Abstract
In this study, short-term liquid nitrogen (LN) storage was used as a strategy to conserve Pyrostegia venusta embryos, and its effects on in vitro germination, phenotypic and biochemical characteristics, and in vitro secondary metabolite production were assessed. Embryos stored in LN for 1 and 7 days presented a higher germination rate and germination speed index compared to those of the control (non-cryostored embryos). Short-term LN storage also favored the phenotypic characteristics of seedlings. LN storage significantly affected the proteins (PTN), soluble sugar (SS) and reducing sugar (RS) contents, oxidative metabolism, and phenylalanine ammonia-lyase (PAL) activity, as well as the total phenolic compound, flavonoid, phytosterol, and alkaloid levels in seedlings regenerated from embryos cryostored for 7 days. Benzoic acid derivatives and flavonoids were observed in regenerated non-acclimatized seedlings. LN storage did not affect the survival rate or phenotypic characteristics of seedlings during acclimatization. Acclimatization promoted significant changes in PTN, SS and RS contents, oxidative metabolism, and PAL activity in seedlings from embryos cryostored for 7 days. Roots from acclimatized seedlings exhibited the highest phenolic, phytosterol, and total alkaloid levels. Differences in the chromatographic profiles of the acclimatized seedlings compared with the non-acclimatized seedlings were observed. LN storage can be an effective means of ex situ conservation of P. venusta genetic resources.
1. Introduction
Pyrostegia venusta (Ker-Gawl.) Miers (Bignoniaceae) is a woody vine with dense branches and pantropical occurrence, popularly known as “orange-trumpet vine” or “cipó-de-São-João” [1]. In folk medicine, it is used to treat coughs, bronchitis, colds, diarrhea, and vitiligo [2,3,4,5]. Ethnopharmacological studies have shown its melanogenic, antitumor, vasodilatory, anthelmintic, antinociceptive, antioxidant, antimicrobial, and immunomodulatory activities [5,6,7,8,9,10,11,12,13,14].
Plant species that produce bioactive compounds are often obtained from predatory collections. The bioprospecting of plant species that produce secondary metabolites of economic and/or medicinal interest stands out as a factor of strategic importance for the economies of developing countries or countries that exhibit abundant biodiversity [15]. However, the advancement of human activities can promote the scarcity or extinction of numerous species that reflect ecological potential and/or pharmaceutical interest, even before they are studied [16]. In vitro culture can be used as a strategy to allow the generation of many propagules from a minimal amount of initial plant material in the natural habitat [17]. The use of these techniques in germplasm conservation has been successfully applied to the conservation of several species, both for propagation and storage [17,18]. In vitro culture allows for continuous production of the plant or plant parts in a short period of time and under aseptic and controlled temperature and light conditions, regardless of climatic and environmental conditions, avoiding predatory collection [18].
Seed and embryo storage are effective methods for the ex situ conservation of plant genetic resources [19]. Ex situ conservation methods consist of conserving the germplasm outside its natural ecosystem under controlled conditions. Cryopreservation, which can be understood as the preservation of cells, tissues, organs, or even complete organisms at very low temperatures, is among the methodologies employed [20,21]. Cryopreservation is often achieved by storing biological materials in liquid nitrogen (LN, −196 °C) or storing them in their vapor phase (<−160 °C) [20]. Low temperatures slow down metabolic processes, such as photosynthesis and respiration, to balance the decrease in sugar production [22]. However, cryopreservation procedures are species-specific and need to be evaluated for a species or genotypes targeted for research [23]. The advantages of cryopreservation include the storage of plant material at small volumes, protection from contamination, very limited maintenance requirements, and a long storage period, without alteration or modification [20,21,24,25]. According to Zevallos et al. [26], short-term LN storage should be tested for each plant material before using cryopreservation for long-term storage, since these studies can predict the effect of long-term LN exposure on seed viability and germination. Several studies have described short- and long-term cryopreservation techniques using different plant materials [17,27,28,29,30,31]. Successful cryopreservation procedures rely on a high-quality propagule source, and its selection depends on the plant species and program goals, i.e., whether population genetic diversity or clones are the program conservation targets [32].
To date, to the best of our knowledge, there are no established protocols for the ex situ conservation of P. venusta germplasm using embryos as a source of propagules, despite, as highlighted before, its use with recognized therapeutic and biotechnological potential [5,33,34,35,36,37], and the plant has been inserted and dispersed in regions of intense anthropogenic action [1,38]. In nature, Pyrostegia venusta regenerates through seeds, which are only produced during a few months of the year (September to December), and the establishment of seedlings is slow, hampered by unfavorable environmental conditions and the attack of pathogens and herbivores [39]. Furthermore, the seeds exhibit low germination rates due to integumentary dormancy [40].
The therapeutic and biotechnological potential of P. venusta, associated with the absence of information on the effects of LN storage of embryos, makes this species a good candidate for in vitro and ex situ conservation studies. Thus, the aims of this study were to evaluate the effects of short-term LN storage of P. venusta zygotic embryos on in vitro germination, phenotypic and biochemical characteristics, and in vitro secondary metabolite production in regenerated seedlings.
2. Materials and Methods
2.1. Chemicals and Reagents
The chemicals used in this study were purchased from different manufacturers. Benzoic acid, caffeic acid, cinnamic acid, chlorogenic acid, gallic acid, quercetin, rutin, allantoin, β-sitosterol, (−)-epicatechin, (±)-catechin, formic acid, tris(hydroxymethyl)-aminomethane, methanol and agar were purchased from Sigma-Aldrich (St. Louis, MO, USA). Ethanol, potassium phosphate, malondialdehyde, hydrogen peroxide, 5,5’-dithiobis-2-nitrobenzoic acid, bismuth nitrate, potassium iodide, acetic anhydride, and the salts that make up the Murashige and Skoog medium [41] were purchased from Vetec Química Fina (Duque de Caxias, Rio de Janeiro, Brazil). Sodium hypochlorite was purchased from Start Química® (Uberlândia, Minas Gerais, Brazil). Vermiculite was purchased from Brasil Minérios (Goiânia, Goiás, Brazil). N-(trichloromethylthio) cyclohex-4-ene-1,2-dicarboximide (Captan SC®) was purchased from Adama Brasil S/A (Londrina, Paraná, Brazil). Ultrapure water was produced by reverse osmosis via the Elga PureLab Option-Q water purification system (Veolia Water Technologies, Lane End, High Wycombe, UK).
2.2. Plant Material
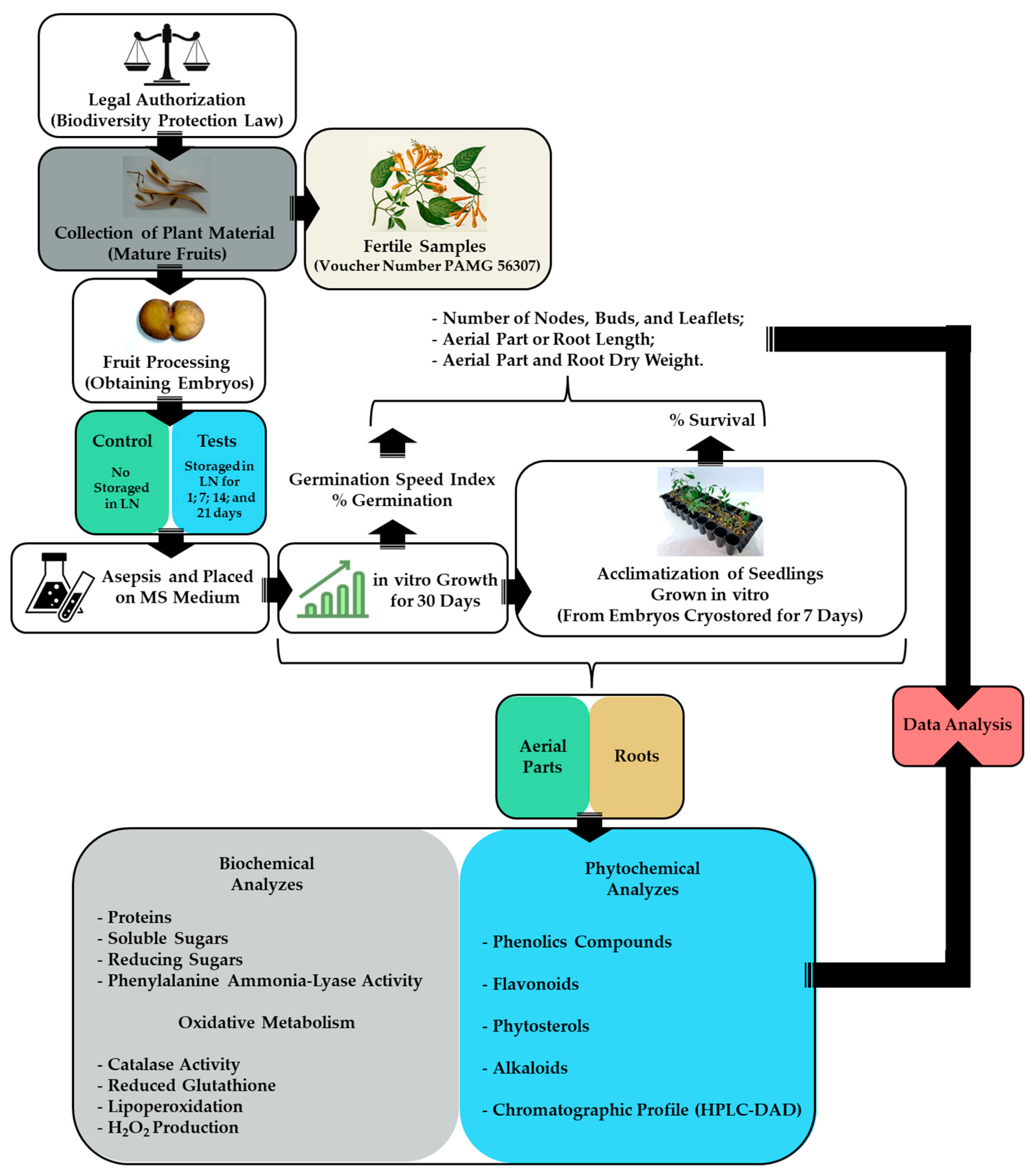
Pyrostegia venusta (Ker-Gawl.) Miers (Bignoniaceae) seeds were obtained from ripe fruits collected in October 2017 (Spring) in the Brazilian Cerrado located in Divinópolis, Minas Gerais State, Brazil (21°11′36.97″ S and 44°55′59.7″ W, at an altitude of 850 m) (SISBIO n◦ 24542-6, IBAMA Registration: 5042260). The fruits were transported from the collection site to the laboratory in styrofoam boxes and were immediately processed to remove the seeds. The seeds were treated for 5 min with Captan SC® at a proportion of 1 g Kg−1 of seeds and stored in a cold room at 4 °C until use [34,40]. Fertile samples were collected, and vouchers were identified by Andreia Fonseca Silva of the PAMG Herbarium (PAMG 56307) at the Agricultural Research Company of Minas Gerais (Belo Horizonte, Brazil). This work is registered on the SisGen Platform (Register A12A940), according to Brazilian Biodiversity Law (13.123/2015). A flowchart of the experimental steps is shown in Figure 1.
Figure 1.
Flowchart of the experimental steps.
2.3. Obtaining Embryos, Cryostorage, and In Vitro Germination
Pyrostegia venusta embryos were obtained by removing the seed coats using a scalpel. The moisture content of P. venusta seeds at the time of the embryo’s storage was 10.81 ± 0.10%, based on the gravimetric method, using an oven (EST.410, Ethiktechnology, Vargem Grande Paulista, Brazil) at 105 ± 2 °C, according to the methods of Brazil [42]. Five embryos (5 mm long) were stored in 5 mL cryovials and immersed directly in liquid nitrogen (LN) at −196 °C for 1, 7, 14, and 21 days. After this period, the cryovials were removed from the LN and thawed directly in water at 40 ± 1 °C for 2 min [43]. Pyrostegia venusta embryos not subjected to LN storage were used as controls.
In a laminar flow chamber (Airstream Class II B2, Esco Lifesciences Group, Singapore), the cryovials were opened, and the embryos were surface sterilized by immersion in 70% ethanol for 1 min and 50% commercial sodium hypochlorite (2.5% active chlorine) for 5 min and then rinsed three times using sterile distilled water. The embryos were placed on 10 mL of MS medium [41] with a 50% salt concentration, supplemented with 30 g L−1 of sucrose, solidified with 7 g L−1 of agar, and pH adjusted to 5.8 ± 0.1 before autoclaving. The experimental design used was completely randomized, with 30 replications per treatment, with each replication consisting of a tube containing an embryo. The material was kept in a controlled environment for 30 days at 25 ± 2 °C, with a photoperiod of 16 h and a photosynthetically active photon flux density of 35 μmol m−2 s−1. At the end of the incubation period, the seedlings were evaluated for: (a) germination percentage (embryos with radicle protrusion were considered germinated); (b) germination speed index (GSI) [44]; (c) number of nodes, buds, and leaflets; (d) aerial part and root length; and (e) aerial part and root dry weight. Seedlings from embryos stored for 7 days in LN and control plants were also evaluated for: (a) soluble protein (PTN), soluble sugar (SS), and reducing sugar (RS) contents; (b) oxidative metabolism; (c) PAL activity; and (d) total phenolic compound, flavonoid, phytosterol, and alkaloid contents. Chromatographic profiles based on high-performance liquid chromatography analyses of phenolic compounds found in hydroethanolic extracts of aerial parts and roots of P. venusta seedlings were obtained. The full description of how these parameters were evaluated is detailed below.
2.4. Acclimatization
Seedlings that were 45 days old, obtained from embryos stored for 7 days and from the control (embryos not stored in LN), were initially subjected to pre-acclimatization for 24 h by opening the culture container inside the growth room under the following conditions: a temperature of 25 ± 2 °C, a photon irradiance of 35 μmol m−2 s−1, and a 16 h photoperiod [45]. For pre-acclimatization, seedlings with aerial parts of 8 to 10 cm, a radicle of 5 to 7 cm, and at least nine leaflets were selected. After this period, the seedlings were transferred to 53 cm3 tubes containing vermiculite, wrapped with transparent plastic bags to maintain humidity, and placed in an external environment with natural light (17 to 26 °C; 11 h photoperiod). Every 3 days, the plastic bags were progressively opened with the aid of scissors to allow for the gradual reduction of humidity inside the bags until the bags were completely removed [46]. The experimental design used was completely randomized, consisting of 20 replications per treatment, with each replication consisting of a tube containing one seedling. At the end of 45 days of acclimatization, the following characteristics were evaluated: (a) seedling survival rate; (b) number of nodes, buds, and leaflets; (c) aerial part and root length; (d) aerial part and root dry weight; (e) PTN, SS, and RS contents; (f) oxidative metabolism; (g) PAL activity; and (h) total phenolic compound, flavonoid, phytosterol, and alkaloid contents. Chromatographic profiles based on HPLC-DAD analyses of phenolic compounds present in hydroethanolic extracts of aerial parts and roots of P. venusta seedlings were obtained.
2.5. Biochemical Analysis
2.5.1. Preparation of Extracts
Approximately 1 g of the fresh sample (aerial part or roots) was crushed with the aid of LN in 4 mL of extraction buffer (0.01 M potassium phosphate). This mixture was centrifuged at 18,000× g at 4 °C for 10 min [47], and the supernatant was used for biochemical assays. All assays were conducted in triplicate.
2.5.2. Proteins, Soluble and Reducing Sugars
Total Soluble Proteins
The determination of PTN levels was carried out using the Bradford [48] method. The samples were subjected to analysis using a spectrophotometer (UV-1800, Shimadzu, São Paulo, Brazil) with a wavelength of 595 nm. PTN quantification was carried out by constructing a calibration curve using a bovine serum albumin solution as a standard, and the results were expressed in milligrams of PTN per gram of fresh biomass (mg PTN g FB−1).
Total Soluble Sugars
To determine total SS levels, the methodology described by Yemm and Willis [49] was used. The samples were subjected to analysis using a wavelength of 620 nm. SS quantification was carried out by constructing a calibration curve using a glucose solution as a standard, and the results were expressed in milligrams of SS per gram of fresh biomass (mg SS g FB−1).
Total Reducing Sugars
To determine the RS levels, the methodology described by Miller [50] was followed. The samples were subjected to analysis using a wavelength of 540 nm. RS quantification was carried out by constructing a calibration curve using a glucose solution as a standard, and the results were expressed in milligrams of RS per gram of fresh biomass (mg RS g FB−1).
2.5.3. Oxidative Metabolism
Catalase Activity
To determine the catalase (CAT) activity, a reaction medium was prepared containing 18 mL of H2O2 solution (10% in distilled water), 1 mL of 1 M Tris-HCl buffer, pH 8.5 mM EDTA, and 800 μL of distilled water. Then, 995 μL of the reaction medium and 5 μL of the prepared extract were placed in a quartz cuvette [51]. The reading was carried out using a spectrophotometer at a wavelength of 230 nm for 1 min. The activity calculation was performed using the following equation: (2.3/Dt) × (a/b) × (log A1/A2), where a = sample volume in the cuvette; b = total volume of the cuvette; A1 = absorbance value at t = 0; and A2 = the absorbance value at the final time, which, in this case, occurred every 15 s during the reaction. All experiments were performed in triplicate, and the results were expressed in units of CAT per milligram of protein (UCAT mg PTN−1).
Reduced Glutathione
The concentration of reduced glutathione (GSH) was determined based on the Ellman reagent reaction, where 5,5’-dithiobis-2-nitrobenzoic acid (DTNB) reacts with the free thiol, generating a more acidic mixed disulfide 2-nitro-5-thiobenzoic acid [52]. After 20 min of reaction, the absorbance was read at 412 nm. Values were expressed in milligrams of GSH per gram of fresh biomass (mg GSH g FB−1).
Lipoperoxidation
Lipid peroxidation (LP) levels were determined by quantifying the thiobarbituric acid reactive substance (TBARS) levels, as described by Kramer et al. [53]. The absorbance was determined at 532 nm, and to obtain the net absorbance values, this value was discounted from the non-specific absorbance at 600 nm. Calculations relating to the release of peroxides through the standard malondialdehyde (MDA) were carried out according to the equation {[(A532 − A600)/157,000] 106} and expressed in milligrams of MDA per gram of fresh biomass (mg MDA g FB−1).
H2O2 Production
The H2O2 concentration was determined using the methodology described by Alexieva et al. [54]. H2O2 production was calculated from a standard curve using hydrogen peroxide PA at concentrations from 0 to 100 nM. The samples were subjected to analysis using a wavelength of 390 nm. Values were expressed in milligrams of H2O2 per gram of fresh biomass (mg H2O2 g FB−1).
2.5.4. Phenylalanine Ammonia-Lyase (PAL) Activity
PAL activity was determined by quantifying the trans-cinnamic acid released from phenylalanine, according to the methodology described by Mori et al. [55]. The absorbance was determined at 290 nm, and the control, composed of 50 μM phenylalanine and 100 μL of 100 mM Tris-HCl buffer (pH 8.8), was heated at 37 °C for 1.5 h, and 50 μL HCl 6 N was added. The results were expressed in units of PAL per milligram of protein (UPAL mg PTN−1, U = nmol cinnamic acid h−1).
2.6. Phytochemical Analysis
2.6.1. Preparation of Extracts
Hydroethanolic extracts were prepared from 1 g of dry sample (aerial part and root) and extracted with 10 mL of 70% ethanol (70% EtOH) by sonication at 35 °C for 30 min. The crude extract (CE) was used for phytochemical analysis. All assays were conducted in triplicate.
2.6.2. Total Phenolic Compounds
The content of total phenolic compounds was determined using the Folin–Ciocalteu method described by Slinkard and Singleton [56], using gallic acid as the standard. The absorbance was measured at 750 nm with a UV-Vis spectrophotometer, and the results were expressed in micrograms of gallic acid equivalents per milligram of crude extract (μg GAEq mg−1 CE).
2.6.3. Total Flavonoids
The total flavonoid content was determined using the aluminum chloride method, employing rutin as a standard [57]. Absorbance was measured at 425 nm, and the results were expressed in micrograms of rutin equivalents per milligram of crude extract (μg RutEq mg−1 CE).
2.6.4. Total Phytosterols
The total phytosterol content was determined using the Liebermann–Buchard method, employing β-sitosterol as the reference substance [58]. The absorbance was measured at 625 nm, and the results were expressed in micrograms of β-sitosterol equivalents per milligram of crude extract (μg β-sitEq mg−1 CE).
2.6.5. Total Alkaloids
The total alkaloid content was determined using the Dragendorff method, as described by Sreevidya and Mehrotra [59], with allantoin as the standard. The absorbance was measured at 435 nm, and the results were expressed in micrograms of allantoin equivalents per milligram of crude extract (μg AllanEq mg−1 CE).
2.6.6. High Performance Liquid Chromatography (HPLC-DAD)
The analyses were carried out on a modular liquid chromatography system, UFLC Prominence (Shimadzu Corp., Kyoto, Japan), consisting of two pumps (LC-20AD), a diode array detector (DAD) (SPD M20A), and a computerized system, employing LabSolutions software (Version 1.2). The compounds were separated on a C18 reversed phase analytical column (Kromasil 5 μm, 4.6 × 5 mm) and stored at room temperature. The mobile phases used, in gradient form, were: A—ultrapure water:0.1% formic acid (99.9:0.1) and B—methanol:0.1% formic acid (99.9:0.1). A 20 µL injection volume and a flow rate of 1.0 mL min−1 were employed. The gradient program was as follows: 0 min, 10% B; 0.01–4 min, 10 to 20% B; 4–6 min, 20 to 50% B; 6–10 min, 50 to 90% B; 10–11 min, 90 to 10% B; 11–15 min, 10% B. The analysis time was 15 min. Separations were monitored at 254, 280, 330, and 358 nm to detect phenolic compounds, phenolic acids, and flavonoids. To determine the compounds in the samples, retention times and spectra in the ultraviolet region of the injected standards were compared, as were data in the literature [60,61,62]. Three replicates were carried out on the same day.
2.7. Data Analyses
A one-way analysis of variance (ANOVA) test was used, followed by a Scott–Knott’s test to compare the results between the groups and the control, with a p-value < 0.05 deemed significant. The results are expressed as the mean ± standard deviation. The software used for the statistics was SISVAR 5.3 [63].
3. Results
3.1. Cryostorage and In Vitro Germination
Exposure to LN significantly affected the germination percentage and GSI of the P. venusta embryos (p < 0.05) (Table 1). Embryos stored in LN for 1 and 7 days showed a higher germination rate and GSI, with the germination percentage being approximately 10% higher in the cryostored embryos. Short-term LN storage also favored an increase in the number of buds in seedlings from embryos stored for 1, 7, and 21 days and did not affect the number of nodes and leaflets in relation to the control seedlings (p > 0.05). There was no contamination in the culture medium or embryos.

Table 1.
Influence of exposure of Pyrostegia venusta embryos to LN storage on germination percentage (G); germination speed index (GSI); number of nodes, buds, and leaflets; aerial part (SL); root length (RL); and dry weight of aerial parts (SDW) and roots (RDW) of seedlings after 45 days of in vitro culture of embryos in the presence of light. Control = seedlings from embryos not stored in LN. The results are presented as the mean ± standard deviation of 10 repetitions.
Significant changes in the aerial part and root length were also observed (p < 0.05) (Table 1). Seedlings from embryos stored in LN for 1 and 7 days showed greater aerial part length when compared to that of the control seedlings (p < 0.05). However, storage in LN for 1, 7, and 14 days reduced the root length, and only seedlings from embryos stored in LN for 21 days showed root lengths equal to those observed for the control seedlings. Storage in LN did not affect the dry weight of the aerial part and roots.
3.2. Acclimatization
LN storage did not affect the seedlings’ survival rate during the acclimatization process. Seedlings from embryos stored in LN for 7 days (+LN) showed a survival rate of 100% after acclimatization, a value slightly higher than the survival rate of seedlings from non-stored embryos (control) (95%) (Table 2). There was no significant variation in the number of nodes, buds, or leaflets; in the length of the aerial part and roots; or in the root dry biomass (p > 0.05). However, seedlings from embryos stored in LN showed a higher dry weight of the aerial part after acclimatization compared to that of the control seedlings (p < 0.05) (Table 2). These results indicate that LN storage of embryos does not drastically affect the phenotypic characteristics of the seedlings during the acclimatization process.

Table 2.
Influence of embryo storage in LN for 7 days on the number of nodes, buds, and leaflets; the length of the aerial parts (SL) and roots (RL); and the dry weight of the aerial parts (SDW) and roots (RDW) in 45-day-old Pyrostegia venusta seedlings after in vitro germination and acclimatization, using vermiculite as a substrate. The results are presented as the mean ± standard deviation of 20 repetitions.
3.3. Biochemical Analyzes
LN storage significantly affected the biochemical characteristics represented by PTN, SS, and RS contents; oxidative metabolism; and PAL activity in seedlings regenerated from embryos cryostored for 7 days (p < 0.05) (Table 3). The roots of seedlings from embryos stored in LN showed high levels of SS and RS and a lower PTN content. High CAT activity in the roots and high GSH, LP, and HPP levels in the aerial parts were also observed, demonstrating that storage in LN affected oxidative metabolism in these organs. Seedlings from embryos stored in LN showed a reduction in PAL activity in the aerial parts and an increase in PAL activity in the roots in relation to those of the control seedlings.

Table 3.
Influence of exposure of embryos to LN on the total soluble protein (PTN), total soluble sugar (SS) and reducing sugar (RS) contents, oxidative metabolism (catalase activity—CAT, reduced glutathione content—GSH, lipoperoxidation levels—LP, hydrogen peroxide production—HPP), and phenylalanine ammonia-lyase (PAL) activity in the aerial parts and roots of Pyrostegia venusta seedlings after 45 days of in vitro culture. The results are presented as the mean ± standard deviation of three repetitions.
Acclimatization promoted significant changes in PTN, SS, and RS contents; oxidative metabolism; and PAL activity in seedlings from embryos stored for 7 days in LN (p < 0.05) (Table 4). In general, the aerial parts and roots of acclimatized seedlings showed significant reductions in PTN, SS, LP, and HPP contents compared to those of the non-acclimatized seedlings. The roots of the acclimatized seedlings showed a higher CAT activity and high levels of RS and GSH compared to those of the non-acclimatized seedlings. Greater PAL activity was observed in the aerial parts of the acclimatized seedlings; this activity was much higher than that seen in the aerial parts of the non-acclimatized seedlings. PAL activity remained unchanged in the roots of both the acclimatized and non-acclimatized seedlings.

Table 4.
Influence of acclimatization on the total soluble protein (PTN), total soluble sugar (SS) and reducing sugar (RS) contents, oxidative metabolism (catalase activity—CAT, reduced glutathione content—GSH, lipoperoxidation levels—LP, hydrogen peroxide production—HPP), and phenylalanine ammonia-lyase (PAL) activity in the aerial parts and roots of Pyrostegia venusta seedlings from embryos stored for 7 days in LN. The results are presented as the mean ± standard deviation of three repetitions.
3.4. Phytochemical Analyzes
Table 5 shows the secondary metabolite contents in seedlings grown under in vitro culture for 30 days. The total phenolic compound, flavonoid, phytosterol, and alkaloid contents varied significantly in seedlings from embryos stored in LN compared to those of the control (p < 0.05). The total phenolic compound content was more pronounced in the roots and aerial parts of seedlings from embryos exposed to LN for 7 days, while the total flavonoid and alkaloid levels were higher in these roots compared with those of the control (p < 0.05). The alkaloid level in the aerial parts remained similar to that of the control (p > 0.05). The total phytosterol level was markedly lower in the aerial parts and roots of seedlings from embryos stored in LN.

Table 5.
Influence of exposure of embryos to liquid nitrogen for 7 days on the total phenolic compound, flavonoid, phytosterol, and alkaloid contents in Pyrostegia venusta seedlings cultivated in vitro for 45 days. The results are presented as the mean ± standard deviation of three repetitions.
Acclimatization significantly influenced the secondary metabolite levels present in the aerial parts and roots of seedlings from embryos stored in LN for 7 days (p < 0.05) (Table 6). Roots from acclimatized seedlings exhibited the highest total phenolics, phytosterols, and alkaloid levels compared to those of the control (p < 0.05). Higher total flavonoid contents were observed in the aerial parts of both the acclimatized and non-acclimatized seedlings than those observed in the roots (p < 0.05). However, compared to the control, it was found that flavonoid levels in the aerial parts remained constant in the acclimatized and non-acclimatized seedlings, and the roots of the acclimatized seedlings showed an increase in total flavonoid contents (p < 0.05).

Table 6.
Influence of acclimatization on the total phenolic compounds, flavonoids, phytosterols, and alkaloid contents in Pyrostegia venusta seedlings from embryos stored for 7 days in liquid nitrogen. The results are presented as the mean ± standard deviation of three repetitions.
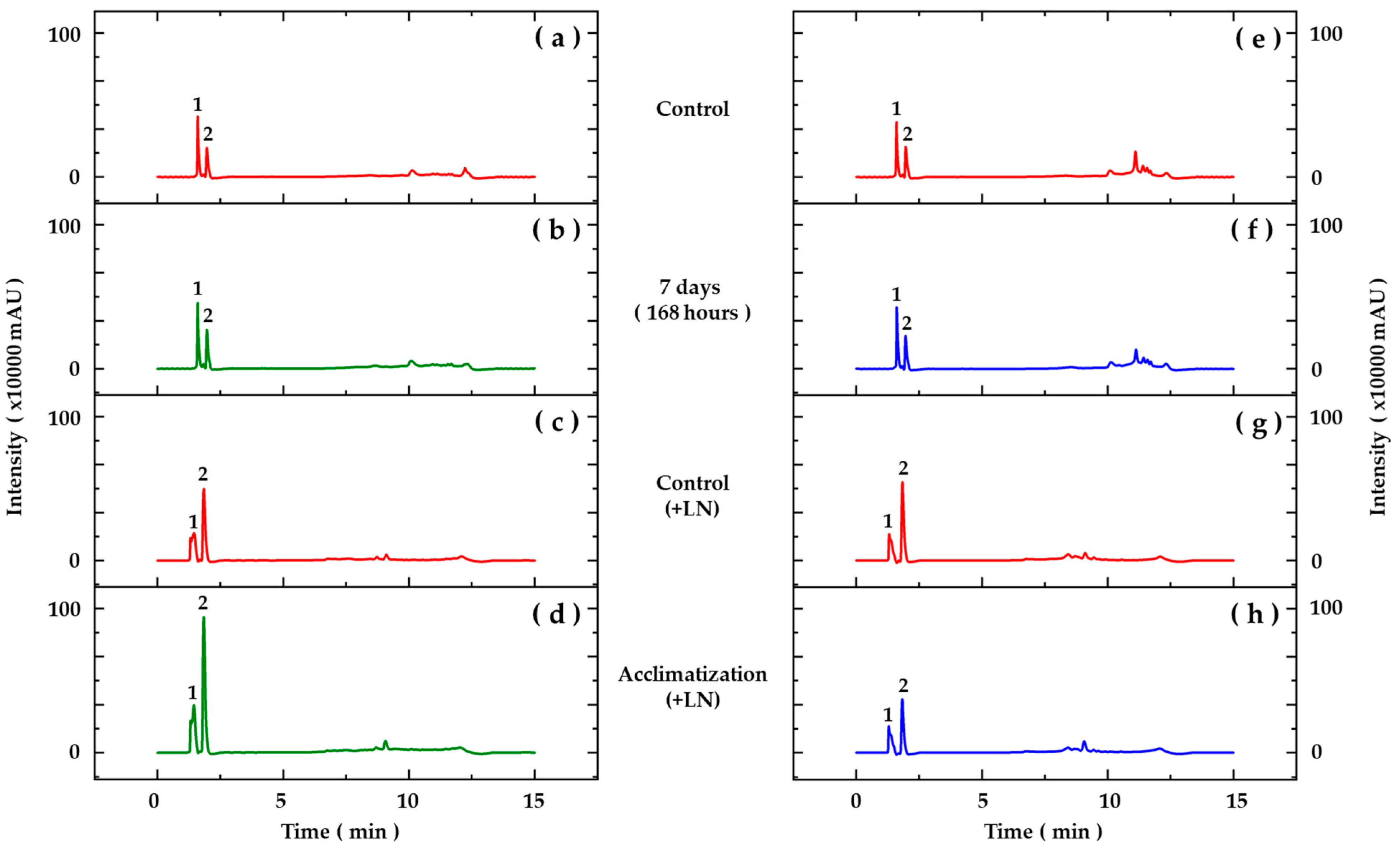
The presence of some phenolic acid derivatives and flavonoids was observed through comparisons with the retention times and UV spectra of previously injected standards and data found in the literature (Figure 2, Table 7). The results obtained for the aerial parts (Figure 2a,b) and roots (Figure 2e,f) of seedlings from cryopreserved and control embryos showed the majority presence of substances characterized as benzoic acid derivatives (Table 7). The retention times and UV spectra obtained suggest that the smaller peaks, with retention times after 7 min, correspond to the presence of flavonoids; however, due to the small amount of these substances in the samples, they were not characterized.
Figure 2.
Chromatographic profiles based on HPLC-DAD analyses of hydroethanolic extracts from aerial parts (a–d) and roots (e–h) of Pyrostegia venusta seedlings grown in vitro (a,b,e,f) and acclimatized for 45 days (c,d,g,h). Detection at 280 nm. Peak numbers correspond to assignation, as described in Table 7.

Table 7.
Profile of phenolic compounds and flavonoids identified by HPLC-DAD analysis for Pyrostegia venusta samples. Rt = retention time.
In acclimatized seedlings, the analyses carried out by HPLC-DAD revealed differences in the chromatographic profiles of the hydroethanolic extracts (Figure 2). The results obtained for the aerial parts (Figure 2c,d) showed the majority presence of substances characterized as benzoic and cinnamic acid derivatives, and in the roots (Figure 2g,h), substances derived from cinnamic acids were characterized (Table 7). Likewise, smaller peaks indicating the presence of flavonoids were observed.
4. Discussion
Until recently, most ex situ conservation strategies regarding plant biodiversity have concentrated on crop species [20]. However, currently, the improvement in techniques for conservation of plants with medicinal potential has also become the target of important studies [64,65,66,67,68]. For medicinal species, a traditional conservation approach involves in vitro conservation using plant tissue culture [69]. Tissue culture techniques are of great interest for the collection, multiplication, and storage of plant germplasm [20]. However, several studies have shown that other ex situ techniques can be used efficiently to complement in vitro methods, representing a good option for the safe conservation of species of pharmacological interest [70,71]. Cryopreservation is one of the main methods currently used for ex situ conservation. Using this technique, seeds, dormant buds, cell suspensions, calli, apices, and zygotic and somatic embryos of numerous plant species have been successfully preserved [20]. At ultra-low temperatures (liquid nitrogen, −196 °C), all cellular divisions and metabolic processes are stopped, and the plant material can thus be stored, without alteration or modification, for a theoretically unlimited period [20]. Successful cryopreservation techniques inhibit metabolic processes and maintain propagules in a latent state, without the formation of ice crystals in the intracellular environment, thereby providing the conditions for indefinite preservation [20,72]. Therefore, for the success of cryopreservation protocols, it is extremely important to maintain an adequate moisture content in the tissues to be cryopreserved [73]. Some materials, such as orthodox seeds, display natural dehydration processes and can be cryopreserved without any pretreatment [20,69].
In the current study, P. venusta embryos were subjected to short-term LN storage and germination, and the phenotypic and biochemical characteristics, as well as in vitro secondary metabolite production, were evaluated. The moisture content of P. venusta seeds at the time of storage was 10.81 ± 0.10%, enabling direct transfer of the embryos to LN, according to the recommendations of Pritchard [19]. An important step for in vitro conservation consists of the establishment of appropriate techniques for the LN storage of seeds and embryos, without affecting their vigor [74]. The low moisture content in seed tissues is extremely important for the success of cryopreservation protocols, as the great challenge of this technique is cryopreservation without the formation of ice crystals inside the cells [20]. According to Kholina and Voronkova [73], using orthodox seeds, with a moisture content between 5 and 12%, is among the most convenient systems available for the long-term storage of genetic information.
Short-term LN storage of P. venusta embryos favored germination, GSI, and phenotypic characteristics of in vitro regenerated seedlings. Phenotypic characteristics were not affected by acclimatization. Additionally, LN storage and acclimatization significantly affected the PTN, SS, and RS levels; oxidative metabolism; PAL activity; secondary metabolite contents; and the chromatographic profile of phenolic compounds in regenerated seedlings. Some studies have been carried out with the aim of understanding the effects of LN storage on phenotypic, biochemical, and phytochemical characteristics [26,29,69,75,76,77]. Phenotypic assessment is probably the easiest way to detect changes following cryopreservation [26]. However, reports regarding phenotypic variations occurring during the in vitro culture of cryopreserved plant materials are scarce. Adu-Gyamfi et al. [78] reported that phenotypic variations may be epigenetic in nature, and could be linked to in vitro culture conditions, cryopreservation, and modifications in DNA methylation. In the common bean, Cejas et al. [76] did not observe phenotypic changes in seedlings recovered from cryopreserved seeds, but several significant effects were recorded at the biochemical level, especially on the roots. Likewise, Arguedas et al. [29] showed that short-term LN storage of maize, common bean, and soybean seeds modified their biochemical composition, without affecting their phenotypic characteristics.
In the seedlings of P. venusta, biochemical changes occurred in a non-specific manner, both in the aerial parts and in the roots. The increase in SS and RS levels observed in the roots of seedlings from embryos stored in LN may indicate a mechanism of tolerance to cryostorage, since sugars not only function as metabolic resources and structural components of cells, but also act as important regulators of processes associated with plant growth and development, as well as protection against abiotic stressors [79]. During cryostorage, metabolic action and cell division stop, as the ultra-low temperature of the LN promotes cell stabilization, ensuring that the germplasm remains viable and does not undergo long-term changes, and can be recovered after storage [80]. However, Walters [81] observed that LN storage can cause oxidative stress because molecular mobility can occur slowly in the cytoplasm, even at cryogenic temperatures, leading to the formation of reactive oxygen species (ROS), which can modify the organization of the cell membrane. These modifications can facilitate the combination of some biochemical compounds that are separated under normal conditions, subsequently causing changes in different metabolic pathways [26]. Oxidative stress is strongly related to cryopreservation [74]. When ROS are produced at levels sufficient to overcome antioxidant defenses, DNA, proteins, and fatty acids in the membrane are oxidized. The latter can result in lipid peroxidation and loss of membrane function [82] In P. venusta, the high CAT activity in the roots and high GSH, LP, and HPP levels in the aerial parts of seedlings from embryos submitted to short-term LN storage suggest that cryostorage was more stressful than acclimatization for P. venusta, where the aerial parts and roots of acclimatized seedlings showed significant reductions in LP and HPP levels compared to non-acclimatized seedlings, despite the high CAT activity observed in the roots. Catalases are the main enzymes that directly eliminate hydrogen peroxide and are essential for the detoxification of ROS during oxidative stress [83].
Plant secondary metabolites are produced in response to the adaptations required for the plant’s interaction with its environment, as well as for its defense [84]. Plants synthesize secondary metabolites that include phenolic antioxidant compounds to help protect against oxidative damage caused by additional ROS [85]. Phenolic compounds are metabolites recognized for their antioxidant activity, and they generally prevent oxidative damage due to their ability to capture ROS when breaking radical chain reactions during lipid peroxidation [86]. Additionally, the stress caused by storage in LN increases the production of phenolic compounds and their subsequent incorporation into the cell wall as suberin or lignin [87]. In P. venusta, we observed an increase in the total phenolic and flavonoid levels in seedlings from cryostored embryos, which corroborates the changes also observed in oxidative metabolism, especially in the roots. These results suggest the establishment of an adaptive response induced by the low storage temperatures to which the embryos were subjected, and this response was also observed in acclimatized seedlings. Changes in the chromatographic profile of P. venusta seedlings during acclimatization occurred in a non-specific manner, both in the aerial parts (Figure 2c,d) and in the roots (Figure 2g,h), suggesting the influence of abiotic factors such as light, temperature, humidity and substrate in the profile of phenolic compounds, which is well described in the literature [88,89,90].
According to Ljubej et al. [89], phenolic compounds are considered to play an important role in the response of plants to abiotic stress. Phenolic compound content can also change during growth and development in response to environmental changes [90]. Phenolic acids (cinnamic and benzoic acid derivatives) and flavonoids observed in the chromatographic profile of P. venusta seedlings are widely described as antioxidant substances [91,92,93,94]. Under low temperatures, phenolic compounds accumulate and contribute to minimizing the effects caused by low temperature stress [93]. Zevallos et al. [26] observed tomato seeds immersed in LN for 7, 14, and 21 days and found that the levels of cell-wall-linked, free, and total phenolics decreased significantly in the roots and stems compared to those of the non-cryopreserved controls. Schulz et al. [95] showed that, in Arabidopsis thaliana, a large amount of flavonols and anthocyanins accumulated after exposure to low temperatures, along with encoding transcription factors and enzymes of the flavonoid biosynthesis pathway. An increase in PAL activity in the roots of P. venusta seedlings from embryos cryopreserved for 7 days was observed, which were probably reflected in the higher levels of total phenolics and flavonoids present in this organ. However, in qualitative terms, the chromatographic profile of phenolics found in the roots remained constant compared to that of the control. In acclimatized seedlings, this relationship was not observed.
PAL is a key enzyme for regulating the biosynthetic pathway of phenylpropanoids and their derivatives [96,97]. PAL catalyzes the transformation by the non-oxidative deamination of the amino acid L-phenylalanine in trans-cinnamic acid, without the requirement of cofactors; this is the first step for the biosynthesis of phenylpropanoid-derived compounds [98]. The produced cinnamic acid is the direct precursor of several phenolic compounds, such as phenolic acids (coumaric, benzoic, and caffeic acids), condensed tannins, flavonoids (flavonones, favones, isoflavones, flavonols, and anthocyanins), lignans, coumarins, and lignins, which play an important role in plant growth and development [99,100,101]. PAL levels can fluctuate significantly over relatively short intervals of time and in response to a wide variety of stimuli, such as photoperiod, pathogenic attacks, wounding, UV irradiation, heavy metals, low nitrogen and phosphate levels, low temperatures, and signaling molecules, including jasmonic acid, salicylic acid, and abscisic acid [102,103,104,105]. An increase in PAL activity may reflect a general demand for the entire phenylpropanoid pathway dedicated to the synthesis of protective compounds, as observed in the work of Sanchez-Ballesta et al. [106]. However, these variations do not depend exclusively on the genotype, but also on the age, development, organ, and tissue of the plant [107].
Phytosterols are among the secondary metabolites most influenced by temperature [108]. Altered phytosterol metabolism has been linked with environmental and chemical plant stressors, including cold temperature stress [109,110]. In P. venusta seedlings from cryostored embryos, reduced phytosterol levels were observed. However, these levels increased after acclimatization. Phytosterols are integral components of the plasma membrane and are involved in many processes occurring in plants [108,110]. One of the principal functions of sterols in plant cells is the maintenance of membrane homeostasis [109]. The sterols predominantly present in the membranes of higher plants are β-sitosterol, stigmasterol, and campesterol [111]. Changes in the concentration of sterols in cell membranes can increase or decrease their permeability [112]. A decreased β-sitosterol/stigmasterol ratio was correlated with increased cold tolerance in banana meristems and bean leaves [113,114].
Alkaloids are nitrogenous compounds, derived from secondary metabolism, found in just over 20% of known vascular plant species [115]. There are no reports of true alkaloids isolated from P. venusta, with only the presence of allantoin reported in its roots, leaves, and flowers [2,4]. In this study, alkaloids were quantified in P. venusta cultures, reaching a value of 0.12 μg AllanEq mg−1 CE in the aerial parts of seedlings from cryostorage embryos. These contents were lower in acclimatized seedlings. There are no reports that show the effects of cryopreservation on the production of alkaloids in plant cells and tissues cultivated in vitro. Currently, the function of defense against mammals is attributed to alkaloids, especially due to their general toxicity and deterrent capacity [116].
5. Conclusions
Short-term LN storage of P. venusta embryos provided important information on the germination, phenotypic and biochemical characteristics, and in vitro production of secondary metabolites, demonstrating that LN storage can be an effective means for the ex situ conservation of genetic resources from P. venusta. However, further studies are needed to clarify the mechanisms involved in the changes recorded here and to evaluate whether the observed effects are maintained after long-term LN storage.
Author Contributions
Conceptualization, M.C.C. and A.H.F.C.; data curation, M.C.C. and A.H.F.C.; formal analysis, M.C.C. and I.J.P.G.; funding acquisition, H.d.L.S. and A.H.F.C.; investigation, M.C.C., I.J.P.G. and A.H.F.C.; methodology, M.C.C., I.J.P.G. and H.d.L.S.; project administration, A.H.F.C.; resources, H.d.L.S. and A.H.F.C.; supervision, A.H.F.C.; validation, M.C.C., I.J.P.G. and A.H.F.C.; writing—original draft preparation, M.C.C. and A.H.F.C.; writing—review and editing, M.C.C. and A.H.F.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (Project 306581/2020-5). This study was also financed, in part, by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES), Finance Code 001.
Data Availability Statement
The data presented in this study are available within the article.
Acknowledgments
We thank to Andreia Fonseca Silva for the botanical identification of this plant. The authors are grateful to the Universidade Federal de São João del-Rei (UFSJ), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for financial support. A.H.F.C. are grateful for Productive Fellowship from CNPq.
Conflicts of Interest
The authors declare that they have no conflicts of interests.
References
- Mostafa, N.M.; El-dahshan, O.; Singab, A.N.B. Pyrostegia venusta (Ker Gawl.) Miers: A botanical, pharmacological and phytochemical review. Med. Aromat. Plants 2013, 2, 100123. [Google Scholar] [CrossRef]
- Ferreira, D.T.; Alvares, P.S.M.; Houghton, P.J.; Braz, R. Chemical constituents from roots of Pyrostegia venusta and considerations about its medicinal importance. Quím. Nova 2000, 23, 42–46. [Google Scholar] [CrossRef]
- Silva, R.M.; Rodrigues, D.T.M.; Augustos, F.S.; Valadares, F.; Neto, P.O.; Dos Santos, L.; Silva, L.P. Antitumor and cytotoxic activity of Kielmeyera coriacea Mart. Zucc. and Pyrostegia venusta (Ker Gawl.) Miers extracts. J. Med. Plants Res. 2012, 6, 4142–4148. [Google Scholar]
- Moreira, C.G.; Horinouchia, C.D.S.; Souza-Filho, C.S.; Campos, F.R.; Barison, A.; Cabrini, D.A.; Otuki, M.F. Hyperpigmentant activity of leaves and flowers extracts of Pyrostegia venusta on murine B16F10 melanoma. J. Ethnopharmacol. 2012, 141, 1005–1011. [Google Scholar] [CrossRef]
- Pereira, A.M.; Hernandes, C.; Pereira, S.I.V.; Bertoni, B.W.; França, S.C.; Pereira, P.S.; Taleb-Contini, S.H. Evaluation of anticandidal and antioxidant activities of phenolic compounds from Pyrostegia venusta (Ker Gawl.) Miers. Chem. Biol. Interact. 2014, 224, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.; Amdekar, S.; Kumar, A.; Singh, V. Preliminary study of the antioxidant properties of flowers and roots of Pyrostegia venusta (Ker Gawl.) Miers. BMC Complement. Altern. Med. 2011, 11, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.; Amdekar, S.; Kumar, A.; Singh, R.; Sharma, P.; Singh, V. In vivo anti-oxidative property, antimicrobial and wound healing activity of flower extracts of Pyrostegia venusta (Ker Gawl.) Miers. J. Ethnopharmacol. 2012, 140, 186–192. [Google Scholar] [CrossRef]
- Silva, P.B.; Medeiros, A.C.M.; Duarte, M.C.T.; Ruiz, A.L.T.G.; Kolb, R.M.; Frei, F.; Santos, C. Evaluation of allelopathic potential, antimicrobial and antioxidant activities of Pyrostegia venusta (Ker Gawl.) Miers (Bignoniaceae) leaf organic extracts. Rev. Bras. Plantas Med. 2011, 13, 447–455. [Google Scholar] [CrossRef]
- Nisha, P.V.; Shruti, N.; Swamy, K.S.; Kumari, M.; Vedamurthy, A.B.; Krishna, V.; Hoskeri, J.H. Anthelmintic activity of Pyrostegia venusta using Pheretima posthuma. Int. J. Pharm. Sci. Drug Res. 2012, 4, 205–208. [Google Scholar]
- Veloso, C.C.; Bitencourt, A.D.; Cabral, L.D.M.; Franqui, L.S.; Dias, D.F.; Santos, M.H.; Soncini, R.; Giusti-Paiva, A. Pyrostegia venusta attenuate the sickness behavior induced by lipopolysaccharide in mice. J. Ethnopharmacol. 2010, 132, 355–358. [Google Scholar] [CrossRef]
- Veloso, C.C.; Cabral, L.D.M.; Bitencourt, A.D.; Franqui, L.S.; Santa-Cecília, F.V.; Dias, D.F.; Soncini, R.; Vilela, F.C.; Giusti-Paiva, A. Anti-inflammatory and anti-nociceptive effects of the hydroethanolic extract of the flowers of Pyrostegia venusta in mice. Braz. J. Pharmacogn. 2012, 22, 162–168. [Google Scholar] [CrossRef]
- Veloso, C.C.; Oliveira, M.C.; Oliveira, C.C.; Rodrigues, V.G.; Giusti-Paiva, A.; Teixeira, M.M.; Duarte, I.D.; Ferreira, A.V.M.; Perez, A.C. Hydroethanolic extract of Pyrostegia venusta (Ker Gawl.) Miers flowers improves inflammatory and metabolic dysfunction induced by high-refined carbohydrate diet. J. Ethnopharmacol. 2014, 151, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Moreira, C.G.; Carrenho, L.Z.B.; Pawloski, P.L.; Soley, B.S.; Cabrini, D.A.; Otuki, M.F. Pre-clinical evidences of Pyrostegia venusta in the treatment of vitiligo. J. Ethnopharmacol. 2015, 168, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Martínez, A.; Valle-Aguilera, J.R.; Antunes-Ricardo, M.; Gutiérrez-Uribe, J.; Gonzalez, C.; Santos-Díaz, M.S. Callus from Pyrostegia venusta (Ker Gawl.) Miers: A source of phenylethanoid glycosides with vasorelaxant activities. Plant Cell Tissue Organ Cult. 2019, 139, 119–129. [Google Scholar] [CrossRef]
- Dutra, R.C.; Campos, M.M.; Santos, A.R.S.; Calixto, J.B. Medicinal plants in Brazil: Pharmacological studies, drug discovery, challenges and perspectives. Pharmacol. Res. 2016, 112, 4–29. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.C.; Paiva, R.; Swennen, R.; Andrè, E.; Panis, B. Cryopreservation of Byrsonima intermedia embryos followed by room temperature thawing. Acta Sci. Agron. 2014, 36, 309–315. [Google Scholar] [CrossRef]
- Kikowska, M.; Sliwinska, E.; Thiem, B. Micropropagation and Production of Somatic Seeds for Short-Term Storage of the Endangered Species Eryngium alpinum L. Plants 2020, 9, 498. [Google Scholar] [CrossRef]
- Espinosa-Leal, C.A.; Puente-Garza, C.A.; García-Lara, S. In vitro plant tissue culture: Means for production of biological active compounds. Planta 2018, 248, 1–18. [Google Scholar] [CrossRef]
- Pritchard, H.W. Cryopreservation of Desiccation-Tolerant Seeds. In Cryopreservation and Freeze-Drying Protocols, Methods in Molecular Biology, 2nd ed.; Day, J.G., Stacey, G.N., Eds.; Humana Press: New York, NY, USA, 2007; Volume 368, pp. 185–201. [Google Scholar]
- Engelmann, F. Use of biotechnologies for the conservation of plant biodiversity. In Vitro Cell. Devel. Biol. Plant 2010, 47, 5–16. [Google Scholar] [CrossRef]
- Pegg, D.E. Principles of Cryopreservation. In Cryopreservation and Freeze-Drying Protocols, Methods in Molecular Biology; Wolkers, W.F., Oldenhof, H., Eds.; Springer: New York, NY, USA, 2015; Volume 1257, pp. 3–19. [Google Scholar]
- Kubota, C.; Seiyama, S.; Kozai, T. Manipulation of photoperiod and light intensity in low temperature storage of eggplant plug seedlings. Sci. Hortic. 2002, 94, 13–20. [Google Scholar] [CrossRef]
- Justus, I.; Kubota, C. Effects of low temperature storage on growth and transplant quality of non-grafted and grafted cantaloupe-type muskmelon seedlings. Sci. Hortic. 2010, 125, 47–54. [Google Scholar] [CrossRef]
- Wang, M.R.; Lambardi, M.; Engelmann, F.; Pathirana, R.; Panis, B.; Volk, G.M.; Wang, Q.C. Advances in cryopreservation of in vitro-derived propagules: Technologies and explant sources. Plant Cell Tissue Organ Cult. 2021, 144, 7–20. [Google Scholar] [CrossRef]
- Mosa, K.A.; Ahmed, A.E.; Hazem, Y.; Kanawati, I.S.; Abdullah, A.; Hernandez-Sori, L.; Ali, M.A.; Vendrame, W. Insights into cryopreservation, recovery and genetic stability of medicinal plant tissues. Fitoterapia 2023, 169, 105555. [Google Scholar] [CrossRef] [PubMed]
- Zevallos, B.; Cejas, I.; Rodríguez, R.C.; Yabor, L.; Aragón, C.; González, J.; Engelmann, F.; Martínez, M.E.; Lorenzo, J.C. Biochemical characterization of ecuadorian wild Solanum lycopersicum MILL. plants produced from non-cryopreserved and cryopreserved seeds. CryoLetters 2013, 34, 413–421. [Google Scholar] [PubMed]
- Kubota, C.; Meng, C.; Son, Y.J.; Lewis, M.; Spalholz, H.; Tronstad, R. Horticultural, systems-engineering and economic evaluations of short-term plant storage techniques as a labor management tool for vegetable grafting nurseries. PLoS ONE 2017, 12, e0170614. [Google Scholar] [CrossRef] [PubMed]
- Teixeira da Silva, J.A.; Engelmann, F. Cryopreservation of oil palm (Elaeis guineensis Jacq.). Cryobiology 2017, 77, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Arguedas, M.; Pérez, A.; Abdelnour, A.; Hernández, M.; Engelmann, F.; Martínez, M.E.; Yabor, L.; Lorenzo, J.C. Short-term liquid nitrogen storage of maize, common bean and soybean seeds modifies their biochemical composition. Agric. Sci. 2016, 4, 6–12. [Google Scholar] [CrossRef]
- Nausch, H.; Buyel, J.F. Cryopreservation of plant cell cultures—Diverse practices and protocols. New Biotechnol. 2021, 62, 86–95. [Google Scholar] [CrossRef]
- Vujović, T.; Anđelić, T.; Vasilijević, B.; Jevremović, D.; Engelmann, F. Cryopreservation of Indigenous Plums and Monitoring of Multiplication and Rooting Capacity of Shoots Obtained from Cryopreserved Specimens. Plants 2023, 12, 3108. [Google Scholar] [CrossRef] [PubMed]
- Bettoni, J.C.; Bonnart, R.; Volk, G.M. Challenges in implementing plant shoot tip cryopreservation technologies. Plant Cell Tissue Organ Cult. 2021, 144, 21–34. [Google Scholar] [CrossRef]
- Loredo-Carrillo, S.E.; Santos-Diáz, M.L.; Leyva, E.; Santos-Diáz, M.S. Establishment of callus from Pyrostegia venusta (Ker Gawl.) Miers and effect of abiotic stress on flavonoids and sterols accumulation. J. Plant Biochem. Biotechnol. 2012, 22, 312–318. [Google Scholar] [CrossRef]
- Braga, K.Q.; Coimbra, M.C.; Castro, A.H.F. In vitro germination, callus induction and phenolic compounds contents from Pyrostegia venusta (Ker Gawl.) Miers. Acta Sci. Biol. Sci. 2015, 37, 151–158. [Google Scholar] [CrossRef]
- Coimbra, M.C.; Chagas, R.C.R.; Duarte-Almeida, J.M.; Castro, A.H.F. Influence of plant growth regulators and light on callus induction and bioactive phenolic compounds production in Pyrostegia venusta (Bignoniaceae). Indian J. Exp. Biol. 2017, 55, 584–590. [Google Scholar]
- Ferreira, N.S.O.; Rosset, M.; Lima, G.; Campelo, P.M.S.; Macedo, R.E.F. Effect of adding Brosimum gaudichaudii and Pyrostegia venusta hydroalcoholic extracts on the oxidative stability of beef burgers. LWT 2019, 108, 145–152. [Google Scholar] [CrossRef]
- Coimbra, M.C.; Chagas, R.C.R.; Vilela, M.S.P.; Castro, A.H.F. Growth, morphology and bioactive phenolic compounds production in Pyrostegia venusta calli. Biocatal. Agric. Biotechnol. 2019, 18, 101036. [Google Scholar] [CrossRef]
- Milani, M.; Heintze, W.; Schafer, G.; Souza, P.V.D. Influence of leaf number and nodes on the rooting of semiwoody cuttings of flame vine. Ornam. Hortic. 2015, 21, 339–344. [Google Scholar] [CrossRef][Green Version]
- Coimbra, M.C.; Castro, A.H.F. Distribution and structural aspects of extrafloral nectaries in leaves of Pyrostegia venusta (Bignoniaceae). Acta Sci. Biol. Sci. 2014, 36, 321–326. [Google Scholar] [CrossRef]
- Rossato, D.R.; Kolb, R.M. Germination of Pyrostegia venusta (Bignoniaceae), seed viability and post-seminal development. Braz. J. Bot. 2010, 33, 51–60. [Google Scholar]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- BRASIL. Regras para Análise de Sementes, 1st ed.; MAPA/ACS: Brasília, Brazil, 2009; 399p.
- Figueiredo, M.A.; Coelho, S.V.B.; Rosa, S.D.V.F.; Vilela, A.L.; Silva, L.C. Exploratory studies for cryopreservation of Coffea arabica L. seeds. J. Seed Sci. 2017, 39, 150–158. [Google Scholar] [CrossRef]
- Maguire, J.D. Speed of germination aid in selection and evaluation for seedling emergence and vigor. Crop Sci. 1962, 2, 176–177. [Google Scholar] [CrossRef]
- Nicioli, P.M.; Paiva, R.; Nogueira, R.C.; Santana, J.R.F.; Silva, L.C.; Silva, D.P.C.; Porto, J.M.P. Adjustment of the process of micropropagation of Stryphnodendron adstringens (Mart.) Coville. Ciênc. Rural 2008, 38, 685–689. [Google Scholar] [CrossRef]
- Schuck, M.R.; Lipski, B.; Da Silva, A.L.L.; De Carvalho, D.C.; Biasi, L.A. Acclimatization of micropropagated plants of fox grape cv. Bordô (Vitis labrusca L.) in different substrates. J. Biotechnol. Biodivers. 2012, 3, 206–212. [Google Scholar] [CrossRef]
- Mollavali, M.; Bolandnazar, S.A.; Schwarz, D.; Rohn, S.; Riehle, P.; Nahandi, F.Z. Flavonol glucoside and antioxidant enzyme biosynthesis affected by mycorrhizal fungi in various cultivars of onion (Allium cepa L.). J. Agric. Food Chem. 2016, 64, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Yemm, E.W.; Willis, A.J. The estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 1954, 57, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Maehly, A.C.; Chance, B. The assay of catalases and peroxidases. Methods Biochem. Anal. 1954, 1, 357–424. [Google Scholar] [PubMed]
- Jollow, D.J.; Mitchell, J.R.; Zampaglione, N.; Gillette, J.R. Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3,4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology 1974, 11, 151–169. [Google Scholar] [CrossRef]
- Kramer, G.F.; Norman, H.A.; Krizek, D.T.; Mirecki, R.M. Influence of UV-B radiation on polyamines, lipid peroxidation and membrane lipids in cucumber. Phytochemistry 1991, 30, 2101–2108. [Google Scholar] [CrossRef]
- Alexieva, V.; Sergiev, I.; Mapelli, S.; Karanov, E. The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ. 2001, 24, 1337–1344. [Google Scholar] [CrossRef]
- Mori, T.; Sakurai, M.; Sakuta, M. Changes in PAL, CHS, DAHP synthase (DS-Co and Ds-Mn) activity during anthocyanin synthesis in suspension culture of Fragaria ananassa. Plant Cell Tissue Organ Cult. 2000, 62, 135–139. [Google Scholar] [CrossRef]
- Slinkard, K.; Singleton, V.L. Total phenol analyses: Automation and comparison with manual methods. Am. J. Enol. Vitic. 1977, 28, 49–55. [Google Scholar] [CrossRef]
- ANVISA. Farmacopeia Brasileira: Plantas Medicinais, 6th ed.; ANVISA: Brasília, Brazil, 2010.
- Araújo, L.B.D.C.; Silva, S.L.; Galvão, M.A.M.; Ferreira, M.R.A.; Araújo, E.L.; Randau, K.P.; Soares, L.A.L. Total phytosterol content in drug materials and extracts from roots of Acanthospermum hispidum by UV-VIS spectrophotometry. Rev. Bras. Farmacogn. 2013, 23, 736–742. [Google Scholar] [CrossRef]
- Sreevidya, N.; Mehrotra, S. Spectrophotometric method for estimation of alkaloids precipitable with Dragendorff’s reagent in plant materials. J. AOAC Int. 2003, 86, 1124–1127. [Google Scholar] [CrossRef] [PubMed]
- Sakakibara, H.; Honda, Y.; Nakagawa, S.; Ashida, H.; Kanazawa, K. Simultaneous determination of all polyphenols in vegetables, fruits, and teas. J. Agric. Food Chem. 2003, 51, 571–581. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Shan, Y.; Wu, Y.; Liu, G.; Chen, B.; Yao, S. Simultaneous determination of flavanones, hydroxycinnamic acids and alkaloids in citrus fruits by HPLC-DAD–ESI/MS. Food Chem. 2011, 127, 880–885. [Google Scholar] [CrossRef]
- Ruiz, A.; Mardones, C.; Vergara, C.; Hermosín-gutiérrez, I.; Von Baer, D.; Hinrichsen, P.; Rodriguez, R.; Arribillaga, D.; Dominguez, E. Analysis of hydroxycinnamic acids derivatives in calafate (Berberis microphylla G. Forst) berries by liquid chromatography with photodiode array and mass spectrometry detection. J. Chromatog. A 2013, 1281, 38–45. [Google Scholar] [CrossRef]
- Ferreira, D.F. Sisvar: A Guide for its bootstrap procedures in multiple comparisons. Ciênc. Agrotecnol. 2014, 38, 109–112. [Google Scholar] [CrossRef]
- Cunningham, A.B.; Brinckmann, J.A.; Yang, X.; He, J. Introduction to the special issue: Saving plants, saving lives: Trade, sustainable harvest and conservation of traditional medicinals in Asia. J. Ethnopharmacol. 2019, 229, 288–292. [Google Scholar] [CrossRef]
- Asgher, M.; Verma, S.; Khan, N.A.; Vyas, D.; Kumari, P.; Rashid, S.; Khan, S.; Qadir, S.; Ajmal Ali, M.; Ahmad, P. Physiological, Biochemical and Reproductive Studies on Valeriana wallichii, a Critically Endangered Medicinal Plant of the Himalayan Region Grown under In-Situ and Ex-Situ Conditions. Plants 2020, 9, 131. [Google Scholar] [CrossRef] [PubMed]
- Bourgou, S.; Ben Haj Jilani, I.; Karous, O.; Megdiche-Ksouri, W.; Ghrabi-Gammar, Z.; Libiad, M.; Khabbach, A.; El Haissoufi, M.; Lamchouri, F.; Greveniotis, V.; et al. Medicinal-Cosmetic Potential of the Local Endemic Plants of Crete (Greece), Northern Morocco and Tunisia: Priorities for Conservation and Sustainable Exploitation of Neglected and Underutilized Phytogenetic Resources. Biology 2021, 10, 1344. [Google Scholar] [CrossRef] [PubMed]
- Ssenku, J.E.; Okurut, S.A.; Namuli, A.; Kudamba, A.; Tugume, P.; Matovu, P.; Wasige, G.; Kafeero, H.M.; Walusansa, A. Medicinal plant use, conservation, and the associated traditional knowledge in rural communities in Eastern Uganda. Trop. Med. Health 2022, 50, 39. [Google Scholar] [CrossRef] [PubMed]
- Asigbaase, M.; Adusu, D.; Anaba, L.; Abugre, S.; Kang-Milung, S.; Acheamfour, S.A.; Adamu, I.; Ackah, D.K. Conservation and economic benefits of medicinal plants: Insights from forest-fringe communities of Southwestern Ghana. Trees For. People 2023, 14, 100462. [Google Scholar] [CrossRef]
- Engelmann, F.; Rao, R. Major Research Challenges and Directions for Future Research. In Conservation of Tropical Plant Species; Normah, M., Chin, H., Reed, B., Eds.; Springer: New York, NY, USA, 2013; pp. 511–526. [Google Scholar]
- Havens, K.; Vitt, P.; Maunder, M.; Guerrant, E.O.; Dixon, K. Ex situ plant conservation and beyond. BioScience 2006, 56, 525–531. [Google Scholar] [CrossRef]
- Chen, S.L.; Yu, H.; Luo, H.M.; Wu, Q.; Li, C.F.; Steinmetz, A. Conservation and sustainable use of medicinal plants: Problems, progress, and prospects. Chin. Med. 2016, 11, 37. [Google Scholar] [CrossRef] [PubMed]
- Walters, C.; Pence, V.C. The unique role of seed banking and cryobiotechnologies in plant conservation. Plants People Planet 2021, 3, 83–91. [Google Scholar] [CrossRef]
- Kholina, A.B.; Voronkova, N.M. Seed cryopreservation of some medicinal legumes. J. Bot. 2012, 2012, 186891. [Google Scholar] [CrossRef]
- Prudente, D.O.; Paiva, R.; Domiciano, D.; Souza, L.B.; Carpentier, S.; Swennen, R.; Silva, L.C.; Nery, F.C.; Máximo, W.P.F.; Panis, B. The cryoprotectant PVS2 plays a crucial role in germinating Passiflora ligularis embryos after cryopreservation by influencing the mobilization of lipids and the antioxidant metabolism. J. Plant Physiol. 2019, 239, 71–82. [Google Scholar] [CrossRef]
- Varghese, B.; Naithani, S.C. Oxidative metabolism-related changes in cryogenically stored neem (Azadirachta indica A. Juss) seeds. J. Plant Physiol. 2008, 165, 755–765. [Google Scholar] [CrossRef]
- Cejas, I.; Méndez, R.; Villalobos, A.; Palau, F.; Aragón, C.; Engelmann, F.; Carputo, D.; Aversano, R.; Martínez, M.E.; Lorenzo, J.C. Phenotypic and molecular characterization of Phaseolus vulgaris plants from non-cryopreserved and cryopreserved seeds. Am. J. Plant Sci. 2013, 4, 844–849. [Google Scholar] [CrossRef]
- Zevallos, B.; Cejas, I.; Valle, B.; Yabor, L.; Aragón, C.; Engelmann, F.; Martínez, M.E.; Lorenzo, J.C. Short-term liquid nitrogen storage of wild tomato (Solanum lycopersicum Mill.) seeds modifies the levels of phenolics in 7 day-old seedlings. Sci. Hortic. 2013, 160, 264–267. [Google Scholar] [CrossRef]
- Adu-Gyamfi, R.; Wetten, A.; López, C.M.R. Effect of cryopreservation and post-cryopreservation somatic embryogenesis on the epigenetic fidelity of cocoa (Theobroma cacao L.). PLoS ONE 2016, 11, e0158857. [Google Scholar] [CrossRef] [PubMed]
- Keunen, E.; Peshev, D.; Vangronsveld, J.; Van Den Ende, W.; Cuypers, A. Plant sugars are crucial players in the oxidative challenge during abiotic stress: Extending the traditional concept. Plant Cell Environ. 2013, 36, 1242–1255. [Google Scholar] [CrossRef] [PubMed]
- Benson, E.E. Cryopreservation of Phytodiversity: A Critical Appraisal of Theory & Practice. CRC Crit. Rev. Plant Sci. 2008, 27, 141–219. [Google Scholar]
- Walters, C. Temperature dependency of molecular mobility in preserved seeds. Biophys. J. 2004, 86, 1253–1258. [Google Scholar] [CrossRef] [PubMed]
- Gutteridge, J.M.C.; Halliwell, B. Free radicals and antioxidants in the year 2000: A historical look to the future. Ann. N. Y. Acad. Sci. 2006, 899, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, P.; Sarwat, M.; Sharma, S. Reactive oxygen species, antioxidants and signaling in plants. J. Plant Biol. 2008, 51, 167–173. [Google Scholar] [CrossRef]
- Ramakrishna, A.; Ravishankar, G.A. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal. Behav. 2011, 6, 1720–1731. [Google Scholar]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Sakihama, Y.; Yamasaki, H. Lipid Peroxidation induced by phenolics in conjunction with aluminum ions. Biol. Plant. 2002, 45, 249–254. [Google Scholar] [CrossRef]
- Griffith, M.; Yaish, M.W.F. Antifreeze proteins in overwintering plants: A tale of two activities. Trends Plant Sci. 2004, 9, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Chiomento, J.L.T.; De Nardi, F.S.; Filippi, D.; Trentin, T.d.S.; Anzolin, A.P.; Bertol, C.D.; Nienow, A.A.; Calvete, E.O. Mycorrhization of strawberry plantlets potentiates the synthesis of phytochemicals during ex vitro acclimatization. Acta Sci. Agron. 2022, 44, e55682. [Google Scholar] [CrossRef]
- Ljubej, V.; Karalija, E.; Salopek-Sondi, B.; Šamec, D. Effects of Short-Term Exposure to Low Temperatures on Proline, Pigments, and Phytochemicals Level in Kale (Brassica oleracea var. acephala). Horticulturae 2021, 7, 341. [Google Scholar] [CrossRef]
- Terletskaya, N.V.; Korbozova, N.K.; Kudrina, N.O.; Kobylina, T.N.; Kurmanbayeva, M.S.; Meduntseva, N.D.; Tolstikova, T.G. The Influence of Abiotic Stress Factors on the Morphophysiological and Phytochemical Aspects of the Acclimation of the Plant Rhodiola semenowii Boriss. Plants 2021, 10, 1196. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Rad. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef] [PubMed]
- Van Hung, P. Phenolic Compounds of Cereals and Their Antioxidant Capacity. Crit. Rev. Food Sci. Nutr. 2016, 56, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Naikoo, M.I.; Dar, M.I.; Raghib, F.; Jaleel, H.; Ahmad, B.; Raina, A.; Khan, F.A.; Naushin, F. Chapter 9—Role and Regulation of Plants Phenolics in Abiotic Stress Tolerance: An Overview. In Plant Signaling Molecules; Khan, M.I.R., Reddy, P.S., Ferrante, A., Khan, N.A., Eds.; Woodhead Publishing: Cambridge, UK, 2019; pp. 157–168. [Google Scholar]
- Kiokias, S.; Proestos, C.; Oreopoulou, V. Phenolic Acids of Plant Origin—A Review on Their Antioxidant Activity In Vitro (O/W Emulsion Systems) Along with Their in Vivo Health Biochemical Properties. Foods 2020, 9, 534. [Google Scholar] [CrossRef]
- Schulz, E.; Tohge, T.; Zuther, E.; Fernie, A.R.; Hincha, D.K. Flavonoids are determinants of freezing tolerance and cold acclimation in Arabidopsis thaliana. Sci. Rep. 2016, 6, 34027. [Google Scholar] [CrossRef]
- MacDonald, M.J.; D’cunha, G.B. A modern view of phenylalanine ammonia lyase. Biochem. Cell Biol. 2007, 85, 273–282. [Google Scholar] [CrossRef]
- Huang, J.; Min, G.; Lai, Z.; Fan, B.; Shi, K.; Zhou, Y.H.; Yu, J.Q.; Chen, Z. Functional analysis of the Arabidopsis PAL gene family in plant growth, development, and response to environmental stress. Plant Physiol. 2010, 153, 1526–1538. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.D.; Qiu, J.Q.; Fan, X.W.; Jia, S.R.; Tan, Z.L. Biotechnological production and applications of microbial henylalanine ammonia lyase: A recent review. Crit. Rev. Biotechnol. 2014, 34, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Khakdan, F.; Alizadeh, H.; Ranjbar, M. Molecular cloning, functional characterization and expression of a drought inducible phenylalanine ammonia-lyase gene (ObPAL) from Ocimum basilicum L. Plant Physiol. Biochem. 2018, 130, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Rahmatabadi, S.S.; Sadeghian, I.; Ghasemi, Y.; Sakhteman, A.; Hemmati, S. Identification and characterization of a sterically robust phenylalanine ammonia-lyase among 481 natural isoforms through association of in silico and in vitro studies. Enzyme Microb. Technol. 2019, 122, 36–54. [Google Scholar] [CrossRef] [PubMed]
- Tobimatsu, Y.; Schuetz, M. Lignin polymerization: How do plants manage the chemistry so well? Curr. Opin. Biotechnol. 2019, 56, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Weisshaar, B.; Jenkins, G.I. Phenylpropanoid biosynthesis and its regulation. Curr. Opin. Plant Biol. 1998, 1, 251–257. [Google Scholar] [CrossRef]
- Janas, K.M.; Zielińska-Tomaszewska, J.; Rybaczek, D.; Maszewski, J.; Posmyk, M.M.; Aramowicz, R.; Kosińska, A. The impact of copper ions on growth, lipid peroxidation, and phenolic compound accumulation and localization in lentil (Lens culinaris Medic.) seedlings. J. Plant Physiol. 2010, 167, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Shang, Q.M.; Li, L.; Dong, C.J. Multiple tandem duplication of the phenylalanine ammonia-lyase genes in Cucumis sativus L. Planta 2012, 236, 1093–1105. [Google Scholar]
- Zhang, X.; Liu, C.J. Multifaceted regulations of gateway enzyme phenylalanine ammonia-lyase in the biosynthesis of phenylpropanoids. Mol. Plant 2015, 8, 17–27. [Google Scholar] [CrossRef]
- Sanchez-Ballesta, M.T.; Zacarias, L.; Granell, A.; Lafuente, M.T. Accumulation of Pal Transcript and Pal Activity as Affected by Heat-Conditioning and Low-Temperature Storage and Its Relation to Chilling Sensitivity in Mandarin Fruits. J. Agric. Food Chem. 2000, 48, 2726–2731. [Google Scholar] [CrossRef]
- Kováčik, J.; Klejdus, B. Tissue and method specificities of Phenylalanine Ammonia-Lyase assay. J. Plant Physiol. 2012, 169, 1317–1320. [Google Scholar] [CrossRef]
- Rogowska, A.; Szakiel, A. The role of sterols in plant response to abiotic stress. Phytochem. Rev. 2020, 19, 1525–1538. [Google Scholar] [CrossRef]
- Moreau, R.A.; Whitaker, B.D.; Hicks, K.B. Phytosterols, phytostanols, and their conjugates in foods: Structural diversity, quantitative analysis, and health-promoting uses. Prog. Lipid Res. 2002, 41, 457–500. [Google Scholar] [CrossRef]
- Rudell, D.R.; Buchanan, D.A.; Leisso, R.S.; Whitaker, B.D.; Mattheis, J.P.; Zhu, Y.; Varanasi, V. Ripening, storage temperature, ethylene action, and oxidative stress alter apple peel phytosterol metabolismo. Phytochemistry 2011, 72, 1328–1340. [Google Scholar] [CrossRef]
- Valitova, J.N.; Sulkarnayeva, A.G.; Minibayeva, F.V. Plant sterols: Diversity, biosynthesis, and physiological functions. Biochemistry 2016, 81, 819–834. [Google Scholar] [CrossRef]
- Grunwald, C. Effects of free sterols, steryl ester, and steryl glycoside on membrane permeability. Plant Physiol. 1971, 48, 653–655. [Google Scholar] [CrossRef]
- Guye, M.G. Phospholipid, sterol composition and ethylene production in relation to choline-induced chill-tolerance in mung bean (Vigna radiata L. Wilcz.) during a chill-warm cycle. J. Exp. Bot. 1989, 40, 369–374. [Google Scholar] [CrossRef]
- Zhu, G.Y.; Geuns, J.; Dussert, S.; Swennen, R.; Panis, B. Change in sugar, sterol and fatty acid composition in banana meristems caused by sucrose-induced acclimation and its effects on cryopreservation. Physiol. Plant. 2006, 128, 80–94. [Google Scholar] [CrossRef]
- Anulika, N.P.; Ignatius, E.O.; Raymond, E.S.; Osasere, O.I.; Abiola, A.H. The Chemistry of Natural Product: Plant Secondary Metabolites. IJTEEE 2016, 4, 1–9. [Google Scholar]
- Hartmann, M.A. Sterol metabolism and functions in higher plants. In Lipid Metabolism and Membrane Biogenesis, Topics in Current Genetics; Daum, G., Ed.; Springer: Berlin/Heidelberg, Germany, 2004; Volume 6, pp. 183–211. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).