Abstract
High-tunnel production is increasing rapidly in the US due to its effectiveness in extending production seasons. Tomato is considered one of the most profitable crops grown in high tunnels. The elevated soil temperature and constant soil moisture in high tunnels lead to the buildup of root-knot nematodes (RKNs). Growing RKN-resistant tomato cultivars or grafting onto RKN-resistant tomato rootstocks is considered effective in managing RKNs. However, all of the RKN-resistant tomato cultivars or rootstocks carry the same resistance gene, Mi-1. This lack of diversity in resistance has led to the emergence of virulent RKN populations breaking tomato Mi-1 resistance. Here, we identified and characterized a virulent population of Meloidogyne incognita from a high tunnel in Indiana. The M. incognita population was confirmed as being able to infect and reproduce on two resistant tomato cultivars, Better Boy and Early Girl, carrying the Mi-1 gene, under a controlled environment at 24 °C. To our knowledge, this is the first report of a virulent M. incognita population breaking Mi-1 resistance in Indiana. Virulent M. incognita populations overcoming Mi-1 resistance were previously reported in California and Georgia in the US. This work emphasizes the importance of regularly monitoring RKN population virulence to sustainably manage the pest.
1. Introduction
Plant-parasitic nematodes pose substantial threats to vegetable production worldwide. Among the most prevalent and destructive are root-knot nematodes (RKNs, Meloidogyne spp.), with a worldwide distribution and host range of more than 3000 plant species [1,2,3]. RKNs can cause yield losses of 15–85% in vegetables, depending on the plant species and population density [1,4]. The infective second-stage juveniles (J2s) of RKNs hatch from eggs and penetrate the roots of host plants. These J2s navigate to the vascular region where the nematodes convert plant cells into large and multinucleated giant cells as their feeding sites to obtain energy and nutrients to further develop into the adult stages and reproduce on host plants [5]. Meanwhile, the hyperplasia of the root cells surrounding the giant cells leads to the formation of galls on the roots, an easily recognized symptom of infection by RKNs [6]. Damaged root systems affect water and nutrient uptake and upward translocation in host plants. The affected plants may have stunted growth, lack vigor and exhibit nutrient deficiency symptoms, leading to crop yield losses [1].
Although over 100 species of RKNs have been identified, the distribution of different species varies due to their adaptation to temperature, environmental conditions and host factors. The three most common and economically important Meloidogyne species, M. incognita, M. javanica and M. arenaria, are typically found in tropical and subtropical regions between 35° S and 35° N latitudes [7]. These species do not have extended survival at temperatures below 10 °C [8]. In contrast, M. hapla is usually found in temperate regions and can survive soil temperatures below 10 °C [9]. A recent summarized distribution of M. incognita in open-field crops in the United States showed the presence of this nematode species in Adams County in Illinois, located at nearly 40° N latitude [10], which is north of the northern limit of the permanent establishment of M. incognita proposed over 40 years ago [7]. The changing climate has and will continue to influence the distribution and damage caused by RKNs in different geographic regions [11,12].
High tunnels have been rapidly adopted and utilized in temperate regions in the United States, particularly because of the Natural Resources Conservation Service (NRCS) cost-share program. High tunnels are covered structures and provide an intermediate level of environmental protection that extends the production season of various specialty crops [13]. The use of high tunnels for extended-season crop production largely increases the air and soil temperatures inside the structures throughout the year. Although the optimal temperature for different RKN species varies, the elevated soil temperature, constant soil moisture and extended cropping seasons in high tunnels facilitate the over-wintering and reproduction of RKNs [14]. A recent soil census of 175 high tunnels in Kentucky found that nearly 55% of soil samples were positive for the presence of RKNs, primarily M. incognita and M. hapla [8]. A survey conducted in high tunnels in Indiana also found that a high percentage of the soil samples tested had RKNs (unpublished data).
In addition to the favorable abiotic factors, RKN-susceptible host crops are grown in high tunnels year-round, which facilitates nematode establishment and population increase. Tomato is considered one of the most economical crops grown in high tunnels in temperate regions. Growing tomatoes in high tunnels extends harvest seasons, increases yield and improves fruit quality compared to growing tomatoes in open fields [15]. Tomatoes, thus, are often repeatedly grown in high tunnels without crop rotation. Tomatoes are excellent hosts for major RKN species. Tomato cultivars with resistance to RKNs are commercially available, but high-tunnel growers are hesitant to use RKN-resistant cultivars if the fruit quality or other fruit characteristics are sacrificed. Therefore, growing grafted tomatoes onto RKN-resistant rootstocks is increasingly becoming popular in managing RKNs and other soilborne pests in high-tunnel tomato production [16].
To our knowledge, all of the commercial tomato cultivars or rootstocks with resistance against RKNs derive their resistance from a single dominant gene, Mi-1, which confers resistance against the three important RKN species: M. incognita, M. javanica and M. arenaria [17,18,19]. It is worth noting that Mi-1-mediated resistance is significantly compromised when the soil temperature is above 28 °C, and J2s of M. incognita, M. javanica or M. arenaria can successfully infect, survive and develop into adult females and lay eggs on the tomato plants carrying the Mi-1 resistance gene [18]. The soil temperature in high tunnels can easily climb above 28 °C in summer. This partially explains why grafted tomatoes with resistant rootstocks occasionally exhibit root galling or have increased RKN populations [20,21,22]. Meanwhile, the lack of diversity in the sources of resistance in tomatoes has led to the emergence of virulent RKN populations that can overcome Mi-1 resistance and reproduce on resistant tomato cultivars or rootstocks regardless of the soil temperatures. Such virulent populations of M. incognita in tomato fields have been reported in California and Georgia in the US [19,23,24,25]. Virulent populations of M. incognita were also found in other parts of the world, including Europe and Asia, under laboratory and field conditions [26,27,28,29]. Although different virulent populations of M. incognita exhibit varied levels of virulence and reproduction on resistant tomato cultivars, the presence of such virulent populations greatly reduces the effectiveness of using Mi-1 tomato resistance to manage the nematode pest. This study reported and characterized, for the first time, a virulent population of M. incognita found in high-tunnel growing tomatoes in Indiana. It shows that monitoring RKN density, species and virulence is critical for establishing management recommendations for high-tunnel growers and guiding tomato or other vegetable breeding efforts.
2. Materials and Methods
2.1. Collection and Propagation of the Root-Knot Nematode Population, RKN-12
Galls caused by RKNs were observed on the roots of the grafted tomatoes scion ‘Red Deuce’ and rootstock ‘DRO141TX’ after harvest. The grafted tomatoes were grown in a high tunnel in Gibson County in Indiana, USA. The RKN population, including J2s and eggs, was collected from the soils where the grafted tomatoes were grown. This RKN population was designated as RKN-12. The extracted J2s and eggs were inoculated on tomato cv. Rutgers (susceptible to RKNs) in a greenhouse (24–28 °C and 16 h light/8 h dark) to enrich and increase the RKN-12 population. Two months after inoculation, the eggs of RKN-12 were extracted from the roots of tomato cv. Rutgers. The root samples were cut into 3–6 cm pieces and vigorously shaken for 4 min in a 10% commercial bleach solution (CloroxTM, Oakland, CA, USA, 8.25% sodium hypochlorite as active ingredient) in a 2 L Nalgene bottle. The eggs were collected by passing through nested sieves of 180 μm, 45 μm and 25 μm, followed by sugar flotation and centrifugation. The eggs were surface-sterilized in 10% commercial bleach solution for 4 min, followed by washing to remove the residual bleach over 25 μm sieves. The collected eggs were used for species identification and tested to investigate whether the RKN-12 population can overcome the tomato Mi-1 resistance gene.
2.2. Species Identification of the RKN-12 Population
DNA was extracted from the RKN-12 eggs isolated on Rutgers tomato roots using the EZ-10 Spin Column DNA Cleanup Miniprep Kit (Bio Basic, Toronto, ON, Canada). In addition, three individual RKN-12 adult females were isolated from the galled roots of the tomato cultivar Better Boy (carrying Mi-1 resistance) inoculated with the RKN-12 eggs under a dissecting microscope. The females were washed in sterile distilled water. The DNA from individual females was extracted using the same procedure as for the eggs. The PCR with species-specific primers for M. incognita [30], M. arenaria and M. javanica [31], M. enterolobii [32] and M. hapla [33] was used to identify the species of the virulent population. Two PCR assays were performed: one using the DNA from the RKN-12 eggs to determine the nematode species, and another from the three individual females to further confirm the RKN species identification. The PCR amplification was performed using a C1000 Touch PCR thermal cycler (Bio-Rad, Hercules, CA, USA) in a total volume of 20 µL. The reaction mixture contained 15.7 µL of nuclease-free water, 1 µL of DNA template, 0.4 µL of 10 mM dNTPs, 2 uL of 10× standard buffer, 0.4 µL of 10 µM of each primer, and 0.1 µL of Taq DNA Polymerase (New England Biolabs, Ipswich, MA, USA). The amplification conditions were as follows: an initial denaturation at 95 °C for 3 min, followed by 40 cycles of 95 °C for 30 s (denaturation), 55 °C for 40 s (annealing) and 72 °C for 40 s (extension), with a final extension cycle of 72 °C for 5 min. The PCR products (10 µL per sample) were run on a 1.5% agarose gel with GelGreen stain (Biotium, Fremont, CA, USA) for 40 min at 120 V. The gels were imaged on a UV gel doc (Bio-Rad, Hercules, CA, USA).
To further confirm the species of RKN-12, DNA sequencing and alignment analysis were conducted using the D2–D3 region of 28S by the PCR employing the primer pairs D2A (5′-ACAAGTACCGTGAGGGAAAGTTG-3′) and D3B (5′-TGCGAAGGAACCAGCTACTA-3′) [34]. The PCR reactions were conducted in a 50 μL reaction volume, comprising the template DNA of an individual adult female (1 μL), 5× PCR buffer (10 μL), 10 mM dNTPs (1 μL), forward and reverse primers (10 µM, 2.5 μL each), Phusion Taq DNA polymerase (10 U/μL, 0.5 μL) and nuclease-free water (32.5 μL). The PCR cycling conditions included an initial denaturation at 98 °C for 30 s, followed by 35 cycles of denaturation at 98 °C for 10 s, annealing at 55 °C for 30 s and extension at 72 °C for 30 s, with a final step at 72 °C for 5 min. Following the PCR completion, samples underwent electrophoretic separation using a 1.5% agarose gel. Subsequently, gel purification of the amplified DNA fragment was conducted using the Gene JET Gel Extraction Kit (Thermo Scientific, Waltham, MA, USA). Each fragment was sequenced bidirectionally using the appropriate PCR primers (MCLAB, San Francisco, CA, USA). Overlapping sequences of the forward and reverse DNA strands were analyzed to generate contigs using Snapgene (v7.1). Each contig quality was manually checked and subsequently subjected to a BLAST search. The sequence was deposited in the GenBank database (PP333903.1).
2.3. Evaluation of Virulence of RKN-12 on Tomatoes Harboring the Resistance Gene Mi-1
The RKN-12 population was assessed for its ability to infect and reproduce on the tomato RKN-resistant cultivars. Two resistant tomato varieties were examined, Early Girl and Better Boy (Burpee Seeds and Plants, Philadelphia, PA, USA), both harboring the Mi-1 resistance gene. Rutgers tomato (Burpee Seeds and Plants, Philadelphia, PA, USA) was used as a susceptible control. Tomato seeds were germinated in plastic cone-tainers (1.5 in. in diameter, 5.5 in. deep) with sand. Four weeks after seeding, 3000 eggs collected from the RKN-12-inoculated Rutgers tomato were inoculated onto each tomato plant in the cone-tainer by making three holes of a 10 cm depth around the tomato stem. The tomato plants were organized in a completely randomized design with 10 replications per cultivar at 24 °C and 16 h light/8 h dark in a plant room. At 35 days and 45 days post-inoculation (dpi), tomato shoots were removed, and the roots were washed to get rid of sand. The root fresh weight of each plant was measured, and the root systems were rated for galling on a 0-to-5 scale, where 0 = no galls, 1 = 1 or 2, 2 = 3 to 10, 3 = 11 to 30, 4 = 31 to 100, and 5 = greater than 100 galls per root system [35]. After the galling evaluation, the roots were cut into 3-to-6 cm pieces, and eggs were extracted, as mentioned previously. The total number of eggs per gram of fresh root was determined. The reproduction factor (Rf), which is the ratio of the total number of nematode eggs extracted at the termination of the experiment to the total number of inoculated eggs at the beginning of the experiment, was calculated. The reproduction factor served as a key indicator for assessing the virulence of the nematode population. The M. incognita avirulent isolate MiVW6 was used as a control.
The eggs from the RKN-12 population were also reared in the resistant tomato variety Better Boy in a greenhouse. Four-week-old Better Boy tomato plants were inoculated with RKN-12 eggs and maintained in the greenhouse for 2–3 months at 24–28 °C. The eggs were collected, as previously mentioned. The eggs collected from the Better Boy tomato cultivar constitute a sub-population of the original population, which was named RKN-12vir. The evaluation of the virulence of this sub-population was conducted using the same method.
2.4. Nematode Penetration and Development Study
Experiments were conducted to study the penetration and development of RKN-12 nematodes in tomato roots. Four-week-old seedlings of the tomato cultivars Rutgers, Early Girl and Better Boy were germinated in yellow cone-tainers, as previously mentioned. Each plant was inoculated with 200 and 500 J2s for penetration and development assays, respectively. The assays were conducted in a plant room at 24 °C in a completely randomized design with 5 replicates for each cultivar. Nematode penetration was carried out by counting the total number of J2s stained by acid fuchsin inside the roots on day 3 post-inoculation (dpi). The development assay of RKN-12 was monitored at 30 dpi and 45 dpi by acid fuchsin and enumerating the various stages of RKN-12 present within the roots. The tomato roots were gently washed with tap water before being subjected to staining with acid fuchsin by the following process: the roots were soaked in 10% commercial bleach for 5 min, followed by a rinse in deionized water (DiH2O). The nematodes were stained by boiling the roots for 1 min in 30 mL of DiH2O water containing 1 mL of acid fuchsin stock solution (0.35% acid fuchsin and 25% acetic acid). After staining, the roots were rinsed with tap water and subsequently de-stained by immersion in acidified glycerol overnight at 4 °C. Using needle and forceps, different developmental stages of the RKN-12 nematodes within the roots were observed and counted under a stereo microscope (Leica Microsystems, Wetzlar, Germany). Nematodes were categorized as infective J2 (vermiform with no swelling), parasitic J2 (vermiform with swelling), J3–J4 (sausage-shaped), young female (pear-shaped) and adult female (with egg mass) [7]. Adult females were enumerated before staining the roots.
2.5. Statistical Analysis
Statistical analysis was performed on the means of the response variables in the RKN-12 virulence experiment, developmental study and J2 penetration experiments. One-way ANOVA was used, followed by Tukey’s HSD for mean comparison (p < 0.05) to identify significant differences among the mean values. The analysis was conducted using GraphPad (version 10.0.2).
3. Results
3.1. Species Identification of the RKN-12 Population
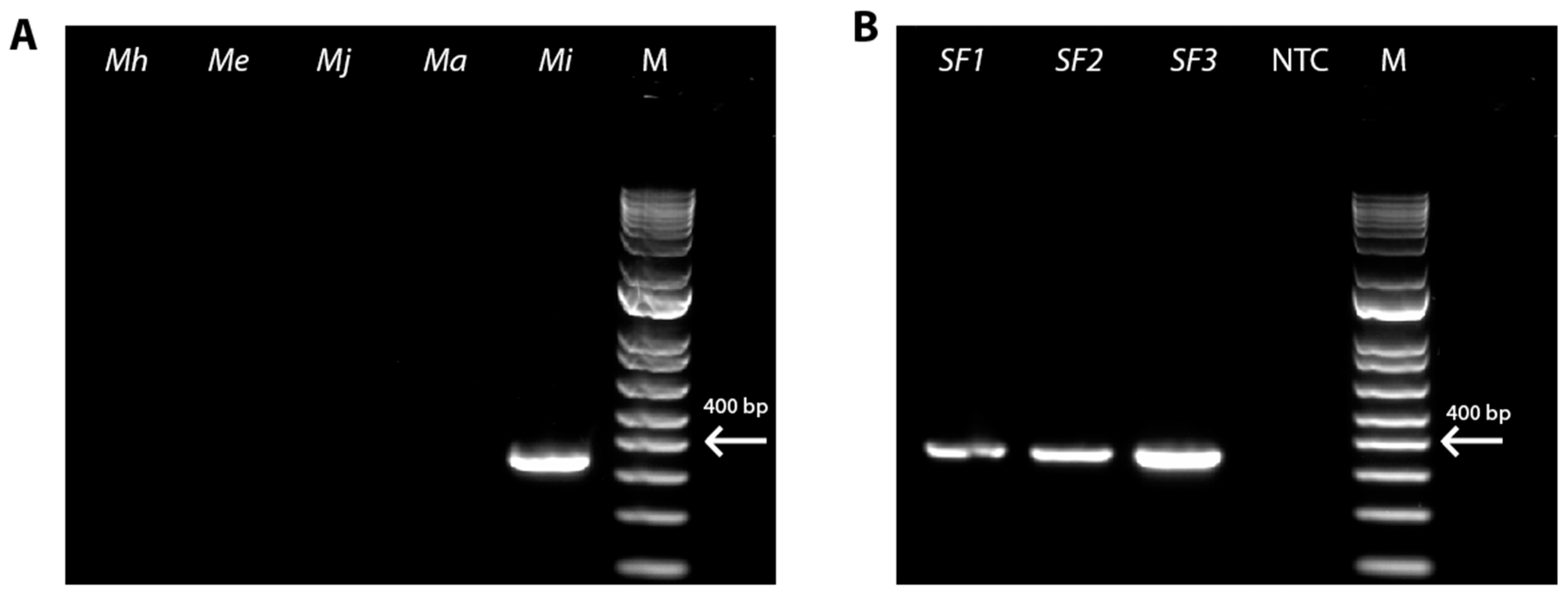
To identify the root-knot nematode species present in the RKN-12 population, we first conducted the PCR on the genomic DNA extracted from the eggs of the RKN-12 population isolated from the galled roots of the Rutgers tomato (susceptible to RKNs) using the species-specific primers of four common RKN species, M. incognita [30], M. javanica, M. arenaria [31] and M. hapla [33], as well as an emerging RKN species M. enterolobii [32]. The PCR result confirms the identity of the RKN-12 population as M. incognita (Figure 1A). Furthermore, we conducted the PCR using the M. incognita species-specific primers on the genomic DNA of individual adult females isolated on the roots of the resistant tomato cultivar Better Boy carrying the Mi-1 gene. The DNA fragment also confirmed that the species of the RKN-12 population is M. incognita (Figure 1B). In addition, the fragment from the D2–D3 region of 28S rDNA amplified from RKN-12 (GenBank: PP333903.1) showed 100% identity (651/651) to M. incognita sequences (MF177877, MT197289, MT159682 and MN847618), further confirming that the RKN-12 population is M. incognita.
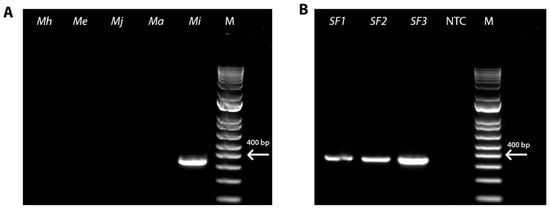
Figure 1.
PCR identification of the RKN-12 population as Meloidogyne incognita. (A) PCR amplification using species-specific primers of different RKN species on egg DNA sample. M, 100-bp DNA ladder; Mi, M. incognita; Ma, M. arenaria; Mj, M. javanica; Me, M. enterolobii; Mh, M. hapla. (B) PCR amplification using M. incognita-specific SCAR primers on single adult female DNA sample. M, 100-bp DNA ladder; NTC, no template control; SF, single female.
3.2. RKN-12 Population Can Overcome the Tomato Mi-1 Resistance Gene
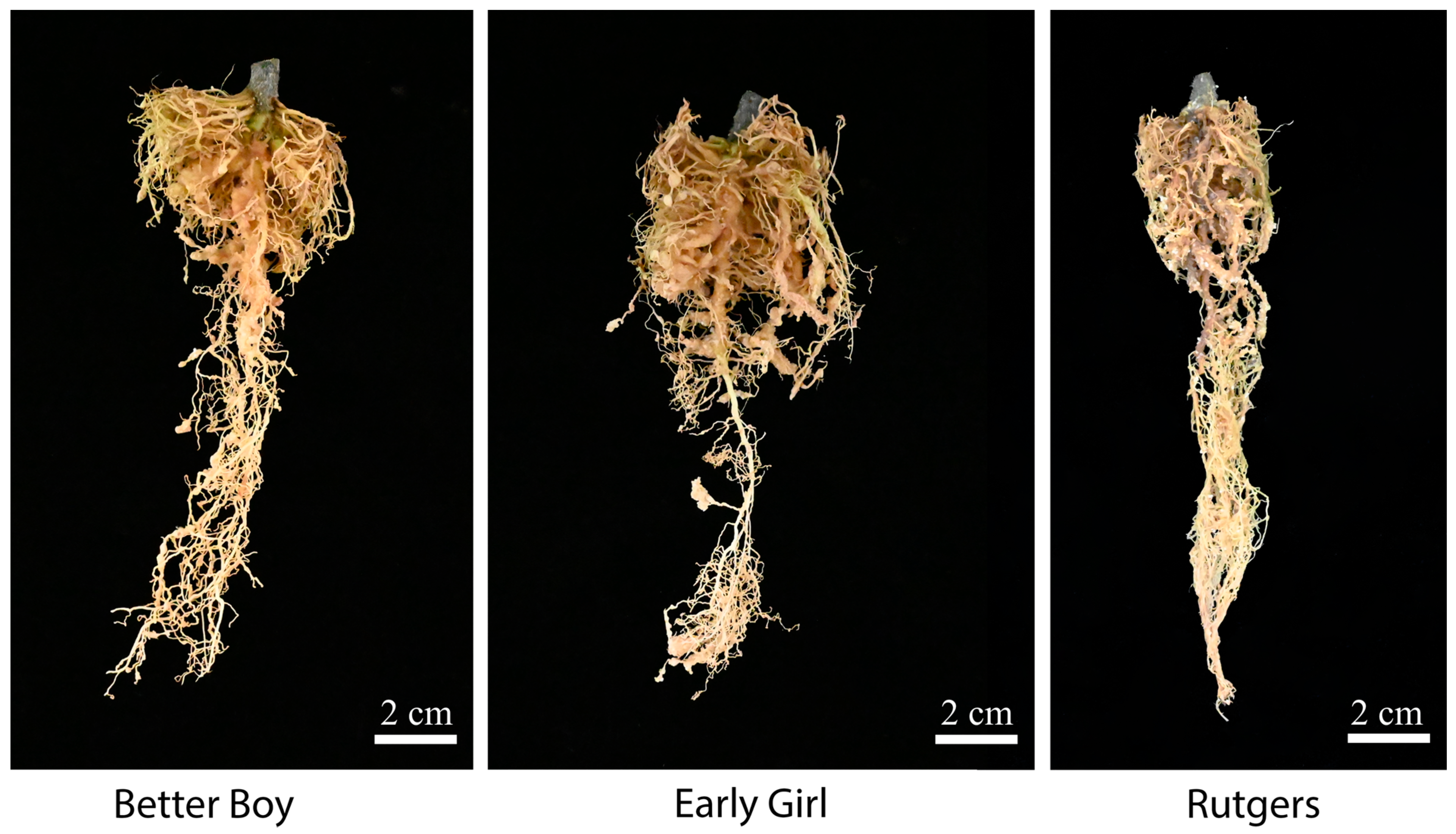
The RKN-12 population was originally collected from the soil in a high tunnel where grafted tomatoes exhibited galls on the roots. As all RKN-resistant rootstocks and cultivars carry Mi-1 resistance, we suspected the RKN-12 population might be able to overcome Mi-1 resistance in tomato. To confirm and study the virulence of the RKN-12 population, two resistant tomato cultivars, Early Girl and Better Boy, carrying the Mi-1 resistance gene were used for a nematode infection assay. As shown in Figure 2, the roots of the two resistant cultivars and the susceptible cultivar inoculated with RKN-12 eggs show obvious galling, an indication of the successful infection of plants by RKNs. The root gall indexes of Early Girl and Better Boy tomatoes inoculated with the RKN-12 population at 35 dpi and 45 dpi were both rated as 4 compared to the root gall index of 5 of the susceptible cultivar Rutgers (Table 1). As for the same groups of tomato cultivars inoculated with the avirulent M. incognita VW6 isolate (MiVW6), the root gall indexes of the two resistant cultivars were rated as 0 (no galls) at 35 dpi, which were significantly lower than the root gall index of 5 of the susceptible cultivar Rutgers at 35 dpi. The results indicate that the RKN-12 population is virulent and able to infect resistant tomato cultivars carrying the Mi-1 resistance gene.
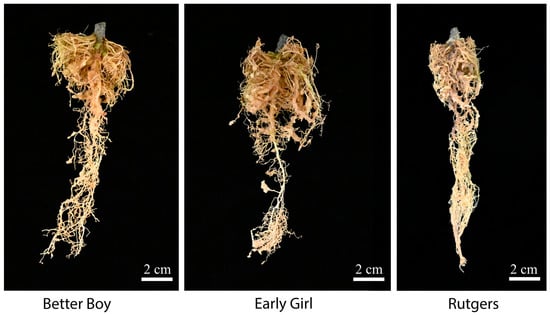
Figure 2.
Galled roots on three tomato cultivars after inoculation with RKN-12: Rutgers (susceptible), Early Girl (resistant) and Better Boy (resistant). Each plant was inoculated with 5000 eggs and maintained in a growth chamber at 24 °C for 3 months.

Table 1.
Virulence of the RKN-12 population on tomato cultivars (Early Girl and Better Boy) carrying the Mi-1 resistance gene.
In order to confirm that the RKN-12 population could successfully reproduce on the resistant tomato cultivars, we evaluated the number of eggs per gram of roots and the reproduction factor (Rf) of the population. For the avirulent isolate MiVW6, at 35 and 45 dpi, the average number of eggs per gram of roots on the susceptible cultivar Rutgers was 39,615 and 68,506, respectively. The reproduction factor on Rutgers reached approximately 50 and 110 at 35 and 45 dpi, respectively (Table 1). In contrast, the number of eggs per gram of roots and Rf of the avirulent isolate MiVW6 on the resistant cultivars were both 0 at 35 dpi (Table 1). The Rf of the avirulent isolate MiVW6 on the resistant cultivars at 45 dpi was not further investigated because the Rf was 0 at 35 dpi, and we usually observe no galls on resistant cultivars at 45 dpi. The results show that the avirulent isolate MiVW6 was not able to reproduce on the two resistant cultivars. At 35 dpi, the RKN-12 population produced an average of 479 and 410 eggs per gram of root on the resistant cultivars Early Girl and Better Boy, respectively. The Rf of the RKN-12 population on Early Girl and Better Boy at 35 dpi was 0.67 and 0.65, respectively. At 45 dpi, the average number of eggs per gram of root of the RKN-12 population increased to 2258 and 1753 on the two resistant cultivars Early Girl and Better Boy, respectively. The Rf of the RKN-12 population on Early Girl and Better Boy tomatoes was 3.28 and 2.67, respectively (Table 1). The RKN-12 population produced a significantly higher number of eggs per gram of root (~57,283), leading to a higher Rf (~75) on the susceptible cultivar Rutgers at 45 dpi compared to 35 dpi. (Table 1). The results indicate that the RKN-12 population is virulent and can successfully reproduce on resistant tomato cultivars carrying the Mi-1 resistance gene, although the population may develop and reproduce slower than the avirulent isolate MiVW6 on the susceptible cultivar Rutgers.
3.3. RKN-12 Penetration and Development in Tomato Roots
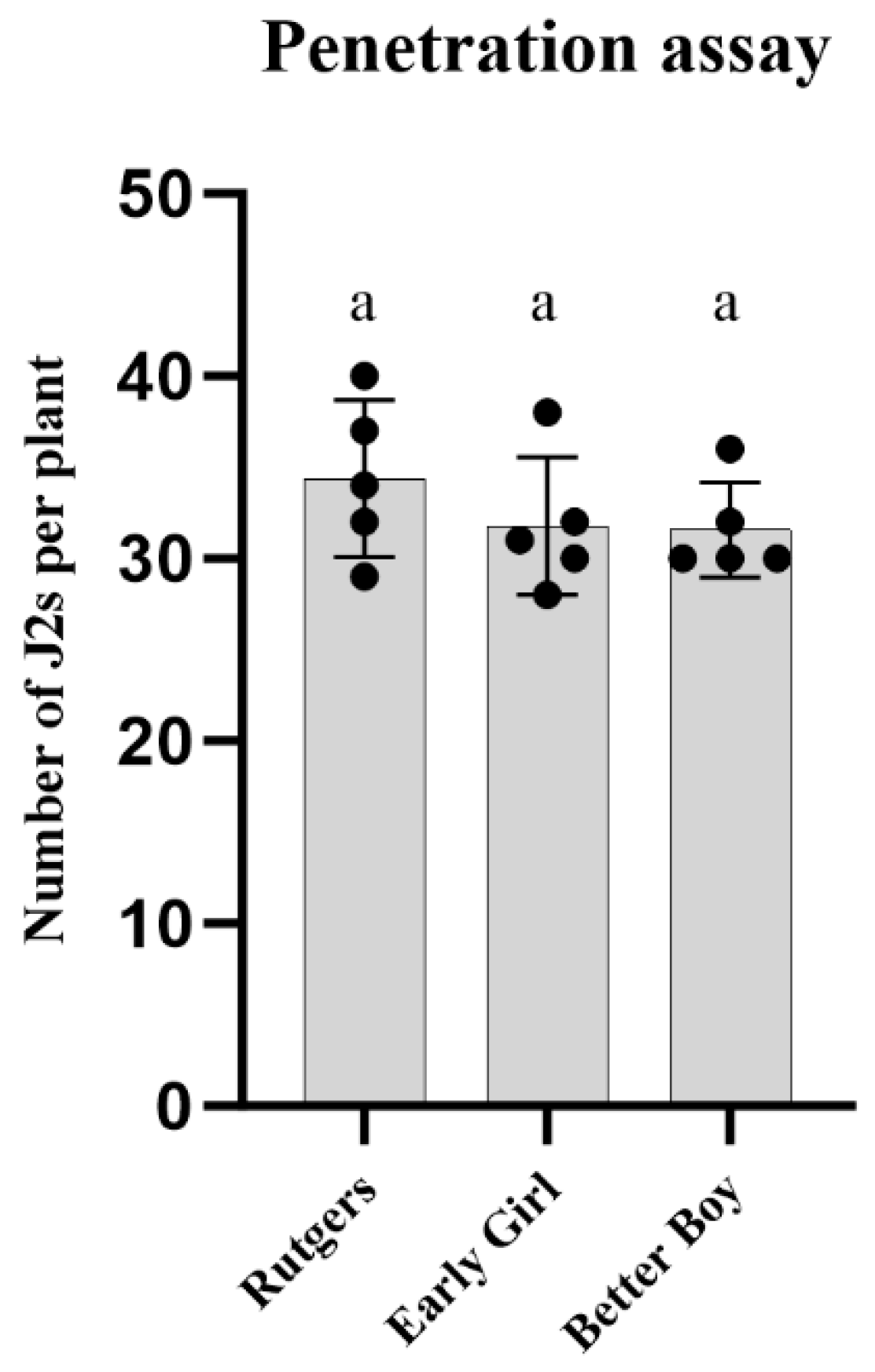
To further study the virulence of the RKN-12 population, we first conducted a root penetration assay to evaluate whether the J2s of RKN-12 invaded the roots of susceptible and resistant tomato cultivars differently. As shown in Figure 3, no difference in the numbers of J2s of RKN-12 was found present in the roots of Rutgers, Early Girl and Better Boy at 3 dpi. The result indicates that the differences in RKN-12 Rf on susceptible and resistant cultivars were not correlated to or caused by the penetration rate at the early stage of infection.
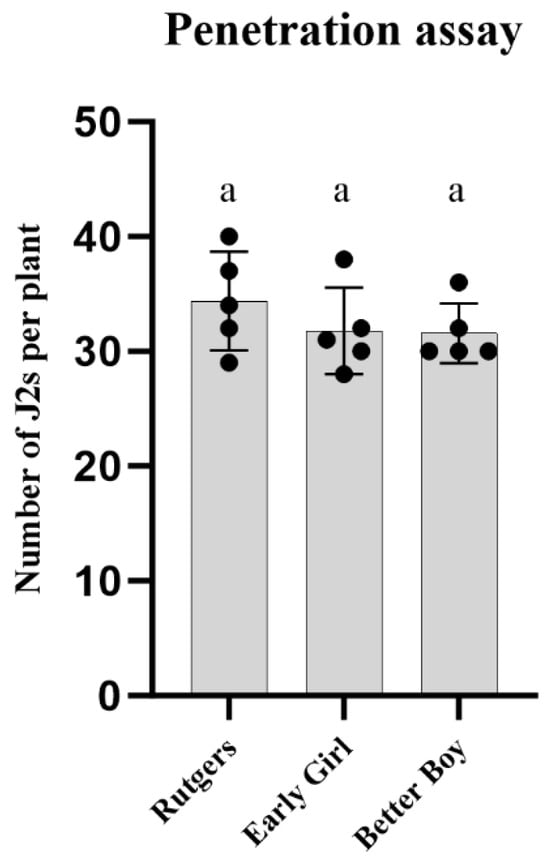
Figure 3.
J2 penetration assay. Number of RKN-12 J2s inside the roots at 3 dpi in different tomato cultivars. Each plant was inoculated with 200 J2s. Bars with same letter are not significantly different (p > 0.05) based on Tukey’s test.
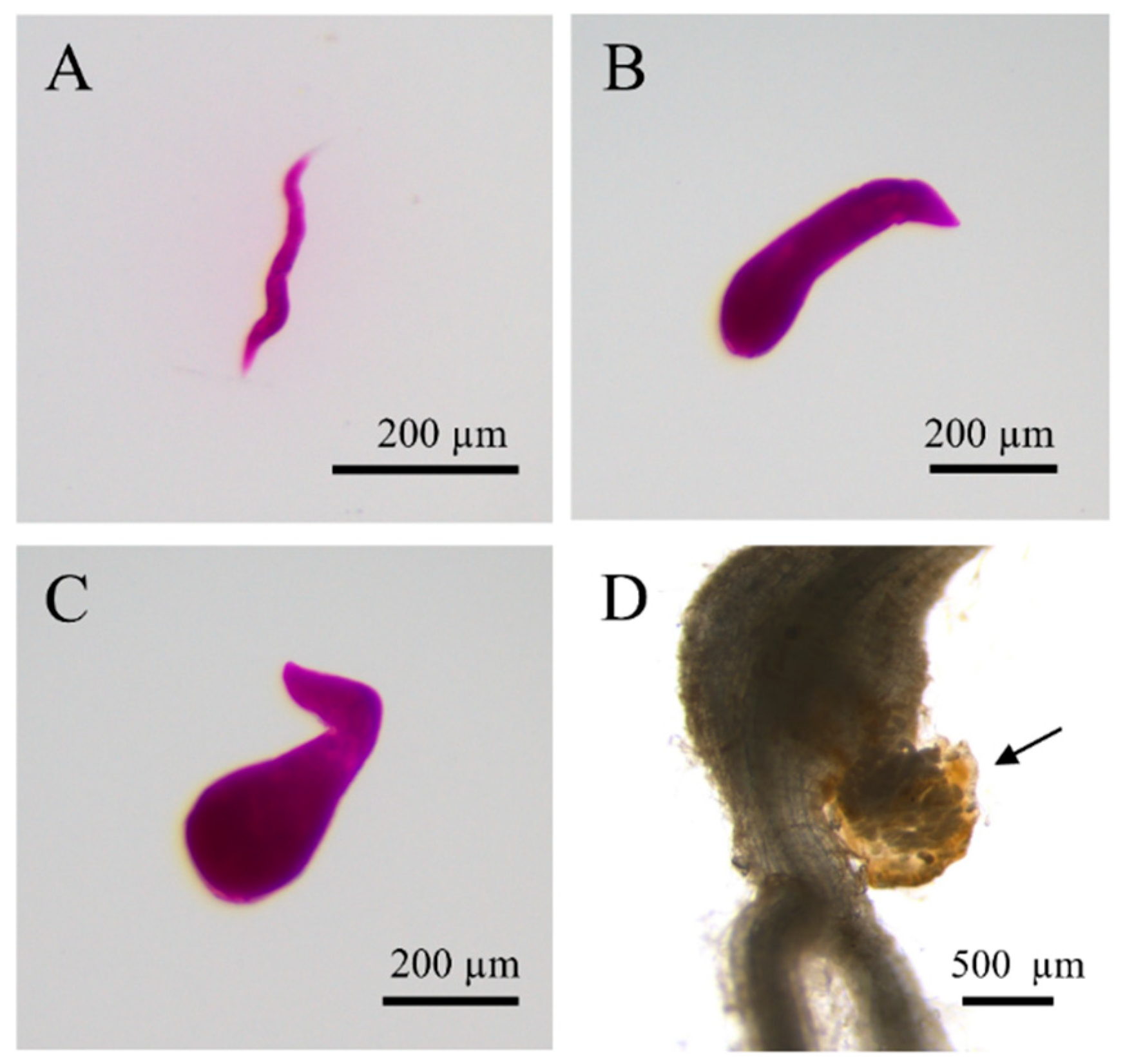
An Rf of RKN-12 greater than 1 on Early Girl and Better Boy resistant cultivars at 45 dpi indicates the successful development and reproduction of the population on the resistant cultivars (Table 1). Therefore, we further studied the development of RKN-12 on resistant and susceptible tomato cultivars at late infection stages. At 30 dpi with J2s of RKN-12, young females were found both on Early Girl and Better Boy, while adult females (egg-laying) were only found on Early Girl. More young females and adult females were found on both Early Girl and Better Boy at 45 dpi (Table 2). The presence of egg-laying adult females on the two resistant cultivars at late infection stages indicates that RKN-12 can overcome Mi-1-mediated resistance and successfully reproduce on resistant tomatoes (Figure 4).

Table 2.
Developmental stages of RKN-12 on tomato cultivars (Early Girl and Better Boy) carrying the Mi-1 resistance gene.
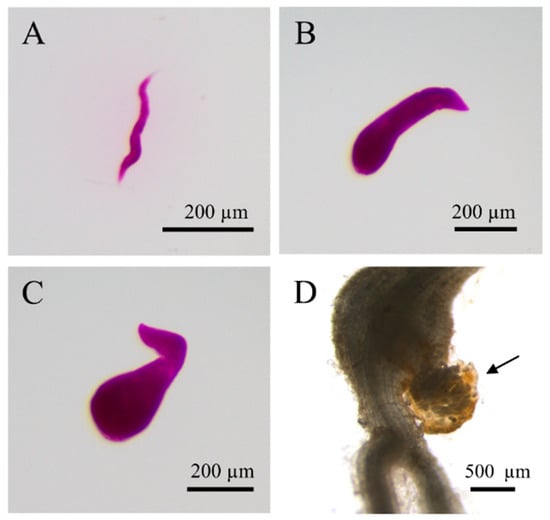
Figure 4.
Developmental stages of RKN-12 on the resistant tomato cultivar Better Boy. The virulent population was able to complete its lifecycle on the resistant cultivar. (A–C) Acid fuchsin staining of RKN-12 on Better Boy at 30 dpi. The nematodes were extracted and imaged. (A) Parasitic J2, (B) J3–J4, (C) young female, (D) unstained root with RKN-12 adult female with egg mass (arrow marked) at 45 dpi.
Interestingly, the number of adult females on the susceptible cultivar Rutgers was 11.7 and 21 times more than on the resistant cultivars Early Girl and Better Boy at 45 dpi, respectively (Table 2), indicating that more individuals of the RKN-12 population were able to develop and reproduce on the susceptible cultivar Rutgers. The total number of the RKN-12 development stages of J3–J4, young and adult females, on Rutgers was 232.6, higher than the numbers on Early Girl (53.8) and Better Boy (50) at 45 dpi. Infective J2s (vermiform with no swelling) and parasitic J2s (vermiform with swelling) found at 45 dpi in the roots of Rutgers were produced from the infection by the J2s hatched from the eggs laid by adult females on the roots that were the second generation of the infection and thus not counted in the analysis of the first generation of infection (Table 2). The results of the development stages on susceptible and resistant tomatoes indicate that the RKN-12 population may be composed of avirulent and virulent sub-populations of M. incognita, with individuals from the virulent sub-population but not the avirulent sub-population being able to break Mi-1 resistance, survive, develop and reproduce on resistant tomato cultivars, although individuals of both the avirulent and virulent sub-populations of RKN-12 can successfully infect, develop and reproduce on the susceptible cultivar Rutgers.
3.4. Enrichment and Study of the Virulent Sub-Population of RKN-12
Based on the data presented above, we speculated that the RKN-12 population collected from soils consisted of a blend of M. incognita isolates. A portion of the population is capable of overcoming Mi-1 resistance, while the remainder is avirulent and unable to break the resistance. In order to enrich the virulent portion of the RKN-12 population that can overcome resistance, we reared the RKN-12 population on the resistant cultivar Better Boy in a greenhouse, and the eggs harvested were presumably the virulent sub-population of the original RKN-12 population. We designated this virulent sub-population as RKN-12vir. We conducted the infection assay using the virulent sub-population RKN-12vir on susceptible and resistant tomato cultivars. At 45 dpi, the gall indexes of all three tomato cultivars reached 5. The Rf of RKN-12vir at 45 dpi on the resistant EG and BB was 14.03 and 13.59, respectively, and the Rf was 57.37 on the susceptible cultivar Rutgers (Table 3). The Rf of RKN-12vir at 45 dpi was higher than that of RKN-12 on the two resistant cultivars (Table 1).

Table 3.
Virulence of RKN-12vir sub-population on tomato cultivars (Early Girl and Better Boy) carrying the Mi-1 resistance gene at 45 dpi.
4. Discussion
High tunnels have quickly gained popularity and have been adopted in temperate regions in the US due to the benefits of extending specialty crop production seasons and thus improving yields and profits. However, the production system has its challenges in disease and pest management [36]. Increased soil temperature and a lack of crop rotation favor the buildup of RKNs, which can limit the profitability and sustainability of crop production in high tunnels. Tomatoes are the most popular crops grown in high tunnels, and they are highly susceptible to RKNs. Growing resistant cultivars has been frequently employed to mitigate yield losses caused by RKNs in the production of processing tomatoes [19]. The resistance in all of the commercially available RKN-resistant tomato cultivars is derived from a single dominant Mi-1 gene, which has been used for more than 70 years. The lack of diversity in resistance sources has led to the occurrence of RKN populations that can reproduce on resistant tomato cultivars [18]. A shift in RKN virulence can make Mi-1 resistance less effective in controlling RKN populations in tomato fields. Tomatoes in high tunnels are grown for fresh markets. Therefore, grafting tomatoes on RKN-resistant rootstock has been a strategy adopted by growers to manage the nematode pest and improve yields [21]. Similarly, all the commercially available RKN-resistant tomato rootstocks are based on the Mi-1 resistance gene. Consistently relying on the same source of resistance in grafted tomatoes can expedite the emergence of virulent RKN populations.
Here, we report the identification and characterization of a virulent population of M. incognita, capable of overcoming Mi-1 resistance, from tomatoes grafted on resistant rootstock in a high tunnel in Indiana. This makes Indiana the third state in the United States to report Mi-1-resistance-breaking M. incognita populations, following California [19,25] and Georgia [23]. Growing resistant tomato cultivars or rootstocks may partially contribute to the occurrence of Mi-1-resistance-breaking populations of M. incognita, although virulent populations were also found in tomato fields with no history of growing resistant cultivars [23]. In the Georgia survey, four virulent M. incognita populations were identified from vegetable fields with no known history of growing resistant tomato cultivars [23]. The initial report on California in 1996 reported two virulent M. incognita populations, one from a field growing resistant tomatoes and the other from a field with no history of resistant tomato crops [25]. A recent study found that sixteen Meloidogyne populations (fifteen identified as M. incognita and one as M. javanica) from tomato fields in California growing resistant cultivars were all virulent and capable of reproducing on resistant tomatoes in greenhouse assays [19]. In addition, a virulent M. incognita population, capable of overcoming pepper Me3 resistance, arose from a single year of selection in a field in France [28]. Consistent with similar scenarios, the high tunnel where the RKN-12 virulent population was identified had grown tomatoes grafted on RKN-resistant rootstocks for three years when the virulent population was identified. Therefore, the frequent monitoring of the presence, population density and virulence of RKNs is recommended when growing RKN-resistant tomato cultivars or rootstocks with Mi-1 resistance in order to sustainably manage the pest.
The Rf of RKN-12 on the two resistant tomato cultivars was greater than 1 at 45 dpi, indicating that the M. incognita population can overcome Mi-1 resistance and successfully reproduce on the resistant tomatoes. The Rf on the susceptible tomato cultivar was approximately 75. It is similar to three of the virulent M. incognita populations reported in Georgia and laboratory-selected virulent populations, which showed higher Rfs on susceptible tomato cultivars than on resistant cultivars [23,28]. However, the Ma6 population from Georgia and the virulent M. incognita field populations of previous studies show comparable reproduction rates between susceptible and resistant tomato cultivars [23,27,28]. The reproduction variability of the same population on susceptible and resistant tomato cultivars may be attributed to the length of time under resistance selection and the heterozygosity of the population. The penetration rate of RKN-12 was equal between susceptible and resistant tomato cultivars, indicating similar behaviors at the early infection stage. The variation in Rf of RKN-12 between susceptible and resistant tomato cultivars was caused by the different number or percent of the RKN-12 inoculums that were able to develop into young and adult females at later stages (30 dpi and 45 dpi). Approximately 8.2% and 6% of the initial inoculum of RKN-12 developed into young and adult females on the two resistant cultivars, Early Girl and Better Boy, respectively, in contrast to 46% on the susceptible cultivar Rutgers at 45 dpi (Table 2). A significantly lower percentage of RKN-12 developed into female stages on resistant cultivars than on the susceptible cultivar, indicating that the population may comprise both avirulent and virulent sub-populations. The heterozygosity of the RKN-12 population may be attributed to the relatively short period of growing grafted tomatoes with resistant rootstocks in the high tunnel. Mi-1-mediated resistance in tomato is hypothesized to operate by eliciting a localized hypersensitive response (HR) in roots when the RKN J2s start to convert plant cells to giant cells as their feeding sites and feed on plant cells. The Mi-1.2 gene encodes a nucleotide-binding leucine-rich repeat (NB-LRR) protein, a class of proteins well-known for their role as resistance (R) genes in plants [37,38]. Furthermore, heat shock protein 90 (HSP90) and a suppressor of the G2 allele of skp1 (SGT1) are critical chaperones that likely stabilize the Mi-1.2 protein, facilitating nematode recognition and downstream signaling events [39]. We speculate that the virulent population RKN-12 can break Mi-1 resistance probably due to its ability to suppress or survive the HR.
To study the reproductive potential of the virulent sub-population in the RKN-12 population, the RKN-12 population was inoculated on the resistant tomato cultivar Better Boy under greenhouse conditions for 3 months to enrich the virulent sub-population referred to as RKN-12vir. The Rf of RKN-12vir at 45 dpi was 3.5 and 6.2 times higher than that of the RKN-12 population on the two resistant cultivars Early Girl and Betty Boy, respectively. The results indicate RKN-12vir would potentially increase if grafted tomatoes were continuously grown in the high tunnel, posing a greater risk to the sustainability of the cropping system. Our findings align with a previous report on progressive increases in virulent RKN populations when reared on resistant tomato cultivars over successive generations [40]. However, the virulence of M. incognita populations has been shown to be stable, with no loss of susceptibility observed, even after more than 20 generations [41].
Our findings highlight the importance of the regular monitoring of RKN virulence in addition to population density when resistant tomato cultivars or rootstocks are used to manage the pest in high tunnels and open fields. Developing diverse and durable resistance in tomatoes against emerging virulent populations of RKNs is needed to control the pest effectively and more sustainably. Although all commercial resistant tomatoes are derived from a single gene, Mi-1, the RKN virulence selected was highly specific to the crop or resistance gene. Crop rotation with other available resistant vegetables, such as RKN-resistant pepper cultivars, is highly recommended to control or reduce the emergence of virulent RKN populations in high tunnels and other vegetable production systems [19,42].
5. Conclusions
Our research identifies and characterizes a virulent population of M. incognita capable of overcoming Mi-1 resistance in tomatoes grown in high tunnels in Indiana. The occurrence of such resistance-breaking populations raises concerns about the sustainability of crop production in high tunnels, particularly given the widespread use of Mi-1-based resistance in commercial tomato cultivars and rootstocks. Our study underscores the need for regular monitoring of the RKN populations in high tunnels, focusing not only on density and species but also on virulence. To address this challenge, efforts need to be directed toward developing diverse and durable resistance in tomatoes, coupled with crop rotation strategies involving RKN-resistant vegetables, such as resistant pepper cultivars. Overall, our findings emphasize the importance of proactive management practices to mitigate the impact of evolving virulent root-knot nematode populations in vegetable production.
Author Contributions
Conceptualization, L.Z.; funding acquisition, L.Z. and W.G.; methodology, L.Z., V.K. and W.G.; investigation, V.K.; project administration, L.Z. and W.G.; resources, L.Z. and W.G.; supervision, L.Z. and W.G.; writing—original draft preparation, L.Z. and V.K.; writing—review and editing, L.Z., V.K. and W.G. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Specialty Crop Research Initiative (SCRI) [grant no. 2021-51181-35904/project accession no. 1027452] from the USDA National Institute of Food and Agriculture.
Data Availability Statement
The original data presented in the study are included in the article. Further inquiries can be directed to the corresponding authors.
Acknowledgments
We thank Marilyn Vargas at Purdue University for helping with growing tomato plants and maintaining nematode cultures in the greenhouse.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Collange, B.; Navarrete, M.; Peyre, G.; Mateille, T.; Tchamitchian, M. Root-knot nematode (Meloidogyne) management in vegetable crop production: The challenge of an agronomic system analysis. Crop Prot. 2011, 30, 1251–1262. [Google Scholar] [CrossRef]
- Koenning, S.R.; Overstreet, C.; Noling, J.W.; Donald, P.A.; Becker, J.O.; Fortnum, B.A. Survey of crop losses in response to phytoparasitic nematodes in the United States for 1994. J. Nematol. 1999, 31, 587–618. [Google Scholar] [PubMed]
- Jones, J.T.; Haegeman, A.; Danchin, E.G.J.; Gaur, H.S.; Helder, J.; Jones, M.G.K.; Kikuchi, T.; Manzanilla-López, R.; Palomares-Rius, J.E.; Wesemael, W.M.L.; et al. Top 10 plant-parasitic nematodes in molecular plant pathology. Mol. Plant Pathol. 2013, 14, 946–961. [Google Scholar] [CrossRef] [PubMed]
- Fullana, A.M.; Expósito, A.; Escudero, N.; Cunquero, M.; Loza-Alvarez, P.; Giné, A.; Sorribas, F.J. Crop rotation with Meloidogyne-resistant germplasm is useful to manage and revert the (a)virulent populations of Mi1.2 gene and reduce yield losses. Front. Plant Sci. 2023, 14, 1133095. [Google Scholar] [CrossRef] [PubMed]
- Favery, B.; Quentin, M.; Jaubert-Possamai, S.; Abad, P. Gall-forming root-knot nematodes hijack key plant cellular functions to induce multinucleate and hypertrophied feeding cells. J. Insect Physiol. 2016, 84, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Mitkowski, N.A.; Abawi, G.S. Root-knot nematodes. Plant Health Instr. 2003, 3. [Google Scholar] [CrossRef]
- Taylor, A.L.; Sasser, J.N. Biology, identification and control of root-knot nematodes (Meloidogyne species); International Nematology Project; North Carolina State University: Raleigh, NC, USA, 1978. [Google Scholar]
- Bajek, V.; Munir, M.; Rudolph, R.E. Soil Census of Kentucky High Tunnels Reveals Statewide Distribution of Two Meloidogyne Species. Plant Health Prog. 2023, 24, 508–515. [Google Scholar] [CrossRef]
- East, K.E.; Zasada, I.A.; Schreiner, R.P.; Moyer, M.M. Developmental Dynamics of Meloidogyne hapla in Washington Wine Grapes. Plant Dis. 2019, 103, 966–971. [Google Scholar] [CrossRef] [PubMed]
- Faske, T.R.; Mueller, J.D.; Becker, J.O.; Bernard, E.; Bradley, C.A.; Bond, J.P.; Desaeger, J.; Eisenback, J.D.; Grabau, Z.J.; Hu, J.; et al. Summarized distribution of the southern root-knot nematode, Meloidogyne incognita, in field crops in the United States. Plant Health Prog. 2023, 24, 522–524. [Google Scholar] [CrossRef]
- Khanal, C.; Land, J. Study on two nematode species suggests climate change will inflict greater crop damage. Sci. Rep. 2023, 13, 14185. [Google Scholar] [CrossRef]
- Dutta, T.K.; Phani, V. The pervasive impact of global climate change on plant-nematode interaction continuum. Front. Plant Sci. 2023, 14, 1143889. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, K.; Ernst, M. High tunnel overview. University of Kentucky Center for Crop Diversification Factsheet CCD-SP-2. 2021. Available online: https://www.uky.edu/ccd/sites/www.uky.edu.ccd/files/hightunneloverview.pdf (accessed on 1 January 2021).
- Giné, A.; López-Gómez, M.; Vela, M.D.; Ornat, C.; Talavera, M.; Verdejo-Lucas, S.; Sorribas, F.J. Thermal requirements and population dynamics of root-knot nematodes on cucumber and yield losses under protected cultivation. Plant Pathol. 2014, 63, 1446–1453. [Google Scholar] [CrossRef]
- O’Connell, S.; Rivard, C.; Peet, M.M.; Harlow, C.; Louws, F. High Tunnel and Field Production of Organic Heirloom Tomatoes: Yield, Fruit Quality, Disease, and Microclimate. HortScience 2012, 47, 1283–1290. [Google Scholar] [CrossRef]
- Kubota, C.; McClure, M.A.; Kokalis-Burelle, N.; Bausher, M.G.; Rosskopf, E.N. Vegetable grafting: History, use, and current technology status in North America. HortScience 2008, 43, 1664–1669. [Google Scholar] [CrossRef]
- Roberts, P.A. Current status of the availability, development, and use of host plant resistance to nematodes. J. Nematol. 1992, 24, 213–227. [Google Scholar] [PubMed]
- Williamson, V.M. Root-knot nematode resistance genes in tomato and their potential for future use. Annu. Rev. Phytopathol. 1998, 36, 277–293. [Google Scholar] [CrossRef] [PubMed]
- Ploeg, A.T.; Stoddard, C.S.; Turini, T.A.; Nunez, J.J.; Miyao, E.M.; Subbotin, S.A. Tomato -gene Resistance-Breaking Populations of Show Variable Reproduction on Susceptible and Resistant Crop Cultivars. J. Nematol. 2023, 55, 20230043. [Google Scholar] [CrossRef] [PubMed]
- Barrett, C.E.; Zhao, X.; McSorley, R. Grafting for Root-knot Nematode Control and Yield Improvement in Organic Heirloom Tomato Production. HortScience 2012, 47, 614–620. [Google Scholar] [CrossRef]
- Bajek, V.; Rudolph, R.E. Managing Southern Root-knot Nematode in Kentucky High Tunnels Using Grafted Tomato. HortScience 2023, 58, 704–713. [Google Scholar] [CrossRef]
- Rivard, C.L.; O’Connell, S.; Peet, M.M.; Louws, F.J. Grafting Tomato with Interspecific Rootstock to Manage Diseases Caused by Sclerotium rolfsii and Southern Root-Knot Nematode. Plant Dis. 2010, 94, 1015–1021. [Google Scholar] [CrossRef]
- Hajihassani, A.; Marquez, J.; Woldemeskel, M.; Hamidi, N. Identification of Four Populations of Meloidogyne incognita in Georgia, United States, Capable of Parasitizing Tomato-Bearing Mi-1.2 Gene. Plant Dis. 2022, 106, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; McGiffen, M.; Kaloshian, I. Reproduction of Mi-Virulent Meloidogyne incognita Isolates on Lycopersicon spp. J. Nematol. 2004, 36, 69–75. [Google Scholar]
- Kaloshian, I.; Williamson, V.M.; Miyao, G.; Lawn, D.A.; Westerdahl, B.B. Resistance-breaking” nematodes identified in California tomatoes. Calif. Agric. 1996, 50, 18–19. [Google Scholar] [CrossRef]
- Guan, T.; Shen, J.; Fa, Y.; Su, Y.; Wang, X.; Li, H. Resistance-breaking population of Meloidogyne incognita utilizes plant peroxidase to scavenge reactive oxygen species, thereby promoting parasitism on tomato carrying Mi-1 gene. Biochem. Biophys. Res. Commun. 2017, 482, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Castagnone-Sereno, P.; Bongiovanni, M.; Dalmasso, A. Reproduction of Virulent Isolates of Meloidogyne incognita on Susceptible and Mi-resistant Tomato. J. Nematol. 1994, 26, 324–328. [Google Scholar]
- Djian-Caporalino, C.; Molinari, S.; Palloix, A.; Ciancio, A.; Fazari, A.; Marteu, N.; Ris, N.; Castagnone-Sereno, P. The reproductive potential of the root-knot nematode Meloidogyne incognita is affected by selection for virulence against major resistance genes from tomato and pepper. Eur. J. Plant Pathol. 2011, 131, 431–440. [Google Scholar] [CrossRef]
- Bucki, P.; Paran, I.; Ozalvo, R.; Iberkleid, I.; Ganot, L.; Braun Miyara, S. Pathogenic Variability of Meloidogyne incognita Populations Occurring in Pepper-Production Greenhouses in Israel Toward Me1, Me3 and N Pepper Resistance Genes. Plant Dis. 2017, 101, 1391–1401. [Google Scholar] [CrossRef]
- Randig, O.; Bongiovanni, M.; Carneiro, R.M.D.G.; Castagnone-Sereno, P. Genetic diversity of root-knot nematodes from Brazil and development of SCAR markers specific for the coffee-damaging species. Genome 2002, 45, 862–870. [Google Scholar]
- Zijlstra, C.; Donkers-Venne, D.T.H.M.; Fargette, M. Identification of Meloidogyne incognita, M. javanica and M. arenaria using sequence characterised amplified region (SCAR) based PCR assays. Nematology 2000, 2, 847–853. [Google Scholar] [CrossRef]
- Ye, W.; Koenning, S.R.; Zeng, Y.; Zhuo, K.; Liao, J. Molecular Characterization of an Emerging Root-Knot Nematode Meloidogyne enterolobii in North Carolina, USA. Plant Dis. 2021, 105, 819–831. [Google Scholar] [CrossRef]
- Wishart, J.; Phillips, M.S.; Blok, V.C. Ribosomal Intergenic Spacer: A Polymerase Chain Reaction Diagnostic for Meloidogyne chitwoodi, M. fallax, and M. hapla. Phytopathology 2002, 92, 884–892. [Google Scholar] [CrossRef] [PubMed]
- Ley, P.D.; Felix, M.-A.; Frisse, L.; Nadler, S.; Sternberg, P.; Thomas, W.K. Molecular and morphological characterisation of two reproductively isolated species with mirror-image anatomy (Nematoda: Cephalobidae). Nematology 1999, 1, 591–612. [Google Scholar] [CrossRef]
- Quesenberry, K.H.; Baltensperger, D.D.; Dunn, R.A.; Wilcox, C.J.; Hardy, S.R. Selection for Tolerance to Root-Knot Nematodes in Red Clover. Crop Sci. 1989, 29, 62–65. [Google Scholar] [CrossRef]
- Bruce, A.B.; Maynard, E.T.; Farmer, J.R. Farmers’ Perspectives on Challenges and Opportunities Associated with Using High Tunnels for Specialty Crops. HortTechnology 2019, 29, 290–299. [Google Scholar] [CrossRef]
- Milligan, S.B.; Bodeau, J.; Yaghoobi, J.; Kaloshian, I.; Zabel, P.; Williamson, V.M. The Root Knot Nematode Resistance Gene Mi from Tomato Is a Member of the Leucine Zipper, Nucleotide Binding, Leucine-Rich Repeat Family of Plant Genes. Plant Cell 1998, 10, 1307–1319. [Google Scholar] [CrossRef] [PubMed]
- El-Sappah, A.H.; M, M.I.; El-awady, H.H.; Yan, S.; Qi, S.; Liu, J.; Cheng, G.-T.; Liang, Y. Tomato Natural Resistance Genes in Controlling the Root-Knot Nematode. Genes 2019, 10, 925. [Google Scholar] [CrossRef]
- Bhattarai, K.K.; Li, Q.; Liu, Y.; Dinesh-Kumar, S.P.; Kaloshian, I. The MI-1-mediated pest resistance requires Hsp90 and Sgt1. Plant Physiol. 2007, 144, 312–323. [Google Scholar] [CrossRef] [PubMed]
- Jarquin-Barberena, H.; Dalmasso, A.; Guiran, G.d.; Cardin, M. Acquired virulence in the plant parasitic nematode Meloidogyne incognita. 1. Biological analysis of the phenomenon. Rev. Nématologie 1991, 14, 261–275. [Google Scholar]
- Castagnone -Sereno, P.; Bongiovanni, M.; Dalmasso, A. Stable Virulence Against the Tomato Resistance Mi Gene in the Parthenogenetic Root-Knot Nematode Meloidogyne incognita. Phytopathology 1993, 83, 803–805. [Google Scholar] [CrossRef]
- Cortada, L.; Sakai, H.; Verdejo-Lucas, S.; Mizukubo, T. Meloidogyne virulence locus molecular marker for characterization of selected mi-virulent populations of Meloidogyne spp. is correlated with several genera of betaproteobacteria. Phytopathology 2011, 101, 410–415. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).