Morphological and Physiological Responses of Weigela florida ‘Eva Rathke’ to Biostimulants and Growth Promoters
Abstract
1. Introduction
2. Materials and Methods
2.1. Substances Used in Experiments
- Kelpak®—0.4% solution;
- Yeald Plus—0.3% solution;
- Bistep 0.5%—solution;
- Control group: received only water at the same time as the treatments.
2.2. Experiment Setup
- A total of 30% crushed calcareous black peat (pH, 6.24; humus content, 17.89%);
- A total of 70% crushed white peat (pH, 5.81; humus content, 25.58%).
2.3. Histological Measurements
2.3.1. Sampling of Plant Material
2.3.2. Histological Analysis
- Microscope: Euromex bScope BS.1153-PLi biological microscope (Euromex Microscopen B.V., Arhem, Netherlands);
- Camera: Euromex CMEX-5f 5 Mp Camera (DC.5000f);
- Lens: due to the sectioning procedure, oil immersion lens blocks cannot be used; therefore, due to the nature of the sections, the PLi Lens PLi 4/0.1 was used, and because of the type of samples, no oil immersion was applied. Magnification: 40 × 4/0.1. Magnification: 40×;
- Ocular: WF120×/20 type and size.
2.4. Physiological Measurements
2.4.1. Transpiration and Evapotranspiration Analysis
2.4.2. Proline Content Determination
2.4.3. Rhizosphere Measurements
2.5. Statistical Evaluation
3. Results
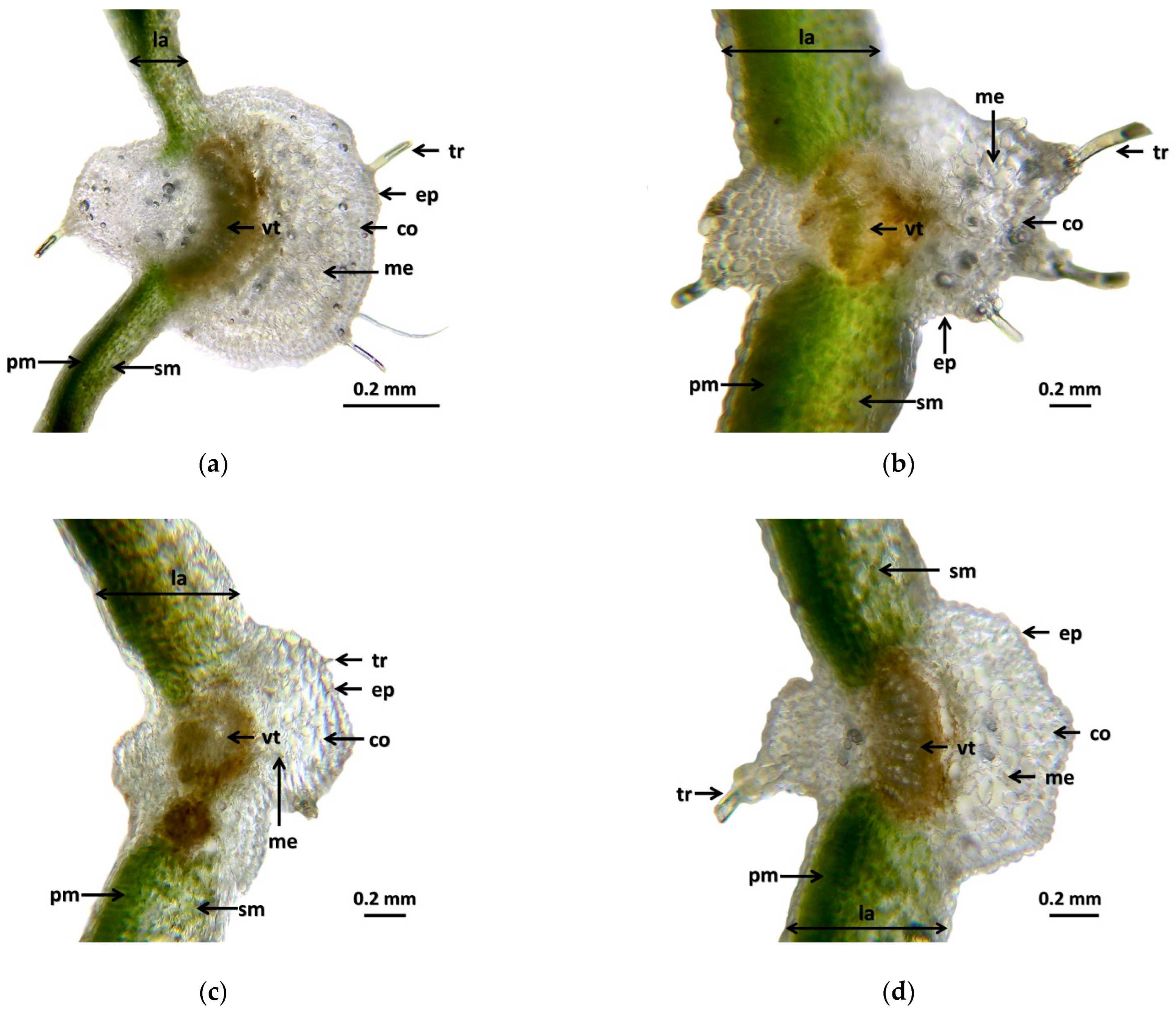
3.1. Histology Analysis of Investigated Plants
3.2. Physiology
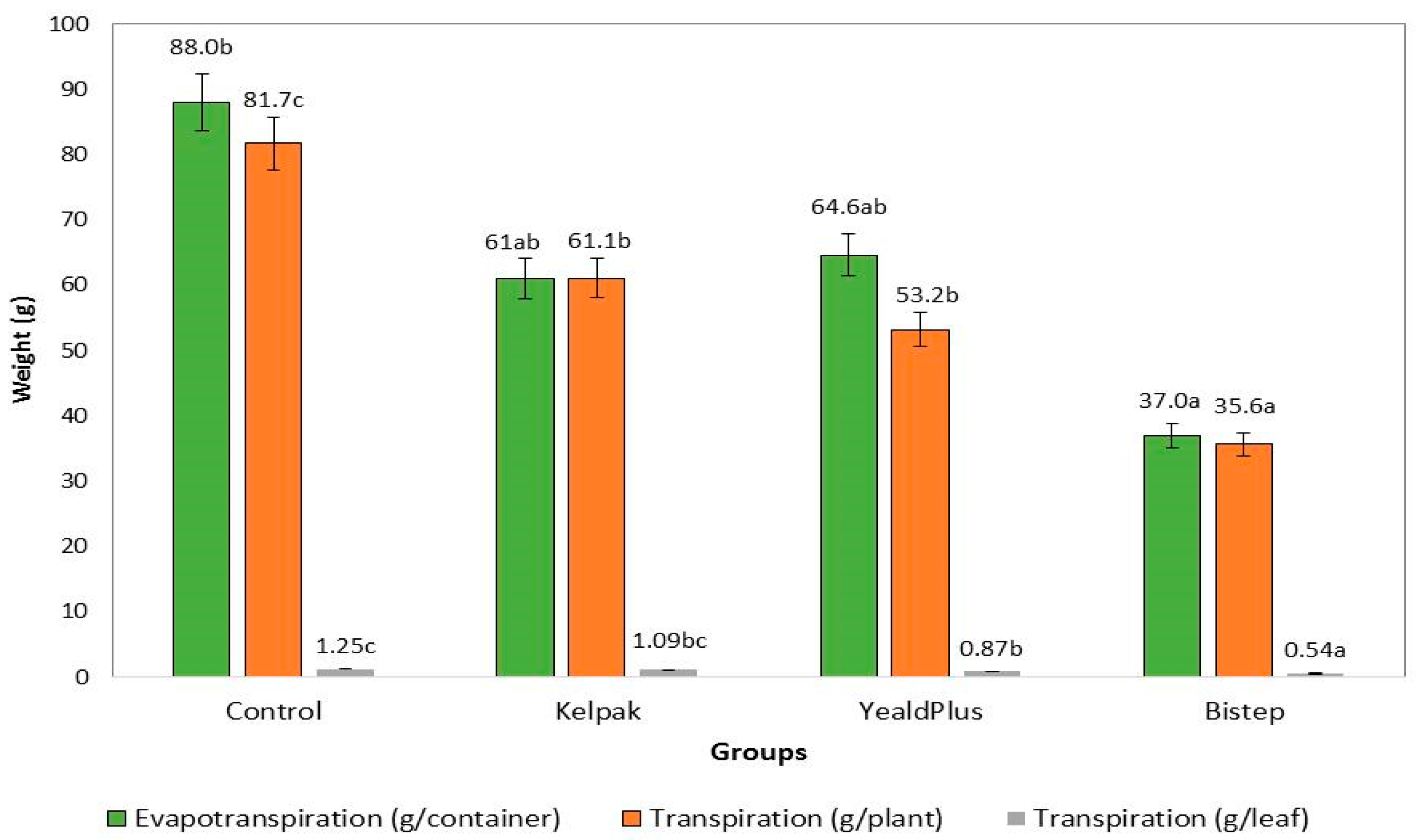
3.2.1. Transpiration and Evapotranspiration Assessment
3.2.2. Proline Content Analysis
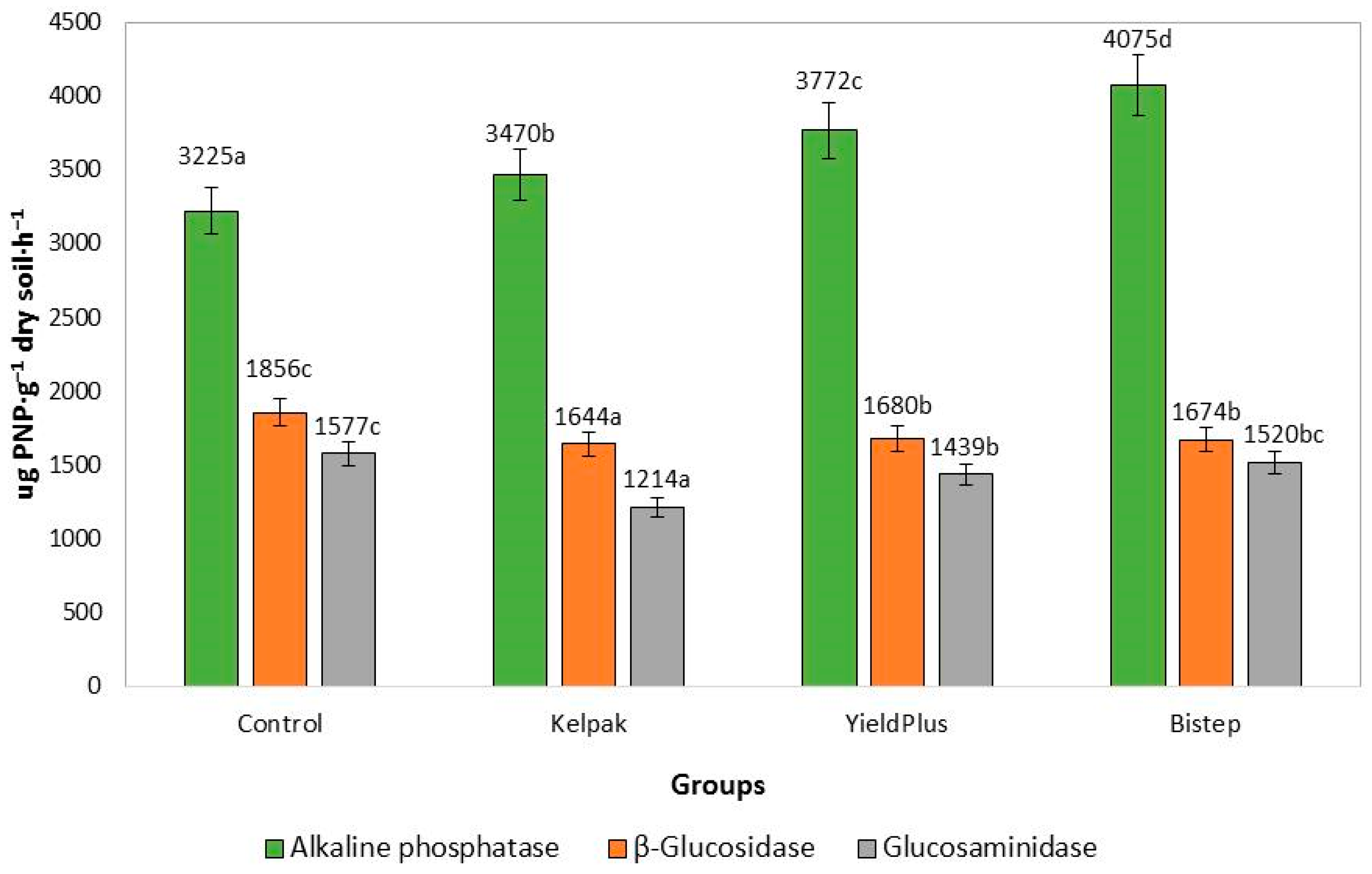
3.2.3. Rhizosphere Measurements
4. Discussion
4.1. Histology
4.2. Transpiration and Evapotranspiration
4.3. Proline Enzyme
4.4. Microbilogical Activity
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guerra, A.J.T.; Fullen, M.A.; Jorge, M.D.C.O.; Bezerra, J.F.R.; Shokr, M.S. Slope processes, mass movement and soil erosion: A review. Pedosphere 2017, 27, 27–41. [Google Scholar] [CrossRef]
- Ferreira, C.S.; Walsh, R.P.; Ferreira, A.J. Degradation in urban areas. Curr. Opin. Environ. Sci. Health 2018, 5, 19–25. [Google Scholar] [CrossRef]
- Cinantya, A. The Effect of Biostimulants on Plant Performance: An Australian Urban Horticultural Perspective. Doctoral Dissertation, Macquarie University, Sydney, Australia, 2023. [Google Scholar]
- Cinantya, A.; Manea, A.; Leishman, M.R. Biostimulants do not affect the performance of urban plant species grown under drought stress. Urban Ecosyst. 2024, 1–11. [Google Scholar] [CrossRef]
- Marschner, H. (Ed.) Marschner’s Mineral Nutrition of Higher Plants; Academic Press: Cambridge, MA, USA, 2011. [Google Scholar] [CrossRef]
- Miceli, A.; Moncada, A.; Vetrano, F.; Esposito, A. Response of Tagetes patula L. and Ageratum houstonianum Mill. to Microbial Biostimulant Inoculation and Organic Fertilization. Agronomy 2023, 13, 2522. [Google Scholar] [CrossRef]
- Parađiković, N.; Teklić, T.; Zeljković, S.; Lisjak, M.; Špoljarević, M. Biostimulants research in some horticultural plant species—A review. Food Energy Secur. 2019, 8, e00162. [Google Scholar] [CrossRef]
- Andreotti, C.; Rouphael, Y.; Colla, G.; Basile, B. Rate and timing of application of biostimulant substances to enhance fruit tree tolerance toward environmental stresses and fruit quality. Agronomy 2022, 12, 603. [Google Scholar] [CrossRef]
- Eef, B.; Marlies, D.; Van Swam, K.; Veen, A.; Burger, L. Identification of the Seaweed Biostimulant Market (Phase1); The North Sea Farm Foundation: The Hague, The Netherlands, 2018. [Google Scholar]
- Werner, T.; Motyka, V.; Strnad, M.; Schmülling, T. Regulation of plant growth by cytokinin. Proc. Natl. Acad. Sci. USA 2001, 98, 10487–10492. [Google Scholar] [CrossRef] [PubMed]
- Lefi, E.; Badri, M.; Hamed, S.B.; Talbi, S.; Mnafgui, W.; Ludidi, N.; Chaieb, M. Influence of Brown Seaweed (Ecklonia maxima) Extract on the Morpho-Physiological Parameters of Melon, Cucumber, and Tomato Plants. Agronomy 2023, 13, 2745. [Google Scholar] [CrossRef]
- Ali, S.; Moon, Y.S.; Hamayun, M.; Khan, M.A.; Bibi, K.; Lee, I.J. Pragmatic role of microbial plant biostimulants in abiotic stress relief in crop plants. J. Plant Interact. 2022, 17, 705–718. [Google Scholar] [CrossRef]
- Kachel, M.; Tratwal, A. Możliwości wykorzystania nanotechnologii dla poprawy jakości kiełków z nasion rzepaku jarego w kontekście wymogów integrowanej produkcji. Prog. Plant Prot. 2022, 62, 3–15. [Google Scholar]
- Kisvarga, S.; Neményi, A.; Farkas, D.; Orlóci, L. Biostimulátorok és mechanikai stressz hatásának vizsgálata Matthiola incana (L.) R. Br. Varsovia fajták esetében. Kertgazdaság Hortic. 2022, 3–15, 277–281. [Google Scholar]
- Yaseen, A.A.; Hajos, M.T. Effect of biostimulants on some bioactive compounds and nitrate level in lettuce (Lactuca sativa L.) grown under unheated plastic tunnel. Iraqi J. Agric. Sci. 2021, 52, 1318–1325. [Google Scholar] [CrossRef]
- Chen, Y.; Aviad, T. Effects of humic substances on plant growth. In Humic Substances in Soil and Crop Sciences: Selected Readings; Wiley: Hoboken, NJ, USA, 1990; pp. 161–186. [Google Scholar]
- Loconsole, D.; Sdao, A.E.; Cristiano, G.; De Lucia, B. Different responses to adventitious rhizogenesis under indole-3-butyric acid and seaweed extracts in ornamental’s cuttings: First Results in Photinia x fraseri ‘Red Robin’. Agriculture 2023, 13, 513. [Google Scholar] [CrossRef]
- Szabó, V.; Sárvári, A.; Hrotkó, K. Treatment of stockplants with biostimulators and their effects on cutting propagation of Prunus marianna’GF 8-1’. Acta Hortic. 2011, 277–281. [Google Scholar] [CrossRef]
- Traversari, S.; Cacini, S.; Nesi, B. Seaweed extracts as substitutes of synthetic hormones for rooting promotion in rose cuttings. Horticulturae 2022, 8, 561. [Google Scholar] [CrossRef]
- De Clercq, P.; Pauwels, E.; Top, S.; Steppe, K.; Van Labeke, M.C. Effect of seaweed-based biostimulants on growth and development of Hydrangea paniculata under continuous or periodic drought stress. Horticulturae 2023, 9, 509. [Google Scholar] [CrossRef]
- Tahir, M.M.; Khurshid, M.; Khan, M.Z.; Abbasi, M.K.; Kazmi, M.H. Lignite-derived humic acid effect on growth of wheat plants in different soils. Pedosphere 2011, 21, 124–131. [Google Scholar] [CrossRef]
- El-Gazzar, T.M.; Tartoura, E.A.; Farid, S.E.; Zake, M.N.; Ebrahim, Y.M. Effect of Different Sources of Organic Manures and Different Levels of Humic and Fulvic Acid on Growth, Flowering, Yield and its Components of Watermelon Plants. J. Plant Prod. 2020, 11, 1135–1143. [Google Scholar] [CrossRef]
- Elkhatib, H.A.; Gabr, S.M.; Roshdy, A.H.; Kasi, R.S. Effects of different nitrogen fertilization rates and foliar application of humic acid, fulvic acid and tryptophan on growth, productivity and chemical composition of common bean plants (Phaseolus vulgaris L.). Alex. Sci. Exch. J. 2020, 41, 191–204. [Google Scholar] [CrossRef]
- Bulgari, R.; Franzoni, G.; Ferrante, A. Biostimulants application in horticultural crops under abiotic stress conditions. Agronomy 2019, 9, 306. [Google Scholar] [CrossRef]
- Guo, R.; Shi, L.; Jiao, Y.; Li, M.; Zhong, X.; Gu, F.; Liu, Q.; Xia, X.; Li, H. Metabolic responses to drought stress in the tissues of drought-tolerant and drought-sensitive wheat genotype seedlings. AoB Plants 2018, 10, ply016. [Google Scholar] [CrossRef]
- Toscano, S.; Gomez-Bellot, M.J.; Romano, D.; Sánchez-Blanco, M.J. Physiological and biochemical changes in response to Moringa oleifera biostimulant in petunia plants under water deficit. Sci. Hortic. 2023, 319, 112187. [Google Scholar] [CrossRef]
- Ozden, M.; Demirel, U.; Kahraman, A. Effects of proline on antioxidant system in leaves of grapevine (Vitis vinifera L.) exposed to oxidative stress by H2O2. Sci. Hortic. 2009, 119, 163–168. [Google Scholar] [CrossRef]
- Rajametov, S.N.; Yang, E.Y.; Cho, M.C.; Chae, S.Y.; Jeong, H.B.; Chae, W.B. Heat-tolerant hot pepper exhibits constant photosynthesis via increased transpiration rate, high proline content and fast recovery in heat stress condition. Sci. Rep. 2021, 11, 14328. [Google Scholar] [CrossRef]
- Gao, G.; Wang, D.; Zha, T.; Wang, L.; Fu, B. A global synthesis of transpiration rate and evapotranspiration partitioning in the shrub ecosystems. J. Hydrol. 2022, 606, 127417. [Google Scholar] [CrossRef]
- Zhang, K.; Kimball, J.S.; Running, S.W. A review of remote sensing based actual evapotranspiration estimation. Wiley Interdiscip. Rev. Water 2016, 3, 834–853. [Google Scholar] [CrossRef]
- Bastug, R.; Karaguzel, O.; Aydinsakir, K.; Buyuktas, D. The effects of drip irrigation on flowering and flower quality of glasshouse gladiolus plant. Agric. Water Manag. 2006, 81, 132–144. [Google Scholar] [CrossRef]
- Qiu, R.J.; Luo, Y.F.; Wu, J.W.; Zhang, B.Z.; Liu, Z.H.; Agathokleous, E.; Hu, W.; Yang, X.; Clothier, B.E. Short–term forecasting of daily evapotranspiration from rice using a modified Priestley–Taylor model and public weather forecasts. Agric. Water Manag. 2023, 277, 108123. [Google Scholar] [CrossRef]
- Wang, K.; Dickinson, R.E. A review of global terrestrial evapotranspiration: Observation, modeling, climatology, and climatic variability. Rev. Geophys 2012, 50, 54. [Google Scholar] [CrossRef]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop Evapotranspiration-Guidelines for Computing Crop Water Requirements-FAO Irrigation and Drainage Paper 56; FAO: Rome, Italy, 1998. [Google Scholar]
- Minhas, P.S.; Ramos, T.B.; Ben-Gal, A.; Pereira, L.S. Coping with salinity in irrigated agriculture: Crop evapotranspiration and water management issues. Agric. Water Manag. 2020, 227, 105832. [Google Scholar] [CrossRef]
- Surendar, K.K.; Devi, D.D.; Ravi, I.; Jeyakumar, P.; Velayudham, K. Effect of water stress on leaf temperature, transpiration rate, stomatal diffusive resistance and yield of banana. Plant Gene Trait. 2013, 4, 43–47. [Google Scholar] [CrossRef]
- Tripathi, B.N.; Gaur, J.P. Relationship between copper-and zinc-induced oxidative stress and proline accumulation in Scenedesmus sp. Planta 2004, 219, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Bohnert, H.J.; Jensen, R.G. Strategies for engineering water-stress tolerance in plants. Trends Biotechnol. 1996, 14, 89–97. [Google Scholar] [CrossRef]
- Sabatino, L.; Consentino, B.B.; Rouphael, Y.; Baldassano, S.; De Pasquale, C.; Ntatsi, G. Ecklonia maxima-derivate seaweed extract supply as mitigation strategy to alleviate drought stress in chicory plants. Sci. Hortic. 2023, 312, 111856. [Google Scholar] [CrossRef]
- Rudani, K.; Vishal, P.; Kalavati, P. The importance of zinc in plant growth—A review. Int. Res. J. Nat. Appl. Sci. 2018, 5, 38–48. [Google Scholar]
- Ramzan, M.; Naveed, N.; Ahmed, M.Z.; Ashraf, H.; Shah, A.A.; Jamil, M.; Ahmad, Z.; Casini, R.; Elansary, H.O. Supplementation of Moringa based zinc oxide nanoparticles mitigates salt stress in Celosia argentea through reduced chloride (Cl−) uptake and modulation in physiochemical attributes. S. Afr. J. Bot. 2023, 157, 457–466. [Google Scholar] [CrossRef]
- Xu, L.; Trinh, H.K.; Geelen, D. Biostimulant mode of action: Impact of PBs on molecular level. In The Chemical Biology of Plant Biostimulants; Wiley: Hoboken, NJ, USA, 2020; pp. 245–259. [Google Scholar] [CrossRef]
- Kumar, D.; Sahu, T.L.; Netam, N.; Patel, S.; Mandavi, G.; Kumar, N. Effect of foliar application of zinc and iron on growth, flowering and yield of gladiolus (Gladiolus grandiflorus L.). Pharma Innov. J. 2022, 11, 2587–2589. [Google Scholar]
- Al-Zurfi, M.T.; Abbass, J.A.; Al-Bayati, A.S.; Abd Alhur, G.H.; Hadi, H.A. Enhancement of Growth, Flowering and Corm Freesia Hybrida Plant via Rice Organic Residue Application and Chelated Zinc Spray. IOP Conf. Ser. Earth Environ. Sci. 2021, 923, 012025. [Google Scholar] [CrossRef]
- Ali, E.F.; Hassan, F.; Abdel-Rahman, S.S.; Siddique, K.H. Foliar application of potassium and zinc enhances the productivity and volatile oil content of damask rose (Rosa damascena miller var. trigintipetala dieck). Acta Sci. Pol. Hortorum Cultus 2021, 20, 101–114. [Google Scholar] [CrossRef]
- Shoaib, A.; Ferdosi, M.F.H.; Saleem, M.A.; Javed, S. Morphological and biochemical variations induced by synergy of salicylic acid and zinc in cockscomb. Folia Hortic. 2021, 33, 79–90. [Google Scholar] [CrossRef]
- Duan, Y.; Zhang, Y.; Zhao, B. Lead, zinc tolerance mechanism and phytoremediation potential of Alcea rosea (Linn.) Cavan. and Hydrangea macrophylla (Thunb.) Ser. and ethylenediaminetetraacetic acid effect. Environ. Sci. Pollut. Res. 2022, 29, 41329–41343. [Google Scholar] [CrossRef] [PubMed]
- Davet, P. Microbial Ecology of the Soil and Plant Grounth; Chapter 2. Science; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Lowe, D.; Sanvictores, T.; Zubair, M.; John, S. Alkaline Phosphatase; StatPearls: St. Petersburg, FL, USA, 2023. [Google Scholar]
- Mulvaney, R.L.; Bremner, J.M. Use of p-benzoquinone and hydroquinone for retardation of urea hydrolysis in soils. Soil Biol. Biochem. 1978, 10, 297–302. [Google Scholar] [CrossRef]
- Hemkemeyer, M.; Schwalb, S.A.; Heinze, S.; Joergensen, R.G.; Wichern, F. Functions of elements in soil microorganisms. Microbiol. Res. 2021, 252, 126832. [Google Scholar] [CrossRef] [PubMed]
- Wade, J.; Li, C.; Vollbracht, K.; Hooper, D.G.; Wills, S.A.; Margenot, A.J. Prescribed pH for soil β-glucosidase and phosphomonoesterase do not reflect pH optima. Geoderma 2021, 401, 115161. [Google Scholar] [CrossRef]
- Adetunji, A.T.; Lewu, F.B.; Mulidzi, R.; Ncube, B. The biological activities of β-glucosidase, phosphatase and urease as soil quality indicators: A review. J. Soil Sci. Plant Nutr. 2017, 17, 794–807. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Findlay, S.; Franchini, P.; Fischer, D. Enzymatic analysis of riverine bacterioplankton production. Limnol. Oceanogr. 1997, 42, 29–38. [Google Scholar] [CrossRef]
- Kopeva, A.; Khrapko, O.; Maslovskaia, O. Features of urban greening for people with visual impairment in Vladivostok. IOP Conf. Ser. Mater. Sci. Eng. 2020, 962, 032050. [Google Scholar] [CrossRef]
- Dogadina, M.A.; Botuz, N.I. Rational nature management of urban flora in urban floristry. IOP Conf. Ser. Earth Environ. Sci. 2019, 315, 052072. [Google Scholar] [CrossRef]
- Lötze, E.; Hoffman, E.W. Nutrient composition and content of various biological active compounds of three South African-based commercial seaweed biostimulants. J. Appl. Phycol. 2016, 28, 1379–1386. [Google Scholar] [CrossRef]
- Kwizda Agro. 2008. Available online: https://kwizda.hu/yeald-plus~p36277 (accessed on 28 April 2024).
- Kovács, D.; Magyar, L.; Diószegi, M.; Hrotkó, K. Biostimulátorok hatása Forsythia x intermedia Zabel.” Beatrix Farrand” konténeres díszcserjék növekedésére = Treatments Affecting the Growth of Forsythia x Intermedia Zabel.’Beatrix Farrand’Container Grown Shrubs. Gradus 2017, 4, 284–289. [Google Scholar]
- Qian, Z.; Lihua, M.; Yajun, S.; Kaihong, X. Cross-breeding and breeding of new varieties of Weigela. For. Eng. 2018, 34, 36–39. [Google Scholar]
- Wang, W.; Shao, Q.; Peng, S.; Xing, W.; Yang, T.; Luo, Y.; Yong, B.; Xu, J. Reference evapotranspiration change and the causes across the Yellow River Basin during 1957–2008 and their spatial and seasonal differences. Water Resour. Res. 2012, 48, W05530. [Google Scholar] [CrossRef]
- Yang, Z.; Jiang, Y.; Qiu, R.; Gong, X.; Agathokleous, E.; Hu, W.; Clothier, B. Heat stress decreased transpiration but increased evapotranspiration in gerbera. Front. Plant Sci. 2023, 14, 1119076. [Google Scholar] [CrossRef] [PubMed]
- Chinard, F.P. Photometric estimation of proline and ornithine. J. Biol. Chem. 1952, 199, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Ábrahám, E.; Hourton-Cabassa, C.; Erdei, L.; Szabados, L. Methods for determination of proline in plants. In Plant Stress Tolerance: Methods and Protocols; Humana Press: Totowa, NJ, USA, 2010; pp. 317–331. [Google Scholar] [CrossRef]
- Tong, A.Z.; Liu, W.; Liu, Q.; Xia, G.Q.; Zhu, J.Y. Diversity and composition of the Panax ginseng rhizosphere microbiome in various cultivation modesand ages. BMC Microbiol. 2021, 21, 18. [Google Scholar] [CrossRef] [PubMed]
- Eivazi, F.; Tabatabai, M.A. Glucosidases and galactosidases in soils. Soil Biol. Biochem. 1988, 20, 601–606. [Google Scholar] [CrossRef]
- Šarapatka, B. Phosphatase Activities (ACP, ALP) in Agroecosystem Soils. Doctoral Thesis, Swedish University of Agricultural Sciences, Uppsala, Sweden, 2003. pp. 36–37. [Google Scholar]
- Tabatabai, M.A.; Bremner, J.M. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Parham, J.A.; Deng, S.P. Detection, quantification and characterization of β-glucosaminidase activity in soil. Soil Biol. Biochem. 2000, 32, 1183–1190. [Google Scholar] [CrossRef]
- Sadok, W.; Lopez, J.R.; Smith, K.P. Transpiration increases under high-temperature stress: Potential mechanisms, trade-offs and prospects for crop resilience in a warming world. Plant Cell Environ. 2021, 44, 2102–2116. [Google Scholar] [CrossRef]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments: A review. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef]
- Ashraf, M.F.M.R.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Li, J.; Xie, T.; Zhu, H.; Zhou, J.; Li, C.; Xiong, W.; Xu, L.; Wu, Y.; He, Z.; Li, X. Alkaline phosphatase activity mediates soil organic phosphorus mineralization in a subalpine forest ecosystem. Geoderma 2021, 404, 115376. [Google Scholar] [CrossRef]
- Zhang, J.; Jin, K.; Luo, Y.; Du, L.; Tian, R.; Wang, S.; Shen, Y.; Zhang, J.; Li, N.; Shao, W.; et al. Responses of soil enzyme activity to long-term nitrogen enrichment and water addition in a typical steppe. Agronomy 2023, 13, 1920. [Google Scholar] [CrossRef]
- Vujinović, T.; Zanin, L.; Venuti, S.; Tomasi, N.; Pinton, R.; Cesco, S. Biostimulant action of dissolved humic substances from a conventionally and an organically managed soil on nitrate acquisition in maize plants. Front. Plant Sci. 2020, 10, 502598. [Google Scholar] [CrossRef]
- Szabó, V.; Magyar, L.; Hrotkó, K. Effect of leaf spray treatments on rooting and quality of Prunus mahaleb (L.) cuttings. Acta Sci. Polonorum. Hortorum Cultus 2016, 15, 77–87. [Google Scholar]


| Parameters | Kelpak | Yeald Plus | Bistep |
|---|---|---|---|
| Manufacturer | KELP PRODUCTS (PTY) Ltd. 7975 Simon’s Town, Blue Water Close South-Afrika | De Sangosse Ltd. Hillside Mill Quarry Lane, Swaffham Bulbeck, Cam-bridge CB5 0LU | UAB ALJARA LT-11219 Vilnius, Geniu str. 16–38. Lithuania |
| pH | 4.5 | 6.5 | 7.4 |
| N (m/v%) | 0.03 | 6 | 0.02 |
| P2O5 (m/v%) | 0.02 | - | 0.03 |
| K2O (m/v%) | 0.65 | - | 0.3 |
| MgO (m/v%) | 0.02 | - | 0.02 |
| As (mg/L) max. | 10 | 10 | 10 |
| Cd (mg/L) max. | 2 | 2 | 2 |
| Co (mg/L) max. | 50 | 50 | 50 |
| Cr (mg/L) max. | 100 | 100 | 100 |
| Cu (mg/L) max. | 100 | 0.25 | 100 |
| Hg (mg/L) max. | 1 | 1 | 1 |
| Ni (mg/L) max. | 50 | 50 | 50 |
| Pb (mg/L) max. | 100 | 100 | 100 |
| Se (mg/L) max. | 5 | 5 | 5 |
| B (m/v%) | - | 0.03 | 0.0002 |
| Fe (m/v%) | - | 0.25 | 0.01 |
| Mn (m/v%) | - | 0.25 | 0.007 |
| Mo (m/v%) | - | 0.001 | 0.09 |
| Zn (m/v%) | - | 5 | 0.008 |
| Number of germ (db/cm3) | - | - | 0.8 × 107 |
| Amount of microbes (db/cm3) | - | - | 1.0 × 102 |
| Ca (mm/m%) | 4.5 | 6.5 | 7.4 |
| B (m/v%) | 0.03 | 6 | 0.02 |
| Parameters | Control | Kelpak | Yeald Plus | Bistep |
|---|---|---|---|---|
| pH (H2O) | 5.37 | 5.1 | 5.05 | 5.3 |
| Organic material (m/m%) | 75.9 | 83 | 82.5 | 79.9 |
| P2O5 (AL soluble) (mg/kg) | 930 | 1390 | 1300 | 1230 |
| K2O (AL soluble) (mg/kg) | 1452 | 1908 | 1988 | 2016 |
| NO3 + NO2-N (KCL soluble) (mg/kg) | 592 | 1182 | 1447 | 776 |
| Na (AL soluble) (mg/kg) | 1680 | 1228 | 1444 | 1392 |
| Cu (EDTA soluble) (mg/kg) | 34.6 | 34.3 | 29.9 | 33.7 |
| Mg (KCL soluble) (mg/kg) | 936 | 1200 | 1144 | 1060 |
| Ca (EDTA soluble) (mg/kg) | 2900 | 2775 | 2625 | 2925 |
| Mn (EDTA soluble) (mg/kg) | 170 | 144 | 143 | 153 |
| SO42−-S (KCL soluble) (mg/kg) | 1625 | 1666 | 2066 | 1528 |
| Zn (EDTA soluble) (mg/kg) | 19 | 23.7 | 40.9 | 41.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovács, D.; Horotán, K.; Orlóci, L.; Makádi, M.; Mosonyi, I.D.; Sütöri-Diószegi, M.; Kisvarga, S. Morphological and Physiological Responses of Weigela florida ‘Eva Rathke’ to Biostimulants and Growth Promoters. Horticulturae 2024, 10, 582. https://doi.org/10.3390/horticulturae10060582
Kovács D, Horotán K, Orlóci L, Makádi M, Mosonyi ID, Sütöri-Diószegi M, Kisvarga S. Morphological and Physiological Responses of Weigela florida ‘Eva Rathke’ to Biostimulants and Growth Promoters. Horticulturae. 2024; 10(6):582. https://doi.org/10.3390/horticulturae10060582
Chicago/Turabian StyleKovács, Dezső, Katalin Horotán, László Orlóci, Marianna Makádi, István Dániel Mosonyi, Magdolna Sütöri-Diószegi, and Szilvia Kisvarga. 2024. "Morphological and Physiological Responses of Weigela florida ‘Eva Rathke’ to Biostimulants and Growth Promoters" Horticulturae 10, no. 6: 582. https://doi.org/10.3390/horticulturae10060582
APA StyleKovács, D., Horotán, K., Orlóci, L., Makádi, M., Mosonyi, I. D., Sütöri-Diószegi, M., & Kisvarga, S. (2024). Morphological and Physiological Responses of Weigela florida ‘Eva Rathke’ to Biostimulants and Growth Promoters. Horticulturae, 10(6), 582. https://doi.org/10.3390/horticulturae10060582






