Interspecific Hardy Geranium Progenies: Morphological Characterization and Genetic Evaluation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Male Fertility and Female Receptivity
2.3. Interspecific Crosses
2.4. Embryo Rescue
2.5. AFLP Analysis
2.6. Morphological Analysis
2.7. Statistical Analysis
3. Results
3.1. Parental Male Fertility and Female Receptivity
3.2. Interspecific Crosses between Geranium Species and Overcoming Post-Fertilization Barriers
3.3. Genetical and Morphological Evaluation of F1 Progeny
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Tuyl, J.M.; Lim, K.B.; Ramanna, M.S. Interspecific Hybridization and Introgression. In Breeding for Ornamentals: Classical and Molecular Approaches; Springer: Dordrecht, The Netherlands, 2002; pp. 85–103. [Google Scholar]
- Horn, W. Breeding Methods and Breeding Research BT. In Breeding For Ornamentals: Classical and Molecular Approaches; Vainstein, A., Ed.; Springer: Dordrecht, The Netherlands, 2002; pp. 47–83. ISBN 978-94-017-0956-9. [Google Scholar]
- Prohens, J.; Gramazio, P.; Plazas, M.; Dempewolf, H.; Kilian, B.; Diez, M.J.; Fita, A.; Herraiz, F.J.; Rodriguez-Burruezo, A.; Soler, S. Introgressiomics: A New Approach for Using Crop Wild Relatives in Breeding for Adaptation to Climate Change. Euphytica 2017, 213, 158. [Google Scholar] [CrossRef]
- Shivanna, K.R.; Bahadur, B. Efficacy of Biotechnological Approaches to Raise Wide Sexual Hybrids BT. In Plant Biology and Biotechnology: Volume II: Plant Genomics and Biotechnology; Bahadur, B., Venkat Rajam, M., Sahijram, L., Krishnamurthy, K.V., Eds.; Springer: New Delhi, India, 2015; pp. 347–362. ISBN 978-81-322-2283-5. [Google Scholar]
- Rogo, U.; Fambrini, M.; Pugliesi, C. Embryo Rescue in Plant Breeding. Plants 2023, 12, 3106. [Google Scholar] [CrossRef] [PubMed]
- Krishna, R.; Khandagale, K.; Benke, A.P.; Soumia, P.S.; Manjunathagowda, D.C.; Ansari, W.A.; Mokat, D.N.; Gawande, S.J.; Ade, A.B.; Singh, M. Embryo Rescue: A Potential Tool for Improvement of Economically Important Crops. In Advances in Plant Tissue Culture; Elsevier: Amsterdam, The Netherlands, 2022; pp. 259–282. [Google Scholar]
- Loyola-Vargas, V.M.; Ochoa-Alejo, N. An Introduction to Plant Cell Culture: The Future Ahead. In Plant Cell Culture Protocols; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2012; pp. 1–8. [Google Scholar]
- Shen, X.; Gmitter, F.G.; Grosser, J.W. Immature Embryo Rescue and Culture. In Plant Embryo Culture; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2011; pp. 75–92. [Google Scholar]
- Sharma, D.R.; Kaur, R.; Kumar, K. Embryo Rescue in Plants—A Review. Euphytica 1996, 89, 325–337. [Google Scholar] [CrossRef]
- Sahijram, L.; Soneji, J.R.; Naren, A.; Rao, B.M. Hybrid Embryo Rescue: A Non-Conventional Breeding Strategy in Horticultural Crops. J. Hortic. Sci. 2013, 8, 1–20. [Google Scholar] [CrossRef]
- Bhatia, R.; Dey, S.S.; Sharma, K.; Singh, S.; Kumar, S.; Pramanik, A.; Parkash, C.; Kumar, R. Back-Cross Introgression of ‘Tour’ Cytoplasm from Brassica napus through in Vitro Embryo Rescue Reveals Partial Restoration of Sterility in B. oleracea. Sci. Hortic. 2021, 282, 110014. [Google Scholar] [CrossRef]
- Giancaspro, A.; Mazzeo, A.; Carlomagno, A.; Gadaleta, A.; Somma, S.; Ferrara, G. Optimization of an In Vitro Embryo Rescue Protocol for Breeding Seedless Table Grapes (Vitis vinifera L.) in Italy. Horticulturae 2022, 8, 121. [Google Scholar] [CrossRef]
- Zhu, W.; Jiang, J.; Chen, S.; Wang, L.; Xu, L.; Wang, H.; Li, P.; Guan, Z.; Chen, F. Intergeneric Hybrid between Chrysanthemum × Morifolium and Artemisia Japonica Achieved via Embryo Rescue Shows Salt Tolerance. Euphytica 2013, 191, 109–119. [Google Scholar] [CrossRef]
- Cheng, X.; Chen, S.; Chen, F.; Fang, W.; Deng, Y.; She, L. Interspecific Hybrids between Dendranthema morifolium (Ramat.) Kitamura and D. nankingense (Nakai) Tzvel. Achieved Using Ovary Rescue and Their Cold Tolerance Characteristics. Euphytica 2010, 172, 101–108. [Google Scholar] [CrossRef]
- Takamura, Y.; Asano, C.; Hikage, T.; Hatakeyama, K.; Takahata, Y. Production of Interspecific Hybrids between Japanese Gentians and Wild Species of Gentiana. Breed. Sci. 2019, 69, 680–687. [Google Scholar] [CrossRef]
- Plaschil, S.; Budahn, H.; Klocke, E.; Wiedemann, M.; Olbricht, K. Spontaneous Polyploidisation of Interspecific and Intersectional Pelargonium Hybrids during Embryo Rescue. J. Appl. Bot. Food Qual. 2021, 94, 206–212. [Google Scholar]
- Aedo, C. Taxonomic Revision of Geranium Sect. Brasiliensia (Geraniaceae). Syst. Bot. 2001, 26, 205–215. [Google Scholar] [CrossRef]
- Marcussen, T.; Meseguer, A.S. Species-Level Phylogeny, Fruit Evolution and Diversification History of Geranium (Geraniaceae). Mol. Phylogenet. Evol. 2017, 110, 134–149. [Google Scholar] [CrossRef] [PubMed]
- Yeo, P.F. Hardy Geraniums; New Edition; BT Batsford: London, UK, 2001. [Google Scholar]
- Armitage, J. Hardy Geraniums–Stage 1; Bulletin Number 2005; Royal Horticultural Society: London, UK, 2005. [Google Scholar]
- Akbarzadeh, M.; Quataert, P.; Van Huylenbroeck, J.; Werbrouck, S.P.O.; Dhooghe, E. Prediction Model for Breeding Hardy Geraniums. Horticulturae 2023, 9, 617. [Google Scholar] [CrossRef]
- Breyne, P.; Boerjan, W.; Gerats, T.; Van Montagu, M.; Van Gysel, A. Applications of AFLP TM in Plant Breeding, Molecular Biology and Genetics. Belgian J. Bot. 1997, 129, 107–117. [Google Scholar]
- Akbarzadeh, M.; Van Laere, K.; Leus, L.; De Riek, J.; Van Huylenbroeck, J.; Werbrouck, S.P.O.; Dhooghe, E. Can Knowledge of Genetic Distances, Genome Sizes and Chromosome Numbers Support Breeding Programs in Hardy Geraniums? Genes 2021, 12, 730. [Google Scholar] [CrossRef] [PubMed]
- Doyle, J.J.; Doyle, J.L. A Rapid DNA Isolation Procedure for Small Quantities of Fresh Leaf Tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- Vos, P.; Hogers, R.; Bleeker, M.; Reijans, M.; Lee, T.V.D.; Hornes, M.; Friters, A.; Pot, J.; Paleman, J.; Kuiper, M.; et al. AFLP: A New Technique for DNA Fingerprinting. Nucleic Acids Res. 1995, 23, 4407–4414. [Google Scholar] [CrossRef] [PubMed]
- Elmer, P. AFLP Plant Mapping Kit Protocol (P/N 402083); Perkin Elmer: Foster City, CA, USA, 1995. [Google Scholar]
- De Riek, J.; Dendauw, J.; Mertens, M.; De Loose, M.; Heursel, J.; Van Bockstaele, E. Validation of Criteria for the Selection of AFLP Markers to Assess the Genetic Variation of a Breeders’ Collection of Evergreen Azaleas. Theor. Appl. Genet. 1999, 99, 1155–1165. [Google Scholar] [CrossRef]
- Denaeghel, H.; Van Laere, K.; Leus, L.; Van Huylenbroeck, J.; Van Labeke, M.C. Interspecific Hybridization in Sarcococca Supported by Analysis of Ploidy Level, Genome Size and Genetic Relationships. Euphytica 2017, 213, 149. [Google Scholar] [CrossRef]
- Dinesh, C. Analysis of the Applicability of Different Color Difference Equations in Printing Industry. Int. J. Sci. Eng. Comput. Technol. 2018, 8, 74–79. [Google Scholar]
- Kamlah, R.; Pinker, I.; Plaschil, S.; Olbricht, K. Hybridization between Pelargonium Acetosum L’Hér. and Pelargonium × peltatum. J. Appl. Bot. Food Qual. 2019, 92, 49–56. [Google Scholar]
- Breman, F.C.; Snijder, R.C.; Korver, J.W.; Pelzer, S.; Sancho-Such, M.; Schranz, M.E.; Bakker, F.T. Interspecific Hybrids between Pelargonium × hortorum and Species from P. Section Ciconium Reveal Biparental Plastid Inheritance and Multi-Locus Cyto-Nuclear Incompatibility. Front. Plant Sci. 2020, 11, 614871. [Google Scholar] [CrossRef]
- Denis-Peixoto, L.; Cadic, A.; Renou, J.-P. Interspecific Crosses between Pelargonium × hortorum and P. quinquelobatum Using Embryo Rescue and Molecular Characterization of Hybrids by an Endogenous Chs Probe. Plant Breed. 1997, 116, 177–180. [Google Scholar] [CrossRef]
- Scemama, C.; Raquin, C. An Improved Method for Rescuing Zygotic Embryos of Pelargonium × hortorum Bailey. J. Plant Physiol. 1990, 135, 763–765. [Google Scholar] [CrossRef]
- Pawar, B.; Prashant, K.; Bahurupe, J.; Jadhav, A.; Anil, K.; Pawar, S. Proline and Glutamine Improve In Vitro Callus Induction and Subsequent Shooting in Rice. Rice Sci. 2015, 22, 283–289. [Google Scholar] [CrossRef]
- Meiners, J.; Debener, T.; Schweizer, G.; Winkelmann, T. Analysis of the Taxonomic Subdivision within the Genus Helleborus by Nuclear DNA Content and Genome-Wide DNA Markers. Sci. Hortic. 2011, 128, 38–47. [Google Scholar] [CrossRef]
- Dickinson, G.R.; Lee, D.J.; Wallace, H.M. The Influence of Pre- and Post-Zygotic Barriers on Interspecific Corymbia Hybridization. Ann. Bot. 2012, 109, 1215–1226. [Google Scholar] [CrossRef]
- Kuligowska, K.; Lütken, H.; Christensen, B.; Skovgaard, I.; Linde, M.; Winkelmann, T.; Müller, R. Evaluation of Reproductive Barriers Contributes to the Development of Novel Interspecific Hybrids in the Kalanchoë Genus. BMC Plant Biol. 2015, 15, 15. [Google Scholar] [CrossRef]

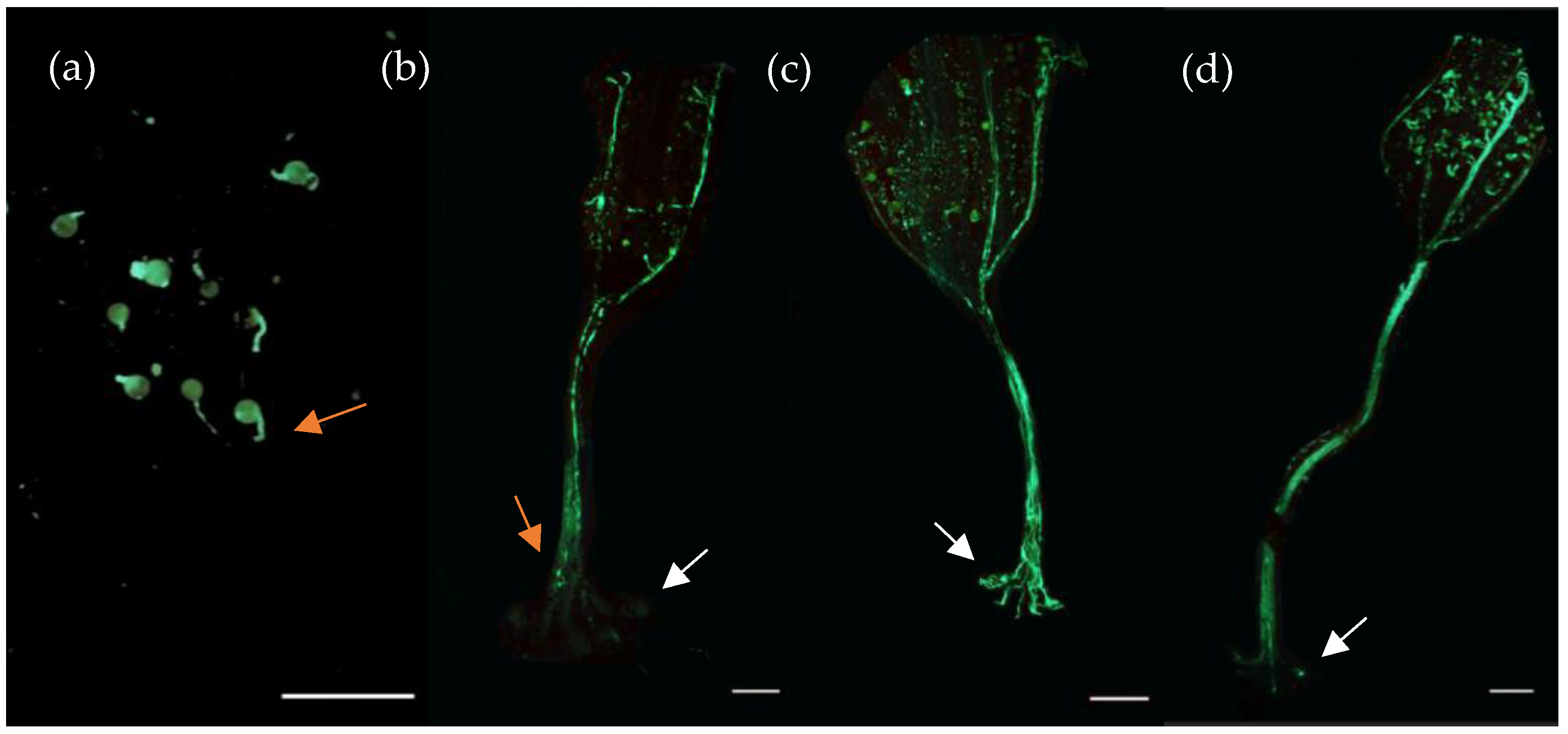
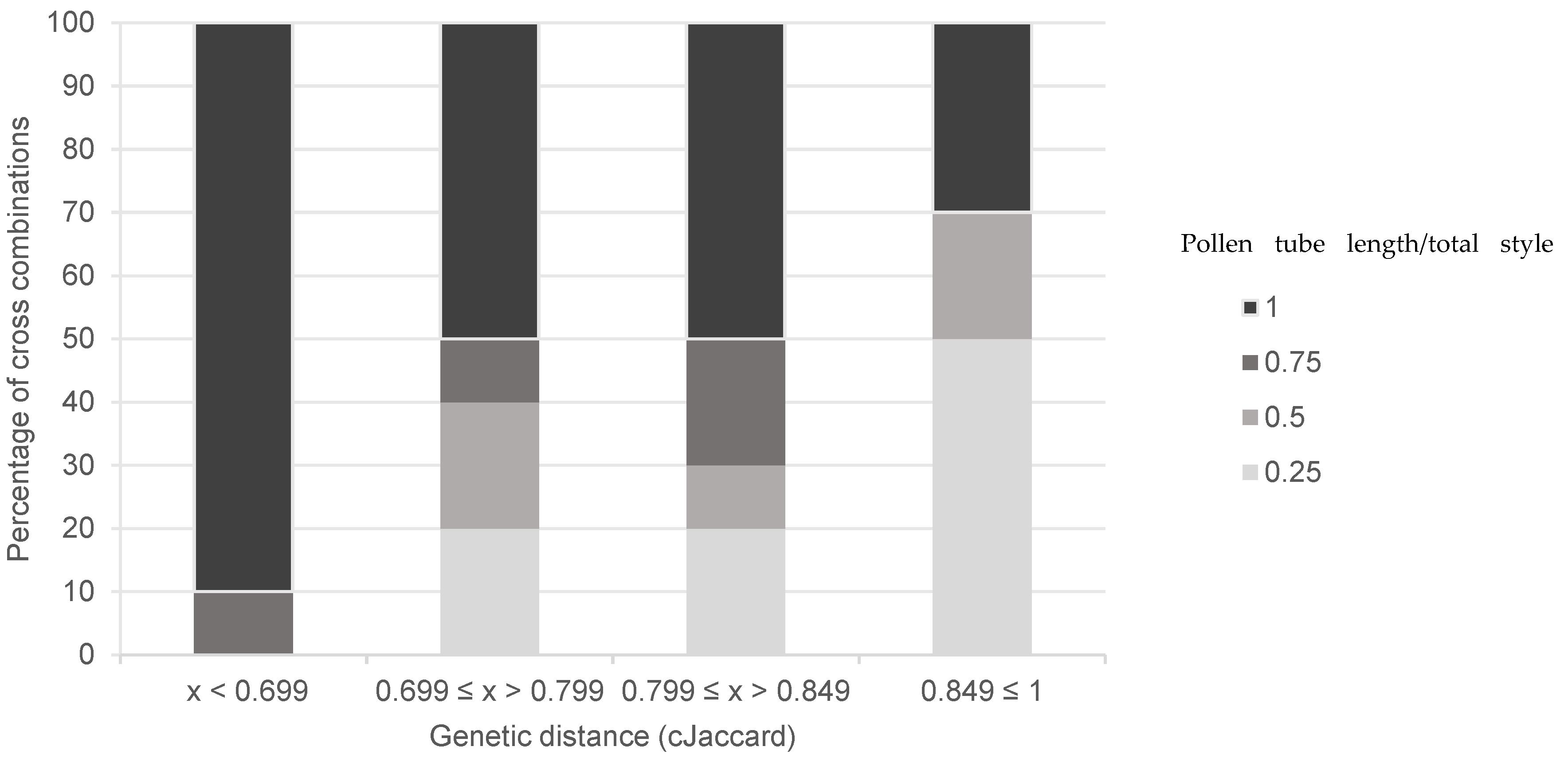
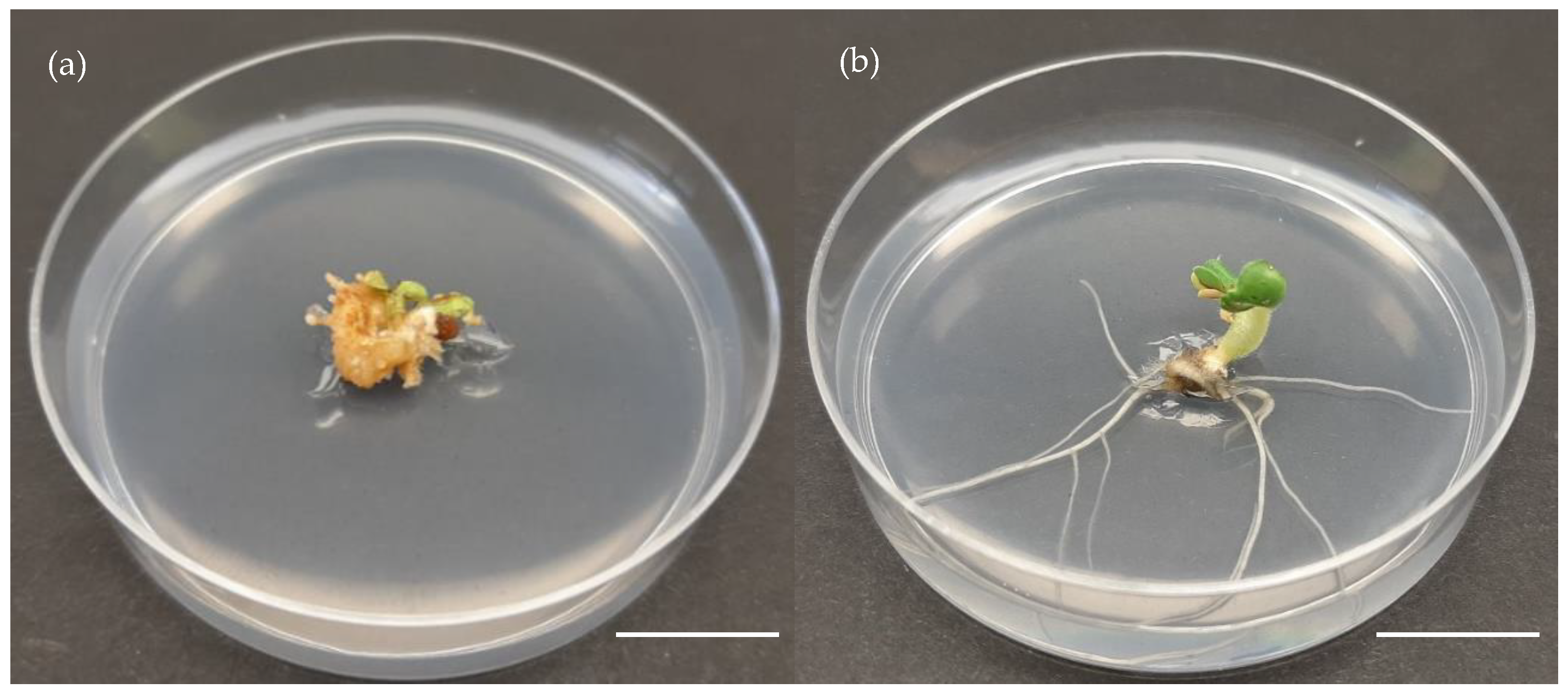
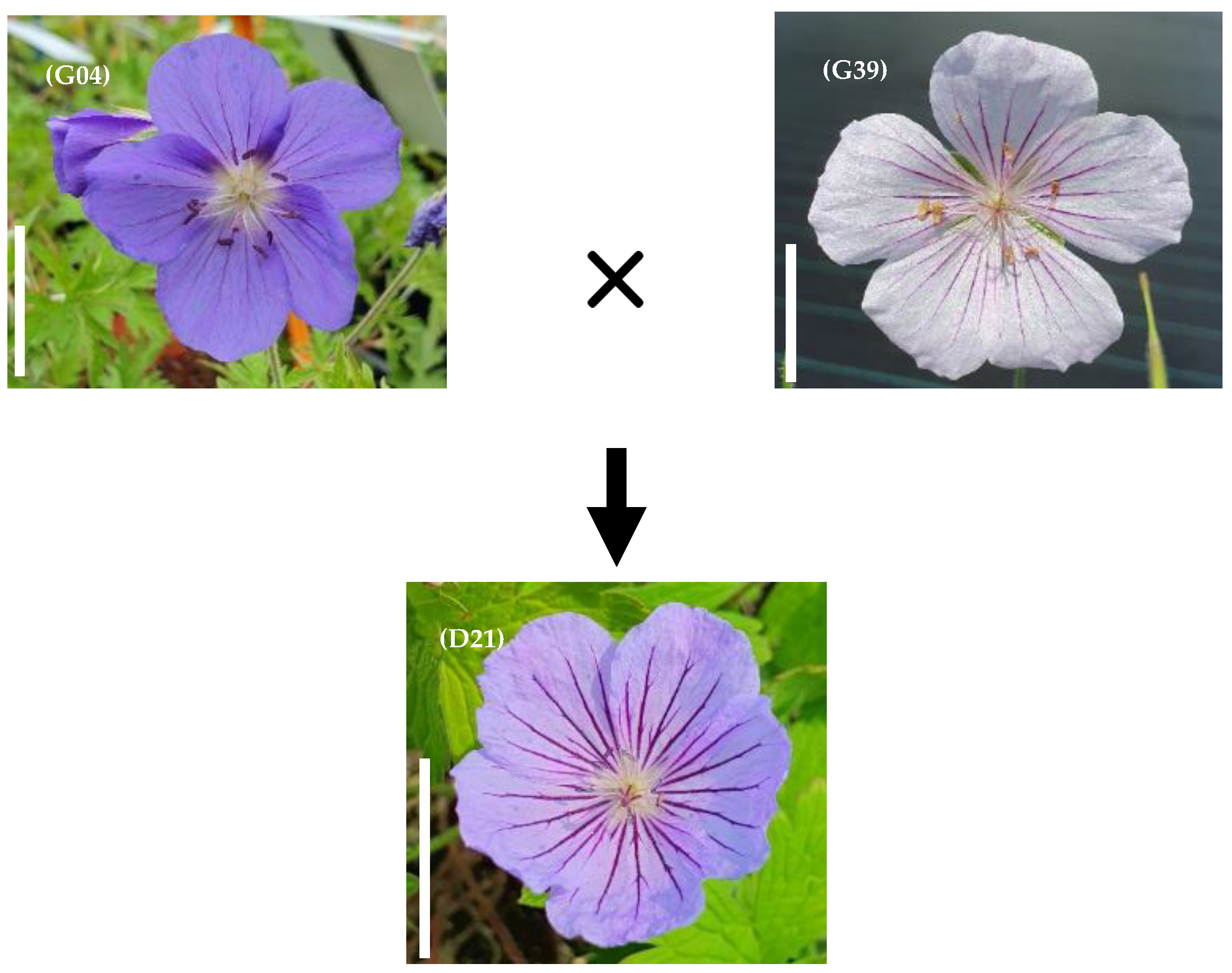
| Medium | Composition |
|---|---|
| M1 | Quoirin and Lepoivre full-strength medium containing sucrose (30 g.L−1), agar (7.5 g.L−1), and activated charcoal (0.05 g.L−1) |
| M2 | M1 supplemented with casein hydrolysate (100 mg.L−1). |
| M3 | M1 supplemented with L-proline (100 mg.L−1). |
| M4 | M1 supplemented with glutamine, asparagine, serine, and arginine (100 mg.L−1 each). |
| Code | Genotype | Female Receptivity Expressed as Seed Set (Open Pollination) * | Male Fertility Expressed as Pollen Viability ± SE | Need for Emasculation |
|---|---|---|---|---|
| G01 | Geranium ‘Ann Thomson’ | No | 0 | × |
| G02 | Geranium ‘Azure Rush’ | Noim | 24.4 ± 2.0 | ✓ |
| G03 | Geranium ‘Bob’s Blunder’ | No | 0 | × |
| G04 | Geranium ‘Brookside’ | Yes | 60.9 ± 5.7 | ✓ |
| G05 | Geranium ‘Catherine Deneuve’ | Noim | 0 | × |
| G06 | Geranium ‘Chantilly’ | No | 24 ± 2.8 | ✓ |
| G07 | Geranium ‘Dragon Heart’ | No | 0 | × |
| G09 | Geranium ‘Galactic’ | Yes | 19 ± 4.6 | ✓ |
| G10 | Geranium ‘Jolly Jewel Red’ | Noim | 37.9 ± 20.8 | ✓ |
| G13 | Geranium ‘Mavis Simpson’ | No | 0 | × |
| G14 | Geranium ‘Orion’ | Yes | 34 ± 2.4 | ✓ |
| G16 | Geranium ‘Rozanne’ | Noim | 26.2 ± 1.9 | ✓ |
| G17 | Geranium ‘Salome’ | No | 7.8 ± 2.7 | ✓ |
| G18 | Geranium ‘Sanne’ | No | 0 | × |
| G19 | Geranium ‘Silverwood’ | Yes | 51.6 ± 0.5 | ✓ |
| G21 | Geranium ‘Sylvia’s Surprise’ | Yes | 36.4 ± 15.2 | ✓ |
| G22 | Geranium ‘Tanya Rendall’ | No | 0 | × |
| G24 | Geranium ‘Tiny Monster’ | Noim | 21 ± 8.0 | × |
| G27 | Geranium × cantabrigiense ‘Biokovo’ | Noim | 60.6 ± 0.9 | ✓ |
| G30 | Geranium cinereum ‘Laurence Flatman’ | No | 61.4 ± 8.6 | ✓ |
| G35 | Geranium endressii | Yes | 75.2 ± 11.7 | ✓ |
| G37 | Geranium endressii ‘Trevor Bath’ | Yes | 39.3 ± 2.1 | ✓ |
| G38 | Geranium himalayense ‘Baby Blue’ | Yes | 56.5 ± 27.2 | ✓ |
| G39 | Geranium himalayense ‘Derrick Cook’ | Yes | 20 ± 9.4 | ✓ |
| G42 | Geranium macrorrhizum ‘Czakor’ | Noim | - * | ✓ |
| G44 | Geranium macrorrhizum ‘White Ness’ | Noim | 45.2 ± 9.6 | ✓ |
| G45 | Geranium maculatum ‘Album’ | Yes | 72.2 ± 13 | ✓ |
| G46 | Geranium maculatum ‘Elizabeth Ann’ | Yes | 79.9 ± 8.7 | ✓ |
| G49 | Geranium × oxonianum ‘Katherine Adele’ | Yes | 49.7 ± 11 | ✓ |
| G50 | Geranium × oxonianum ‘Southcombe Double’ | Yes | 0 | × |
| G54 | Geranium phaeum ‘Angelina’ | Yes | 54.8 ± 5.9 | ✓ |
| G57 | Geranium pratense ‘Algera Double’ | No | 0 | × |
| G61 | Geranium pratense ‘Purple Ghost’ | Yes | 10.6 ± 3.1 | ✓ |
| G62 | Geranium psilostemon | No | - * | × |
| G64 | Geranium renardii | Noim | 70 ± 3.2 | ✓ |
| G69 | Geranium sanguineum ‘Album’ | Yes | 46.6 ± 17.6 | ✓ |
| G71 | Geranium sylvaticum ‘Album’ | Yes | 26.2 ± 7.3 | ✓ |
| G73 | Geranium versicolor | Yes | 49.2 ± 14.7 | ✓ |
| G75 | Geranium ‘Bloom Time’ | Yes | 10.4 ± 1.2 | ✓ |
| G76 | Geranium wallichianum ‘Havana Blue’ | Noim | 22.1 ± 7.5 | ✓ |
| G77 | Geranium wlassovianum | No | 0 | × |
| G80 | Geranium ‘Blushing Turtle’ | Noim | 30.6 ± 8.9 | × |
| Medium | Embryo Age | Germinated Embryo (%) | Callused Embryo (%) | Non-Reactive Embryo (%) |
|---|---|---|---|---|
| M1 | A1 | 2/18 ab | 1/29 a | 3/37 a |
| A2 | 4/18 a | 0 a | 1/37 a | |
| A3 | 1/18 b | 4/29 a | 5/37 a | |
| M2 | A1 | - * | - * | - * |
| A2 | 2/18 ab | 3/29 a | 6/37 a | |
| A3 | 2/18 ab | 5/29 a | 1/37 a | |
| M3 | A1 | 1/18 ab | 4/29 a | 4/37 a |
| A2 | 0 b | 3/29 a | 3/37 a | |
| A3 | 0 b | 4/29 a | 2/37 a | |
| M4 | A1 | 1/18 ab | 1/29 a | 3/37 a |
| A2 | 2/18 ab | 1/29 a | 6/37 a | |
| A3 | 3/18 ab | 3/29 a | 3/37 a |
| Growing Season | 2020 | 2021 | 2022 |
|---|---|---|---|
| Number of all crosses | 349 | 1155 | 934 |
| Number of different cross combinations | 96 | 236 | 61 |
| Number of harvested seeds | 15 | 175 | 255 |
| Number of combinations that produced seeds | 5 | 36 | 33 |
| Number of combinations that produced seedlings | 2 | 15 | 13 |
| Number of seedlings | 3 | 32 | 47 |
| Germination rate (%) | 0.17 | 0.57 | 1.01 |
| ♀ × ♂ | cJaccard | No. of Crosses | No. of Seeds | No. of Seedlings | Germination Rate (%) | True Hybrid | Partial Hybrid | Non-Hybrid |
|---|---|---|---|---|---|---|---|---|
| G49 × G35 | 0.486 | 22 | 40 | 10 | 25 | 10 | 0 | 0 |
| G50 × G49 | 0.512 | 18 | 7 | 1 | 14 | 1 | 0 | 0 |
| G73 × G35 | 0.528 | 2 | 4 | 1 | 25 | 1 | 0 | 0 |
| G21 × G75 | 0.563 | 10 | 1 | 1 | 100 | 0 | 1 | 0 |
| G14 × G39 | 0.631 | 37 | 38 | 5 | 13 | 5 | 0 | 0 |
| G04 × G39 | 0.641 | 29 | 35 | 4 | 11 | 4 | 0 | 0 |
| G04 × G09 | 0.686 | 33 | 26 | 20 | 77 | 18 | 0 | 2 |
| G09 × G04 | 0.686 | 31 | 10 | 4 | 40 | 2 | 0 | 2 |
| G80 × G69 | 0.689 | 48 | 11 | 2 | 18 | 2 | 0 | 0 |
| G04 × G61 | 0.690 | 4 | 3 | 2 | 67 | 2 | 0 | 0 |
| G09 × G39 | 0.721 | 16 | 33 | 2 | 6 | 2 | 0 | 0 |
| G39 × G09 | 0.721 | 19 | 18 | 4 | 22 | 4 | 0 | 0 |
| G49 × G62 | 0.767 | 5 | 2 | 1 | 50 | 0 | 0 | 1 |
| G39 × G62 | 0.778 | 1 | 2 | 2 | 100 | 0 | 0 | 2 |
| G05 × G39 | 0.795 | 43 | 54 | 1 | 2 | 0 | 1 | 0 |
| G38 × G62 | 0.798 | 2 | 3 | 3 | 100 | 0 | 3 | 0 |
| G35 × G75 | 0.852 | 1 | 3 | 3 | 100 | 3 | 0 | 0 |
| G45 × G69 | 0.866 | 5 | 5 | 3 | 60 | 0 | 0 | 3 |
| G50 × G21 | 0.871 | 5 | 1 | 1 | 100 | 0 | 0 | 1 |
| G45 × G06 | 0.881 | 26 | 5 | 3 | 60 | 0 | 0 | 3 |
| G54 × G44 | 0.918 | 35 | 21 | 9 | 43 | 0 | 0 | 9 |
| Combinations | Flower Diameter (mm) | Style Length (mm) | Number of Flowers | Flowering Period (Weeks) | ΔE*ab (Flower) | ΔE*ab (Leaf) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Code | ♀ × ♂ | ♀ | ♂ | Progeny | ♀ | ♂ | Progeny | ♀ | ♂ | Progeny | ♀ | ♂ | Progeny | ♀ | ♂ | ♀ | ♂ |
| D21 | G04 × G39 | 46 | 56 | 39 | 14 | 15 | 11.4 | 245 | 53 | 25 | 6 | 3 | 8 | 27 | 38 | 10 | 13 |
| D25 | G04 × G61 | 46 | 35 | 39 | 14 | 12 | 11 | 245 | na * | 85 | 6 | 3 | 14 | 61 | 22 | 8 | 14 |
| D26 | G04 × G61 | 46 | 35 | 39 | 14 | 12 | 11 | 245 | na * | 365 | 6 | 3 | 14 | 52 | 31 | 3 | 19 |
| D34 | G14 × G39 | 57 | 56 | 45 | - | - | - | - | - | - | - | - | - | 4 | 64 | 18 | 17 |
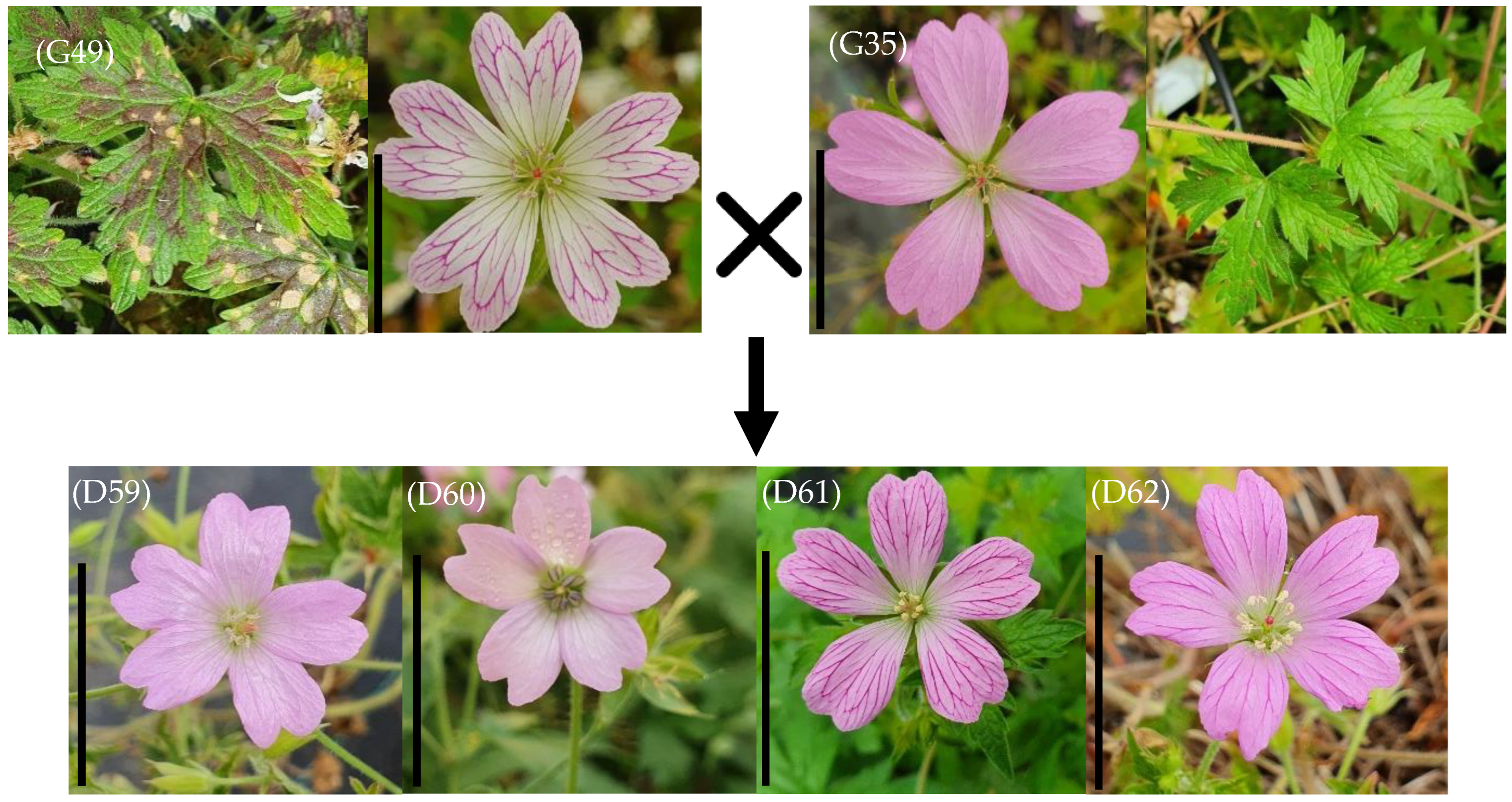
| D59 | G49 × G35 | 32 | 41 | 27 | 10 | 10 | 7.3 | 298 | 128 | 490 | 26 | 14 | 16 | 24 | 37 | 2 | 5 |
| D60 | G49 × G35 | 32 | 41 | 30 | 10 | 10 | 8.4 | 298 | 128 | 630 | 26 | 14 | 16 | 19 | 32 | 4 | 8 |
| D61 | G49 × G35 | 32 | 41 | 36 | 10 | 10 | 8.5 | 298 | 128 | 818 | 26 | 14 | 16 | 43 | 56 | 2 | 4 |
| D62 | G49 × G35 | 32 | 41 | 27 | 10 | 10 | 8 | 298 | 128 | 465 | 26 | 14 | 16 | 44 | 57 | 3 | 8 |
| D80 | G73 × G35 | 26 | 41 | 30 | 8 | 10 | 8 | na | na | 195 | 11 | 14 | 10 | 21 | 43 | 33 | 25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akbarzadeh, M.; Van Laere, K.; De Keyser, E.; Van Huylenbroeck, J.; Werbrouck, S.P.O.; Dhooghe, E. Interspecific Hardy Geranium Progenies: Morphological Characterization and Genetic Evaluation. Horticulturae 2024, 10, 723. https://doi.org/10.3390/horticulturae10070723
Akbarzadeh M, Van Laere K, De Keyser E, Van Huylenbroeck J, Werbrouck SPO, Dhooghe E. Interspecific Hardy Geranium Progenies: Morphological Characterization and Genetic Evaluation. Horticulturae. 2024; 10(7):723. https://doi.org/10.3390/horticulturae10070723
Chicago/Turabian StyleAkbarzadeh, Mehrdad, Katrijn Van Laere, Ellen De Keyser, Johan Van Huylenbroeck, Stefaan P. O. Werbrouck, and Emmy Dhooghe. 2024. "Interspecific Hardy Geranium Progenies: Morphological Characterization and Genetic Evaluation" Horticulturae 10, no. 7: 723. https://doi.org/10.3390/horticulturae10070723







