Nutritional Value, Major Chemical Compounds, and Biological Activities of Petromarula pinnata (Campanulaceae)—A Unique Nutraceutical Wild Edible Green of Crete (Greece)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Extractions
2.3. Nuclear Magnetic Resonance (NMR)
2.4. Nutritional Value
2.5. Total Phenolic Content (TPC)
2.6. Total Flavonoid (TF) Assay
2.7. Total Antioxidant Capacity (TAC)
2.8. DPPH Scavenging Assay
2.9. Inhibition of Linoleic Acid Lipid Peroxidation
2.10. Inhibition of Soybean Lipoxygenase (LOX)
2.11. Antimicrobial Activity
2.12. Statistical Analysis
3. Results
3.1. Nutritional Value of Wild-Growing Petromarula pinnata
3.2. Extraction Yields of Ex Situ Cultivated Petromarula pinnata Harvested in December and June
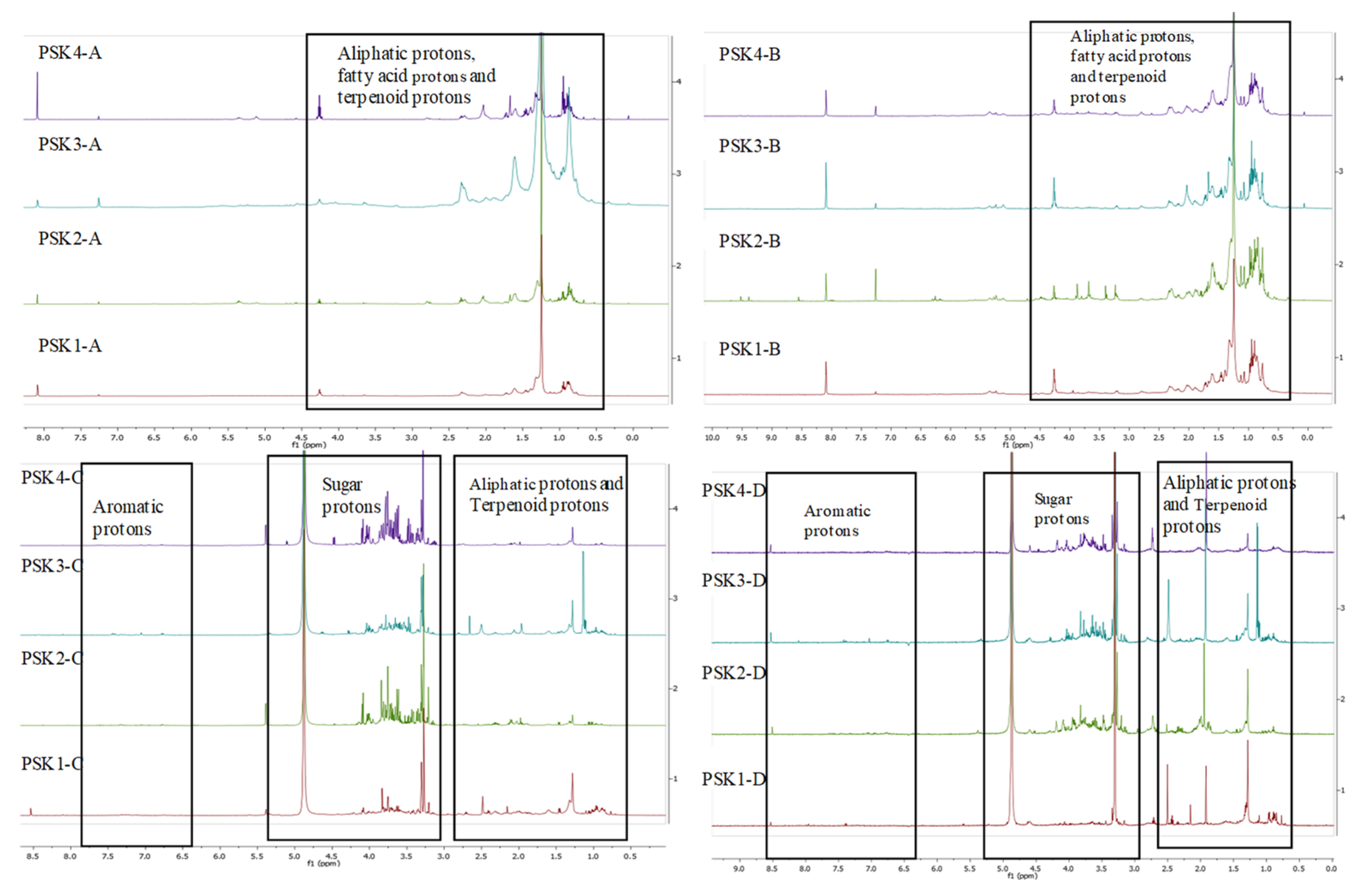
3.3. 1H NMR of the Extracts
3.4. In Vitro Tests
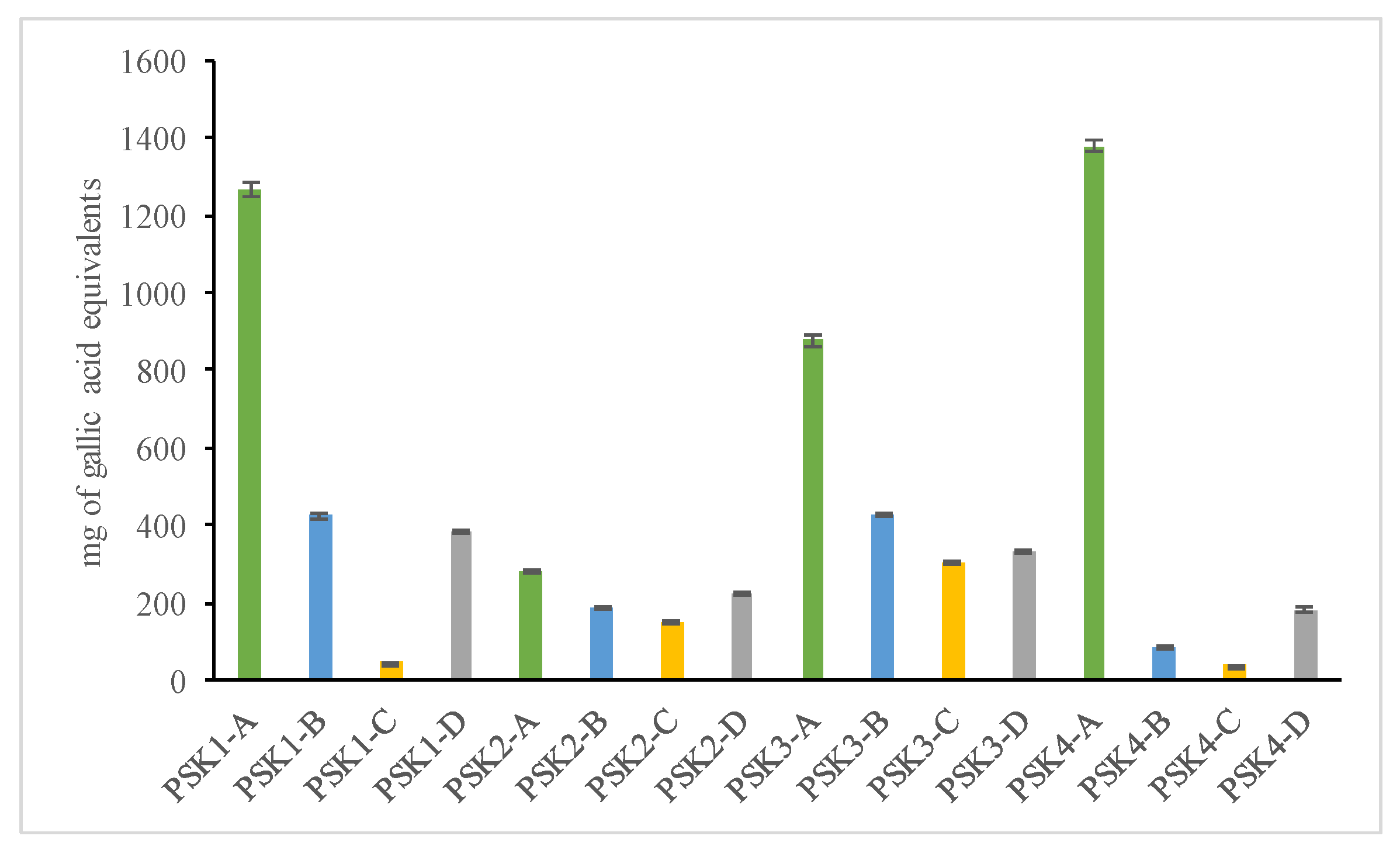
3.4.1. Total Phenolic Content
3.4.2. Total Flavonoid Assay
3.4.3. Phosphomolybdate Assay
3.4.4. DPPH
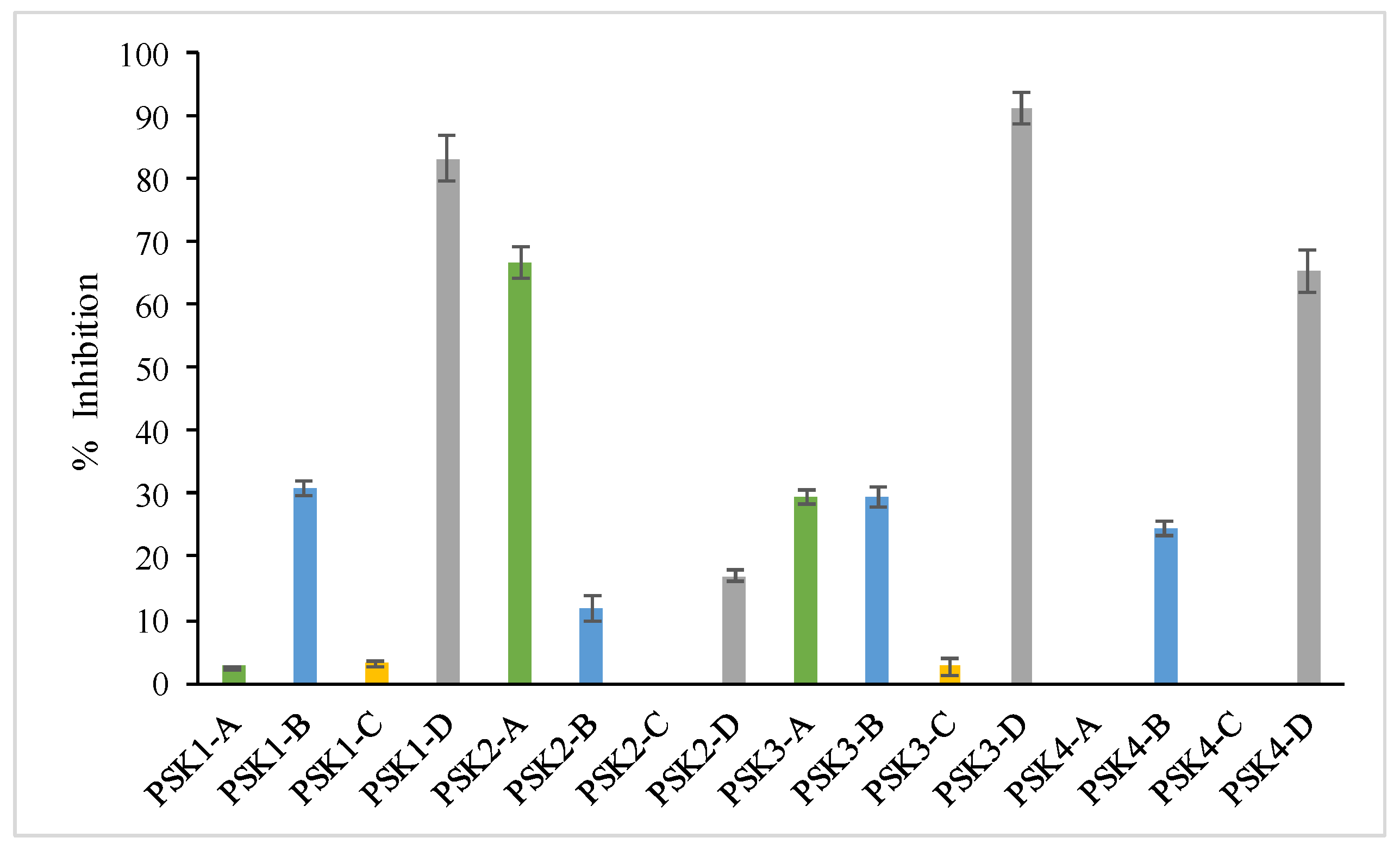
3.4.5. Inhibition of Linoleic Acid Lipid Peroxidation
3.4.6. Inhibition of Soybean Lipoxygenase
3.4.7. Antimicrobial Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anderson, J.J.B.; Nieman, D.C. Diet quality—The Greeks had it right! Nutrients 2016, 8, 636. [Google Scholar] [CrossRef]
- Martínez-González, A.M.; Salas-Salvadó, J.; Estruch, R.; Corella, D.; Fitó, M.; Ros, M. Benefits of the Mediterranean Diet: Insights from the PREDIMED study. Progr. Cardiovasc. Dis. 2015, 58, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.; Bryan, J.; Hodgson, J.; Murphy, K. Definition of the Mediterranean Diet; A literature review. Nutrients 2015, 7, 9139–9153. [Google Scholar] [CrossRef] [PubMed]
- Chib, A.; Gupta, N.; Bhat, A.; Anjum, N.; Yadav, G. Role of antioxidants in food. Int. J. Chem. Stud. 2020, 8, 2354–2361. [Google Scholar] [CrossRef]
- Liveri, E.; Crowl, A.A.; Cellinese, N. Past, present, and future of Campanula (Campanulaceae) systematics—A review. Bot. Chron. 2019, 22, 209–222. [Google Scholar]
- Park, S.; Seong, D.H.; Park, D.; Kim, S.; Gou, J.; Ahn, J.; Yoon, W.B.; Lee, H.Y. Chemical compositions of fermented Codonopsis lanceolata. J. Korean Soc. Food Sci. Nutr. 2009, 38, 396–400. [Google Scholar] [CrossRef]
- Scott, T. ABC Biologie; Walter de Gruyter: Berlin, Germany, 1996; p. 207. ISBN 978-3-11-010661-9. [Google Scholar]
- Jiang, L.; Niu, H.; Chen, Y.; Li, X.; Zhao, Y.; Zhang, C.; Li, M. Quality control of Platycodon grandiflorum (Jacq.) A. DC. based on value chains and food chain analysis. Sci. Rep. 2023, 13, 14048. [Google Scholar] [CrossRef] [PubMed]
- Ji, M.Y.; Bo, A.; Yang, M.; Xu, J.F.; Jiang, L.L.; Zhou, B.C.; Li, M.H. The pharmacological effects and health benefits of Platycodon grandiflorus—A medicine food homology species. Foods 2020, 9, 142. [Google Scholar] [CrossRef] [PubMed]
- Guarrera, P.M.; Savo, V. Wild food plants used in traditional vegetable mixtures in Italy. J. Ethnopharmacol. 2016, 185, 202–234. [Google Scholar] [CrossRef] [PubMed]
- Cellinese, N.; Smith, S.A.; Edwards, E.J.; Kim, S.T.; Haberle, R.C.; Avramakis, M.; Donoghue, M.J. Historical biogeography of the endemic Campanulaceae of Crete. J. Biogeogr. 2009, 36, 1253–1269. [Google Scholar] [CrossRef]
- Pieroni, A.; Sulaiman, N.; Sõukand, R. Chorta (Wild Greens) in central Crete: The bio-cultural heritage of a hidden and resilient ingredient of the Mediterranean Diet. Biology 2022, 11, 673. [Google Scholar] [CrossRef] [PubMed]
- Libiad, M.; Khabbach, A.; El Haissoufi, M.; Anestis, I.; Lamchouri, F.; Bourgou, S.; Megdiche-Ksouri, W.; Ghrabi-Gammar, Z.; Greveniotis, V.; Tsiripidis, I.; et al. Agro-Alimentary potential of the neglected and underutilized local endemic plants of Crete (Greece), Rif-Mediterranean coast of Morocco and Tunisia: Perspectives and challenges. Plants 2021, 10, 1770. [Google Scholar] [CrossRef] [PubMed]
- Vasilopoulou, E.; Trichopoulou, A. Green pies: The flavonoid rich Greek snack. Food Chem. 2011, 126, 855–858. [Google Scholar] [CrossRef]
- Sarropoulou, V.; Krigas, N.; Tsoktouridis, G.; Maloupa, E.; Grigoriadou, K. Seed germination trials and ex situ conservation of local prioritized endemic plants of Crete (Greece) with commercial interest. Seeds 2022, 1, 279–302. [Google Scholar] [CrossRef]
- Koutsovoulou, K.; Daws, M.I.; Thanos, C.A. Campanulaceae: A family with small seeds that require light for germination. Ann. Bot. 2014, 113, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Kalpoutzakis, E.; Chatzimitakos, T.; Athanasiadis, V.; Mitakou, S.; Aligiannis, N.; Bozinou, E.; Gortzi, O.; Skaltsounis, L.A.; Lalas, S.I. Determination of the total phenolics content and antioxidant activity of extracts from parts of plants from the Greek Island of Crete. Plants 2023, 12, 1092. [Google Scholar] [CrossRef] [PubMed]
- Shivraj, H.N.; Khobragade, C.N.N. Determination of nutritive value and mineral elements of some important medicinal plants from Western part of India. J. Med. Plants 2009, 8, 79–88. [Google Scholar]
- Papagrigoriou, T.; Iliadi, P.; Mitić, M.N.; Mrmošanin, J.M.; Papanastasi, K.; Karapatzak, E.; Maloupa, E.; Gkourogianni, A.V.; Badeka, A.V.; Krigas, N.; et al. Wild-Growing and conventionally or organically cultivated Sambucus nigra germplasm: Fruit phytochemical profile, total phenolic content, antioxidant activity, and leaf elements. Plants 2023, 12, 1701. [Google Scholar] [CrossRef] [PubMed]
- Khorasani Esmaeili, A.; Mat Taha, R.; Mohajer, S.; Banisalam, B. Antioxidant activity and total phenolic and flavonoid content of various solvent extracts from in vivo and in vitro grown Trifolium pratense L. (Red Clover). Biomed. Res. Int. 2015, 2015, 643285. [Google Scholar] [CrossRef] [PubMed]
- Jan, S.; Khan, M.R.; Rashid, U.; Bokhari, J. Assessment of antioxidant potential, total phenolics and flavonoids of different solvent fractions of Monotheca buxifolia fruit. Osong Public Health Res. Perspect. 2013, 4, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Tsiftsoglou, O.; Stefanakis, M.; Kalpourtzi, E.; Hadjipavlou-Litina, D.; Lazari, D. Chemical constituents isolated from the aerial parts of Helleborus cyclophyllus (A. Braun) Boiss. (Ranunculaceae), evaluation of their antioxidant and anti-inflammatory activity in vitro and virtual screening of molecular properties and bioactivity score. Nat. Prod. Res. 2022, 36, 6031–6038. [Google Scholar] [CrossRef] [PubMed]
- Hodaj-Çeliku, E.; Tsiftsoglou, O.; Shuka, L.; Abazi, S.; Hadjipavlou-Litina, D.; Lazari, D. Antioxidant activity and chemical composition of essential oils of some aromatic and medicinal plants from Albania. Nat. Prod. Commun. 2017, 12, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Peperidou, A.; Kapoukranidou, D.; Kontogiorgis, C.; Hadjipavlou-Litina, D. Multitarget molecular hybrids of cinnamic acids. Molecules 2014, 19, 20197–20226. [Google Scholar] [CrossRef] [PubMed]
- Charalambous, D.; Christoforou, M.; Kitiri, E.; Andreou, M.; Partassides, D.; Papachrysostomou, C.; Frantzi, M.; Karikas, G.A.; Pantelidou, M. Antimicrobial activities of Saponaria cypria Boiss root extracts and identification of nine saponins and six phenolic compounds. Molecules 2022, 27, 5812. [Google Scholar] [CrossRef] [PubMed]
- Lammers, T.G. Campanulaceae. In The Families and Genera of Vascular Plants; Flowering Plants Eudicots. The Families and Genera of Vascular Plants; Kadereit, J.W., Jeffrey, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; Volume 8, pp. 30–31. [Google Scholar] [CrossRef]
- Folquitto, D.G.; Swiech, J.N.D.; Pereira, C.B.; Bobek, V.B.; Halila Possagno, G.C.; Farago, P.V.; Miguel, M.D.; Duarte, J.L.; Miguel, O.G. Biological activity, phytochemistry and traditional uses of genus Lobelia (Campanulaceae): A systematic review. Fitoterapia 2019, 134, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Tsiftsoglou, O.S.; Lagogiannis, G.; Psaroudaki, A.; Vantsioti, A.; Mitić, M.N.; Mrmošanin, J.M.; Lazari, D. Phytochemical analysis of the aerial parts of Campanula pelviformis Lam. (Campanulaceae): Documenting the dietary value of a local endemic plant of Crete (Greece) traditionally used as wild edible green. Sustainability 2023, 15, 7404. [Google Scholar] [CrossRef]
- Schweingruber, F.H.; Říha, P.; Doležal, J. Variation in stem anatomical characteristics of Campanuloideae species in relation to evolutionary history and ecological preferences. PLoS ONE 2014, 9, e88199. [Google Scholar] [CrossRef] [PubMed]
- Grohar, M.C.; Medic, A.; Ivancic, T.; Veberic, R.; Jogan, J. Color variation and secondary metabolites’ footprint in a taxonomic complex of Phyteuma sp. (Campanulaceae). Plants 2022, 11, 2894. [Google Scholar] [CrossRef] [PubMed]
- Abbet, C.; Slacanin, I.; Hamburger, M.; Potterat, O. Comprehensive analysis of Phyteuma orbiculare L., a wild Alpine food plant. Food Chem. 2013, 136, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Abbet, C.; Neuburger, M.; Wagner, T.; Quitschau, M.; Hamburger, M.; Potterat, O. Phyteumosides A and B: New saponins with unique triterpenoid aglycons from Phyteuma orbiculare L. Org. Lett. 2011, 13, 1354–1357. [Google Scholar] [CrossRef] [PubMed]
- Jaradat, N.A.; Abualhasan, M. Comparison in vitro of antioxidant activity between Fifteen Campanula Species (Bellflower) from Palestinian flora. Phcog. J. 2015, 7, 276–279. [Google Scholar] [CrossRef]
- Dumlu, M.U.; Gurkan, E.; Tuzlaci, E. Chemical composition and antioxidant activity of Campanula alliariifolia. Nat. Prod. Res. 2008, 22, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, B.; Fandakli, S.; Barut, B.; Yildirim, S.; Ozlem Sener, S.; Ozturk, E.; Terzioglu, S.; Yayli, N. Volatile and phenolic components and antioxidant, acetylcholinesterase, tyrosinase, α-Glucosidase inhibitory effects of extracts obtained from Campanula latifolia L. subsp. latifolia. J. Essent. Oil-Bear. Plants 2020, 23, 1118–1131. [Google Scholar] [CrossRef]
- Sarikurkcu, C.; Sarikurkcu, R.T.; Tepe, B. Campanula macrostachya: Biological activity and identification of phenolics using a liquid chromatography electrospray ionization tandem mass spectrometry system. Environ. Sci. Pollut. Res. 2021, 28, 21812–21822. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, F.S.; Uysal, I.; Şabik, A.E.; Doğan, M.; Sevindik, M. Total phenolic and flavonoid contents, antimicrobial and antioxidant potentials of Campanula strigosa Banks & Sol. J. Microb. Biotech. Food Sci. 2023, 13, e10057. [Google Scholar] [CrossRef]
- Politeo, O.; Skocibusic, M.; Burcul, F.; Maravic, A.; Carev, I.; Ruscic, M.; Milos, M. Campanula portenschlagiana Roem. & Schult.: Chemical and antimicrobial activities. Chem. Biodivers. 2013, 10, 1072–1080. [Google Scholar] [CrossRef] [PubMed]
- Alhage, J.; Elbitar, H.; Taha, S.; Benvegnu, T. In vitro assessment of antioxidant, antimicrobial, cytotoxic, anti inflammatory, and antidiabetic activities of Campanula retrorsa crude extracts. Pharmacogn. Res. 2018, 10, 397–403. [Google Scholar] [CrossRef]
- Bourgou, S.; Ben Haj Jilani, I.; Karous, O.; Megdiche-Ksouri, W.; Ghrabi-Gammar, Z.; Libiad, M.; Khabbach, A.; El Haissoufi, M.; Lamchouri, F.; Greveniotis, V.; et al. Medicinal-cosmetic potential of the local endemic plants of Crete (Greece), Northern Morocco and Tunisia: Priorities for conservation and sustainable exploitation of neglected and underutilized phytogenetic resources. Biology 2021, 10, 1344. [Google Scholar] [CrossRef] [PubMed]
- Krigas, N.; Tsoktouridis, G.; Anestis, I.; Khabbach, A.; Libiad, M.; Megdiche-Ksouri, W.; Ghrabi-Gammar, Z.; Lamchouri, F.; Tsiripidis, I.; Tsiafouli, M.A.; et al. Exploring the Potential of Neglected Local Endemic Plants of Three Mediterranean Regions in the Ornamental Sector: Value Chain Feasibility and Readiness Timescale for Their Sustainable Exploitation. Sustainability 2021, 13, 2539. [Google Scholar] [CrossRef]
- Anestis, I.; Pipinis, E.; Kostas, S.; Karapatzak, E.; Dariotis, E.; Paradeisopoulou, V.; Greveniotis, V.; Tsoktouridis, G.; Hatzilazarou, S.; Krigas, N. GIS-Facilitated germination of stored seeds from four wild-growing populations of Petromarula pinnata (L.) A. DC.—A valuable, yet vulnerable local endemic plant of Crete (Greece). Agronomy 2024, 14, 274. [Google Scholar] [CrossRef]
- Arfini, F.; Bellassen, V. (Eds.) Sustainability of European Food Quality Schemes: Multi-Performance, Structure, and Governance of PDO, PGI, and Organic Agri-Food Systems; Springer Nature AG: Cham, Switzerland, 2019; p. 567. [Google Scholar] [CrossRef]

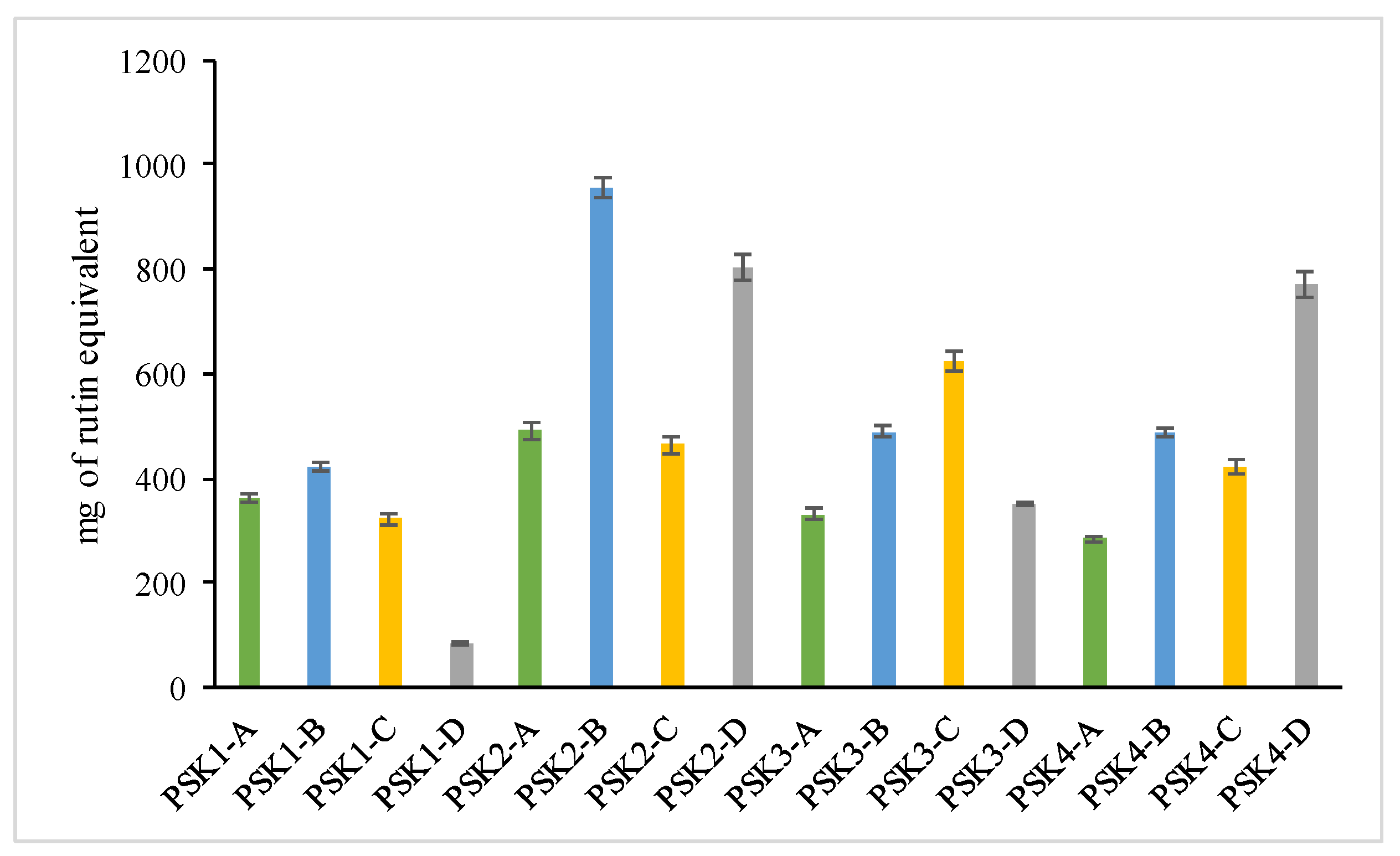

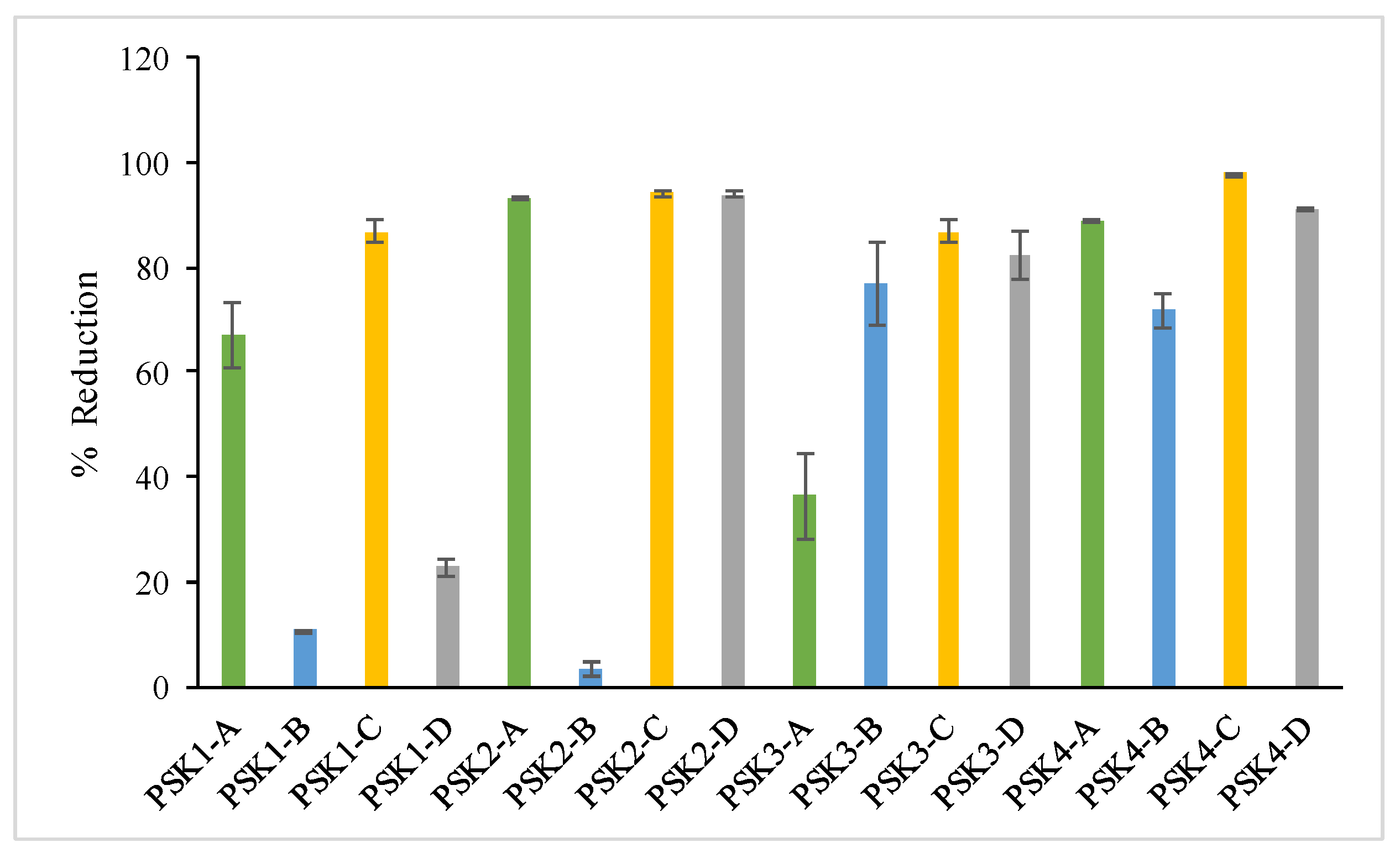
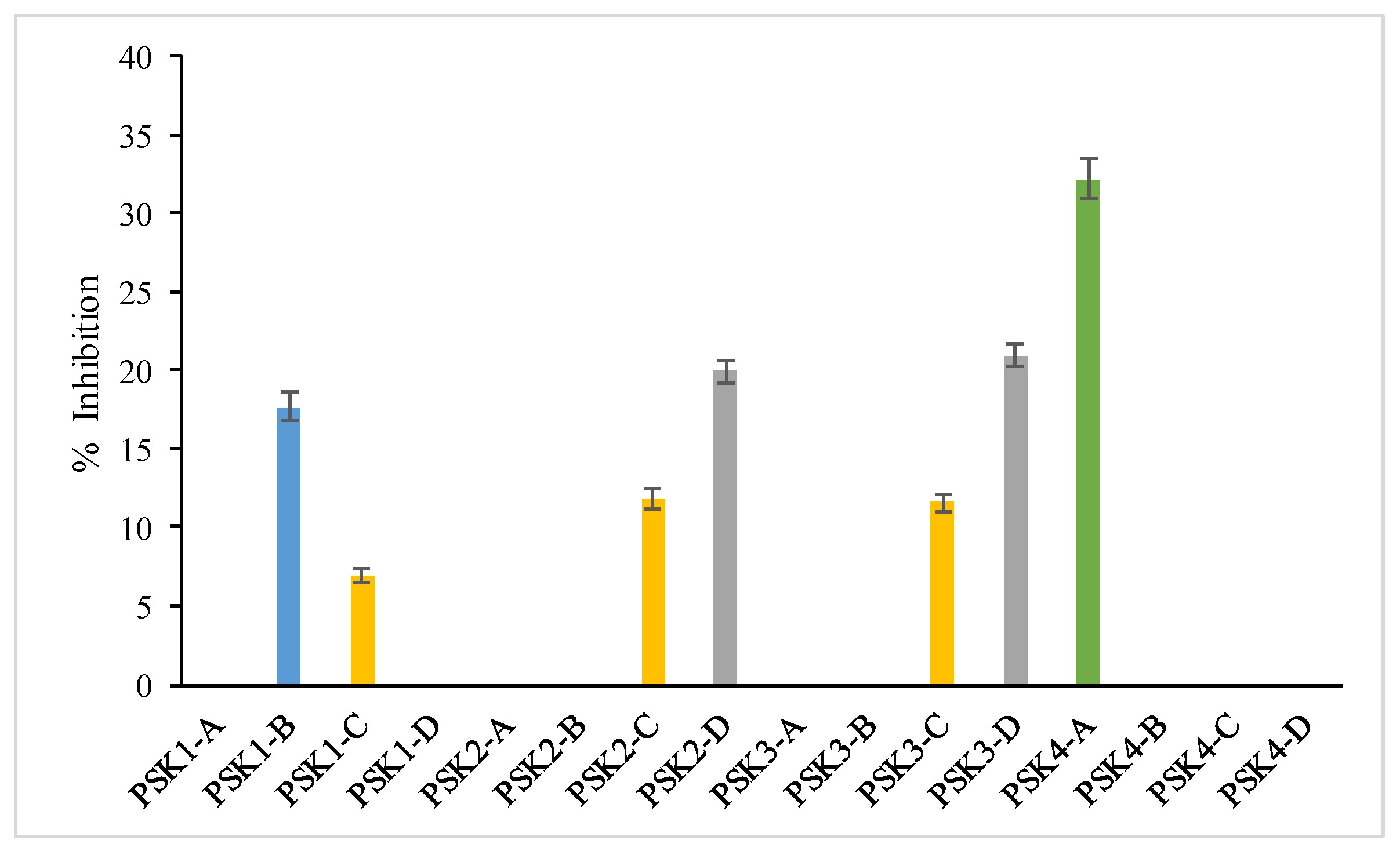
| Parameter | Results |
|---|---|
| Neutral detergent fiber (NDF As) | 40.04 ± 0.11% |
| Acid detergent fiber (ADF As) | 21.44 ± 0.06% |
| Moisture content | 10.19 ± 0.08% |
| Ash content | 9.02 ± 0.04% |
| Crude fiber | 4.14 ± 0.04% |
| Protein content | 13.27 ± 0.03% |
| Fat content | 2.98 ± 0.01% |
| Carbohydrate content | 64.54 ± 0.12% |
| Calcium | 0.40 ± 0.00% |
| Phosphorus | 0.32 ± 0.00% |
| Nutritive value | 338.08 ± 0.55 cal/100 g plant material |
| Processing Method | Code Name | Extraction Solvent | Weight of Extracts | Extraction Yield |
|---|---|---|---|---|
| Ground with liquid nitrogen | PSK1-A | Hexane | 0.413 ± 0.09 g | 0.35 ± 0.07% g |
| PSK1-B | Dichloromethane | 0.973 ± 0.12 g | 0.82 ± 0.10% f,g | |
| PSK1-C | Methanol | 4.053 ± 0.25 g | 3.52 ± 0.21% d | |
| PSK1-D | Methanol:H2O 70:30 | 3.245 ± 0.34 g | 2.73 ± 0.28% d,e,f | |
| Air-dried and then ground | PSK2-A | Hexane | 3.799 ± 0.20 g | 2.45 ± 0.13% d,e,f |
| PSK2-B | Dichloromethane | 5.595 ± 0.29 g | 3.61 ± 0.18% d | |
| PSK2-C | Methanol | 45.923 ± 2.95 g | 29.61 ± 1.90% a | |
| PSK2-D | Methanol:H2O 70:30 | 35.825 ± 2.50 g | 23.10 ± 1.61% b | |
| Ground with liquid nitrogen | PSK3-A | Hexane | 0.332 ± 0.07 g | 0.30 ± 0.06% g |
| PSK3-B | Dichloromethane | 0.789 ± 0.11 g | 0.71 ± 0.10% f,g | |
| PSK3-C | Methanol | 3.273 ± 0.26 g | 2.92 ± 0.23% d,e | |
| PSK3-D | Methanol:H2O 70:30 | 1.174 ± 0.17 g | 1.05 ± 0.15% e,f,g | |
| Air-dried and then ground | PSK4-A | Hexane | 1.303 ± 0.12 g | 2.98 ± 0.27% d,e |
| PSK4-B | Dichloromethane | 1.153 ± 0.04 g | 2.63 ± 0.10% d,e,f | |
| PSK4-C | Methanol | 10.876 ± 0.92 g | 24.83 ± 2.09% b | |
| PSK4-D | Methanol:H2O 70:30 | 6.314 ± 0.68 g | 14.41 ± 1.55% c |
| Sample | Total Phenolic Content (mg of Gallic Acid Equivalents per 100 g of Extract) | Total Flavonoids (mg of Rutin Equivalents per 100 g of Extract) | Percentage (%) TAC | Percentage (%) Interaction with the DPPH (20 min) Free Radical | Percentage (%) Inhibition of Lipid Peroxidation | Percentage (%) Inhibition of LOX |
|---|---|---|---|---|---|---|
| PSK1-A | 1267.10 ± 34.22 b | 363.18 ± 16.69 f | 57.34 ± 1.90 g,h | 67.03 ± 8.81 e | 2.53 ± 0.34 i | na |
| PSK1-B | 424.20 ± 12.30 d | 421.56 ± 15.04 e | 114.64 ± 8.95 d | 10.71 ± 0.26 h | 30.8 ± 2.13 e | 17.69 ± 1.46 c |
| PSK1-C | 45.85 ± 2.98 l | 322.03 ± 20.69 f,g | 72.75 ± 0.80 e,f | 86.81 ± 3.31 a,b,c | 2.99 ± 0.77 i | 6.88 ± 0.85 e |
| PSK1-D | 382.15 ± 6.83 e | 83.73 ± 5.38 h | 12.02 ± 1.29 i | 22.73 ± 2.25 g | 65.53 ± 6.39 d | na |
| PSK2-A | 280.40 ± 10.62 g | 490.46 ± 25.81 d | 104.72 ± 2.42 d | 93.4 ± 0.10 a,b | 83.12 ± 4.29 b | na |
| PSK2-B | 185.25 ± 5.76 i | 955.56 ± 36.58 a | 151.37 ± 5.48 a | 3.29 ± 1.61 h | 11.95 ± 3.45 h | na |
| PSK2-C | 149.85 ± 12.56 j | 464.62 ± 27.33 e | 102.32 ± 8.48 d | 94.16 ± 0.81 a,b | na | 11.79 ± 1.01 d |
| PSK2-D | 222.85 ± 9.74 h | 802.44 ± 44.63 a | 79.57 ± 4.59 e | 93.83 ± 0.57 a,b | 17.01 ± 1.87 g | 19.9 ± 1.38 b |
| PSK3-A | 877.70 ± 24.71 c | 331.60 ± 17.63 a | 100.88 ± 3.04 d | 36.26 ± 11.44 f | 29.43 ± 2.12 e | na |
| PSK3-B | 426.40 ± 10.04 d | 489.50 ± 18.77 d | 124.28 ± 1.83 c | 76.92 ± 11.24 c,d,e | 29.43 ± 2.97 e | na |
| PSK3-C | 304.70 ± 8.34 f,g | 622.53 ± 33.03 c | 109.71 ± 4.38 c,d | 86.81 ± 2.90 a,b,c | 2.53 ± 2.41 i | 11.55 ± 0.86 d |
| PSK3-D | 333.45 ± 4.19 f | 349.78 ± 6.23 f | 66.33 ± 1.30 f,g | 82.42 ± 6.49 b,c,d | 91.32 ± 4.32 a | 20.93 ± 1.34 b |
| PSK4-A | 1377.70 ± 20.94 a | 284.70 ± 8.34 g | 102.44 ± 9.24 d | 89.01 ± 0.17 a,b | na | 32.19 ± 2.13 a |
| PSK4-B | 81.25 ± 5.64 k | 486.63 ± 11.23 d | 129.06 ± 11.98 b,c | 71.75 ± 4.65 d,e | 24.6 ± 1.97 f | na |
| PSK4-C | 39.25 ± 1.23 l | 421.56 ± 24.81 e | 135.28 ± 5.28 b | 97.8 ± 0.03 a | na | na |
| PSK4-D | 180.8 ± 9.09 i | 770.86 ± 39.56 a | 52.58 ± 1.29 h | 91.21 ± 0.14 a,b | 71.69 ± 5.86 c | na |
| NGDA | - | - | - | 81.00 | 93.00 | - |
| Trolox | - | - | - | - | - | 95.00 |
| Ascorbic acid (1 mg/mL) | - | - | 100 | - | - | - |
| MIC (mg/mL) | Escherichia coli | Staphylococcus aureus | Enterococcus faecalis | Salmonella enteritidis |
|---|---|---|---|---|
| PSK1-A | 2.50 ± 0.025 b | 5.00 ± 0.010 e | 2.50 ± 0.046 c | 2.50 ± 0.026 b |
| PSK1-B | 5.00 ± 0.103 c | 5.00 ± 0.032 e | 5.00 ± 0.030 d | 5.00 ± 0.035 c |
| PSK1-C | 2.50 ± 0.025 b | 1.25 ± 0.015 c | 2.50 ± 0.021 c | 2.50 ± 0.015 b |
| PSK1-D | 5.00 ± 0.025 c | 2.50 ± 0.035 d | 5.00 ± 0.035 d | 5.00 ± 0.036 a |
| PSK2-A | 2.50 ± 0.025 b | 2.50 ± 0.010 d | 5.00 ± 0.010 d | 5.00 ± 0.038 c |
| PSK2-B | 5.00 ± 0.020 c | 5.00 ± 0.035 e | 5.00 ± 0.035 d | 5.00 ± 0.050 c |
| PSK2-C | 2.50 ± 0.025 b | 1.25 ± 0.025 c | 2.50 ± 0.030 c | 2.50 ± 0.025 b |
| PSK2-D | 2.50 ± 0.040 b | 2.50 ± 0.064 d | 5.00 ± 0.040 d | 5.00 ± 0.055 c |
| PSK3-A | 1.25 ± 0.025 a | 1.25 ± 0.040 c | 1.25 ± 0.025 b | 1.25 ± 0.035 a |
| PSK3-B | 5.00 ± 0.021 c | 2.50 ± 0.020 d | 2.50 ± 0.031 c | 2.50 ± 0.051 b |
| PSK3-C | 1.25 ± 0.046 a | 0.63 ± 0.010 b | 1.25 ± 0.035 b | 1.25 ± 0.015 a |
| PSK3-D | 1.25 ± 0.025 a | 1.25 ± 0.046 c | 2.50 ± 0.044 c | 2.50 ± 0.035 b |
| PSK4-A | 1.25 ± 0.015 a | 1.25 ± 0.025 c | 2.50 ± 0.040 c | 2.50 ± 0.025 b |
| PSK4-B | 2.50 ± 0.055 b | 2.50 ± 0.055 d | 2.50 ± 0.046 c | 2.50 ± 0.020 b |
| PSK4-C | 1.25 ± 0.025 a | 0.31 ± 0.015 a | 0.63 ± 0.025 a | 1.25 ± 0.015 a |
| PSK4-D | 2.50 ± 0.032 b | 2.50 ± 0.040 b | 2.50 ± 0.040 c | 2.50 ± 0.020 b |
| Ampicillin | 0.03 ± 0.001 | - | - | 0.02 ± 0.002 |
| Gentamycin | - | 0.02 ± 0.001 | 0.02 ± 0.003 | - |
| Superfood (Nutraceutical) Potential | Identified Ethnobotanical Uses | Poisonousness-Toxicity | Identified Phytochemical Compounds | European Mdicines Agency (EMA) Monograph Status | Approved Indications | Medicinal Potential | Distrinct Ethnobotanical Uses | Distrinct Medicinal Properties | Overall Medicinal Potential |
|---|---|---|---|---|---|---|---|---|---|
| 0 → 6 | 6 | 6 | 0 → 6 | 0 | 0 | 6 | 1 → 3 | 1 → 3 | Average (37.04%) → High (66.67%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dimitriadis, K.M.; Karavergou, S.; Tsiftsoglou, O.S.; Karapatzak, E.; Paschalidis, K.; Hadjipavlou-Litina, D.; Charalambous, D.; Krigas, N.; Lazari, D. Nutritional Value, Major Chemical Compounds, and Biological Activities of Petromarula pinnata (Campanulaceae)—A Unique Nutraceutical Wild Edible Green of Crete (Greece). Horticulturae 2024, 10, 689. https://doi.org/10.3390/horticulturae10070689
Dimitriadis KM, Karavergou S, Tsiftsoglou OS, Karapatzak E, Paschalidis K, Hadjipavlou-Litina D, Charalambous D, Krigas N, Lazari D. Nutritional Value, Major Chemical Compounds, and Biological Activities of Petromarula pinnata (Campanulaceae)—A Unique Nutraceutical Wild Edible Green of Crete (Greece). Horticulturae. 2024; 10(7):689. https://doi.org/10.3390/horticulturae10070689
Chicago/Turabian StyleDimitriadis, Kyriakos Michail, Sofia Karavergou, Olga S. Tsiftsoglou, Eleftherios Karapatzak, Konstantinos Paschalidis, Dimitra Hadjipavlou-Litina, Despina Charalambous, Nikos Krigas, and Diamanto Lazari. 2024. "Nutritional Value, Major Chemical Compounds, and Biological Activities of Petromarula pinnata (Campanulaceae)—A Unique Nutraceutical Wild Edible Green of Crete (Greece)" Horticulturae 10, no. 7: 689. https://doi.org/10.3390/horticulturae10070689









