Synergistic Effects of Salicylic Acid and Bacillus butanolivorans KJ40 for Enhancing Napa Cabbage (Brassica napa subsp. pekinensis) Resilience to Water-Deficit Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Bacterial Strain and Salicylic Acid
2.2. Plant Growth Assay
2.3. Contents of Malondialdehyde and Proline, and Antioxidant Enzyme Activity
2.4. Salicylic Acid and Glucosinolate Analysis
2.5. Soil Microbial Physiological Profile Using EcoPlate and Activity
2.6. Effect of Salicylic Acid on Growth and Colonization of KJ40
2.7. Statistical Analysis
3. Results and Discussion
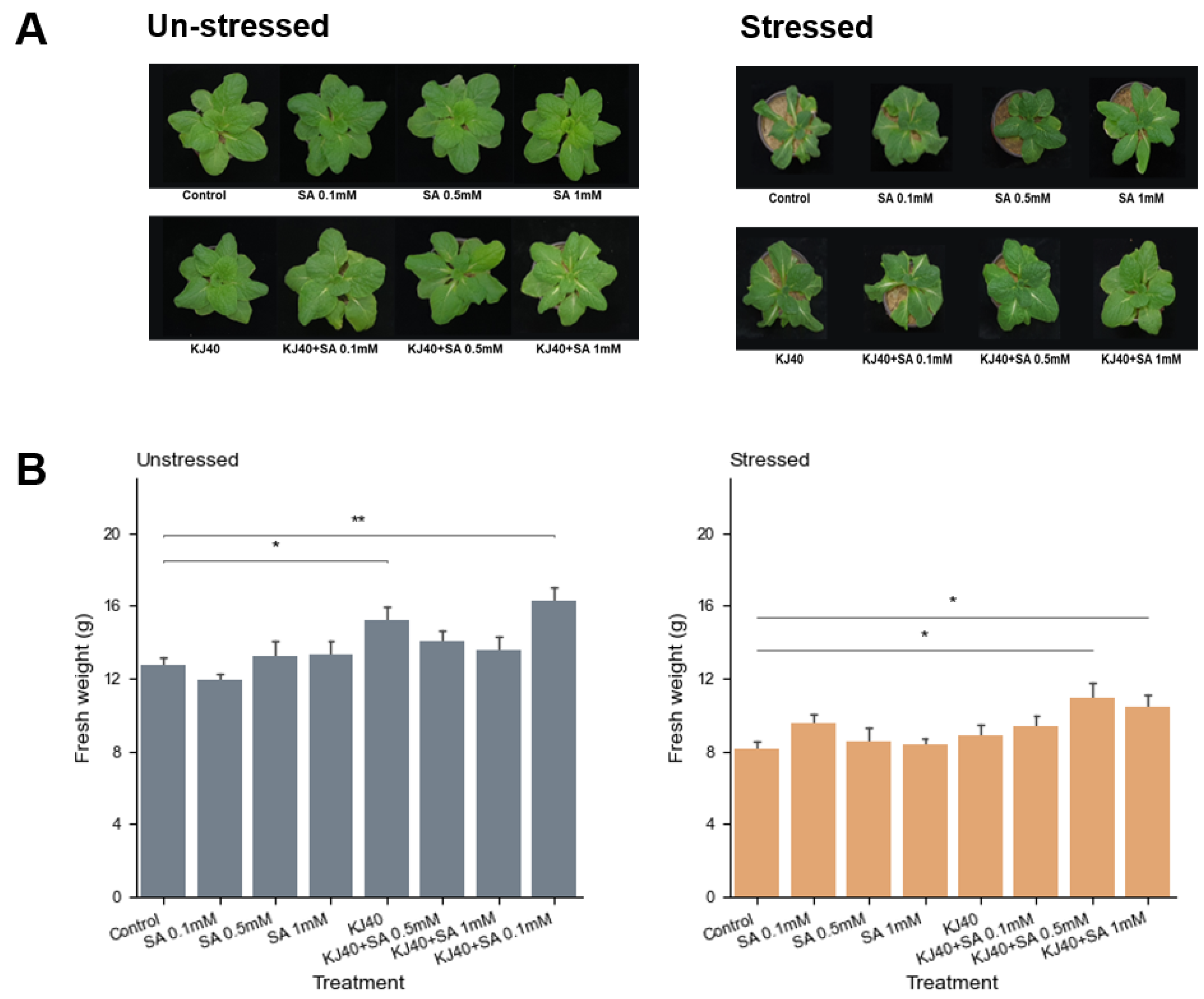
3.1. Synergistic Effects of Salicylic Acid and Bacillus Butanolivorans KJ40 (KJ40) on Plant Growth under Water-Deficit Stress
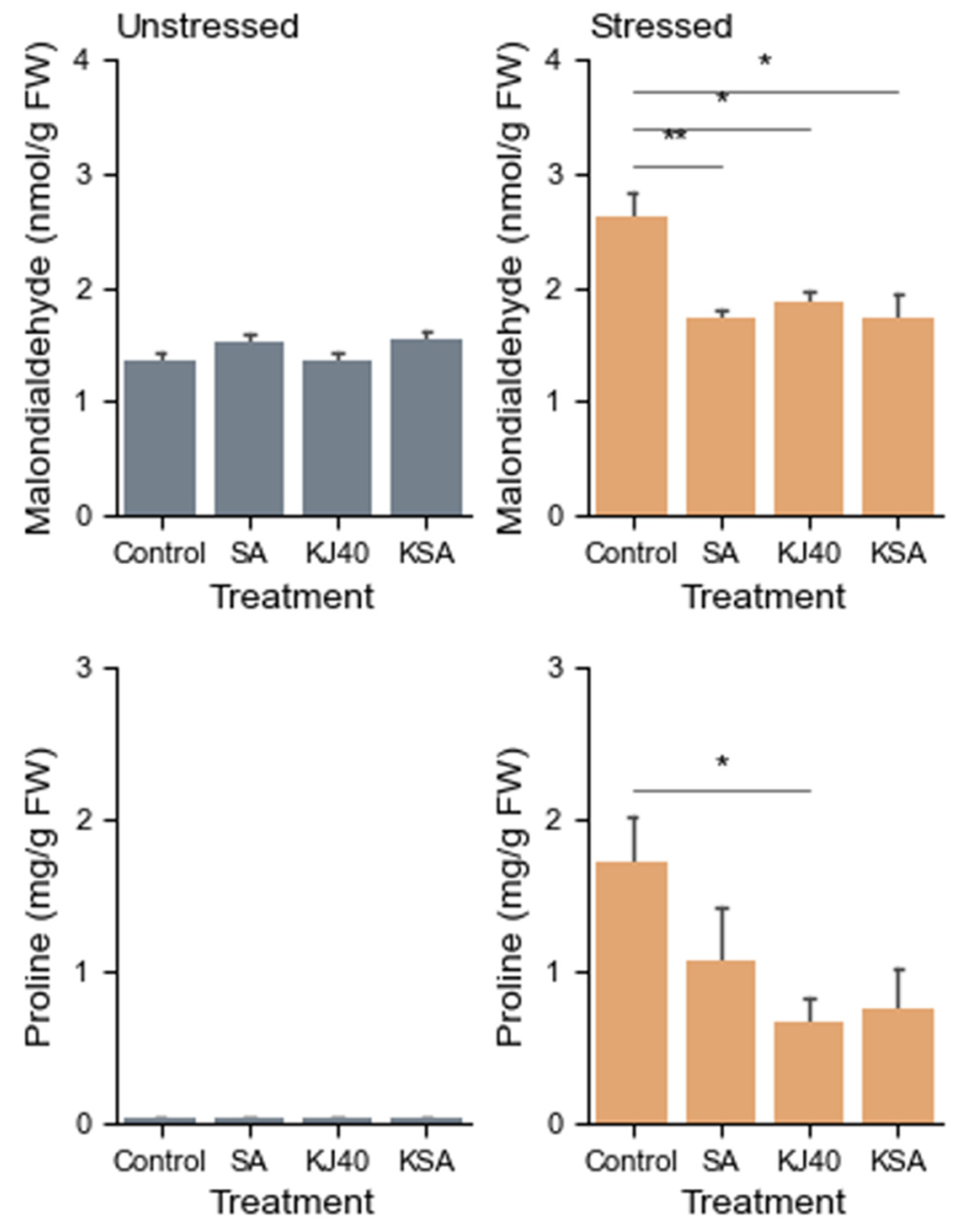
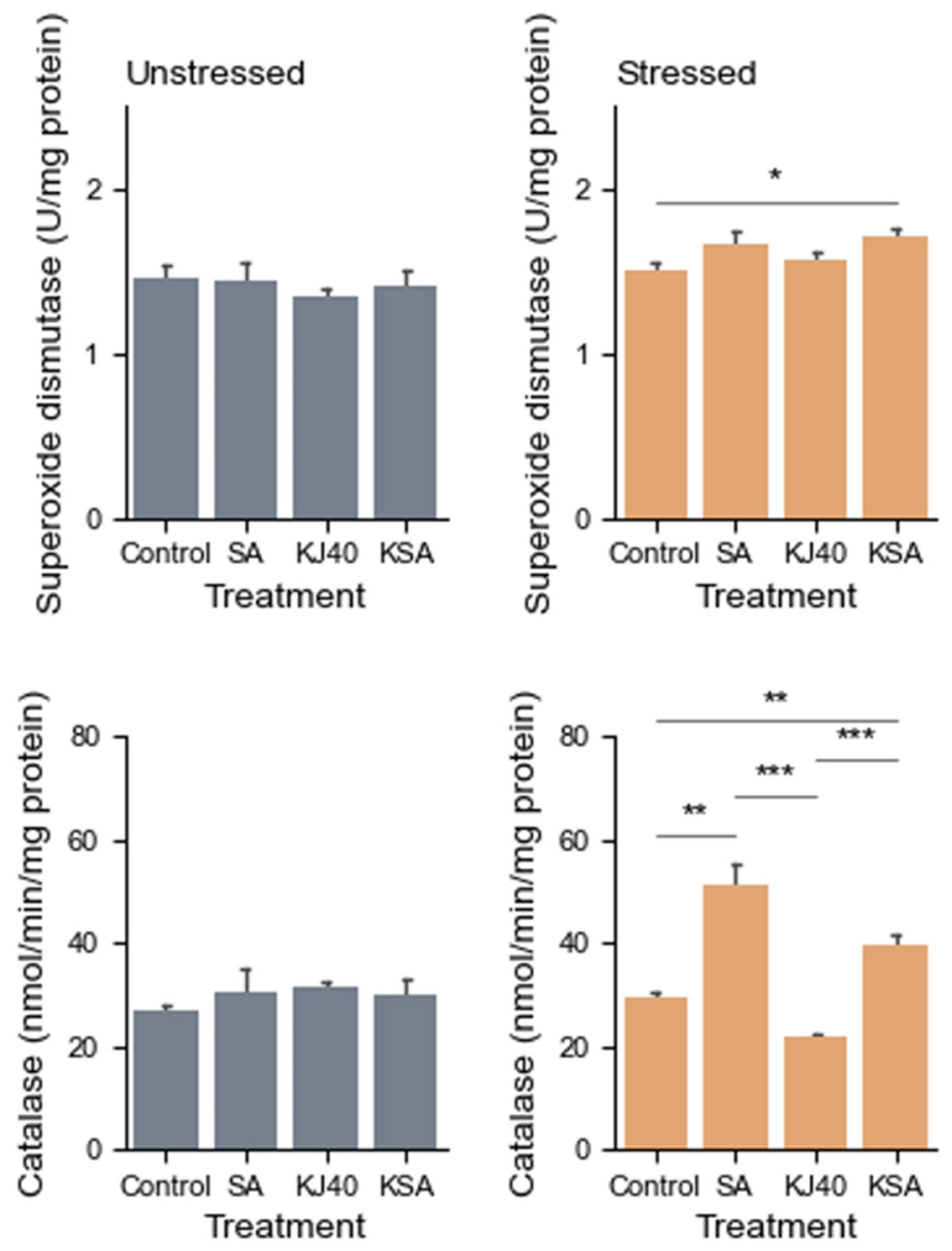
3.2. Reduction in Lipid Peroxidation and Enhancement of Antioxidant Enzyme Activity with KJ40 and SA Combination
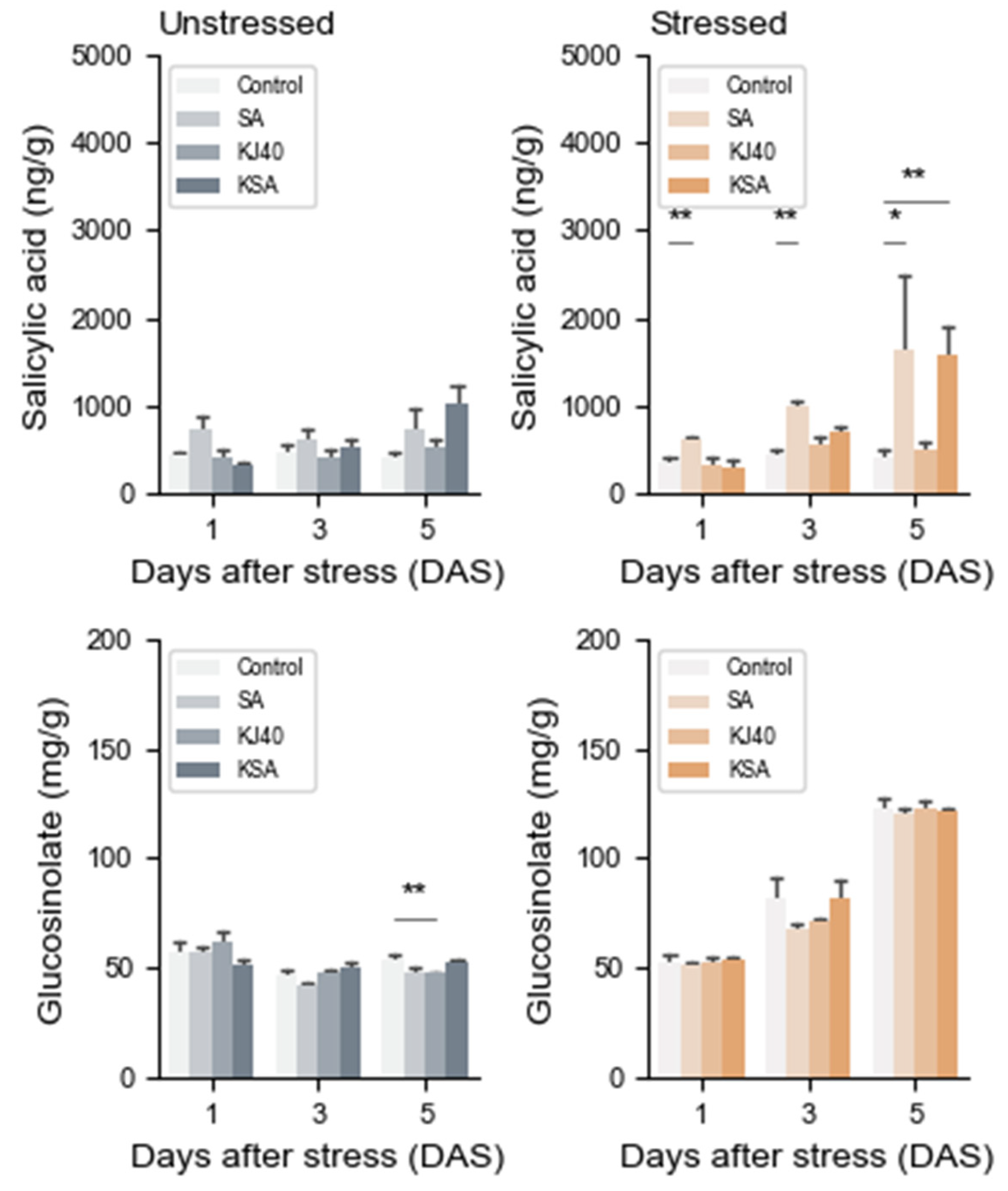
3.3. Single Salicylic Acid and Combination Treatments Induce Changes in Salicylic Acid Contents, Excluding Glucosinolate, in Napa Cabbage
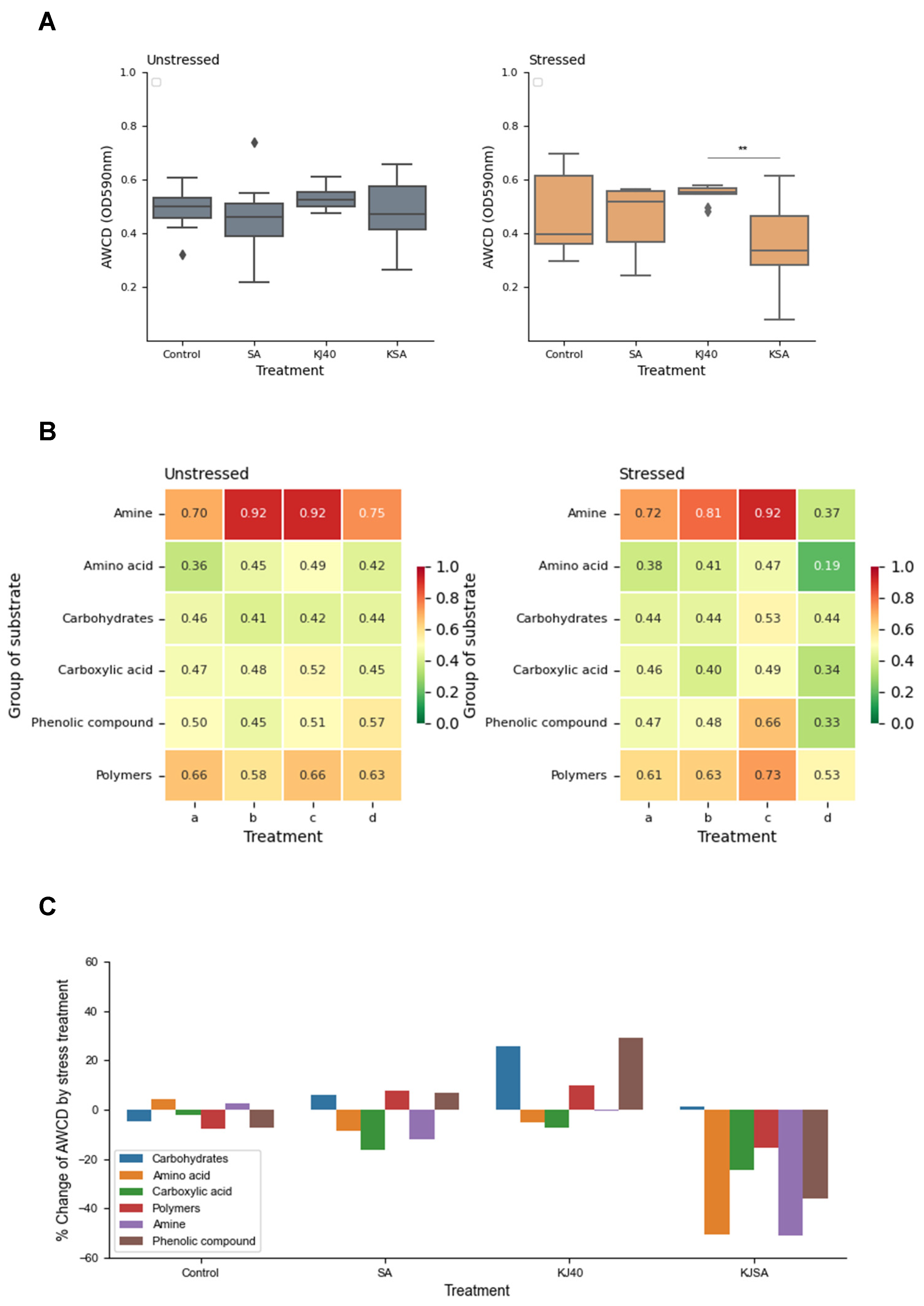
3.4. Impact on Soil Microbial Profiles and Activity
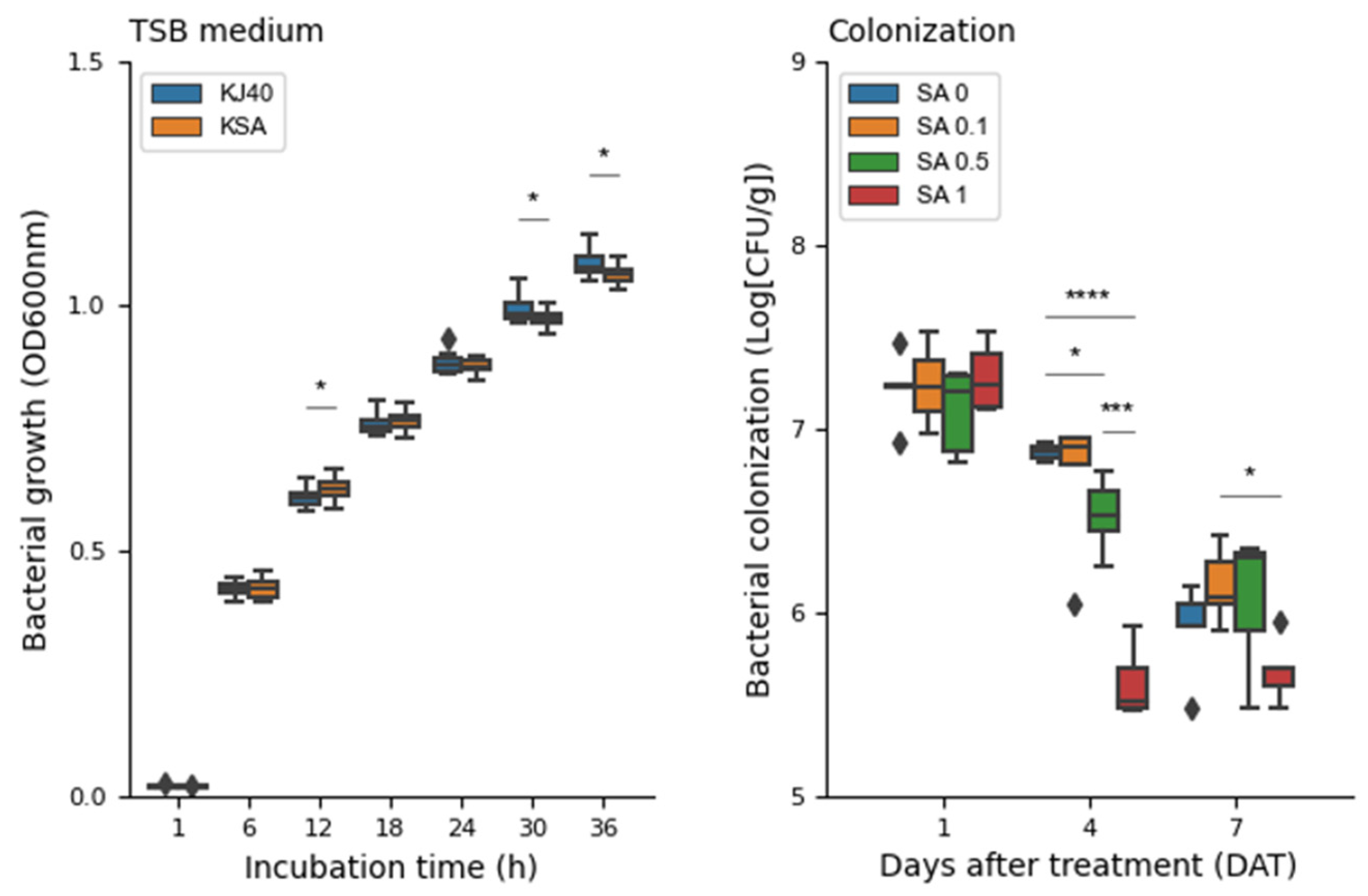
3.5. Impact of Salicylic Acid on the Population Dynamics of KJ40
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pörtner, H.-O.; Roberts, D.C.; Poloczanska, E.S.; Mintenbeck, K.; Tignor, M.; Alegría, A.; Craig, M.; Langsdorf, S.; Löschke, S.; Möller, V.; et al. (Eds.) IPCC, 2022: Summary for Policymakers. In Climate Change 2022: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2022; pp. 3–33. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, G.; Long, W.; Zou, X.; Li, F.; Nishio, T. Recent progress in drought and salt tolerance studies in Brassica crops. Breed. Sci. 2014, 64, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhang, J. Drought hazard assessment in typical corn cultivated areas of China at present and potential climate change. Nat. Hazards 2016, 81, 1323–1331. [Google Scholar] [CrossRef]
- Hu, Z.; Wu, Z.; Zhang, Y.; Li, Q.; Islam, A.T.; Pan, C. Risk assessment of drought disaster in summer maize cultivated areas of the Huang-Huai-Hai plain, Eastern China. Environ. Monit. Assess. 2021, 193, 441. [Google Scholar] [CrossRef]
- Prabnakorn, S.; Maskey, S.; Suryadi, F.X.; de Fraiture, C. Assessment of drought hazard, exposure, vulnerability, and risk for rice cultivation in the Mun River Basin in Thailand. Nat. Hazards 2019, 97, 891–911. [Google Scholar] [CrossRef]
- Maggio, A.; De Pascale, S.; Ruggiero, C.; Barbieri, G. Physiological response of field-grown cabbage to salinity and drought stress. Eur. J. Agron. 2005, 23, 57–67. [Google Scholar] [CrossRef]
- Khan, A.; Anwar, Y.; Hasan, M.M.; Iqbal, A.; Ali, M.; Alharby, H.F.; Hakeem, K.R.; Hasanuzzaman, M. Attenuation of drought stress in Brassica seedlings with exogenous application of Ca2+ and H2O2. Plants 2017, 6, 20. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S. Plant drought stress: Effects, mechanisms and management. In Sustainable Agriculture; Springer: Dordrecht, The Netherlands, 2009; pp. 153–188. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Reynolds, M.P.; Quilligan, E.; Aggarwal, P.K.; Bansal, K.C.; Cavalieri, A.J.; Chapman, S.C.; Chapotin, S.M.; Datta, S.K.; Duveiller, E.; Gill, K.S. An integrated approach to maintaining cereal productivity under climate change. Glob. Food Secur. 2016, 8, 9–18. [Google Scholar] [CrossRef]
- Verma, K.K.; Song, X.-P.; Lin, B.; Guo, D.-J.; Singh, M.; Rajput, V.D.; Singh, R.K.; Singh, P.; Sharma, A.; Malviya, M.K.; et al. Silicon induced drought tolerance in crop plants: Physiological adaptation strategies. Silicon 2022, 14, 2473–2487. [Google Scholar] [CrossRef]
- Yan, M. Seed priming stimulate germination and early seedling growth of Chinese cabbage under drought stress. S. Afr. J. Bot. 2015, 99, 88–92. [Google Scholar] [CrossRef]
- Haghighi, M.; Saadat, S.; Abbey, L. Effect of exogenous amino acids application on growth and nutritional value of cabbage under drought stress. Sci. Hortic. 2020, 272, 109561. [Google Scholar] [CrossRef]
- Yildirim, E.; Ekinci, M.; Turan, M. Impact of biochar in mitigating the negative effect of drought stress on cabbage seedlings. J. Soil. Sci. Plant Nutr. 2021, 21, 2297–2309. [Google Scholar] [CrossRef]
- Fleming, T.R.; Fleming, C.C.; Levy, C.C.; Repiso, C.; Hennequart, F.; Nolasco, J.B.; Liu, F. Biostimulants enhance growth and drought tolerance in Arabidopsis thaliana and exhibit chemical priming action. Ann. Appl. Biol. 2019, 174, 153–165. [Google Scholar] [CrossRef]
- Bonini, P.; Rouphael, Y.; Miras-Moreno, B.; Lee, B.; Cardarelli, M.; Erice, G.; Cirino, V.; Lucini, L.; Colla, G. A microbial-based biostimulant enhances sweet pepper performance by metabolic reprogramming of phytohormone profile and secondary metabolism. Front. Plant Sci. 2020, 11, 567388. [Google Scholar] [CrossRef] [PubMed]
- Nephali, L.; Moodley, V.; Piater, L.; Steenkamp, P.; Buthelezi, N.; Dubery, I.; Burgess, K.; Huyser, J.; Tugizimana, F. A metabolomic landscape of maize plants treated with a microbial biostimulant under well-watered and drought conditions. Front. Plant Sci. 2021, 12, 676632. [Google Scholar] [CrossRef] [PubMed]
- Koo, Y.M.; Heo, A.Y.; Choi, H.W. Salicylic acid as a safe plant protector and growth regulator. Plant Pathol. J. 2020, 36, 1. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Chakraborty, D. Salicylic acid increases tolerance of Vigna mungo cv. T9 to short-term drought stress. Acta Physiol. Plant 2023, 45, 25. [Google Scholar] [CrossRef]
- Kiliç, T. Seed treatments with salicylic and succinic acid to mitigate drought stress in flowering kale cv. ‘Red Pigeon F1’. Sci. Hortic. 2023, 313, 111939. [Google Scholar] [CrossRef]
- Appu, M.; Muthukrishnan, S. Foliar application of salicylic acid stimulates flowering and induce defense related proteins in finger millet plants. Univers. J. Plant Sci. 2014, 2, 14–18. [Google Scholar] [CrossRef]
- Kim, S.T.; Yoo, S.; Weon, H.; Song, J.; Sang, M.K. Bacillus butanolivorans KJ40 contributes alleviation of drought stress in pepper plants by modulating antioxidant and polyphenolic compounds. Sci. Hortic. 2022, 301, 111111. [Google Scholar] [CrossRef]
- Dhindsa, R.S.; Plumb-Dhindsa, P.; Thorpe, T.A. Leaf senescence: Correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Bao, A.; Wang, S.; Wu, G.; Xi, J.; Zhang, J.; Wang, C. Overexpression of the Arabidopsis H+-PPase enhanced resistance to salt and drought stress in transgenic alfalfa (Medicago sativa L.). Plant Sci. 2009, 176, 232–240. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil. 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Seskar, M.; Shulaev, V.; Raskin, I. Endogenous methyl salicylate in pathogen-inoculated tobacco plants. Plant Physiol. 1998, 116, 387–392. [Google Scholar] [CrossRef]
- Jung, H.W.; Tschaplinski, T.J.; Wang, L.; Glazebrook, J.; Greenberg, J.T. Priming in systemic plant immunity. Science 2009, 324, 89–91. [Google Scholar] [CrossRef] [PubMed]
- Mawlong, I.; Sujith Kumar, M.S.; Gurung, B.; Singh, K.H.; Singh, D. A simple spectrophotometric method for estimating total glucosinolates in mustard de-oiled cake. Int. J. Food Prop. 2017, 20, 3274–3281. [Google Scholar] [CrossRef]
- Schnurer, J.; Rosswall, T. Fluorescein diacetate hydrolysis as a measure of total microbial activity in soil and litter. Appl. Environ. Microbiol. 1982, 43, 1256–1261. [Google Scholar] [CrossRef]
- Sang, M.K.; Kim, K.D. Plant growth-promoting rhizobacteria suppressive to Phytophthora blight affect microbial activities and communities in the rhizosphere of pepper (Capsicum annuum L.) in the field. Appl. Soil. Ecol. 2012, 62, 88–97. [Google Scholar] [CrossRef]
- Wani, A.B.; Chadar, H.; Wani, A.H.; Singh, S.; Upadhyay, N. Salicylic acid to decrease plant stress. Environ. Chem. Lett. 2017, 15, 101–123. [Google Scholar] [CrossRef]
- Li, A.; Sun, X.; Liu, L. Action of salicylic acid on plant growth. Front. Plant Sci. 2022, 13, 878076. [Google Scholar] [CrossRef]
- Cao, S.; Hu, Z.; Zheng, Y.; Lu, B. Synergistic effect of heat treatment and salicylic acid on alleviating internal browning in cold-stored peach fruit. Postharvest Biol. Technol. 2010, 58, 93–97. [Google Scholar] [CrossRef]
- Li, Z.; Xu, J.; Gao, Y.; Wang, C.; Guo, G.; Luo, Y.; Huang, Y.; Hu, W.; Sheteiwy, M.S.; Guan, Y. The synergistic priming effect of exogenous salicylic acid and H2O2 on chilling tolerance enhancement during maize (Zea mays L.) seed germination. Front. Plant Sci. 2017, 8, 1153. [Google Scholar] [CrossRef] [PubMed]
- Saurabh, V.; Barman, K.; Singh, A.K. Synergistic effect of salicylic acid and chitosan on postharvest life and quality attributes of jamun (Syzygium cumini Skeels) fruit. Acta Physiol. Plant 2019, 41, 89. [Google Scholar] [CrossRef]
- Kong, J.; Xie, Y.; Yu, H.; Guo, Y.; Cheng, Y.; Qian, H.; Yao, W. Synergistic antifungal mechanism of thymol and salicylic acid on Fusarium solani. LWT 2021, 140, 110787. [Google Scholar] [CrossRef]
- Talaat, N.B.; Shawky, B.T. Synergistic effects of salicylic acid and melatonin on modulating ion homeostasis in salt-stressed wheat (Triticum aestivum L.) plants by enhancing root H+-pump activity. Plants 2022, 11, 416. [Google Scholar] [CrossRef] [PubMed]
- Ali, Q.; Ahmad, M.; Kamran, M.; Ashraf, S.; Shabaan, M.; Babar, B.H.; Zulfiqar, U.; Haider, F.U.; Ali, M.A.; Elshikh, M.S. Synergistic effects of Rhizobacteria and salicylic acid on maize salt-stress tolerance. Plants 2023, 12, 2519. [Google Scholar] [CrossRef] [PubMed]
- Sahin, U.; Ekinci, M.; Ors, S.; Turan, M.; Yildiz, S.; Yildirim, E. Effects of individual and combined effects of salinity and drought on physiological, nutritional and biochemical properties of cabbage (Brassica oleracea var. capitata). Sci. Hortic. 2018, 240, 196–204. [Google Scholar] [CrossRef]
- Yang, X.; Lu, M.; Wang, Y.; Wang, Y.; Liu, Z.; Chen, S. Response mechanism of plants to drought stress. Horticulturae 2021, 7, 50. [Google Scholar] [CrossRef]
- Singh, B.K.; Sharma, S.R.; Singh, B. Antioxidant enzymes in cabbage: Variability and inheritance of superoxide dismutase, peroxidase and catalase. Sci. Hortic. 2010, 124, 9–13. [Google Scholar] [CrossRef]
- Cha, Y.R.; Kim, S.; Lee, J.H.; Shim, I. Enhanced drought tolerance in Chinese cabbage (Brassica campestris L.) seedlings upon pretreatment with exogenous salicylic acid. Hortic. Sci. Technol. 2020, 38, 9–20. [Google Scholar] [CrossRef]
- Choudhary, S.; Bhat, T.M.; Alwutayd, K.M.; Abd El-Moneim, D.; Naaz, N. Salicylic acid enhances thermotolerance and antioxidant defense in Trigonella foenum graecum L. under heat stress. Heliyon 2024, 10, E27227. [Google Scholar] [CrossRef] [PubMed]
- Melo, A.S.d.; Costa, R.R.d.; Sá, F.V.d.S.; Dias, G.F.; Alencar, R.S.d.; Viana, P.M.d.O.; Peixoto, T.D.C.; Suassuna, J.F.; Brito, M.E.B.; Ferraz, R.L.d.S. Modulation of Drought-Induced Stress in Cowpea Genotypes Using Exogenous Salicylic Acid. Plants 2024, 13, 634. [Google Scholar] [CrossRef] [PubMed]
- Del Carmen Martínez-Ballesta, M.; Moreno, D.A.; Carvajal, M. The physiological importance of glucosinolates on plant response to abiotic stress in Brassica. Int. J. Mol. Sci. 2013, 14, 11607–11625. [Google Scholar] [CrossRef] [PubMed]
- Santisree, P.; Jalli, L.C.L.; Bhatnagar-Mathur, P.; Sharma, K.K. Emerging Roles of Salicylic Acid and Jasmonates in Plant Abiotic Stress Responses. In Protective Chemical Agents in the Amelioration of Plant Abiotic Stress: Biochemical and Molecular Perspectives; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2020; pp. 342–373. [Google Scholar] [CrossRef]
- Ai, X.; El-Badri, A.M.; Batool, M.; Lou, H.; Gao, G.; Bai, C.; Wang, Z.; Jiang, C.; Zhao, X.; Wang, B. Morpho-Physiochemical Indices and Transcriptome Analysis Reveal the Role of Glucosinolate and Erucic Acid in Response to Drought Stress during Seed Germination of Rapeseed. Int. J. Mol. Sci. 2024, 25, 3308. [Google Scholar] [CrossRef]
- Padilla, G.; Cartea, M.E.; Velasco, P.; de Haro, A.; Ordás, A. Variation of glucosinolates in vegetable crops of Brassica rapa. Phytochemistry 2007, 68, 536–545. [Google Scholar] [CrossRef] [PubMed]
- Shawon, R.A.; Kang, B.S.; Lee, S.G.; Kim, S.K.; Lee, H.J.; Katrich, E.; Gorinstein, S.; Ku, Y.G. Influence of drought stress on bioactive compounds, antioxidant enzymes and glucosinolate contents of Chinese cabbage (Brassica rapa). Food Chem. 2020, 308, 125657. [Google Scholar] [CrossRef]
- Yi, G.; Robin, A.H.K.; Yang, K.; Park, J.; Hwang, B.H.; Nou, I. Exogenous methyl jasmonate and salicylic acid induce subspecies-specific patterns of glucosinolate accumulation and gene expression in Brassica oleracea L. Molecules 2016, 21, 1417. [Google Scholar] [CrossRef]
- Ben Ammar, H.; Arena, D.; Treccarichi, S.; Di Bella, M.C.; Marghali, S.; Ficcadenti, N.; Lo Scalzo, R.; Branca, F. The effect of water stress on the glucosinolate content and profile: A comparative study on roots and leaves of Brassica oleracea L. Crops. Agronomy 2023, 13, 579. [Google Scholar] [CrossRef]
- Chen, W.; Wang, Y.; Xu, L.; Dong, J.; Zhu, X.; Ying, J.; Wang, Q.; Fan, L.; Li, C.; Liu, L. Methyl jasmonate, salicylic acid and abscisic acid enhance the accumulation of glucosinolates and sulforaphane in radish (Raphanus sativus L.) taproot. Sci. Hortic. 2019, 250, 159–167. [Google Scholar] [CrossRef]
- Ozturk, M.; Turkyilmaz Unal, B.; García-Caparrós, P.; Khursheed, A.; Gul, A.; Hasanuzzaman, M. Osmoregulation and its actions during the drought stress in plants. Physiol. Plant. 2021, 172, 1321–1335. [Google Scholar] [CrossRef]
- Gargallo-Garriga, A.; Preece, C.; Sardans, J.; Oravec, M.; Urban, O.; Peñuelas, J. Root exudate metabolomes change under drought and show limited capacity for recovery. Sci. Rep. 2018, 8, 12696. [Google Scholar] [CrossRef] [PubMed]
- Bobille, H.; Fustec, J.; Robins, R.J.; Cukier, C.; Limami, A.M. Effect of water availability on changes in root amino acids and associated rhizosphere on root exudation of amino acids in Pisum sativum L. Phytochemistry 2019, 161, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Tomar, U.; Baishya, R. Seasonality and moisture regime control soil respiration, enzyme activities, and soil microbial biomass carbon in a semi-arid forest of Delhi, India. Ecol. Process 2020, 9, 50. [Google Scholar] [CrossRef]
- Abdul Rahman, N.S.N.; Abdul Hamid, N.W.; Nadarajah, K. Effects of abiotic stress on soil microbiome. Int. J. Mol. Sci. 2021, 22, 9036. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.T.; Sang, M.K. Synergistic Effects of Salicylic Acid and Bacillus butanolivorans KJ40 for Enhancing Napa Cabbage (Brassica napa subsp. pekinensis) Resilience to Water-Deficit Stress. Horticulturae 2024, 10, 618. https://doi.org/10.3390/horticulturae10060618
Kim ST, Sang MK. Synergistic Effects of Salicylic Acid and Bacillus butanolivorans KJ40 for Enhancing Napa Cabbage (Brassica napa subsp. pekinensis) Resilience to Water-Deficit Stress. Horticulturae. 2024; 10(6):618. https://doi.org/10.3390/horticulturae10060618
Chicago/Turabian StyleKim, Sang Tae, and Mee Kyung Sang. 2024. "Synergistic Effects of Salicylic Acid and Bacillus butanolivorans KJ40 for Enhancing Napa Cabbage (Brassica napa subsp. pekinensis) Resilience to Water-Deficit Stress" Horticulturae 10, no. 6: 618. https://doi.org/10.3390/horticulturae10060618
APA StyleKim, S. T., & Sang, M. K. (2024). Synergistic Effects of Salicylic Acid and Bacillus butanolivorans KJ40 for Enhancing Napa Cabbage (Brassica napa subsp. pekinensis) Resilience to Water-Deficit Stress. Horticulturae, 10(6), 618. https://doi.org/10.3390/horticulturae10060618






