Characterization of Physiological and Biochemical Attributes of Neem (Azadirachta indica A. Juss) under Salinity Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Properties and Treatments
2.2. Growth Conditions
2.3. Harvesting and Growth Measurements
2.4. Determination of Antioxidants
2.5. Total Chlorophyll, Protein Contents, and Reactive Oxygen Species (H2O2)
2.6. Determination of Ion Contents
2.7. Statistical Analysis
3. Results
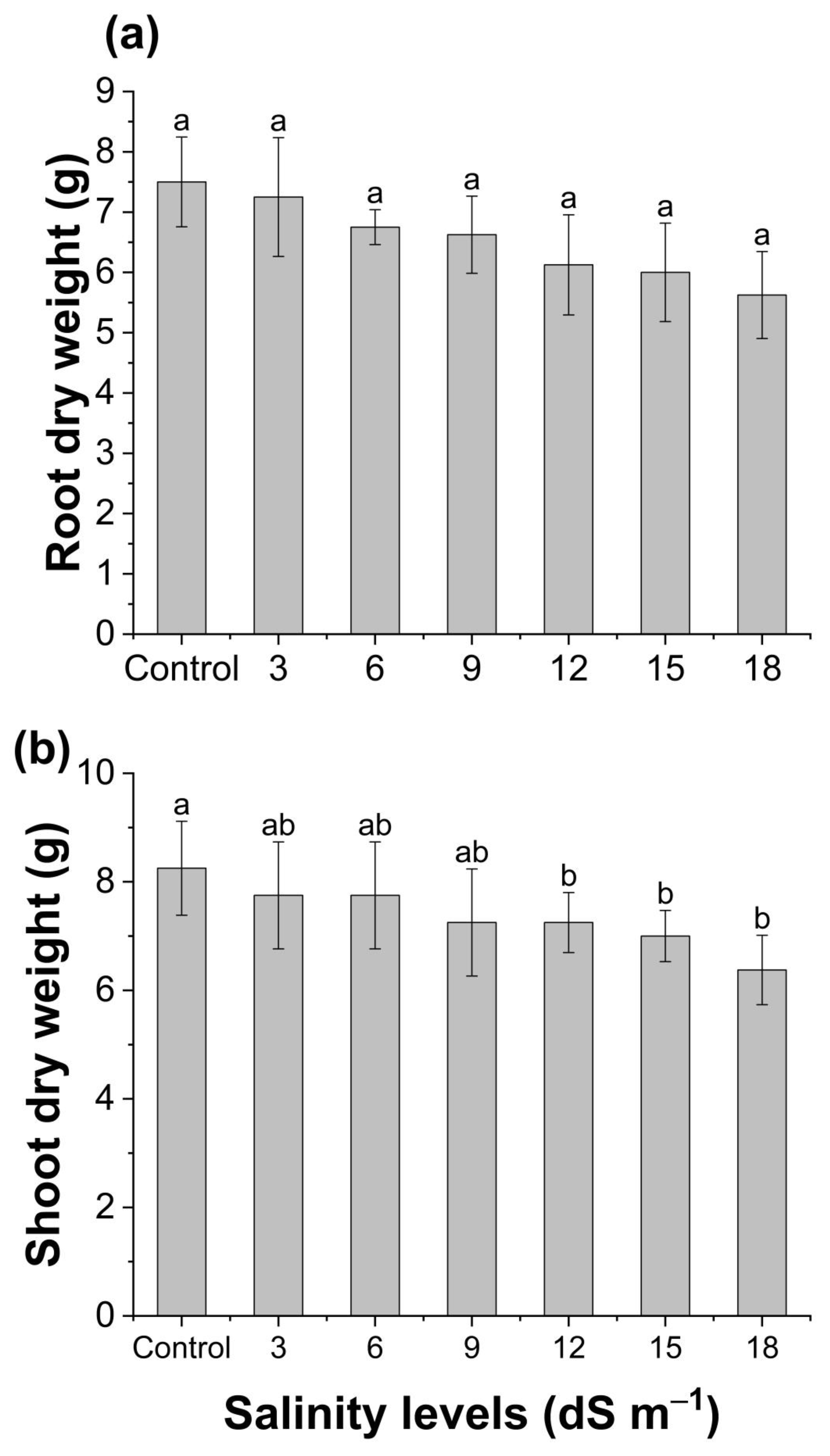
3.1. Plant Growth
3.2. Antioxidant Activity
3.3. Total Chlorophyll, Protein, and H2O2 Contents
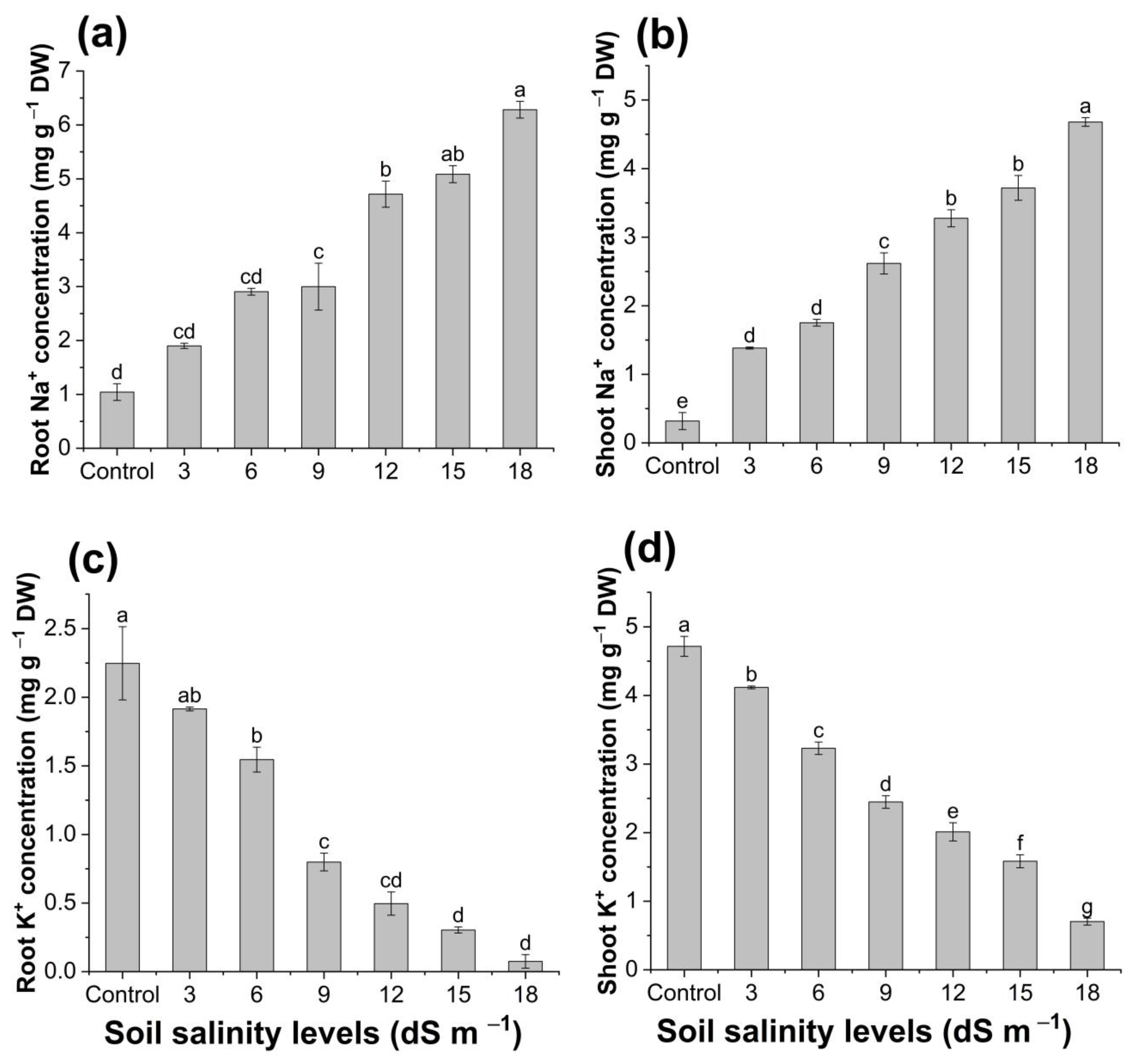
3.4. Root and Shoot Ion Contents
3.5. Correlation Analysis
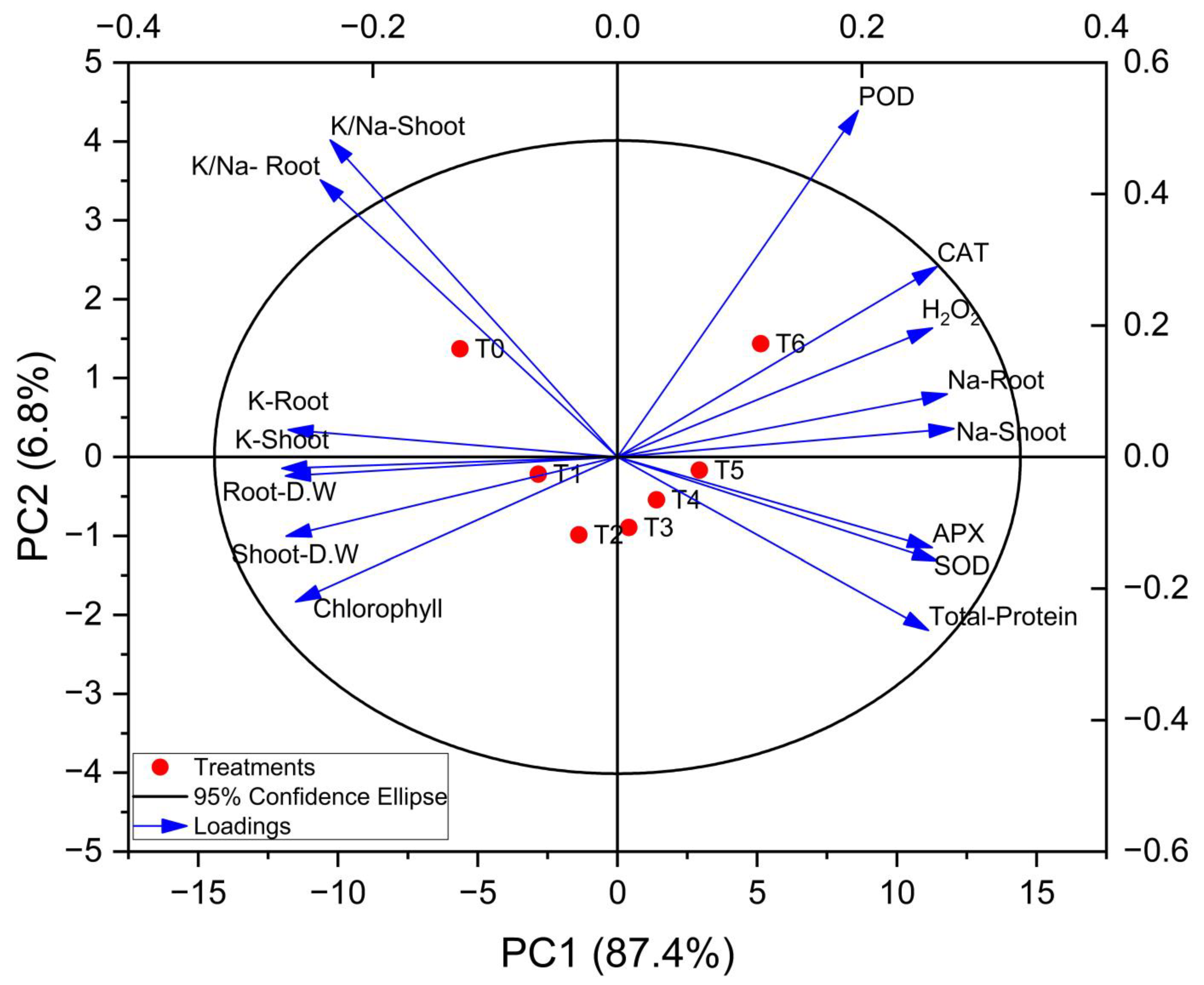
3.6. Principal Component Analysis (PCA)
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iqbal, W.; Afridi, M.Z.; Jamal, A.; Mihoub, A.; Saeed, M.F.; Székely, Á.; Zia, A.; Khan, M.A.; Jarma-Orozco, A.; Pompelli, M.F. Canola seed priming and its effect on gas exchange, chlorophyll photobleaching, and enzymatic activities in response to salt stress. Sustainability 2022, 14, 9377. [Google Scholar] [CrossRef]
- Zia, A.; Munsif, F.; Jamal, A.; Mihoub, A.; Saeed, M.F.; Fawad, M.; Ahmad, I.; Ali, A. Morpho-physiological attributes of Different maize (Zea mays L.) genotypes under varying salt stress conditions. Gesunde Pflanz. 2022, 74, 661–673. [Google Scholar] [CrossRef]
- Ahsan, M.; Zulfiqar, H.; Farooq, M.A.; Ali, S.; Tufail, A.; Kanwal, S.; Shaheen, M.R.; Sajid, M.; Gul, H.; Jamal, A. Strigolactone (GR24) Application positively regulates photosynthetic attributes, stress-related metabolites and antioxidant enzymatic activities of ornamental sunflower (Helianthus annuus cv. Vincent’s Choice) under salinity stress. Agriculture 2022, 13, 50. [Google Scholar] [CrossRef]
- Lekka, C.; Detsikas, S.E.; Petropoulos, G.P.; Chalkias, C. Mapping and monitoring of salt-affected soils: The contribution of geoinformation. In Remote Sensing in Precision Agriculture; Elsevier: Amsterdam, The Netherlands, 2024; pp. 71–91. [Google Scholar]
- Ahmad, I.; Munsif, F.; Mihoub, A.; Jamal, A.; Saeed, M.F.; Babar, S.; Fawad, M.; Zia, A. Beneficial effect of melatonin on growth and chlorophyll content in wheat (Triticum aestivum L.) grown under salt stress conditions. Gesunde Pflanz. 2022, 74, 997–1009. [Google Scholar] [CrossRef]
- Singh, K.; Chatrath, R. Salinity tolerance. In Application of Physiology in Wheat Breeding; CIMMYT: Méx, Mexico, 2001; pp. 101–110. [Google Scholar]
- Chele, K.H.; Tinte, M.M.; Piater, L.A.; Dubery, I.A.; Tugizimana, F. Soil salinity, a serious environmental issue and plant responses: A metabolomics perspective. Metabolites 2021, 11, 724. [Google Scholar] [CrossRef] [PubMed]
- Abbas, G.; Amjad, M.; Saqib, M.; Murtaza, B.; Asif Naeem, M.; Shabbir, A.; Murtaza, G. Soil sodicity is more detrimental than salinity for quinoa (Chenopodium quinoa Willd.): A multivariate comparison of physiological, biochemical and nutritional quality attributes. J. Agron. Crop Sci. 2021, 207, 59–73. [Google Scholar] [CrossRef]
- Zia-ur-Rehman, M.; Anayatullah, S.; Irfan, E.; Hussain, S.M.; Rizwan, M.; Sohail, M.I.; Jafir, M.; Ahmad, T.; Usman, M.; Alharby, H.F. Nanoparticles assisted regulation of oxidative stress and antioxidant enzyme system in plants under salt stress: A review. Chemosphere 2023, 314, 137649. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Bano, A.; Khan, N. Climate change and salinity effects on crops and chemical communication between plants and plant growth-promoting microorganisms under stress. Front. Sustain. Food Syst. 2021, 5, 618092. [Google Scholar] [CrossRef]
- Vasilik, M.P.; Belova, N.I.; Lazareva, E.M.; Kononenko, N.V.; Fedoreyeva, L.I. Salt Tolerance Assessment in Triticum aestivum and Triticum durum. Front. Biosci. 2024, 29, 150. [Google Scholar] [CrossRef]
- Balasubramaniam, T.; Shen, G.; Esmaeili, N.; Zhang, H. Plants’ response mechanisms to salinity stress. Plants 2023, 12, 2253. [Google Scholar] [CrossRef]
- Pompelli, M.F.; Ferreira, P.P.; Chaves, A.R.; Figueiredo, R.C.; Martins, A.O.; Jarma-Orozco, A.; Bhatt, A.; Batista-Silva, W.; Endres, L.; Araújo, W.L. Physiological, metabolic, and stomatal adjustments in response to salt stress in Jatropha curcas. Plant Physiol Biochem. 2021, 168, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Pompelli, M.F.; Jarma-Orozco, A.; Rodríguez-Páez, L.A. Salinity in Jatropha curcas: A review of physiological, biochemical, and molecular factors involved. Agriculture 2022, 12, 594. [Google Scholar] [CrossRef]
- Lu, Y.; Fricke, W. Salt Stress—Regulation of root water uptake in a whole-plant and diurnal context. Int. J. Mol. Sci. 2023, 24, 8070. [Google Scholar] [CrossRef] [PubMed]
- Jēkabsone, A.; Karlsons, A.; Osvalde, A.; Ievinsh, G. Effect of Na, K and Ca Salts on Growth, Physiological Performance, Ion Accumulation and Mineral Nutrition of Mesembryanthemum crystallinum. Plants 2024, 13, 190. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, F.A.; da Silva, D.D.; dos Santos, S.T.; de Oliveira, M.K.; Nascimento, L.; Silva, R.T.; de Sousa Neto, O.N.; Pinto, F.F.B. Mineral nutrition and hydroponic kale production under saline stress and calcium nitrate. Hortic. Bras. 2023, 41, e2615. [Google Scholar] [CrossRef]
- Faisal, M.; Faizan, M.; Tonny, S.H.; Rajput, V.D.; Minkina, T.; Alatar, A.A.; Pathirana, R. Strigolactone-mediated mitigation of negative effects of salinity stress in Solanum lycopersicum through reducing the oxidative damage. Sustainability 2023, 15, 5805. [Google Scholar] [CrossRef]
- Dos Santos, D.B.; de Sousa Medeiros, S.; Rocha, T.A.L.C.G.; Batista, R.O.; Júnior, J.A.S.; de Oliveira Mesquita, F.; Macedo, R.S.; de Vasconcelos, E.S.A.G.; de Bakker, A.P. Chemical characteristics of a bean-cultivated acrisol irrigated with saline water. Agric. Water Manag. 2022, 263, 107397. [Google Scholar] [CrossRef]
- Ziyatdinova, G.; Kalmykova, A. Electrochemical characterization of the antioxidant properties of medicinal plants and products: A review. Molecules 2023, 28, 2308. [Google Scholar] [CrossRef] [PubMed]
- Spormann, S.; Nadais, P.; Sousa, F.; Pinto, M.; Martins, M.; Sousa, B.; Fidalgo, F.; Soares, C. Accumulation of proline in plants under contaminated soils—Are we on the same page? Antioxidants 2023, 12, 666. [Google Scholar] [CrossRef]
- Nadarajah, K.K. ROS homeostasis in abiotic stress tolerance in plants. Int. J. Mol. Sci. 2020, 21, 5208. [Google Scholar] [CrossRef]
- Wylie, M.R.; Merrell, D.S. The antimicrobial potential of the neem tree Azadirachta indica. Front. Pharmacol. 2022, 13, 891535. [Google Scholar] [CrossRef]
- Wasim, A.; Bushra, H.; Rakhi, R.; Ashish, V. Comprehensive Review of the Neem Plant’s Attributes and Applications. Int. J. Res. Dev. Technol. 2023, 1, 1. Available online: http://ijrdt.com/index.php/files/article/view/83 (accessed on 14 May 2024).
- Shishupala, S. Ethnomedicinal and Current Applications of Neem (Azadirachta indica). In Ethnic Knowledge and Perspectives of Medicinal Plants; Taylor & Francis Group: Oxford, UK, 2024; pp. 287–309. [Google Scholar]
- Nagini, S.; Palrasu, M.; Bishayee, A. Limonoids from neem (Azadirachta indica A. Juss.) are potential anticancer drug candidates. Med. Res. Rev. 2024, 44, 457–496. [Google Scholar] [CrossRef]
- Pramanik, P.; Tewari, S.; Mukherjee, P.; Banka, R.; Sen, S. Indian traditional plants: Medicinal properties and Human health. J. Surv. Fish. 2023, 10, 6170–6179. [Google Scholar] [CrossRef]
- Hashem, M.M.; Attia, D.; Hashem, Y.A.; Hendy, M.S.; AbdelBasset, S.; Adel, F.; Salama, M.M. Rosemary and neem: An insight into their combined anti-dandruff and anti-hair loss efficacy. Sci. Rep. 2024, 14, 7780. [Google Scholar] [CrossRef] [PubMed]
- Omar, S.A.; Ashokhan, S.; Yaacob, J.S. Salinity-induced modulation of growth and accumulation of phytochemicals composition in in vitro root cultures of Azadirachta indica. Biocatal. Agric. Biotechnol. 2023, 50, 102748. [Google Scholar] [CrossRef]
- Omar, S.A.; Ashokhan, S.; Majid, N.A.; Karsani, S.A.; Lau, B.Y.C.; Yaacob, J.S. Enhanced azadirachtin production in neem (Azadirachta indica) callus through NaCl elicitation: Insights into differential protein regulation via shotgun proteomics. Pestic Biochem Physiol. 2024, 199, 105778. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Singh, P.; Dhillon, G.; Sharma, S.; Singh, B.; Gill, R. Differential impacts of soil salinity and water logging on Eucalyptus growth and carbon sequestration under mulched vs. unmulched soils in south-western Punjab, India. Plant Soil. 2023, 482, 401–425. [Google Scholar] [CrossRef]
- Arif, A.; Ashraf, I.; Hussain, M.S.; Iqbal, R. Salinity Tolerance and Reclamation Potential of two Widely Distributed Subtropical Tree Species. Pak. J. Bot 2024, 56, 1255–1261. [Google Scholar] [CrossRef]
- Lalitha, M.; Dharumarajan, S.; Kumar, K.A.; Srinivasan, R.; Kaliraj, S.; Senthilvalavan, P.; Hegde, R.; Singh, S. Mapping of Mesquite (Prosopis juliflora) invasion in salt-affected soils of semiarid tropics: A case study. In Remote Sensing of Soils; Elsevier: Amsterdam, The Netherlands, 2024; pp. 469–475. [Google Scholar]
- Joseph, S.; Murphy, D.J.; Bhave, M. Identification of salt tolerant Acacia species for saline land utilisation. Biologia 2015, 70, 174–182. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1984; Volume 105, pp. 121–126. [Google Scholar]
- Amako, K.; Chen, G.-X.; Asada, K. Separate assays specific for ascorbate peroxidase and guaiacol peroxidase and for the chloroplastic and cytosolic isozymes of ascorbate peroxidase in plants. Plant Cell Physio. 1994, 35, 497–504. [Google Scholar] [CrossRef]
- Hemeda, H.; Klein, B. Effects of naturally occurring antioxidants on peroxidase activity of vegetable extracts. J. Food Sci. 1990, 55, 184–185. [Google Scholar] [CrossRef]
- Dhindsa, R.S.; Plumb-Dhindsa, P.; Thorpe, T.A. Leaf senescence: Correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1987; Volume 148, pp. 350–382. [Google Scholar]
- Islam, M.M.; Hossain, M.A.; Jannat, R.; Munemasa, S.; Nakamura, Y.; Mori, I.C.; Murata, Y. Cytosolic alkalization and cytosolic calcium oscillation in Arabidopsis guard cells response to ABA and MeJA. Plant Cell Physiol. 2010, 51, 1721–1730. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- AOAC International. AOAC International Guidelines for Laboratories Performing Microbiological and Chemical Analyses of Food and Pharmaceuticals: An Aid to Interpretation of ISO/IEC 17025: 2005; AOAC International: Rockville, MD, USA, 2006. [Google Scholar]
- Shahid, M.A.; Sarkhosh, A.; Khan, N.; Balal, R.M.; Ali, S.; Rossi, L.; Gómez, C.; Mattson, N.; Nasim, W.; Garcia-Sanchez, F. Insights into the physiological and biochemical impacts of salt stress on plant growth and development. Agronomy 2020, 10, 938. [Google Scholar] [CrossRef]
- Iftikhar, A.; Abbas, G.; Saqib, M.; Shabbir, A.; Amjad, M.; Shahid, M.; Ahmad, I.; Iqbal, S.; Qaisrani, S.A. Salinity modulates lead (Pb) tolerance and phytoremediation potential of quinoa: A multivariate comparison of physiological and biochemical attributes. Environ. Geochem. Health 2022, 44, 257–272. [Google Scholar] [CrossRef] [PubMed]
- Parvez, S.; Abbas, G.; Shahid, M.; Amjad, M.; Hussain, M.; Asad, S.A.; Imran, M.; Naeem, M.A. Effect of salinity on physiological, biochemical and photostabilizing attributes of two genotypes of quinoa (Chenopodium quinoa Willd.) exposed to arsenic stress. Ecotoxicol. Environ. Saf. 2020, 187, 109814. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. The response of salinity stress-induced A. tricolor to growth, anatomy, physiology, non-enzymatic and enzymatic antioxidants. Front Plant Sci. 2020, 11, 559876. [Google Scholar] [CrossRef]
- Xi, Y.; Cai, J.; Li, G.; Huang, H.; Peng, X.; Zhu, G. High CO2 facilitates fatty acid biosynthesis and mitigates cellular oxidative stress caused by CAC2 dysfunction in Arabidopsis. Plant J. 2023, 115, 1316–1330. [Google Scholar] [CrossRef]
- Alam, P.; Balawi, T.A.; Faizan, M. Salicylic acid’s impact on growth, photosynthesis, and antioxidant enzyme activity of Triticum aestivum when exposed to salt. Molecules 2022, 28, 100. [Google Scholar] [CrossRef] [PubMed]
- Ryang, S.; Woo, S.; Kwon, S.Y.; Kim, S.; Lee, S.; Kim, K.; Lee, D. Changes of net photosynthesis, antioxidant enzyme activities, and antioxidant contents of Liriodendron tulipifera under elevated ozone. Photosynthetica 2009, 47, 19–25. [Google Scholar] [CrossRef]
- Mishra, N.; Jiang, C.; Chen, L.; Paul, A.; Chatterjee, A.; Shen, G. Achieving abiotic stress tolerance in plants through antioxidative defense mechanisms. Front. Plant Sci. 2023, 14, 1110622. [Google Scholar] [CrossRef] [PubMed]
- Derbali, I.; Derbali, W.; Gharred, J.; Manaa, A.; Slama, I.; Koyro, H.-W. Mitigating Salinity Stress in Quinoa (Chenopodium quinoa Willd.) with Biochar and Superabsorber Polymer Amendments. Plants 2023, 13, 92. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Li, G.; Yang, J.; Huang, X.; Ji, Q.; Liu, Z.; Ke, W.; Hou, H. Effect of salt stress on growth, physiological parameters, and ionic concentration of water dropwort (Oenanthe javanica) cultivars. Front. Plant Sci. 2021, 12, 660409. [Google Scholar] [CrossRef] [PubMed]
- Jahan, I.; Parvin, S.; Miah, M.G.; Ahmed, J.U. Effect of salinity on the physiological and biochemical responses of neem. Int. J. Environ. Agric. Res 2018, 4, 47–54. [Google Scholar]
- Mansour, M.M.F.; Hassan, F.A. How salt stress-responsive proteins regulate plant adaptation to saline conditions. Plant Mol. Biol. 2022, 108, 175–224. [Google Scholar] [CrossRef]
- Kausar, R.; Komatsu, S. Proteomic approaches to uncover salt stress response mechanisms in crops. Int. J. Mol. Sci. 2022, 24, 518. [Google Scholar] [CrossRef]
- Athar, H.-U.-R.; Zulfiqar, F.; Moosa, A.; Ashraf, M.; Zafar, Z.U.; Zhang, L.; Ahmed, N.; Kalaji, H.M.; Nafees, M.; Hossain, M.A. Salt stress proteins in plants: An overview. Front. Plant Sci. 2022, 13, 999058. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, T.; Zhu, K.; Wang, W.; Zhang, W.; Zhang, H.; Liu, L.; Zhang, Z.; Wang, Z.; Wang, B. Effects of salt stress on grain yield and quality parameters in rice cultivars with differing salt tolerance. Plants 2023, 12, 3243. [Google Scholar] [CrossRef]
- Masarmi, A.G.; Solouki, M.; Fakheri, B.; Kalaji, H.M.; Mahgdingad, N.; Golkari, S.; Telesiński, A.; Lamlom, S.F.; Kociel, H.; Yousef, A.F. Comparing the salinity tolerance of twenty different wheat genotypes on the basis of their physiological and biochemical parameters under NaCl stress. PLoS ONE 2023, 18, e0282606. [Google Scholar] [CrossRef] [PubMed]
- Gupta, B.; Huang, B. Mechanism of salinity tolerance in plants: Physiological, biochemical, and molecular characterization. Int. J. Genom. 2014, 2014, 701596. [Google Scholar] [CrossRef] [PubMed]
- Volkov, V. Salinity tolerance in plants. Quantitative approach to ion transport starting from halophytes and stepping to genetic and protein engineering for manipulating ion fluxes. Front. Plant Sci. 2015, 6, 155182. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; Day, D.A.; Fricke, W.; Watt, M.; Arsova, B.; Barkla, B.J.; Bose, J.; Byrt, C.S.; Chen, Z.H.; Foster, K.J. Energy costs of salt tolerance in crop plants. New Phytol. 2020, 225, 1072–1090. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, S.; Premkumar, A. Ion changes and signaling under salt stress in wheat and other important crops. Plants 2023, 13, 46. [Google Scholar] [CrossRef] [PubMed]
- Tran, D.Q.; Pham, A.C.; Nguyen, T.T.T.; Vo, T.C.; Vu, H.D.; Ho, G.T.; Mohsin, S.M. Growth, Physiological, and Biochemical Responses of a Medicinal Plant Launaea sarmentosa to Salinity. Horticulturae 2024, 10, 388. [Google Scholar] [CrossRef]
- Wang, C.-F.; Han, G.-L.; Qiao, Z.-Q.; Li, Y.-X.; Yang, Z.-R.; Wang, B.-S. Root Na+ content negatively correlated to salt tolerance determines the salt tolerance of Brassica napus L. inbred seedlings. Plants 2022, 11, 906. [Google Scholar] [CrossRef]
- Saqib, M.; Abbas, G.; Akhtar, J. Root-mediated acidification and resistance to low calcium improve wheat (Triticum aestivum) performance in saline-sodic conditions. Plant. Physiol. Biochem. 2020, 156, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Abrar, M.M.; Saqib, M.; Abbas, G.; Atiq-ur-Rahman, M.; Mustafa, A.; Shah, S.A.A.; Mehmood, K.; Maitlo, A.A.; Sun, N.; Xu, M. Evaluating the contribution of growth, physiological, and ionic components towards salinity and drought stress tolerance in Jatropha curcas. Plants 2020, 9, 1574. [Google Scholar] [CrossRef]
- Guo, H.; Huang, Z.; Li, M.; Hou, Z. Growth, ionic homeostasis, and physiological responses of cotton under different salt and alkali stresses. Sci. Rep. 2020, 10, 21844. [Google Scholar] [CrossRef]
- Liu, J.; Shabala, S.; Shabala, L.; Zhou, M.; Meinke, H.; Venkataraman, G.; Chen, Z.; Zeng, F.; Zhao, Q. Tissue-specific regulation of Na+ and K+ transporters explains genotypic differences in salinity stress tolerance in rice. Front. Plant Sci. 2019, 10, 1361. [Google Scholar] [CrossRef] [PubMed]



| Parameters | CAT | APX | POD | SOD | Chlorophyll | H2O2 | Root DW | Shoot DW | Na+ Root | Na+ Shoot | K+ Root | K+ Shoot | K+/Na+ Root | K+/Na+ Shoot |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CAT | 1 | |||||||||||||
| APX | 0.65 ** | 1 | ||||||||||||
| POD | 0.54 * | 0.36 NS | 1 | |||||||||||
| SOD | 0.79 ** | 0.67 * | 0.23 NS | 1 | ||||||||||
| Chlorophyll | −0.52 * | −0.39 NS | −0.26 NS | −0.49 | 1 | |||||||||
| H2O2 | 0.67 * | 0.55 * | 0.48 | 0.59 * | −0.44 | 1 | ||||||||
| Root DW | −0.40 | −0.52 * | −0.11 NS | −0.39 NS | 0.29 NS | −0.45 | 1 | |||||||
| Shoot DW | −0.42 | −0.36 NS | −0.13 NS | −0.21 NS | 0.62 * | −0.44 | 0.42 | 1 | ||||||
| Na+ Root | 0.88 ** | 0.83 ** | 0.52 * | 0.89 ** | −0.59 * | 071 ** | −055 * | −0.38 | 1 | |||||
| Na+ Shoot | 0.89 ** | 0.78 ** | 0.44 | 0.91 ** | −0.64 * | 0.67 * | −0.47 NS | −0.41 NS | 0.96 ** | 1 | ||||
| K+ Root | −0.86 ** | −0.81 ** | −0.36 NS | −0.90 ** | 0.51 * | −0.62 * | 0.49 | 0.35 NS | −0.94 ** | −0.96 ** | 1 | |||
| K+ Shoot | −0.67 * | −0.74 ** | −0.30 NS | −0.70 ** | 0.34 NS | −0.56 * | 0.22 NS | 0.15 NS | −0.76 ** | −0.76 ** | 0.76 ** | 1 | ||
| K+/Na+ Root | −0.34 NS | −0.21 NS | 0.07 NS | −0.48 | 0.36 NS | −0.27 NS | 0.30 NS | 0.24 NS | −0.77 ** | −0.42 NS | 0.35 NS | 0.15 NS | 1 | |
| K+/Na+ Shoot | −0.53 * | −0.52 * | −0.12 NS | −0.55 * | 0.44 | −0.43 | 0.49 | 0.36 NS | −0.63 * | −0.63 * | 0.63 * | 0.41 | 0.40 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akram, M.; Sajid, Z.; Farooq, A.B.U.; Ahmad, I.; Jamal, A.; Rizwana, H.; Almunqedhi, B.M.; Ronga, D. Characterization of Physiological and Biochemical Attributes of Neem (Azadirachta indica A. Juss) under Salinity Stress. Horticulturae 2024, 10, 702. https://doi.org/10.3390/horticulturae10070702
Akram M, Sajid Z, Farooq ABU, Ahmad I, Jamal A, Rizwana H, Almunqedhi BM, Ronga D. Characterization of Physiological and Biochemical Attributes of Neem (Azadirachta indica A. Juss) under Salinity Stress. Horticulturae. 2024; 10(7):702. https://doi.org/10.3390/horticulturae10070702
Chicago/Turabian StyleAkram, Muhammad, Zunera Sajid, Abu Bakr Umer Farooq, Iftikhar Ahmad, Aftab Jamal, Humaira Rizwana, Bandar M. Almunqedhi, and Domenico Ronga. 2024. "Characterization of Physiological and Biochemical Attributes of Neem (Azadirachta indica A. Juss) under Salinity Stress" Horticulturae 10, no. 7: 702. https://doi.org/10.3390/horticulturae10070702
APA StyleAkram, M., Sajid, Z., Farooq, A. B. U., Ahmad, I., Jamal, A., Rizwana, H., Almunqedhi, B. M., & Ronga, D. (2024). Characterization of Physiological and Biochemical Attributes of Neem (Azadirachta indica A. Juss) under Salinity Stress. Horticulturae, 10(7), 702. https://doi.org/10.3390/horticulturae10070702








