645. [Google Scholar]
- Shi, S.; Zhao, D.; Xing, J.; Pan, K.; Ma, J.; Wang, X.; Liu, J.; Cao, C.; Jiang, Y. Relationship between Rice Grain Protein Content and Key Phenotypes in Rice. Agron. J. 2023, 115, 1257–1264. [Google Scholar] [CrossRef]
- Kondić-Špika, A.; Mladenov, N.; Grahovac, N.; Zorić, M.; Mikić, S.; Trkulja, D.; Marjanović-Jeromela, A.; Miladinović, D.; Hristov, N. Biometric Analyses of Yield, Oil and Protein Contents of Wheat (Triticum Aestivum L.) Genotypes in Different Environments. Agronomy 2019, 9, 270. [Google Scholar] [CrossRef]
- de la Barca, A.M.C.; Rojas-Martínez, M.E.; Islas-Rubio, A.R.; Cabrera-Chávez, F. Gluten-Free Breads and Cookies of Raw and Popped Amaranth Flours with Attractive Technological and Nutritional Qualities. Plant Foods Hum. Nutr. 2010, 65, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Jimoh, M.O.; Afolayan, A.J.; Lewu, F.B. Suitability of Amaranthus Species for Alleviating Human Dietary Deficiencies. S. Afr. J. Bot. 2018, 115, 65–73. [Google Scholar] [CrossRef]
- Wudil, A.H.; Usman, M.; Rosak-Szyrocka, J.; Pilař, L.; Boye, M. Reversing Years for Global Food Security: A Review of the Food Security Situation in Sub-Saharan Africa (SSA). Int. J. Environ. Res. Public Health 2022, 19, 14836. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Wen, Y.; Choi, Y.; Zhang, X. Global Trends on Food Security Research: A Bibliometric Analysis. Land 2021, 10, 119. [Google Scholar] [CrossRef]
- Balana, B.B.; Ogunniyi, A.; Oyeyemi, M.; Fasoranti, A.; Edeh, H.; Andam, K. COVID-19, Food Insecurity and Dietary Diversity of Households: Survey Evidence from Nigeria. Food Secur. 2022, 15, 219–241. [Google Scholar] [CrossRef] [PubMed]
- Otekunrin, O.A. Investigating Food Insecurity, Health and Environment-Related Factors, and Agricultural Commercialization in Southwestern Nigeria: Evidence from Smallholder Farming Households. Environ. Sci. Pollut. Res. 2022, 29, 51469–51488. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, H.U. Factors militating against agricultural productivity of crop farmers in Niger state, Nigeria. J. Agripreneurship Sustain. Dev. 2023, 6, 15–23. [Google Scholar] [CrossRef]
- Abdulai, A. Information Acquisition and the Adoption of Improved Crop Varieties. Am. J. Agric. Econ. 2023, 105, 1049–1062. [Google Scholar] [CrossRef]
- Uduji, J.I.; Okolo-Obasi, E.N. Adoption of Improved Crop Varieties by Involving Farmers in the E-Wallet Program in Nigeria. J. Crop Improv. 2018, 32, 717–737. [Google Scholar] [CrossRef]
- Ajadi, B.; Adeniyi, A.; Afolabi, M. Impact of Climate on Urban Agriculture: Case Study of Ilorin City, Nigeria. Glob. J. Hum. Soc. Sci. 2011, 11, 25–29. [Google Scholar]
- Feller, C.; Bleiholder, H.; Buhr, L.; Hack, H.; Hess, M.; Klose, R.; Meier, U.; Stauss, R.; van den Boom, T.; Weber, E. Phenological development stages of vegetable plants I. Bulb, root, tuber and leafy vegetables: Coding and description according to the extended BBCH scale with illustrations. Nachrichtenbl. Deut. Pflanzenschutzd. 1995, 8, 193–206. Available online: https://www.openagrar.de/receive/openagrar_mods_00067067 (accessed on 13 November 2023).
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis; AOAC: Washington, DC, USA, 2000. [Google Scholar]
- Animasaun, D.A.; Oyedeji, S.; Musa, L.B.; Adedibu, P.A.; Adekola, O.F. Performance and Genetic Diversity of Some Sesame (Sesamum Indicum L.) Accessions Based on Morpho-Agronomic Traits and Seed Proximate Composition in Kwara State of Nigeria. Acta Agric. Slov. 2022, 118, 1–15. [Google Scholar] [CrossRef]
- Bao, J.; Cai, Y.; Sun, M.; Wang, G.; Corke, H. Anthocyanins, Flavonols, and Free Radical Scavenging Activity of Chinese Bayberry (Myrica Rubra) Extracts and Their Color Properties and Stability. J. Agric. Food Chem. 2005, 53, 2327–2332. [Google Scholar] [CrossRef] [PubMed]
- Akin-Idowu, P.; Ademoyegun, O.; Olagunju, Y.; Aduloju, A.; Adebo, G. Phytochemical Content and Antioxidant Activity of Five Grain Amaranth Species. Am. J. Food Sci. Technol 2017, 5, 249–255. [Google Scholar]
- Williams, L.; Abdi, H. Fisher’s Least Significant Difference Test. In The SAGE Encyclopedia of Research Design; Salkind, N., Ed.; SAGE Publications, Inc.: Thousand Oaks, CA, USA, 2010. [Google Scholar]
- Hammer, D.; Harper, D.; Ryan, P. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Azeez, M.A.; Olowookere, M.B.; Animasaun, D.A.; Bello, B.O. Utility of Some Floral Characters in the Assessment of Genetic Diversity in Sesame (Sesamum Indicum L.). Acta Agric. Slov. 2017, 109, 61–70. [Google Scholar] [CrossRef]
- Maranna, S.; Nataraj, V.; Kumawat, G.; Chandra, S.; Rajesh, V.; Ramteke, R.; Patel, R.M.; Ratnaparkhe, M.B.; Husain, S.M.; Gupta, S.; et al. Breeding for Higher Yield, Early Maturity, Wider Adaptability and Waterlogging Tolerance in Soybean (Glycine Max L.): A Case Study. Sci. Rep. 2021, 11, 22853. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.S.; Khaskheli, M.A.; Uzair, M.; Xu, Y.; Wattoo, F.M.; Rehman, O.U.; Amatus, G.; Fatima, H.; Khan, S.A.; Fiaz, S.; et al. Inquiring the Inter-Relationships amongst Grain-Filling, Grain-Yield, and Grain-Quality of Japonica Rice at High Latitudes of China. Front. Genet. 2022, 13, 988256. [Google Scholar] [CrossRef]
- Jahan, N.; Sarker, U.; Hasan Saikat, M.M.; Hossain, M.M.; Azam, M.G.; Ali, D.; Ercisli, S.; Golokhvast, K.S. Evaluation of Yield Attributes and Bioactive Phytochemicals of Twenty Amaranth Genotypes of Bengal Floodplain. Heliyon 2023, 9, e19644. [Google Scholar] [CrossRef]
- Animasaun, D.A.; Morakinyo, J.A.; Krishnamurthy, R.; Mustapha, O.T. Genetic Divergence of Nigerian and Indian Pearl Millet Accessions Based on Agronomical and Morphological Traits. J. Agric. Sci. Belgrade 2017, 62, 115–131. [Google Scholar] [CrossRef]
- Kulakow, P.A. Simply inherited genetic variation in grain amaranth. In Advances in New Crops; Janick, J., Simon, J.E., Eds.; Timber Press: Portland, OR, USA, 1990. [Google Scholar]
- Oboh, B. Multivariate Analysis of the Diversity among Some Nigerian Accessions of Amaranthus hybridus. Int. J. Plant Breed. Genet. 2007, 1, 89–94. [Google Scholar] [CrossRef]
- Gerrano, A.S.; van Rensburg, W.S.J.; Adebola, P.O. Genetic Diversity of Amaranthus species in South Africa. S. Afr. J. Plant Soil 2015, 32, 39–46. [Google Scholar] [CrossRef]
- Akin-Idowu, P.; Gbadegesin, M.; Orkpeh, U.; Ibitoye, D.; Odunola, O. Characterization of Grain Amaranth (Amaranthus spp.) Germplasm in South West Nigeria Using Morphological, Nutritional, and Random Amplified Polymorphic DNA (RAPD) Analysis. Resources 2016, 5, 6. [Google Scholar] [CrossRef]
- Oduwaye, O.A.; Ayo-Vaughan, M.A.; Porbeni, J.B.O.; Oyelakin, O.O. Genetic Diversity in Amaranth (Amaranthus spp.) Based on Phenotypic and RAPD Markers. Niger. J. Biotechnol. 2019, 36, 62. [Google Scholar] [CrossRef]
- Thapa, R.; Blair, M. Morphological Assessment of Cultivated and Wild Amaranth Species Diversity. Agronomy 2018, 8, 272. [Google Scholar] [CrossRef]
- Varalakshmi, B. Characterization and Preliminary Evaluation of a Vegetable Amaranth (Amarnthus sp.), Germplasm. PGR Newsl. 2004, 137, 55–57. [Google Scholar]
- Hailu, A.; Alameraw, S. Estimation of Association Characters in Amaranths Germplasm Accessions (Amaranthus spp.) under Mizan and Tepi Condtions, South-west Ethiopia. Int. J. Sci. Res. 2015, 2, 1–24. [Google Scholar]
- Showemimo, F.; Soyombo, M.A.; Amira, J.O.; Porbeni, J.B.O. Traits Selection Criteria for Genetic Improvement of Grain and Leafy Amaranth (Amaranthus spp.) Using Principal Component Analysis. Egypt. J. Agric. Res. 2021, 99, 170–179. [Google Scholar] [CrossRef]
- Zafar, N.; Aziz, S.; Masood, S. Phenotypic divergence for agro-morphological traits among landrace genotypes of rice (Oryza sativa L.) from Pakistan. Inter. J. Agri. Biol. 2008, 6, 335–339. Available online: http://www.ijab.org (accessed on 13 November 2023).
- Animasaun, D.A.; Afeez, A.; Adedibu, P.A.; Akande, F.P.; Oyedeji, S.; Olorunmaiye, K.S. Morphometric Variation, Genetic Diversity and Allelic Polymorphism of an Underutilised Species Thaumatococcus Daniellii Population in Southwestern Nigeria. J. Plant Biotechnol. 2020, 47, 298–308. [Google Scholar] [CrossRef]
- Baturaygil, A.; Schmid, K. Characterization of Flowering Time in Genebank Accessions of Grain Amaranths and Their Wild Relatives Reveals Signatures of Domestication and Local Adaptation. Agronomy 2022, 12, 505. [Google Scholar] [CrossRef]
- Khan, A.W.; Garg, V.; Roorkiwal, M.; Golicz, A.A.; Edwards, D.; Varshney, R.K. Super-Pangenome by Integrating the Wild Side of a Species for Accelerated Crop Improvement. Trends Plant Sci. 2020, 25, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Animasaun, D.A.; Adedibu, P.A.; Shkryl, Y.; Emmanuel, F.O.; Tekutyeva, L.; Balabanova, L. Modern Plant Biotechnology: An Antidote against Global Food Insecurity. Agronomy 2023, 13, 2038. [Google Scholar] [CrossRef]
- Ogrodowska, D.; Zadernowski, R.; Czaplicki, S.; Derewiaka, D.; Wronowska, B. Amaranth Seeds and Products—The Source of Bioactive Compounds. Pol. J. Food Nutr. Sci. 2014, 64, 165–170. [Google Scholar] [CrossRef]
- Singh, A.; Punia, D. Characterization and Nutritive Values of Amaranth Seeds. Curr. J. Appl. Sci. Technol. 2020, 39, 27–33. [Google Scholar] [CrossRef]
- Oko, A.; Famurewa, A. Estimation of Nutritional and Starch Characteristics of Dioscorea Alata (Water Yam) Varieties Commonly Cultivated in the South-Eastern Nigeria. Br. J. Appl. Sci. Technol. 2015, 6, 145–152. [Google Scholar] [CrossRef]
- Goufo, P.; Trindade, H. Rice Antioxidants: Phenolic Acids, Flavonoids, Anthocyanins, Proanthocyanidins, Tocopherols, Tocotrienols, oryzanol, and Phytic Acid. Food Sci. Nutr. 2014, 2, 75–104. [Google Scholar] [CrossRef]
- Singh, S.K.; Singh, P.; Khaire, A.R.; Korada, M.; Singh, D.K.; Majhi, P.K.; Jayasudha, S. Genetic Variability, Character Association and Path Analysis for Yield and Its Related Traits in Rice (Oryza Sativa L.) Genotypes. Int. J. Plant Soil Sci. 2021, 33, 437–446. [Google Scholar] [CrossRef]



| Cultivar | PH (cm) | NL | LL (cm) | LW (g) | PL (cm) | SG (cm) |
|---|---|---|---|---|---|---|
| AM-1 | 60.70 a ± 21.8 | 19.40 bc ± 3.95 | 14.00 a ± 2.5 | 6.04 a ± 1.8 | 6.22 a ± 0.68 | 2.80 a ± 0.18 |
| AM-2 | 41.30 ab ± 19.1 | 16.40 c ± 7.8 | 10.50 b ± 2.5 | 6.06 a ± 1.5 | 4.38 b ± 0.74 | 3.20 a ± 0.68 |
| AM-3 | 59.30 a ± 5.7 | 23.74 ab ± 3.2 | 11.82 ab ± 1.3 | 6.12 a ± 0.9 | 6.80 a ± 0.40 | 2.78 a ± 0.18 |
| AM-4 | 51.40 ab ± 12.7 | 21.48 bc ± 5.4 | 9.90 bc ± 0.8 | 4.74 ab ± 0.8 | 1.60 c ± 0.06 | 2.54 a ± 0.22 |
| AM-5 | 37.70 b ± 5.0 | 29.48 a ± 3.7 | 7.88 c ± 1.6 | 3.68 b ± 0.4 | 3.60 b ± 0.48 | 2.76 a ± 0.28 |
| Average | 50.08 | 22.10 | 10.82 | 5.33 | 4.52 | 2.82 |
| LSD | 19.40 | 6.69 | 2.48 | 1.43 | 1.57 | 1.06 |
| CV % | 32.56 | 29.23 | 24.86 | 29.83 | 48.34 | 27.30 |
| p value | 0.05 | 0.008 | 0.001 | 0.03 | 0.00 | 0.78 * |
| Cultivar Code | Accession Number | Leaf Color | Seed Shape | Seed Color | Fluorescence Color | Stem Color | Leaf Type |
|---|---|---|---|---|---|---|---|
| AM-1 | NG/AO/11/08/040 | green | oval | golden brown | green | green | entire and round |
| AM-2 | NG/AO/11/08/039 | green | oval | golden brown | pink | purple | entire and round |
| AM-3 | NG/AO/11/042 | green | oval | deep brown | pink | purple | entire and round |
| AM-4 | NHGB/09/09 | green | oval | black | white | green | entire and spiral |
| AM-5 | NG/TO/AUG/09/007 | green | oval | brown | pink | purple | entire and round |
| Cultivars | TCC (%) | TPC (%) | TLC (%) | CFC (%) | MC (%) | AC (%) | TP (mg/100 g GAE) | TA (mg/100 g AAE) |
|---|---|---|---|---|---|---|---|---|
| AM-1 | 66.52 bc ± 7.83 | 12.42 a ± 0.37 | 4.98 c ± 0.13 | 9.16 a ± 0.46 | 3.33 b ± 0.16 | 3.59 a ± 0.64 | 29.64 a ± 1.79 | 181.59 a ± 12.06 |
| AM-2 | 72.33 ab ± 6.25 | 10.02 b ± 1.51 | 5.36 bc ± 0.69 | 6.25 bc ± 0.45 | 3.29 b ± 0.43 | 2.75 a ± 0.81 | 27.04 a ± 2.74 | 163.77 b ± 16.02 |
| AM-3 | 65.01 c ± 7.19 | 11.35 a ± 1.01 | 6.55 a ± 0.57 | 9.81 a ± 0.42 | 4.22 a ± 0.53 | 3.06 a ± 0.77 | 28.99 a ± 5.10 | 180.53 a ± 10.23 |
| AM-4 | 76.28 a ± 4.07 | 6.57 c ± 0.93 | 4.51 c ± 1.09 | 5.56 c ± 1.25 | 4.16 a ± 0.62 | 2.92 a ± 0.52 | 26.65 a ± 3.49 | 131.64 c ± 6.86 |
| AM-5 | 73.65 ab ± 4.21 | 7.29 c ± 0.67 | 6.13 ab ± 0.90 | 6.96 b ± 1.09 | 3.24 b ± 0.98 | 2.73 a ± 1.13 | 23.36 a ± 7.40 | 140.77 c ± 9.91 |
| Average | 70.76 | 9.53 | 5.51 | 7.55 | 3.65 | 3.01 | 27.14 | 159.66 |
| LSD | 8.06 | 1.29 | 1.00 | 1.08 | 0.80 | 1.06 | 6.29 | 15.07 |
| CV % | 11.63 | 26.02 | 18.51 | 24.50 | 19.51 | 26.25 | 17.43 | 14.59 |
| p value | 0.003 | 0.00 | 0.002 | 0.00 | 0.03 | 0.45 * | 0.25 * | 0.00 |
| Traits | σ2p | σ2g | σ2e | GCV |
|---|---|---|---|---|
| Germination (%) | 52.4 | 46.59 | 5.81 | 7.21 |
| No of leaves | 49.08 | 18.08 | 31.01 | 19.24 |
| Plant height (cm) | 244.56 | 73.49 | 171.08 | 17.12 |
| Leaf width (cm) | 2.63 | 0.82 | 1.81 | 16.98 |
| Leaf length (cm) | 7.62 | 4.52 | 3.09 | 19.65 |
| Petioles length (cm) | 5.41 | 4.05 | 1.35 | 44.40 |
| Stem girth(cm) | 0.58 | −0.08 | 0.65 | 9.79 |
| TCC (g/100 g) | 43.44 | 17.66 | 25.77 | 5.94 |
| TPC (g/100 g) | 7.01 | 6.27 | 0.74 | 26.25 |
| TLC (%) | 1.16 | 0.57 | 0.59 | 13.75 |
| CFC (%) | 3.88 | 3.30 | 0.58 | 24.08 |
| MC (%) | 0.53 | 0.17 | 0.35 | 11.46 |
| AC (%) | 0.44 | 0.04 | 0.39 | 7.04 |
| TP (mg/100 g GAE) | 26.77 | 0.87 | 25.90 | 3.45 |
| TA (mg/100 g AAE) | 622.46 | 492.86 | 129.60 | 13.90 |
| Traits | Plant Height | Leaf Width | Leaf Length | Petioles Length | Stem Girth | TCC | TPC | TLC | CFC | MC | AC | TP | TA | GCLY |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Plant height | 0.80 | −0.32 | −0.45 | 0.95 | −0.10 | −0.56 | −0.70 | −0.04 | 0.28 | −0.23 | 0.34 | 0.09 | −0.68 | −0.64 |
| Leaf width | 0.45 | −0.57 | −0.44 | 1.20 | 0.00 | −0.58 | −0.91 | −0.01 | 0.21 | −0.07 | 0.19 | 0.08 | −0.88 | −1.34 |
| Leaf length | 0.78 | −0.55 | −0.46 | 1.20 | −0.02 | −0.57 | −0.83 | −0.12 | 0.23 | −0.05 | 0.29 | 0.09 | −0.78 | −0.80 |
| Petioles length | 0.49 | −0.44 | −0.36 | 1.55 | 0.02 | −0.68 | −0.86 | 0.29 | 0.32 | 0.05 | 0.18 | 0.06 | −0.83 | −0.21 |
| Stem girth | −0.77 | 0.01 | 0.09 | 0.35 | −0.10 | −0.11 | −0.38 | 0.17 | −0.01 | 0.22 | 0.01 | −0.02 | −0.37 | 0.26 |
| TCC | −0.73 | 0.53 | 0.43 | −1.72 | −0.02 | 0.62 | 0.92 | −0.25 | −0.36 | 0.06 | −0.26 | −0.07 | 0.93 | 0.08 |
| TPC | 0.63 | −0.59 | −0.43 | 1.51 | 0.04 | −0.64 | −0.89 | 0.11 | 0.27 | 0.05 | 0.21 | 0.08 | −0.86 | −0.49 |
| TLC | −0.07 | 0.02 | 0.12 | 1.02 | 0.04 | −0.35 | −0.21 | 0.45 | 0.18 | −0.06 | −0.07 | −0.03 | −0.31 | 0.73 |
| CFC | 0.69 | −0.37 | −0.33 | 1.52 | 0.00 | −0.69 | −0.75 | 0.25 | 0.32 | −0.06 | 0.21 | 0.06 | −0.74 | 0.11 |
| MC | 0.53 | −0.12 | −0.07 | −0.21 | −0.07 | −0.11 | 0.12 | 0.08 | 0.05 | −0.35 | −0.04 | 0.07 | 0.10 | −0.03 |
| AC | 1.54 | −0.63 | −0.76 | 1.61 | 0.01 | −0.94 | −1.07 | −0.17 | 0.39 | 0.08 | 0.17 | 0.13 | −0.90 | −0.55 |
| TP | 2.02 | −1.25 | −1.16 | 2.68 | −0.06 | −1.20 | −1.95 | −0.40 | 0.56 | −0.64 | 0.62 | 0.04 | −1.67 | −2.41 |
| TA | 0.65 | −0.61 | −0.43 | 1.56 | 0.05 | −0.69 | −0.92 | 0.17 | 0.29 | 0.04 | 0.19 | 0.07 | −0.83 | −0.46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions nor products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adedibu, P.A.; Animasaun, D.A.; Tekutyeva, L.; Son, O.; Abubakar, M.A.; Adamu, U.M.; Balabanova, L.; Yugay, Y.; Shkryl, Y. Variability in Morphological, Biochemical, and Proximate Yield Composition among Predominant Amaranthus hybridus Cultivars in South-West Nigeria. Horticulturae 2024, 10, 461. https://doi.org/10.3390/horticulturae10050461
Adedibu PA, Animasaun DA, Tekutyeva L, Son O, Abubakar MA, Adamu UM, Balabanova L, Yugay Y, Shkryl Y. Variability in Morphological, Biochemical, and Proximate Yield Composition among Predominant Amaranthus hybridus Cultivars in South-West Nigeria. Horticulturae. 2024; 10(5):461. https://doi.org/10.3390/horticulturae10050461
Chicago/Turabian StyleAdedibu, Peter Adeolu, David Adedayo Animasaun, Liudmila Tekutyeva, Oksana Son, Mujahid Ado Abubakar, Ubaida Muhammad Adamu, Larissa Balabanova, Yulia Yugay, and Yury Shkryl. 2024. "Variability in Morphological, Biochemical, and Proximate Yield Composition among Predominant Amaranthus hybridus Cultivars in South-West Nigeria" Horticulturae 10, no. 5: 461. https://doi.org/10.3390/horticulturae10050461
APA StyleAdedibu, P. A., Animasaun, D. A., Tekutyeva, L., Son, O., Abubakar, M. A., Adamu, U. M., Balabanova, L., Yugay, Y., & Shkryl, Y. (2024). Variability in Morphological, Biochemical, and Proximate Yield Composition among Predominant Amaranthus hybridus Cultivars in South-West Nigeria. Horticulturae, 10(5), 461. https://doi.org/10.3390/horticulturae10050461










