Chitosan and GRAS Substances: An Alternative for the Control of Neofusicoccum parvum In Vitro, Elicitor and Maintenance of the Postharvest Quality of Avocado Fruits
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Isolation
2.2. Preparation of Formulations
2.3. In Vitro Evaluation
2.3.1. Inhibition of N. parvum with Basic or GRAS Substance Formulations
2.3.2. Scanning Electron Microscopy
2.4. In Vivo Evaluation—Application of Treatments
2.4.1. Postharvest Quality
Skin Color
Firmness
Weight Loss
2.4.2. Apparent Fruit Quality
2.4.3. Measurement of Enzymatic Activity
2.5. Statistical Analysis
3. Results and Discussion
3.1. Identification of Pathogen Causing Black Spot on Avocado Fruit
3.2. In Vitro Inhibition of Non-Chemical Treatments Based on Chitosan and Natural Compounds on N. parvum
3.3. Postharvest Quality
3.3.1. Skin Color
3.3.2. Weight Loss
3.3.3. Firmness
3.3.4. Apparent Quality of the Fruit
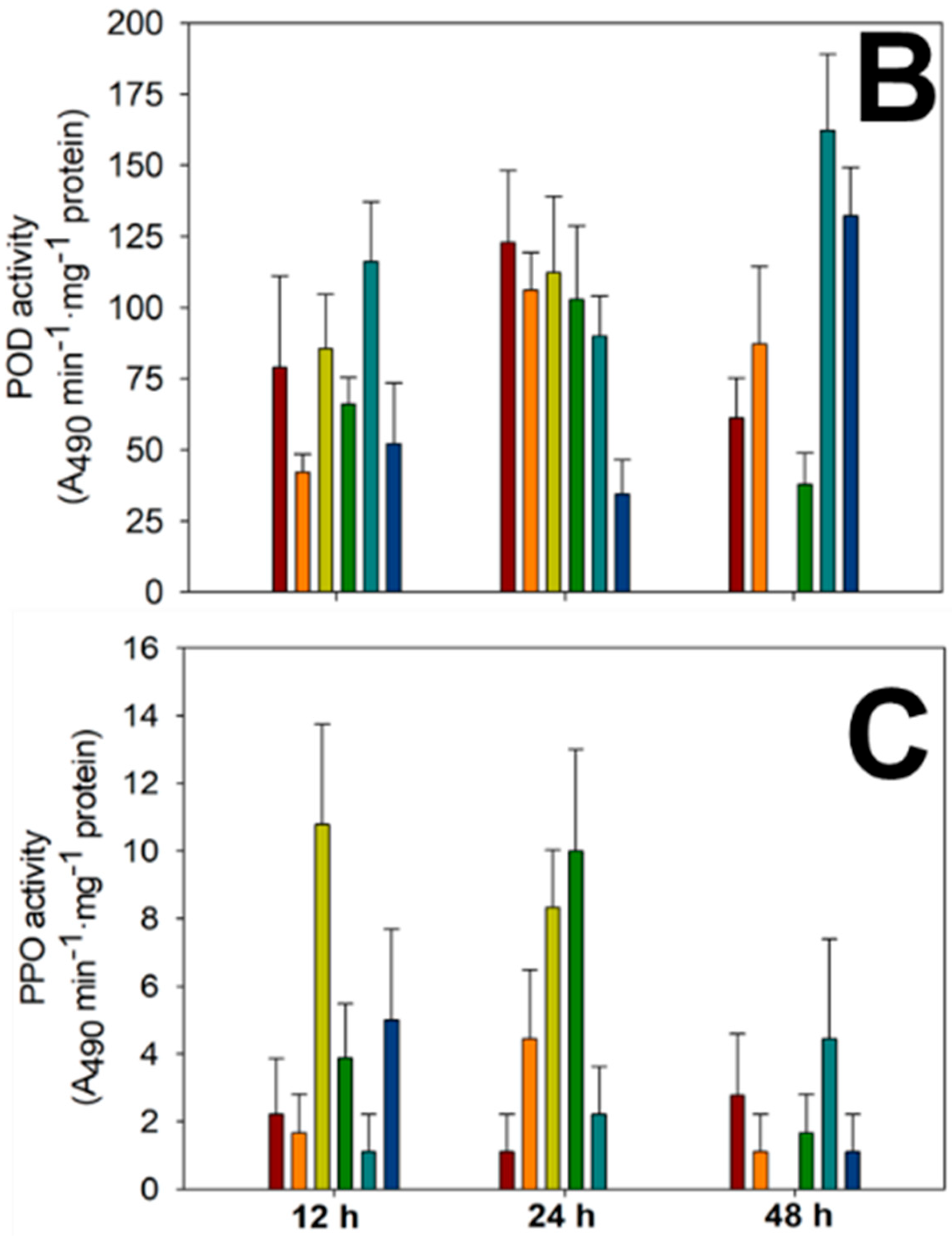
3.3.5. Enzymatic Activity of PAL, POD and PPO
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations. FAOSTAD. Available online: https://www.fao.org/faostat/es/#data (accessed on 13 July 2022).
- SIAP. Producción Agrícola. Available online: https://nube.siap.gob.mx/cierreagricola/ (accessed on 13 July 2022).
- Fuentes-Aragón, D.; Silva-Rojas, H.V.; Guarnaccia, V.; Mora-Aguilera, J.A.; Aranda-Ocampo, S.; Bautista-Martínez, N.; Téliz-Ortíz, D. Colletotrichum Species Causing Anthracnose on Avocado Fruit in Mexico: Current Status. Plant Pathol. 2020, 69, 1513–1528. [Google Scholar] [CrossRef]
- Rodríguez-Gálvez, E.; Hilário, S.; Batista, E.; Lopes, A.; Alves, A. Lasiodiplodia Species Associated with Dieback of Avocado in the Coastal Area of Peru. Eur. J. Plant Pathol. 2021, 161, 219–232. [Google Scholar] [CrossRef]
- Molina-Gayosso, E.; Silva-Rojas, H.V.; García-Morales, S.; Avila-Quezada, G. First Report of Black Spots on Avocado Fruit Caused by Neofusicoccum Parvum in Mexico. Plant Dis. 2012, 96, 287. [Google Scholar] [CrossRef] [PubMed]
- Becerra-Morales, D.; Corrales-García, J.E.; Almaraz-Sánchez, A.; Ayala-Escobar, V.; Nieto-Angel, D. First Report of Neofusicoccum Parvum, Causal Agent of Dieback on ‘Hass’ Avocado Branches (Persea americana Cv. Hass) in Atlixco, México. Plant Dis. 2019, 103, 3283. [Google Scholar] [CrossRef]
- Hartill, W.F.T.; Everett, K.R. Inoculum Sources and Infection Pathways of Pathogens Causing Stem-End Rots of ‘Hass’ Avocado (Persea americana). N. Z. J. Crop Hortic. Sci. 2002, 30, 249–260. [Google Scholar] [CrossRef]
- Herrera-González, J.A.; Bautista-Baños, S.; Salazar-García, S.; Gutiérrez-Martínez, P. Current Situation of Postharvest Handling and Fungal Diseases of Avocado ‘Hass’ for Export in Michoacán, Mexico. Rev. Mex. Cienc. Agric. 2020, 11, 1647–1660. [Google Scholar] [CrossRef]
- Comisión Europea. Reglamento (CE) No 1107/2009 del Parlamento Europeo y del Consejo, de 21 de Octubre de 2009, Relativo a la Comercialización de Productos Fitosanitarios y por el que se Derogan las Directivas 79/117/CEE y 91/414/CEE del Consejo; Comisión Europea: Brussels, Belgium, 2009; Volume 309. [Google Scholar]
- Romanazzi, G.; Moumni, M. Chitosan and Other Edible Coatings to Extend Shelf Life, Manage Postharvest Decay, and Reduce Loss and Waste of Fresh Fruits and Vegetables. Curr. Opin. Biotechnol. 2022, 78, 102834. [Google Scholar] [CrossRef] [PubMed]
- Herrera-González, J.A.; Bautista-Baños, S.; Serrano, M.; Romanazzi, G.; Gutiérrez-Martínez, P. Non-Chemical Treatments for the Pre- and Post-Harvest Elicitation of Defense Mechanisms in the Fungi–Avocado Pathosystem. Molecules 2021, 26, 6819. [Google Scholar] [CrossRef]
- Coronado-Partida, L.D.; Serrano, M.; Romanazzi, G.; González-Estrada, R.R.; Gutiérrez-Martínez, P. Aplicación de Compuestos GRAS para el Control de la Pudrición Blanda en Frutos de Jaca (Artocarpus heterophyllus L.) Causado por Rhizopus Stolonifer. TIP Rev. Espec. Cienc. Químico-Biol. 2021, 24, e327. [Google Scholar] [CrossRef]
- Ramos-Bell, S.; Hernández-Montiel, L.G.; Velázquez-Estrada, R.M.; Sánchez-Burgos, J.A.; Bautista-Rosales, P.U.; Gutiérrez-Martínez, P. Additive Effect of Alternative Treatment to Chemical Control of Botrytis cinerea in Blueberries. Rev. Mex. Ing. Quim. 2022, 21, Bio2839. [Google Scholar] [CrossRef]
- Rayón-Díaz, E.; Birke-Biewendt, A.B.; Velázquez-Estrada, R.M.; González-Estrada, R.R.; Ramírez-Vázquez, M.; Rosas-Saito, G.H.; Gutierrez-Martinez, P. Sodium Silicate and Chitosan: An Alternative for the In Vitro Control of Colletotrichum gloeosporioides Isolated from Papaya (Carica papaya L.). Rev. Bio Cienc. 2021, 8, e1059. [Google Scholar] [CrossRef]
- Chávez-Magdaleno, M.E.; González-Estrada, R.R.; Ramos-Guerrero, A.; Plascencia-Jatomea, M.; Gutiérrez-Martínez, P. Effect of Pepper Tree (Schinus molle) Essential Oil-Loaded Chitosan Bio-Nanocomposites on Postharvest Control of Colletotrichum gloeosporioides and Quality Evaluations in Avocado (Persea americana) Cv. Hass. Food Sci. Biotechnol. 2018, 27, 1871–1875. [Google Scholar] [CrossRef] [PubMed]
- Chávez-Magdaleno, M.; Luque-Alcaraz, A.; Gutiérrez-Martínez, P.; Cortez-Rocha, M.; Burgos-Hernández, A.; Lizardi-Mendoza, J.; Plascencia-Jatomea, M. Effect of Chitosan-Pepper Tree (Schinus molle) Essential Oil Biocomposites on the Growth Kinetics, Viability and Membrane Integrity of Colletotrichum gloeosporioides. Rev. Mex. Ing. Quim. 2018, 17, 29–45. [Google Scholar] [CrossRef]
- Zea-Bonilla, T.; González-Sánchez, M.A.; Martín-Sánchez, P.M.; Pérez-Jiménez, R.M. Avocado Dieback Caused by Neofusicoccum parvum in the Andalucia Region, Spain. Plant Dis. 2007, 91, 1052. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Hernández, C.; Zambrano-Zaragoza, M.L.; Velázquez-Estrada, R.M.; Sánchez-Burgos, J.A.; Gutierrez-Martinez, P. Identification of a Colletotrichum Species from Mango Fruit and Its in Vitro Control by GRAS Compounds. Rev. Mex. Ing. Quim. 2022, 21, 2777. [Google Scholar] [CrossRef]
- Silva-Rojas, H.V.; Ávila-Quezada, G.D. Phylogenetic and Morphological Identification of Colletotrichum boninense: A Novel Causal Agent of Anthracnose in Avocado. Plant Pathol. 2011, 60, 899–908. [Google Scholar] [CrossRef]
- Herrera-González, J.A.; Hernández-Sánchez, D.A.; Bueno-Rojas, D.; Ramos-Bell, S.; Velázquez-Estrada, R.M.; Bautista-Rosales, P.U.; Gutiérrez-Martínez, P. Effect of Commercial Chitosan on In Vitro Inhibition of Colletotrichum siamense, Fruit Quality and Elicitor Effect on Postharvest Avocado Fruit. Rev. Mex. Ing. Quim. 2022, 21, 2706. [Google Scholar] [CrossRef]
- Peralta-Ruiz, Y.; Tovar, C.D.G.; Sinning-Mangonez, A.; Coronell, E.A.; Marino, M.F.; Chaves-Lopez, C. Reduction of Postharvest Quality Loss and Microbiological Decay of Tomato “Chonto” (Solanum lycopersicum L.) Using Chitosan-E Essential Oil-Based Edible Coatings under Low-Temperature Storage. Polymers 2020, 12, 1822. [Google Scholar] [CrossRef] [PubMed]
- López-Mora, L.I.; Gutiérrez-Martínez, P.; Bautista-Baños, S.; Jiménez-García, L.F.; Zavaleta-Mancera, H.A. Evaluación de la Actividad Antifúngica del Quitosano en Alternaria alternata y en la Calidad de Mango Cv. ‘Tommy Atkins’ Durante el Almacenamiento. Rev. Chapingo Ser. Hortic. 2013, 19, 315–331. [Google Scholar] [CrossRef]
- White, A.; Woolf, A.B.; Arpaia, M.L. The International Avocado Quality Manual, 1st ed.; HortResearch: Auckland, New Zealand, 2005. [Google Scholar]
- Medda, S.; Dessena, L.; Mulas, M. Monitoring of the PAL Enzymatic Activity and Polyphenolic Compounds in Leaves and Fruits of Two Myrtle Cultivars during Maturation. Agriculture 2020, 10, 389. [Google Scholar] [CrossRef]
- Sellamuthu, P.S.; Sivakumar, D.; Soundy, P.; Korsten, L. Essential Oil Vapours Suppress the Development of Anthracnose and Enhance Defence Related and Antioxidant Enzyme Activities in Avocado Fruit. Postharvest Biol. Technol. 2013, 81, 66–72. [Google Scholar] [CrossRef]
- Tian, S.; Wan, Y.; Qin, G.; Xu, Y. Induction of Defense Responses against Alternaria Rot by Different Elicitors in Harvested Pear Fruit. Appl. Microbiol. Biotechnol. 2006, 70, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- StatSoft, Inc. STATISTICA (Data Analysis Software System); StatSoft, Inc.: Tulsa, OK, USA, 2014. [Google Scholar]
- Crous, P.W.; Slippers, B.; Wingfield, M.J.; Rheeder, J.; Marasas, W.F.O.; Philips, A.J.L.; Alves, A.; Burgess, T.; Barber, P.; Groenewald, J.Z. Phylogenetic Lineages in the Botryosphaeriaceae. Stud. Mycol. 2006, 55, 235–253. [Google Scholar] [CrossRef] [PubMed]
- Sakalidis, M.L.; Slippers, B.; Wingfield, B.D.; Hardy, G.E.S.J.; Burgess, T.I. The Challenge of Understanding the Origin, Pathways and Extent of Fungal Invasions: Global Populations of the Neofusicoccum parvum-N. ribis Species Complex. Divers. Distrib. 2013, 19, 873–883. [Google Scholar] [CrossRef]
- Twizeyimana, M.; Förster, H.; McDonald, V.; Wang, D.H.; Adaskaveg, J.E.; Eskalen, A. Identification and Pathogenicity of Fungal Pathogens Associated with Stem-End Rot of Avocado in California. Plant Dis. 2013, 97, 1580–1584. [Google Scholar] [CrossRef]
- Junior, A.K.; Marino, J.S.P.; Magalhães, K.M.; Mattiuz, B.-H. Chitosan-Propolis Combination Inhibits Anthracnose in “Hass” Avocados. Emir. J. Food Agric. 2018, 30, 681–687. [Google Scholar] [CrossRef]
- Chávez-Magdaleno, M.E.; Gutiérrez-Martínez, P.; Montaño-Leyva, B.; González-Estrada, R.R. Evaluación In Vitro del Quitosano y Aceites Esenciales para el Control de dos Especies Patógenas de Colletotrichum Aisladas de Aguacate (Persea americana Mill). TIP Rev. Espec. Cienc. Químico-Biol. 2019, 22, 51–58. [Google Scholar] [CrossRef]
- Deliopoulos, T.; Kettlewell, P.S.; Hare, M.C. Fungal Disease Suppression by Inorganic Salts: A Review. Crop Prot. 2010, 29, 1059–1075. [Google Scholar] [CrossRef]
- Ge, Y.; Chen, Y.; Li, C.; Wei, M.; Lv, J.; Meng, K. Inhibitory Effects of Sodium Silicate on the Fungal Growth and Secretion of Cell Wall-Degrading Enzymes by Trichothecium roseum. J. Phytopathol. 2017, 165, 620–625. [Google Scholar] [CrossRef]
- Guimarães, J.E.R.; de la Fuente, B.; Pérez-Gago, M.B.; Andradas, C.; Carbó, R.; Mattiuz, B.-H.; Palou, L. Antifungal Activity of GRAS Salts against Lasiodiplodia theobromae In Vitro and as Ingredients of Hydroxypropyl Methylcellulose-Lipid Composite Edible Coatings to Control Diplodia Stem-End Rot and Maintain Postharvest Quality of Citrus Fruit. Int. J. Food Microbiol. 2019, 301, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Herrera-González, J.A.; Venegas-González, E.; Madrigal-Huendo, L. Proporciones de daños mecánicos y su efecto en calidad postcosecha de aguacate ‘Hass’. Rev. Mex. Cienc. Agric. 2017, 19, 3897–3909. [Google Scholar] [CrossRef][Green Version]
- Mendieta, B.; Olaeta, J.A.; Pedreschi, R.; Undurraga, P. Reduction of Cold Damage during Cold Storage of Hass Avocado by a Combined Use of Pre-Conditioning and Waxing. Sci. Hortic. 2016, 200, 119–124. [Google Scholar] [CrossRef]
- Romanazzi, G.; Feliziani, E.; Baños, S.B.; Sivakumar, D. Shelf Life Extension of Fresh Fruit and Vegetables by Chitosan Treatment. Crit. Rev. Food Sci. Nutr. 2017, 57, 579–601. [Google Scholar] [CrossRef]
- Perato, S.M.; Martínez-zamora, M.G.; Salazar, S.M.; Díaz-ricci, J.C. The Elicitor AsES Stimulates Ethylene Synthesis, Induce Ripening and Enhance Protection against Disease Naturally Produced in Avocado Fruit. Sci. Hortic. 2018, 240, 288–292. [Google Scholar] [CrossRef]
- Bill, M.; Sivakumar, D.; Korsten, L.; Thompson, A.K. The Efficacy of Combined Application of Edible Coatings and Thyme Oil in Inducing Resistance Components in Avocado (Persea americana Mill.) against Anthracnose during Post-Harvest Storage. Crop Prot. 2014, 64, 159–167. [Google Scholar] [CrossRef]
- Xoca-Orozco, L.-Á.; Cuellar-Torres, E.A.; González-Morales, S.; Gutiérrez-Martínez, P.; López-García, U.; Herrera-Estrella, L.; Vega-Arreguín, J.; Chacón-López, A. Transcriptomic Analysis of Avocado Hass (Persea americana Mill) in the Interaction System Fruit-Chitosan-Colletotrichum. Front. Plant Sci. 2017, 8, 956. [Google Scholar] [CrossRef]
- Hajiboland, R. Reactive Oxygen Species and Photosynthesis; Elsevier Inc.: Amsterdam, The Netherlands, 2014; ISBN 9780127999630. [Google Scholar]
- Moosa, A.; Farzand, A.; Sahi, S.T.; Khan, S.A.; Aslam, M.N.; Zubair, M. Salicylic Acid and Cinnamomum verum Confer Resistance against Penicillium Rot by Modulating the Expression of Defense Linked Genes in Citrus Reticulata Blanco. Postharvest Biol. Technol. 2021, 181, 111649. [Google Scholar] [CrossRef]
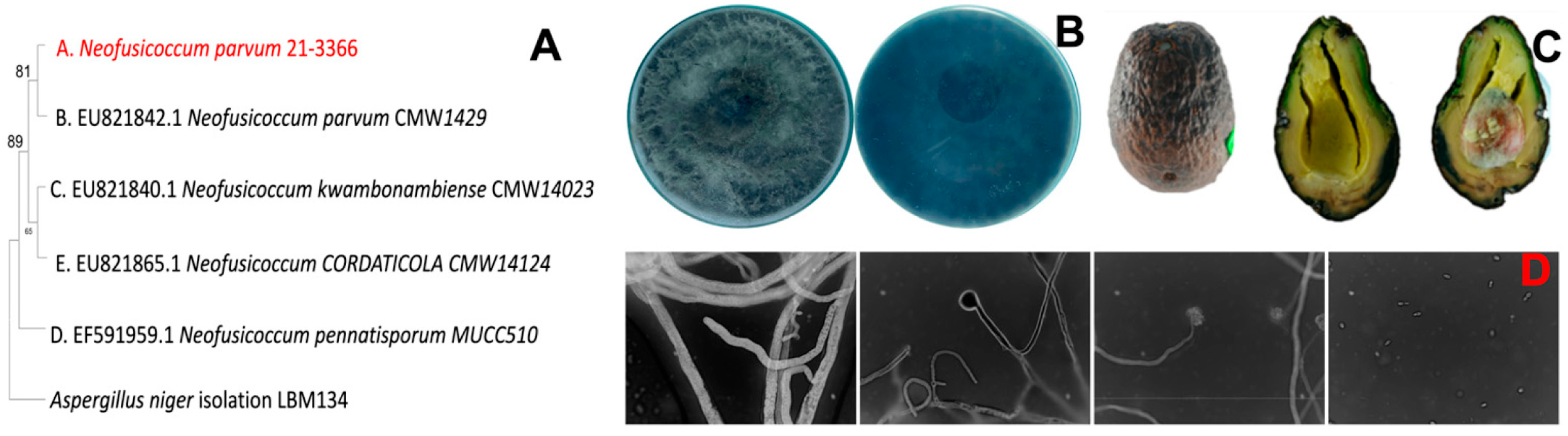

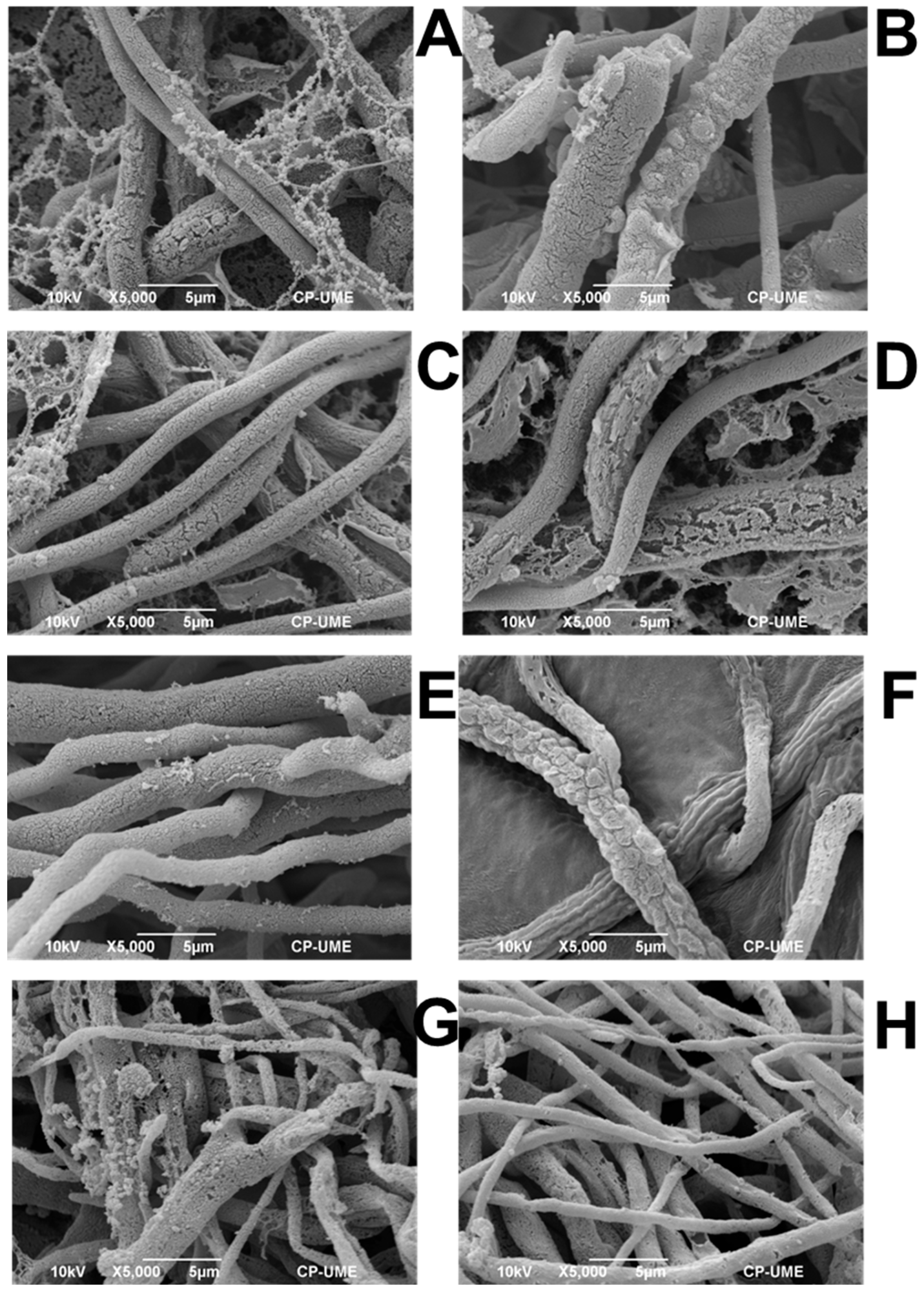


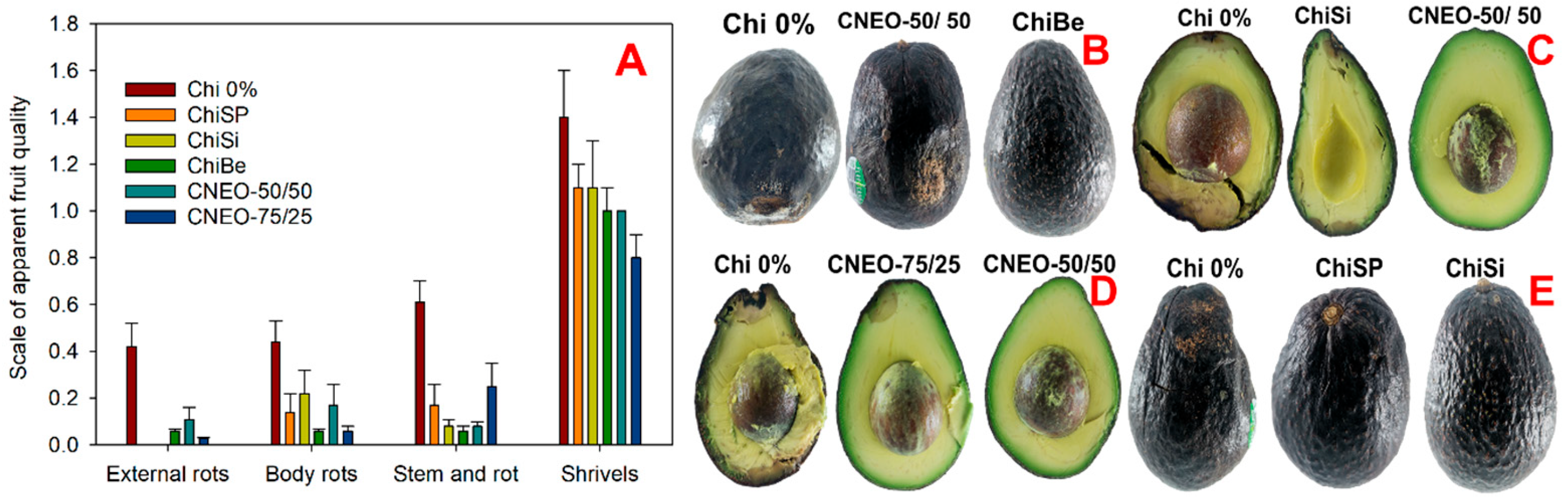
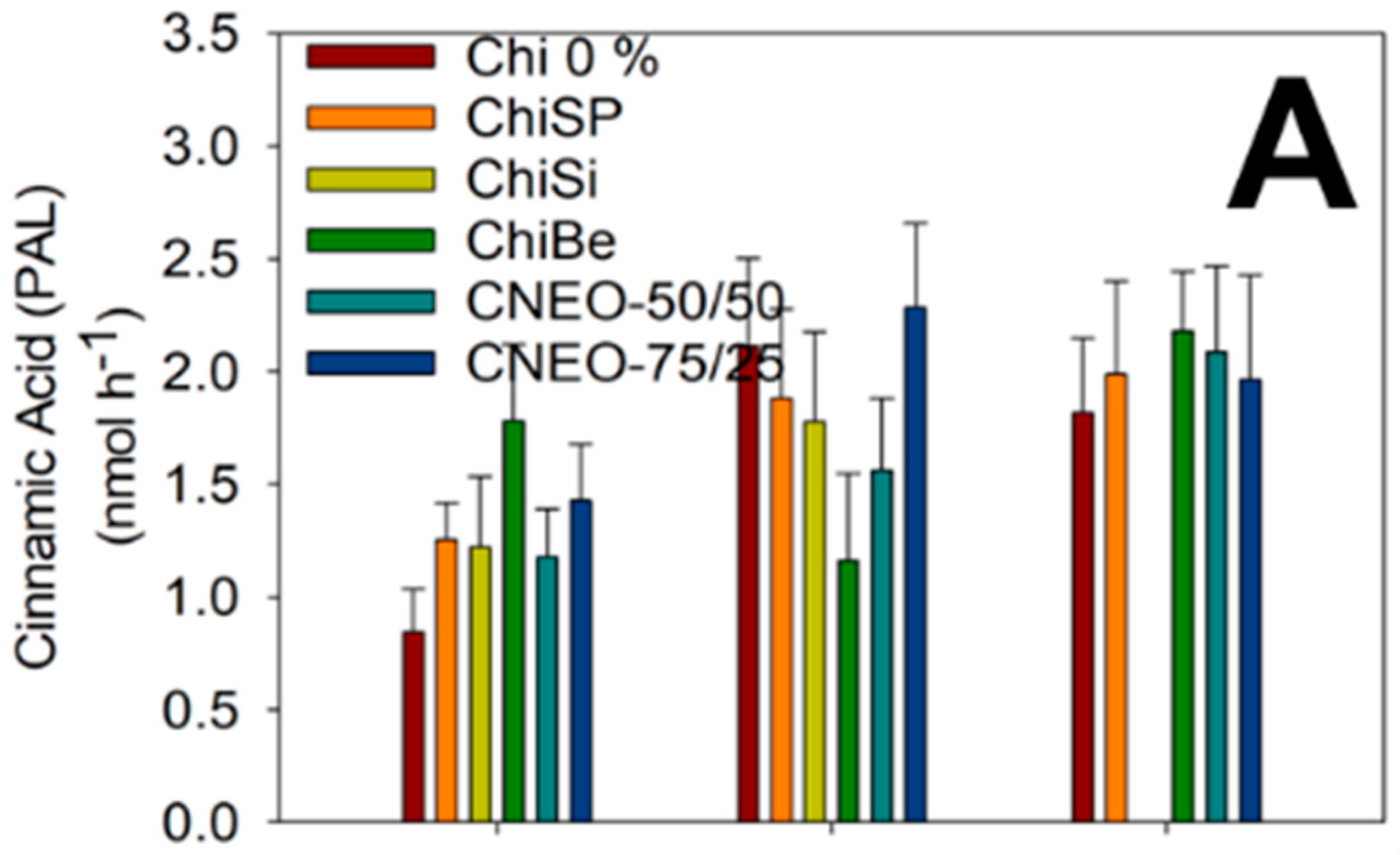
| No. | Formulation | Code |
|---|---|---|
| 1 | Control (PDA) | Control |
| 2 | Chitosan 0.5% | Chi 0.5% |
| 3 | Chitosan 1.0% | Chi 1.0% |
| 4 | Chitosan 1.5% | Chi 1.5% |
| 5 | Chitosan 0.5% + potassium sorbate 1% | ChiSP |
| 6 | Chitosan 0.5% + Salicylic acid 2% | ChiSA |
| 7 | Chitosan 0.5% + Sodium silicate 2% | ChiSi |
| 8 | Chitosan 0.5% + sodium benzoate 2% | ChiBe |
| 9 | 50/50% [Chitosan 0.5% + Emulsion (Chi 2% and cinnamon essential oil 2.5%)] | CNEO-50/50 |
| 10 | 75/25% [Chitosan 0.5% + Emulsion (Chi 2% and cinnamon essential oil 2.5%)] | CNEO-75/25 |
| 11 | Synthetic fungicide (azoxistrobin 20.51% and fludioxonil 20.51%) | Fungicide |
| 12 | Emulsion 100% (Chi 2% and cinnamon essential oil 2.5%) | CNEO |
| Treatments | Inhibition of Mycelial Growth (%) | Sporulation (1 × 106 Spores/mL) | Spore Size (µm) |
|---|---|---|---|
| 1 | 0 ± 0 f 1 | 12.8 a | 4.8 ± 0.3 ab |
| 2 | 90.3 ± 2.2 cd | 0.171 b | 4.8 ± 1.1 ab |
| 3 | 89.9 ± 2.7 cd | 0.0291 b | 7.5 ± 2.7 a |
| 4 | 90.9 ± 3.9 c | 0.176 b | 4.4 ± 0.9 ab |
| 5 | 96.5 ± 3.9 b | 0 b | ns |
| 6 | 87.7 ± 1.2 e | 0.148 b | 5.7 ± 0.7 ab |
| 7 | 100 ± 0 a | 0 b | ns |
| 8 | 89.4 ± 3.1 d | 0.0943 b | 3.8 ± 0.4 b |
| 9 | 100 ± 0 a | 0 b | ns |
| 10 | 100 ± 0 a | 0 b | - |
| 11 | 95.5 ± 3.6 b | 0.0352 b | 5.7 ± 1.3 ab |
| 12 | 100 ± 0 a | 0 b | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herrera-González, J.A.; Ramos-Bell, S.; Bautista-Baños, S.; Velázquez-Estrada, R.M.; Rayón-Díaz, E.; Martínez-Batista, E.; Gutiérrez-Martínez, P. Chitosan and GRAS Substances: An Alternative for the Control of Neofusicoccum parvum In Vitro, Elicitor and Maintenance of the Postharvest Quality of Avocado Fruits. Horticulturae 2024, 10, 687. https://doi.org/10.3390/horticulturae10070687
Herrera-González JA, Ramos-Bell S, Bautista-Baños S, Velázquez-Estrada RM, Rayón-Díaz E, Martínez-Batista E, Gutiérrez-Martínez P. Chitosan and GRAS Substances: An Alternative for the Control of Neofusicoccum parvum In Vitro, Elicitor and Maintenance of the Postharvest Quality of Avocado Fruits. Horticulturae. 2024; 10(7):687. https://doi.org/10.3390/horticulturae10070687
Chicago/Turabian StyleHerrera-González, Juan Antonio, Surelys Ramos-Bell, Silvia Bautista-Baños, Rita María Velázquez-Estrada, Edson Rayón-Díaz, Estefania Martínez-Batista, and Porfirio Gutiérrez-Martínez. 2024. "Chitosan and GRAS Substances: An Alternative for the Control of Neofusicoccum parvum In Vitro, Elicitor and Maintenance of the Postharvest Quality of Avocado Fruits" Horticulturae 10, no. 7: 687. https://doi.org/10.3390/horticulturae10070687
APA StyleHerrera-González, J. A., Ramos-Bell, S., Bautista-Baños, S., Velázquez-Estrada, R. M., Rayón-Díaz, E., Martínez-Batista, E., & Gutiérrez-Martínez, P. (2024). Chitosan and GRAS Substances: An Alternative for the Control of Neofusicoccum parvum In Vitro, Elicitor and Maintenance of the Postharvest Quality of Avocado Fruits. Horticulturae, 10(7), 687. https://doi.org/10.3390/horticulturae10070687








