Effect of Foliar Selenate Supplementation on Biochemical Characteristics of Purslane Weed (Portulaca oleracea L.)
Abstract
:1. Introduction
2. Material and Methods
2.1. Experimental Protocol and Growing Conditions
2.2. Photosynthetic Pigments
2.3. Selenium
2.4. Polyphenols (TP)
2.5. Antioxidant Activity (AOA)
2.6. Ascorbic Acid
2.7. Proline
2.8. Organic Acids
2.9. Lipid Extraction
2.10. Determination of Fatty Acid Composition
2.11. Squalene Determination
2.12. Sterol Analysis
2.13. Sterol Identification
2.14. Statistical Analysis
3. Results and Discussion
3.1. Leaves
3.1.1. Antioxidants and Photosynthetic Pigments
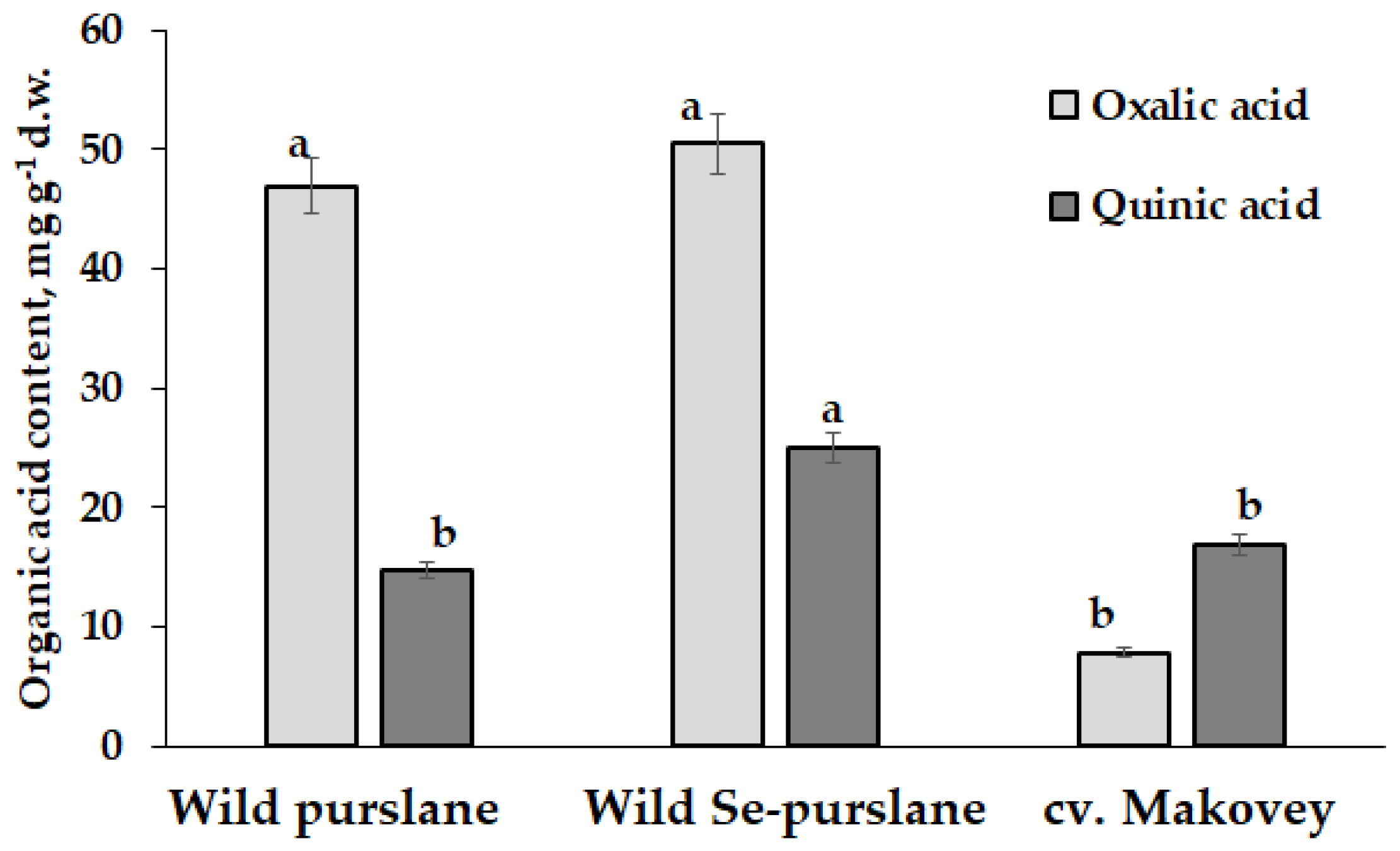
3.1.2. Organic Acids
3.2. Seeds
3.2.1. Antioxidant Status
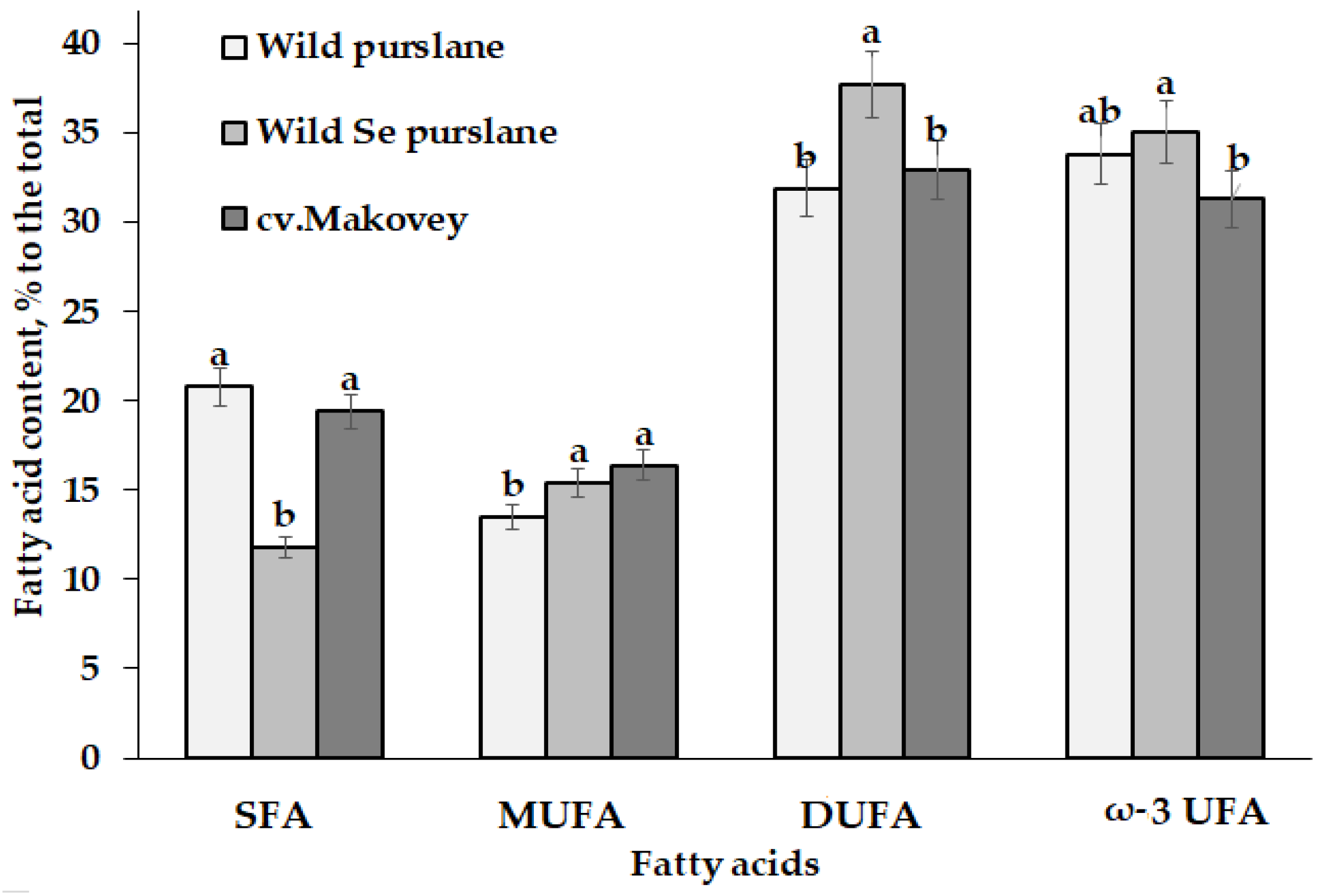
3.2.2. Fatty Acid Profile of Purslane Seed Oil
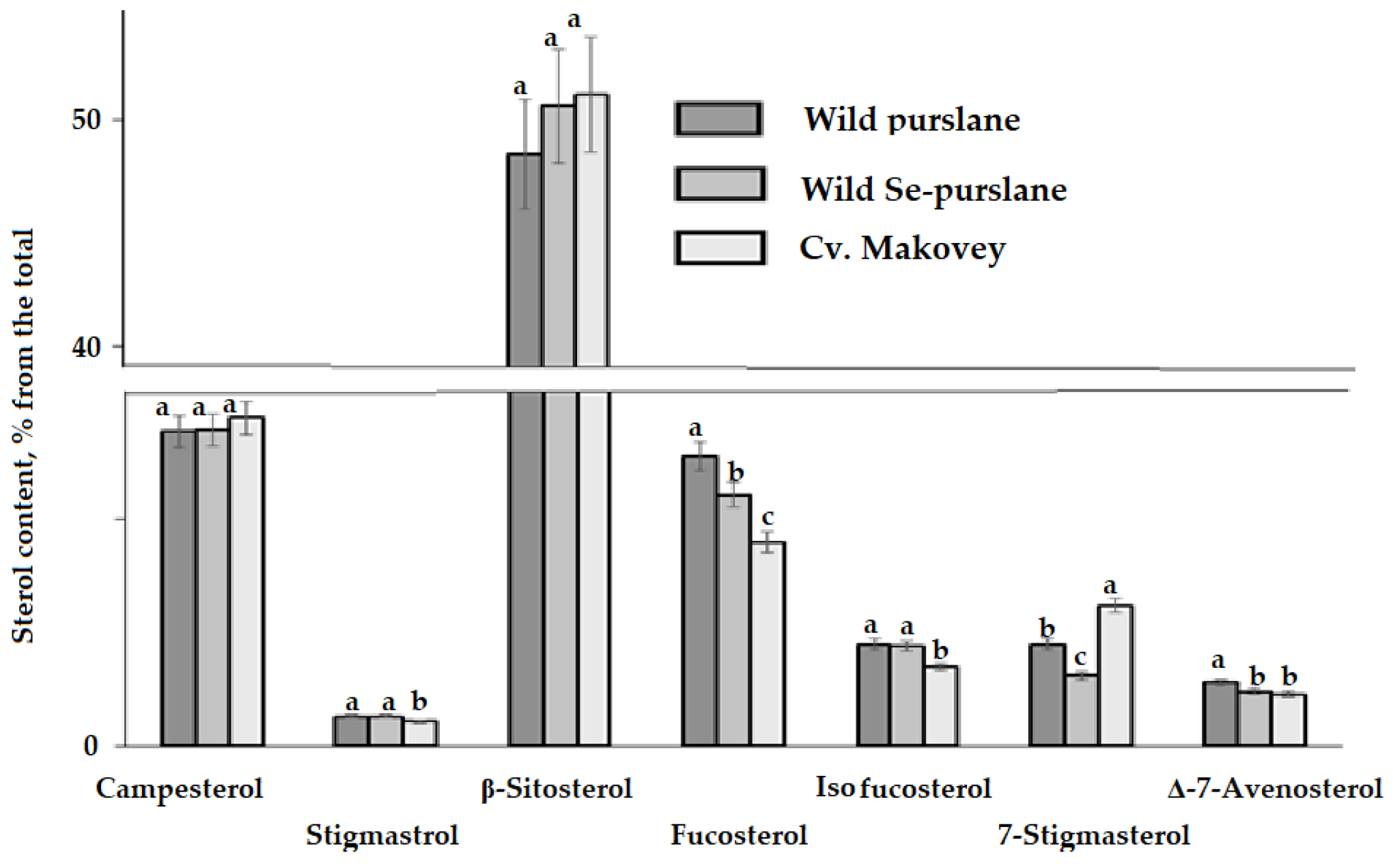
3.3. Phytosterol Composition
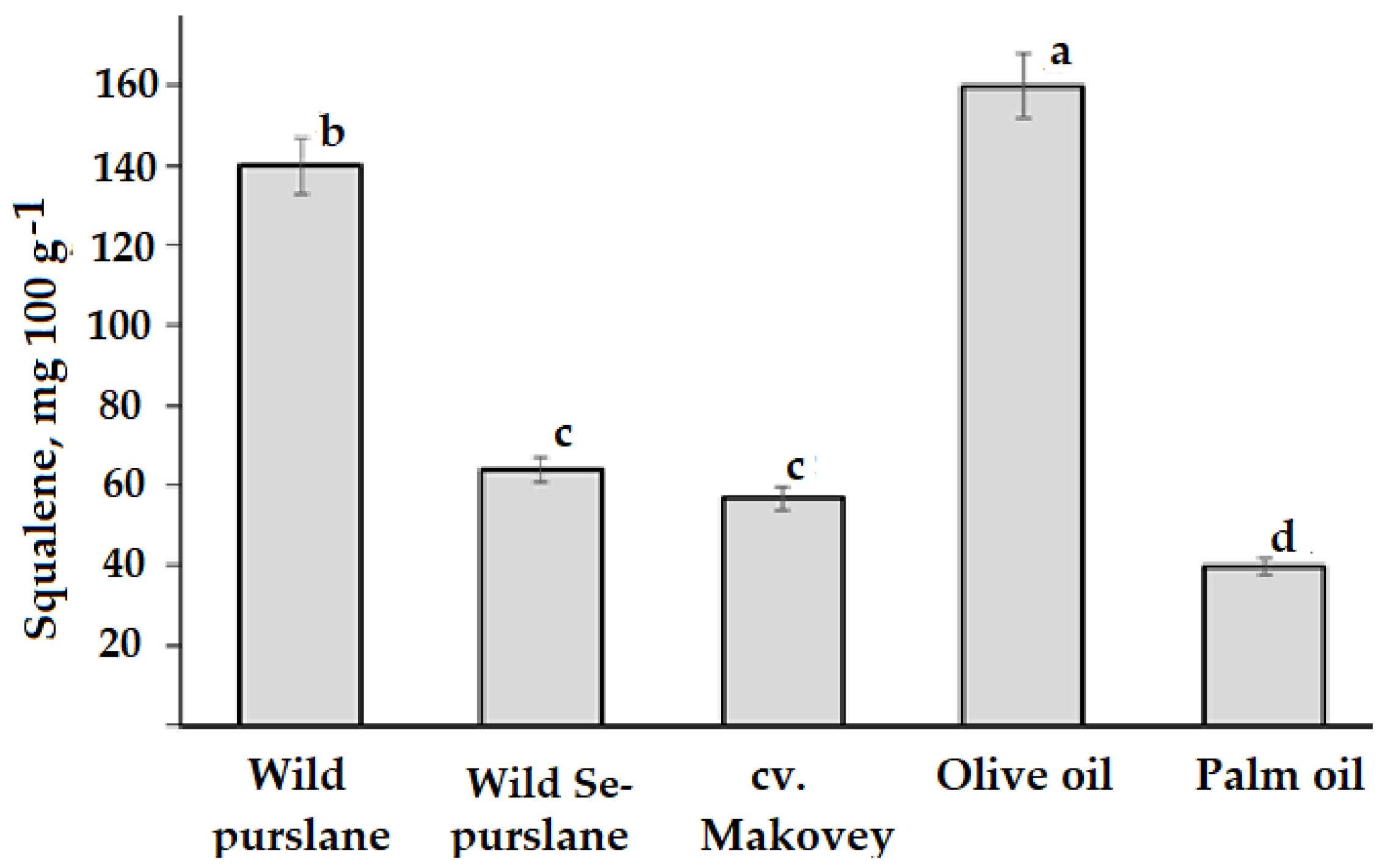
3.4. Squalene
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Genchi, G.; Lauria, G.; Catalano, A.; Sinicropi, M.S.; Carocci, A. Biological Activity of Selenium and Its Impact on Human Health. Int. J. Mol. Sci. 2023, 24, 2633. [Google Scholar] [CrossRef] [PubMed]
- Moulick, D.; Mukherjee, A.; Das, A.; Roy, A.; Majumdar, A.; Dhar, A.; Pattanaik, B.K.; Chowardhara, B.; Ghosh, D.; Upadhyay, M.K.; et al. Selenium—An environmentally friendly micronutrient in agroecosystem in the modern era: An overview of 50-year findings. Ecotoxicol. Environ. Saf. 2024, 270, 115832. [Google Scholar] [CrossRef] [PubMed]
- El-Ramady, H.; Faizy, S.E.-D.; Abdalla, N.; Taha, H.; Domokos-Szabolcsy, É.; Fari, M.; Elsakhawy, T.; Omara, A.E.D.; Shalaby, T.; Bayoumi, Y.; et al. Selenium and Nano-Selenium Biofortification for Human Health: Opportunities and Challenges. Soil Syst. 2020, 4, 57. [Google Scholar] [CrossRef]
- Chugh, V.; Mishra, V.; Dwivedi, D.V.; Sharma, K.D. Purslane (Portulaca oleracea L.): An underutilized wonder plant with potential pharmacological value. Pharma Innov. J. 2019, 8, 236–246. [Google Scholar]
- Yazici, I.; Turkan, A.; Sekmen, H.; Demiral, T. Salinity tolerance of purslane (Portulaca oleracea L.) is achieved by enhanced antioxidative system, lower level of lipid peroxidation and proline accumulation. Environ. Exp. Bot. 2007, 61, 49–57. [Google Scholar] [CrossRef]
- Srivastava, R.; Srivastava, V.; Singh, A. Multipurpose Benefits of an Underexplored Species Purslane (Portulaca oleracea L.): A Critical Review. Environ. Manag. 2023, 72, 309–320. [Google Scholar] [CrossRef]
- Agil, R.; Gilbert, C.; Tavakoli, H.; Hosseinian, F. Redefining Unusable Weeds to Beneficial Plants: Purslane as a Powerful Source of Omega-3 for the Future. J. Food Res. 2015, 4, 39–47. [Google Scholar] [CrossRef]
- Uddin, M.K.; Juraimi, A.S.; Hossain, M.S.; Nahar, M.A.; Ali, M.E.; Rahman, M.M. Purslane Weed (Portulaca oleracea): A Prospective Plant Source of Nutrition, Omega-3 Fatty Acid, and Antioxidant Attributes. Sci. World J. 2014, 2014, 951019. [Google Scholar] [CrossRef]
- Masoodi, M.H.; Ahmad, B.; Mir, S.R.; Zargar, B.A.; Tabasum, N. Portulaca oleracea L. A Review. J. Pharm. Res. 2011, 4, 3044–3048. [Google Scholar]
- Simopoulos, A.P.; Salem, N. Purslane: A terrestrial source of omega-3 fatty acids. N. Engl. J. Med. 1986, 315, 833. [Google Scholar]
- Simopoulos, A.P. Omega-3 fatty acids and antioxidants in edible wild plants. Biol. Res. 2004, 37, 263–277. [Google Scholar] [CrossRef]
- Ojah, E.O.; Oladele, E.O.; Chukwuemeka, P. Phytochemical and antibacterial properties of root extracts from Portulaca oleracea Linn. (Purslane) utilised in the management of diseases in Nigeria. J. Med. Plant Econ. Dev. 2021, 5, a103. [Google Scholar] [CrossRef]
- Mishra, V.; Chugh, V.; Dwivedi, S.V.; Sharma, K.D. Food and nutraceuticals value of purslane (Portulaca oleracea L.): An overview. Pharma Innov. J. 2020, 9, 419–424. [Google Scholar]
- Rayan, A.M.; Swailam, H.M.; Hamed, Y.S. Composition, Structure, and Techno-Functional Characteristics of the Flour, Protein Concentrate, and Protein Isolate from Purslane (Portulaca oleracea L.) Seeds. Plant Food Hum. Nutr. 2023, 7, 117–123. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Fernandes, Â.; Dias, M.I.; Vasilakoglou, I.B.; Petrotos, K.; Barros, L.; Ferreira, I.C.F.R. Nutritional Value, Chemical Composition and Cytotoxic Properties of Common Purslane (Portulaca oleracea L.) in Relation to Harvesting Stage and Plant Part. Antioxidants 2019, 8, 293. [Google Scholar] [CrossRef]
- Golubkina, N.; Kharchenko, V.; Moldovan, A.; Antoshkina, M.; Ushakova, O.; Sękara, A.; Stoleru, V.; Murariu, O.C.; Tallarita, A.V.; Sannino, M.; et al. Effect of Selenium and Garlic Extract Treatments of Seed-Addressed Lettuce Plants on Biofortification Level, Seed Productivity and Mature Plant Yield and Quality. Plants 2024, 13, 1190. [Google Scholar] [CrossRef]
- Guardado-Félix, D.; Serna-Saldivar, S.O.; Gutiérrez-Uribe, J.A.; Chuck-Hernández, C. Selenium in Germinated Chickpea (Cicer arietinum L.) Increases the Stability of Its Oil Fraction. Plants 2019, 8, 113. [Google Scholar] [CrossRef]
- D’Amato, R.; Regni, L.; Falcinelli, B.; Mattioli, S.; Benincasa, P.; Dal Bosco, A.; Pacheco, P.; Proietti, P.; Troni, E.; Santi, C.; et al. Current Knowledge on Selenium Biofortification to Improve the Nutraceutical Profile of Food: A Comprehensive Review. J. Agric. Food Chem. 2020, 68, 4075–4097. [Google Scholar] [CrossRef] [PubMed]
- Golubkina, N.; Amagova, Z.; Matsadze, V. Monitoring of the selenium content in the environment of the Chechen repubic. Adv. Mod. Sci. 2017, 2, 28–33. (In Russian) [Google Scholar]
- Cipriano, P.E.; da Silva, R.F.; de Lima, F.R.D.; de Oliveira, C.; de Lima, A.B.; Celante, G.; Dos Santos, A.A.; Archilha, M.V.L.R.; Pinatto-Botelho, M.F.; Faquin, V.; et al. Selenium biofortification via soil and its effect on plant metabolism and mineral content of sorghum plants. J. Food Comp. Anal. 2022, 109, 104505. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic bio-membranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Alfthan, G.V. A micromethod for the determination of selenium in tissues and biological fluids by single-test-tube fluorimetry. Anal. Chim. Acta 1984, 165, 187–194. [Google Scholar] [CrossRef]
- Golubkina, N.A.; Kekina, H.G.; Molchanova, A.V.; Antoshkina, M.S.; Nadezhkin, S.; Soldatenko, A.V. Plants Antioxidants and Methods of Their Determination; Infra-M: Moscow, Russia, 2020; (In Russian). [Google Scholar] [CrossRef]
- AOAC Association Official Analytical Chemists. The Official Methods of Analysis of AOAC International; 22 ‘Vitamin C’; AOAC: Rockville, MD, USA, 2012. [Google Scholar]
- Ouertani, R.N.; Abid, G.; Karmous, C.; Chikha, M.B.; Boudaya, O.; Mahmoudi, H.; Mejri, S.; Jansen, K.; Ghorbel, A. Evaluating the contribution of osmotic and oxidative stress components on barley growth under salt stress. AoB Plants 2021, 13, plab034. [Google Scholar] [CrossRef] [PubMed]
- Guide for the Evaluation of Quality and Safety of Food Biologically Active Additives; Organic Acids Determination; Health Ministry of RF P 4.1.1672-03: Moscow, Russia, 2004; pp. 109–111.
- GOST 31663-2012; Vegetable Oils and Animal Fats. Determination of Methyl Esters of Fatty Acids by Gas Chromatography Method. ISO: Moscow, Russia, 2012.
- Golay, P.A.; Moulin, J. Determination of labeled fatty acids content in milk products, infant formula, and adult/pediatric nutritional formula by capillary gas chromatography: Collaborative study, final action 2012.13. J. AOAC Int. 2012, 99, 210–222. [Google Scholar] [CrossRef] [PubMed]
- Golay, P.A.; Dong, Y. Determination of labeled fatty acids content in milk products, infant formula, and adult/pediatric nutritional formula by capillary gas chromatography: Single-Laboratory Validation, First Action 2012.13. J. AOAC Int. 2015, 98, 1679–1696. [Google Scholar] [CrossRef] [PubMed]
- Budge, S.M.; Barry, C. Determination of squalene in edible oils by transmethylation and GC analysis. MethodsX 2018, 6, 15–21. [Google Scholar] [CrossRef]
- ISO 12228-1-2014; Determination of Individual and Total Sterols Contents—Gas Chromatographic Method—Part 1: Animal and Vegetable Fats and Oils. ISO: Moscow, Russia, 2014.
- National Institute of Standards and Technology. NIST Chemistry WebBook, SRD 69. Available online: https://webbook.nist.gov/chemistry/ (accessed on 26 February 2024).
- Goad, L.J.; Akihisa, T. Analysis of Sterols; Blackie Academic and Professional: London, UK, 1997. [Google Scholar]
- The Lipid Web. Available online: https://www.lipidmaps.org/resources/lipidweb/lipidweb_html/ms/others/others-arch/index1.htm#sterol (accessed on 26 February 2024).
- Cunha, S.S.; Fernandes, J.O.; Oliveira, M.B.P.P. Quantification of free and esterified sterols in Portuguese olive oils by solid-phase extraction and gas chromatography–mass spectrometry. J. Chromatogr. A 2006, 1128, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Li, T.S.C.; Beveridge, T.H.J.; Drover, J.C.G. Phytosterol content of sea buckthorn (Hippophae rhamnoides L.) seed oil: Extraction and identification. Food Chem. 2007, 101, 1633–1639. [Google Scholar] [CrossRef]
- Semeniuc, C.A.; Cardenia, V.; Mandrioli, M.; Muste, S.; Borsari, A.; Rodriguez-Estrada, M.T. Stability of flavored phytosterol-enriched drinking yogurts during storage as affected by different packaging materials. J. Sci. Food Agric. 2016, 96, 2782–2787. [Google Scholar] [CrossRef]
- Márquez, V.G.; Moreno, Á.M.; Mendoza, A.B.; Macías, J.M. Ionic Selenium and Nanoselenium as Biofortifiers and Stimulators of Plant Metabolism. Agronomy 2020, 10, 1399. [Google Scholar] [CrossRef]
- Sdouga, D.; Branca, F.; Kabtni, S.; Di Bella, M.C.; Trifi-Farah, N.; Marghali, S. Morphological Traits and Phenolic Compounds in Tunisian Wild Populations and Cultivated Varieties of Portulaca oleracea L. Agronomy 2020, 10, 948. [Google Scholar] [CrossRef]
- Liu, H.; Xiao, C.; Qiu, T.; Deng, J.; Cheng, H.; Cong, X.; Cheng, S.; Rao, S.; Zhang, Y. Selenium Regulates Antioxidant, Photosynthesis, and Cell Permeability in Plants under Various Abiotic Stresses: A Review. Plants 2022, 12, 44. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X.; Wang, L.; Zhang, R.; Zhang, Y.; Cao, L. Purslane-induced oxalate nephropathy: Case report and literature review. BMC Nephrol. 2023, 24, 207. [Google Scholar] [CrossRef]
- Benali, T.; Bakrim, S.; Ghchime, R.; Benkhaira, N.; El Omari, N.; Balahbib, A.; Bouyahya, A. Pharmacological insights into the multifaceted biological properties of quinic acid. Biotechnol. Genet. Eng. Rev. 2022, 1–30. [Google Scholar] [CrossRef]
- Soviguidi, D.R.J.; Pan, R.; Liu, Y.; Rao, L.; Zhang, W.; Yang, X. Chlorogenic acid metabolism: The evolution and roles in plant response to abiotic stress. Phyton Int. J. Exp. Bot. 2022, 91, 239–255. [Google Scholar] [CrossRef]
- Cervera-Mata, A.; Sahu, P.K.; Chakradhari, S.; Sahu, Y.K.; Patel, K.S.; Singh, S.; Towett, E.K.; Martín-Ramos, P.; Quesada-Granados, J.J.; Rufián-Henares, J.A. Plant seeds as source of nutrients and phytochemicals for the Indian population. Int. J. Food Sci. Technol. 2022, 57, 525–532. [Google Scholar] [CrossRef]
- Jalali Mousavi, S.R.; Niazmand, R.; Shahidi Noghabi, M. Noghabi Antioxidant Activity of Purslane (Portulaca oleracea L.) Seed Hydro-alcoholic Extract on the Stability of Soybean Oil. J. Agric. Sci. Technol. 2015, 17, 1473–1480. [Google Scholar]
- Iqbal, M.; Hussain, I.; Liaqat, H.; Ashraf, M.A.; Rasheed, R.; Rehman, A.U. Exogenously applied selenium reduces oxidative stress and induces heat tolerance in spring wheat. Plant Physiol. Biochem. 2015, 94, 95–103. [Google Scholar] [CrossRef]
- Ros, E.; Hu, F.B. Consumption of plant seeds and cardiovascular health: Epidemiological and clinical trial evidence. Circulation 2013, 128, 553–565. [Google Scholar] [CrossRef]
- Khan, M.A.; Castro-Guerrero, N.; Mendoza-Cozatl, D.G. Moving toward a precise nutrition: Preferential loading of seeds with essential nutrients over non-essential toxic elements. Front. Plant Sci. 2014, 5, 51. [Google Scholar] [CrossRef]
- Esmaillzadeh, A.; Zakizadeh, E.; Faghihimani, E.; Gohari, M.; Jazayeri, S. The effect of purslane seeds on glycemic status and lipid profiles of persons with type 2 diabetes: A randomized controlled cross-over clinical trial. J. Res. Med. Sci. 2015, 20, 47–53. [Google Scholar] [PubMed]
- Petropoulosa, S.A.; Fernandesb, A.; Arampatzisa, D.A.; Tsiropoulosa, N.G.; Petrovićc, J.; Sokovićc, M.; Barrosb, L.; Ferreirab, I.C.F.R. Seed oil and seed oil byproducts of common purslane (Portulaca oleracea L): A new insight to plant-based sources rich in omega-3 fatty acids. LWT-Food Sci. Technol. 2020, 123, 109099. [Google Scholar] [CrossRef]
- Desta, M.; Molla, A.; Yusuf, Z. Characterization of physico-chemical properties and antioxidant activity of oil from seed, leaf and stem of purslane (Portulaca oleracea L.). Biotechnol. Rep. 2020, 27, e00512. [Google Scholar] [CrossRef] [PubMed]
- Lidon, F.C.; Oliveira, K.; Ribeiro, M.M.; Pelica, J.; Pataco, I.; Ramalho, J.C.; Leitão, A.E.; Almeida, A.S.; Campos, P.S.; Ribeiro-Barros, A.I.; et al. Selenium biofortification of rice grains and implications on macronutrients quality. J. Cereal Sci. 2018, 81, 22–29. [Google Scholar] [CrossRef]
- Kieliszek, M.; Błażejak, S.; Bzducha-Wróbel, A. Effect of Selenium on Lipid and Amino Acid Metabolism in Yeast Cells. Biol. Trace Elem. Res. 2019, 187, 316–327. [Google Scholar] [CrossRef] [PubMed]
- Davoudi, B.; Mirshekari, A.; Shirani-Rad, F.; Farahvash, V. Rashidi Effect of selenium foliar application on oil yield, fatty acid composition and glucosinolate content of rapeseed cultivars under late-season thermal stress. Oilseeds Fats Crops Lipids (OCL) 2019, 26, 43. [Google Scholar] [CrossRef]
- Mousavi, S.R.J.; Niazmand, R. Fatty Acids Composition and Oxidation Kinetic Parameters of Purslane (Portulaca oleracea) Seed Oil. Agric. Res. 2017, 6, 421–426. [Google Scholar] [CrossRef]
- Mahla, H.R.; Rathore, S.S.; Venkatesan, K.; Sharma, R. Analysis of fatty acid methyl esters and oxidative stability of seed purpose watermelon (Citrullus lanatus) genotypes for edible oil. J. Food Sci. Technol. 2018, 55, 1552–1561. [Google Scholar] [CrossRef]
- Nehdi, I. Characteristics, chemical composition and utilization of Albizia julibrissin seed oil. Ind. Crops Prod. 2011, 33, 30–34. [Google Scholar] [CrossRef]
- Oomah, B.; Dumon, D.; Cardador-Martinez, A.; Godfrey, D. Characteristics of Echinacea seed oil. Food Chem. 2006, 96, 304–312. [Google Scholar] [CrossRef]
- Li, X.; Xin, Y.; Mo, Y.; Marozik, P.; He, T.; Guo, H. The Bioavailability and Biological Activities of Phytosterols as Modulators of Cholesterol Metabolism. Molecules 2022, 27, 523. [Google Scholar] [CrossRef] [PubMed]
- Babu, S.; Jayaraman, S. An update on β-sitosterol: A potential herbal nutraceutical for diabetic management. Biomed. Pharmacother. 2020, 131, 110702. [Google Scholar] [CrossRef] [PubMed]
- Bai, G.; Ma, C.; Chen, X. Phytosterols in edible oil: Distribution, analysis and variation during processing. Grain Oil Sci. Technol. 2021, 4, 33–44. [Google Scholar] [CrossRef]
- Lozano-Grande, M.A.; Gorinstein, S.; Espitia-Rangel, E.; Dávila-Ortiz, G.; Martínez-Ayala, A.L. Plant Sources, Extraction Methods, and Uses of Squalene. Int. J. Agron. 2018, 2018, 1829160. [Google Scholar] [CrossRef]
- Piironen, V.; Lindsay, D.G.; Miettinen, T.A.; Toivo, J. Plant sterols: Biosynthesis, biological function and their importance to human nutrition. J. Sci. Food Agric. 2000, 80, 939–966. [Google Scholar] [CrossRef]
- LoRicco, J.G.; Hoffmann, I.; Caliò, A.; Peters, J. The membrane regulator squalane increases membrane rigidity under high hydrostatic pressure in archaeal membrane mimics. Soft Matter 2023, 19, 6280–6286. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Fu, X.; Chu, Y.; Wu, P.; Liu, Y.; Ma, L.; Tian, H.; Zhu, B. Biosynthesis and the Roles of Plant Sterols in Development and Stress Responses. Int. J. Mol. Sci. 2022, 23, 2332. [Google Scholar] [CrossRef]
- Popa, O.; Băbeanu, N.E.; Popa, I.; Niță, S.; Dinu-Pârvu, C.E. Methods for obtaining and determination of squalene from natural sources. BioMed Res. Int. 2015, 2015, 367202. [Google Scholar] [CrossRef] [PubMed]
- Acedo, J.Z.; Reyes, C.T.; Rodriguez, E.B. Health-promoting lipids from purslane (Portulaca oleraceae). Isolation, characterization, quantification and in vivo assay of angiogenic activity. Philipp. Agric. Sci. 2012, 95, 327–334. [Google Scholar]
- Akgün, N.A. Separation of squalene from olive oil deodorizer distillate using supercritical fluids. Eur. J. Lipid Sci. Technol. 2011, 113, 1558–1565. [Google Scholar] [CrossRef]
- Posada, L.R.; Shi, J.; Kakuda, Y.; Xue, S.J. Extraction of tocotrienols from palm fatty acid distillates using molecular distillation. Sep. Purif. Technol. 2007, 57, 220–229. [Google Scholar] [CrossRef]
- Sun, H.; Wiesenborn, D.; Rayas-Duarte, P.; Mohamed, A.; Hagen, K. Bench-scale processing of amaranth seed for oil. J. Am. Oil Chem. Soc. 1995, 72, 1551–1555. [Google Scholar] [CrossRef]
- Gilmore, S.F.; Uao, A.I.; Tietel, Z.; Facciotti, K.T.; Parikh, A.N. Role of Squalene in the Organization of Monolayers Derived from Lipid Extracts of Halobacterium salinarum. Langmuir 2013, 29, 7922–7930. [Google Scholar] [CrossRef] [PubMed]

| Month | 2022 | 2023 | ||
|---|---|---|---|---|
| Mean Temperature (°C) | Rainfall (mm) | Mean Temperature (°C) | Rainfall (mm) | |
| May | 16.6 | 97.5 | 16.5 | 66.0 |
| June | 21.7 | 31.8 | 21.2 | 131.0 |
| July | 23.9 | 40.3 | 23.7 | 80.0 |
| August | 23.6 | 9.0 | 26.1 | 12.0 |
| September | 18.1 | 31.0 | 21.0 | 84.0 |
| Peak No | Compound | M | M-15 1 | M-90 2 | M-105 | M-129 3 | M-122 3 | M-SC-2H 4 | Other Ions |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Cholestanol (internal standard) | 460 | 445 | 370 | 355 | - | - | - | 215; 75 |
| 2 | Kampesterol | 472 | 457 | 382 | 367 | 343 | 261 | - | 129 |
| 3 | Stigmasterol | - | - | 394 | - | 355 | - | - | 129 |
| 4 | β-Sitosterol | 486 | 471 | 396 | 381 | 357 | 275 | - | 129 |
| 5 | Isofucosterol | 484 | 469 | 394 | 379 | 355 | 273 | - | 386; 296; 129 |
| 6 | Δ7-Stigmastenol | 486 | 471 | 396 | 381 | - | 275 | 343 | 255; 75 |
| 7 | Δ7-Avenasterol | - | - | - | - | - | 273 | 343 | 386; 281; 253; 213 |
| Peak Number | Compound | Rt, min | RI | RI in a Mixture of Standards | Values According to [37] |
|---|---|---|---|---|---|
| 1 | Cholestanol (internal standard) | 20.992 | 3144 | 3148 | 3169 |
| 2 | Kampesterol | 23.480 | 3238 | 3242 | 3253 |
| 3 | Stigmasterol | 24.316 | 3269 | 3274 | 3274 |
| 4 | β-Sitosterol | 25.841 | 3327 | 3329 | 3348 |
| 5 | Isofucosterol | 26.275 | 3343 | - | - |
| 6 | Δ7-Stigmastenol | 27.300 | 3382 | - | - |
| 7 | Δ7-Avenasterol | 27.747 | 3398 | - | - |
| Parameter | Wild Purslane | Wild Se-Purslane | cv. Makovey |
|---|---|---|---|
| Plant biomass (g) | 129 ± 10 b | 153 ± 11 a | 110 ± 10 b |
| Stem length (cm) | 17.8 ± 1.1 b | 23.1 ± 1.9 a | 18.0 ± 1.0 b |
| Number of stems per plant | 20.5 ± 1.7 b | 24.6 ± 1.0 a | 16.0 ± 1.0 b |
| Plant diameter (cm) | 37.4 ± 3.5 b | 46.9 ± 4.2 a | 38.0 ± 3.0 b |
| Weight of 1000 seeds (g) | 0.123 ± 0.01 b | 0.118 ± 0.01 b | 0.336 ± 0.01 a |
| Seed weight per plant (g) | 1.79 ab | 1.84 a | 1.52 b |
| Seed productivity (number of seeds per plant) | 14,553 a | 15,593 a | 4524 b |
| Parameter | Wild Purslane | Wild Se-Purslane | cv. Makovey |
|---|---|---|---|
| AA (mg 100 g−1 f.w.) | 25.5 ± 1.5 b | 30.5 ± 2.5 a | 24.8 ± 1.9 b |
| AOA (mg GAE g−1 d.w.) | 47.7 ± 4.0 a | 43.2 ± 3.4 a | 45.3 ± 3.9 a |
| TP (mg GAE g−1 d.w.) | 22.4 ± 2.2 a | 18.1 ± 1.8 a | 19.3 ± 1.7 a |
| Se (µg kg−1 d.w.) | 108 ± 10 b | 621 ± 56 a | 93 ± 9 b |
| Chl a (mg g−1 f.w.) | 0.66 ± 0.03 b | 0.84 ± 0.06 a | 0.51 ± 0.03 c |
| Chl b (mg g−1 f.w.) | 0.48 ± 0.03 b | 0.76 ± 0.06 a | 0.24 ± 0.02 c |
| Total Chl (mg g−1 f.w.) | 1.14 ± 0.1 b | 1.60 ± 0.1 a | 0.75 ± 0.04 c |
| Carotene (mg g−1 f.w.) | 0.069 ± 0.01 a | 0.087 ± 0.01 a | 0.043 ± 0.01 b |
| Chl a/chl b | 1.38 | 1.11 | 2.125 |
| Chl/carotene | 14.4 | 18.4 | 17.4 |
| Parameter | Wild Purslane | Wild Se-Purslane | cv. Makovey |
|---|---|---|---|
| AOA (mg GAE g−1 d.w.) | 19.5 ± 1.7 a | 17.0 ± 1.6 a | 9.9 ± 1.0 b |
| TP (mg GAE g−1 d.w.) | 9.3 ± 0.9 a | 10.5 ± 0.9 a | 2.9 ± 0.2 b |
| AA (mg 100 g−1 d.w.) | 4.3 ± 0.4 b | 6.0 ± 0.5 a | 4.7 ± 0.4 b |
| Se (µg kg−1 d.w.) | 87 ± 8.0 b | 570 ± 50 a | 78 ± 7.1 b |
| Total lipids (g 100 g−1) | 14.8 ± 1.3 a | 16.2 ± 1.4 a | 17.4 ± 1.5 a |
| Pro (mg g−1 d.w.) | 0.188 ± 0.01 b | 0.235 ± 0.02 a | 0.236 ± 0.02 a |
| MDA (mg g−1 d.w.) | 0.200 ± 0.02 a | 0.086 ± 0.008 b | 0.070 ± 0.007 b |
| Fatty Acid | Wild Purslane | Wild Se Purslane | cv. Makovey | Literature Data [55] | |
|---|---|---|---|---|---|
| Myristic 14:0 | 1 a | 1 a | 1 a | 0.7 | |
| Pentadecanic 15:0 | 1 a | 0 b | 0 b | 0.2 | |
| Palmitic 16:0 | 103 b | 72 c | 134 a | 164.3 | |
| Margaric 17:0 | 0 | 0 | 0 | 2.2 | |
| Stearic 18:0 | 26 b | 23 b | 32 a | 30.9 | |
| Arachidic 20:0 | 64 a | 5 b | 6 b | 1.4 | |
| Behenic 22:0 | 4 a | 4 a | 2 b | 9.4 | |
| Lignoceri 24:0 | 3 a | 3 a | 2 b | 1.5 | |
| Saturated acids | 202 | 108 | 177 | 210.9 | |
| Hexadecenic 16:1 | 1 a | 1 a | 1 a | 4.8 | |
| Palmitoleinic 16:1 cis | 1 a | 1 a | 1 a | ||
| Elaidic | 18:1 9-trans | 1 a | 1 a | 0 b | |
| Oleic | 18:1 9 cis | 109 a | 116 a | 127 a | 163.7 |
| Vaccenic | 18:1 11-trans | 18 a | 20 a | 19 a | |
| Gondoic (sum of isomers) 20:1 | 1 | 2 | 1 | ||
| Mono-unsaturated | 131 | 141 | 149 | 168.5 | |
| Cis, trans linoleic 18:2 9-cis, 12-trans | 1 | 1 | 1 | ||
| Linoleic 18:2 | 309 b | 344 a | 299 b | 336.3 | |
| Di-unsaturated acids | 310 | 345 | 300 | 336.5 | |
| Octadecatrienoic 18:3 cis-9, trans-12 Trans-15 + 18:3 cis-9. Cis-12. Trans-15 | 3 a | 2 b | 2 b | 14.8 | |
| α-Linolenic | 18:3 ω-3 | 325 a | 320 a | 283 a | 267.7 |
| PUSFA | 328 | 322 | 285 | 282.5 | |
| Total lipids (g 100 g−1) | 14.8 | 16.2 | 17.4 | - | |
| P/S ratio (PI index) | 3.81 | 7.48 | 4.15 | 2.93 | |
| Sterols (mg 100 g−1) | Wild Purslane | Wild Se-Purslane | cv. Makovey |
|---|---|---|---|
| Campesterol | 28.5 ± 0.9 b | 27.8 ± 1.1 b | 34.2 ± 1.0 a |
| Stigmasterol | 2.6 ± 0.1 a | 2.6 ± 0.1 a | 2.6 ± 0.1 a |
| β-Sitosterol | 99.2 ± 3.0 b | 100.8 ± 4.0 b | 120.4 ± 3.6 a |
| Fucosterol | 26.1 ± 0.8 a | 22.1 ± 0.9 b | 21.2 ± 0.6 b |
| Isofucosterol | 9.1 ± 0.3 a | 8.7 ± 0.3 ab | 8.2 ± 0.2 b |
| 7-Stigmasterol | 9.2 ± 0.3 b | 6.2 ± 0.2 c | 14.5 ± 0.4 a |
| ∆-7-Avenosterol | 5.9 ± 0.2 a | 4.8 ± 0.2 b | 5.5 ± 0.2 a |
| Total | 204.4 ± 6.1 b | 199.2 ± 8.0 b | 235.5 ± 7.1 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golubkina, N.; Amagova, Z.; Kharchenko, V.; Bogachuk, M.; Makarenko, M.; Paleeva, M.; Malinkin, A.; Andreeva, K.; Kavarnakaeva, Z.; Matsadze, V.; et al. Effect of Foliar Selenate Supplementation on Biochemical Characteristics of Purslane Weed (Portulaca oleracea L.). Horticulturae 2024, 10, 708. https://doi.org/10.3390/horticulturae10070708
Golubkina N, Amagova Z, Kharchenko V, Bogachuk M, Makarenko M, Paleeva M, Malinkin A, Andreeva K, Kavarnakaeva Z, Matsadze V, et al. Effect of Foliar Selenate Supplementation on Biochemical Characteristics of Purslane Weed (Portulaca oleracea L.). Horticulturae. 2024; 10(7):708. https://doi.org/10.3390/horticulturae10070708
Chicago/Turabian StyleGolubkina, Nadezhda, Zarema Amagova, Viktor Kharchenko, Maria Bogachuk, Maria Makarenko, Maria Paleeva, Alexey Malinkin, Katherine Andreeva, Zulfia Kavarnakaeva, Visita Matsadze, and et al. 2024. "Effect of Foliar Selenate Supplementation on Biochemical Characteristics of Purslane Weed (Portulaca oleracea L.)" Horticulturae 10, no. 7: 708. https://doi.org/10.3390/horticulturae10070708
APA StyleGolubkina, N., Amagova, Z., Kharchenko, V., Bogachuk, M., Makarenko, M., Paleeva, M., Malinkin, A., Andreeva, K., Kavarnakaeva, Z., Matsadze, V., Murariu, O. C., & Caruso, G. (2024). Effect of Foliar Selenate Supplementation on Biochemical Characteristics of Purslane Weed (Portulaca oleracea L.). Horticulturae, 10(7), 708. https://doi.org/10.3390/horticulturae10070708










