Combining Ability and Hybrid Breeding in Tunisian Melon (Cucumis melo L.) for Fruit Traits
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
| Parents | Descriptive Features | Biotic Stress and Resistant Genes | Reference |
|---|---|---|---|
| P1 | Ovate shape, light-orange skin, white flesh, big fruit size. | Resistant to powdery mildew and carrying both the Vat gene and Fom-1 gene in homozygosis. | [23,24] |
| P2 | Elliptical shape, yellow skin. | Resistant to powdery mildew | [22] |
| P3 | Oblate shape, greenish-orange skin. | - | [25] |
| P4 | Elliptical shape, orange skin. | Resistant to powdery mildew | [26] |
| P5 | Oblate shape, greenish skin. | Carrying Fom-1 gene in homozygosis | [23] |
| P6 | Elliptical shape, greenish skin. | Resistant to powdery mildew and carrying the Fom-1 gene in homozygosis. | [22,23] |
| P7 | Flattened shape, greenish skin. | Carrying the Fom-1 gene in homozygosis. | [23] |
2.2. Fruit Traits and Yield Related Parameters
2.3. Data Analysis
= [F1 − (P1 + P2)/2]/[(P1 + P2)/2] × 100,
= [(F1 − PB)/PB] × 100,
3. Results
3.1. Hybrid Performance by Parent
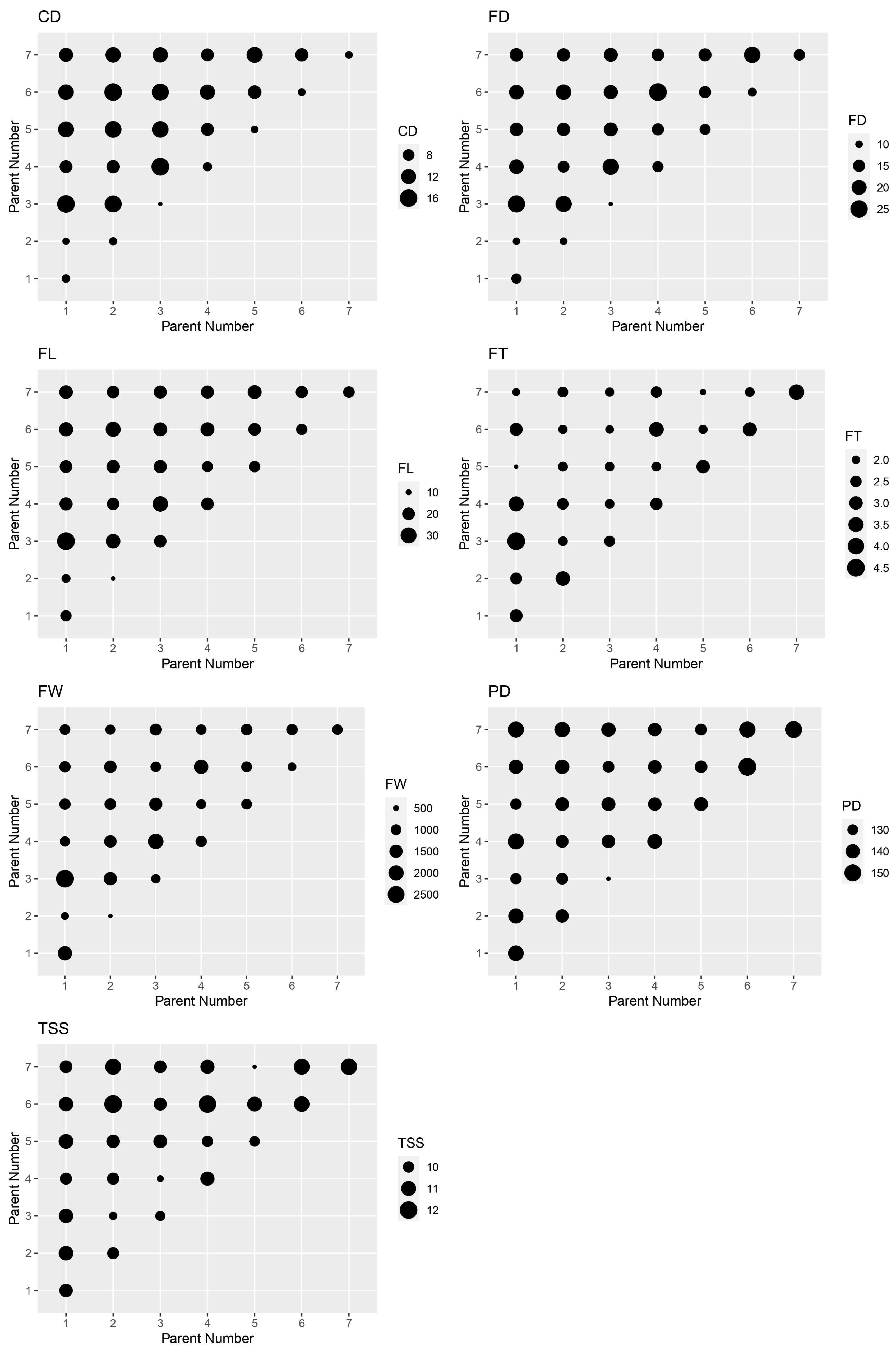
3.2. Combining Ability
| DF | PD a | FW | FL | FD | CD | RT | TSS | |
|---|---|---|---|---|---|---|---|---|
| GCA | 6 | 641.732 ** | 726,120.616 ** | 49.149 ns | 118.379 ** | 19.505 ** | 0.797 * | 3.522 * |
| SCA | 21 | 773.208 ** | 542,237.899 ** | 75.228 ** | 660.073 ** | 58.176 ** | 1.392 * | 0.951 * |
| Error | 54 | 32.649 | 116,607.226 | 31.527 | 15.885 | 3.331 | 0.015 | 0.016 |
| σ2GCA | 9.045 | 32,620.055 | 1.675 | 0.279 | 0.342 | 0.004 | 0.079 | |
| σ2SCA | 24.429 | 32,620.055 | 18.751 | 17.931 | 12.765 | 0.383 | 0.326 | |
| σ2 Residual | 0.266 | 40,681.210 | 0.033 | 0.131 | 0.023 | 0.020 | 0.051 | |
| GPR | 0.425 | 0.667 | 0.152 | 0.030 | 0.051 | 0.020 | 0.326 | |
| H2 | 0.992 | 0.615 | 0.998 | 0.993 | 1.000 | 0.951 | 0.890 | |
| h2 | 0.270 | 0.500 | 0.082 | 0.015 | 0.026 | 0.011 | 0.173 |
3.3. General and Specific Combining Ability Effects

| Cross | PD | FW | FL | FD | CD | RT | TSS |
|---|---|---|---|---|---|---|---|
| 1 × 2 | 2.946 * | −209.241 * | −7.190 | −6.577 * | −5.432 * | 0.000 * | 0.263 ** |
| 1 × 3 | −4.606 * | 486.220 ** | 14.400 * | 8.230 ** | 5.526 ** | 1.884 ** | 0.493 ** |
| 1 × 4 | 6.157 * | −166.897 * | −0.037 | 2.372 ** | −1.029 * | 0.874 ** | −0.368 * |
| 1 × 5 | −7.305 * | −56.662 * | −0.383 | 0.465 ** | 2.824 ** | −0.829 * | 0.485 ** |
| 1 × 6 | −2.737 * | −58.822 * | 2.936 | 2.469 ** | 2.319 ** | 0.278 * | −0.174 * |
| 1 × 7 | 3.158 * | −82.547 * | 1.709 | 0.269 * | 0.431 * | −0.658 * | −0.379 * |
| 2 × 3 | −1.413 * | 103.458 ** | 3.987 * | 5.843 ** | 4.325 ** | −0.398 * | −0.757 |
| 2 × 4 | −3.656 * | 89.175 ** | −0.606 | −2.286 * | −0.684 * | −0.054 * | −0.306 * |
| 2 × 5 | 1.614 * | 71.326 ** | 2.305 | 0.286 * | 4.001 ** | −0.347 * | 0.112 * |
| 2 × 6 | −0.222 * | 135.166 ** | 7.120 * | 4.202 ** | 5.649 ** | −0.472 * | 1.017 ** |
| 2 × 7 | 2.346 * | −13.934 * | 0.375 | −0.110 * | 2.241 ** | −0.209 * | 0.593 * |
| 3 × 4 | 2.675 * | 239.089 ** | 5.747 * | 5.824 ** | 5.747 ** | −0.394 * | −0.830 * |
| 3 × 5 | 6.105 * | 60.241 ** | −0.393 | 1.127 ** | 3.413 ** | −0.365 * | 0.529 * |
| 3 × 6 | −4.240 * | −129.044 * | 0.768 | 1.213 ** | 4.235 ** | −0.565 * | −0.243 * |
| 3 × 7 | 2.775 * | −9.644 * | −0.968 | 0.920 * | 1.660 ** | −0.455 * | −0.084 * |
| 4 × 5 | −0.147 * | −114.417 * | −4.181 | −1.905 * | −1.027 * | −0.378 * | −0.296 * |
| 4 × 6 | −4.242 * | 224.298 ** | 2.331 * | 9.298 ** | 1.757 ** | 0.762 ** | 0.947 ** |
| 4 × 7 | −5.017 * | −64.302 * | 0.352 | −1.237 * | −1.065 * | −0.082 * | 0.079 * |
| 5 × 6 | −4.733 * | −19.551 * | −0.521 | −1.854 * | −0.457 * | −0.449 * | 0.183 * |
| 5 × 7 | −7.341 * | 43.099 ** | 3.371 * | −0.383 * | 3.044 ** | −0.736 * | −1.204 |
| 6 × 7 | 2.441 * | 32.939 ** | −1.695 | 5.921 ** | −0.748 * | −0.399 * | 0.220 * |
3.4. Heterosis Performance
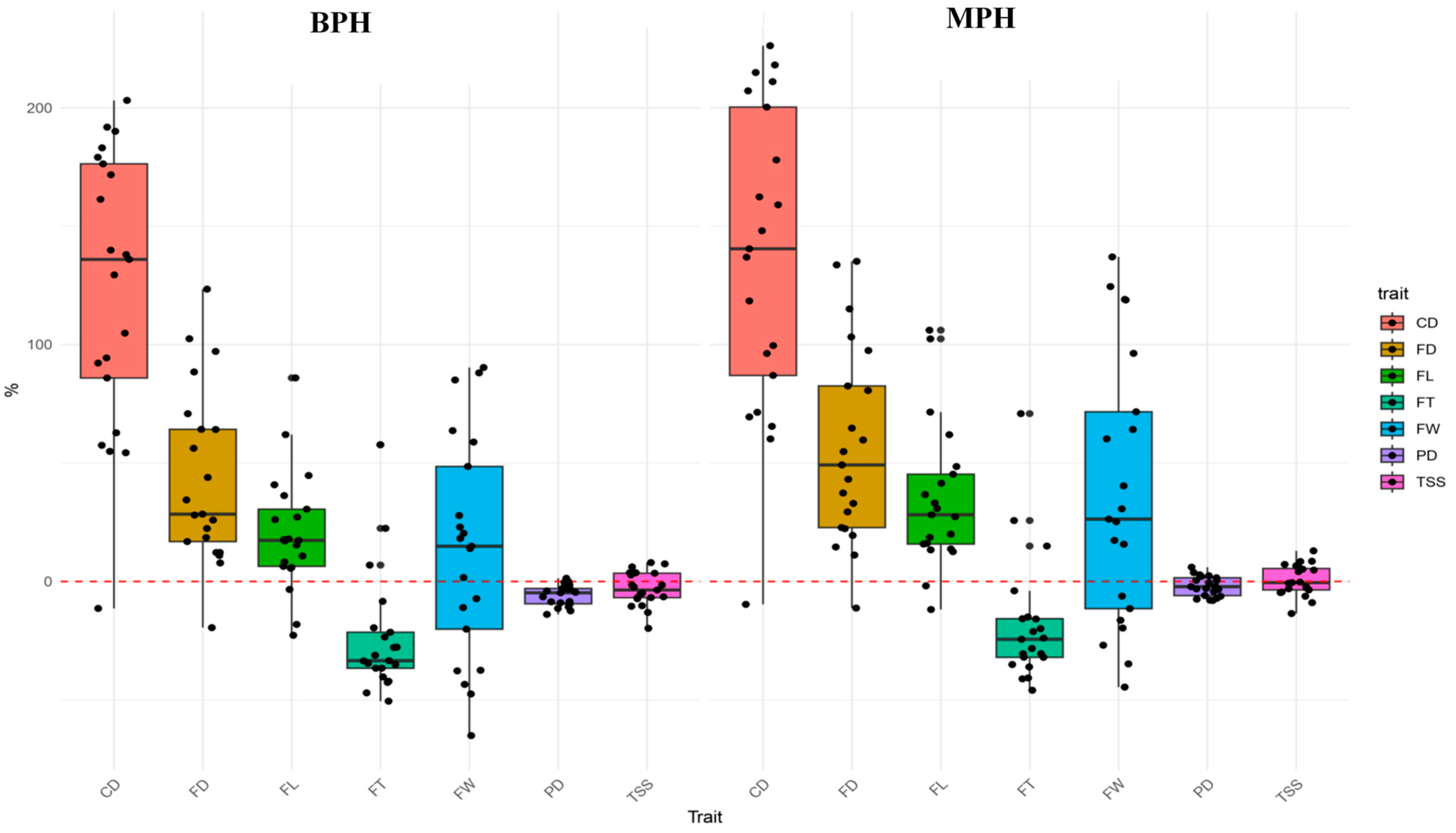
3.5. Correlation Analysis of Phenotypic Traits

3.6. Correlation between Parent Performance, F1 Performance and Heterosis

4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garcia-Mas, J.; Benjak, A.; Sanseverino, W.; Bourgeois, M.; Mir, G.; González, V.M. The Genome of melon (Cucumis melo L.). Proc. Natl. Acad. Sci. USA 2012, 109, 11872–11877. [Google Scholar] [CrossRef] [PubMed]
- Pitrat, M. Melon genetic resources: Phenotypic diversity and horticultural taxonomy. In Genetics and Genomics of Cucurbitaceae; Grumet, R., Katzir, N., Garcia-Mas, J., Eds.; Springer: New York, NY, USA, 2016; pp. 1–36. [Google Scholar] [CrossRef]
- Manchali, S.; Kotamballi, N.; Chidambara, M.V.; Bhimanagoud, S.P. Nutritional composition and health benefits of various botanical types of melon (Cucumis melo L.). Plants 2021, 10, 1755. [Google Scholar] [CrossRef] [PubMed]
- Maietti, A.; Tedeschi, P.; Stagno, C.; Bordiga, M.; Travaglia, F.; Locatelli, M.; Arlorio, M.; Brandolini, V. Analytical traceability of melon (Cucumis melo var reticulatus): Proximate composition, bioactive compounds, and antioxidant capacity in relation to cultivar, plant physiology state, and seasonal variability. J. Food. Sci. 2012, 77, C646–C652. [Google Scholar] [CrossRef] [PubMed]
- Chikh-Rouhou, H.; Abdedayem, W.; Solmaz, I.; Sari, N.; Garcés-Claver, A. Melon (Cucumis melo L.): Genomics and Breeding. In Smart Plant Breeding for Vegetable Crops in Post-Genomics Era; Singh, S., Sharma, D., Sharma, S.K., Singh, R., Eds.; Springer: Singapore, 2023; pp. 25–52. [Google Scholar] [CrossRef]
- El-Sayed, A.; Gharib, A.H.A.M.; El-Tahawey, M.A.F.A. Heterosis and combining ability in melon (Cucumis melo L.). Menoufia J. Plant Prod. 2019, 4, 429–441. [Google Scholar] [CrossRef]
- Fasahat, P.; Rajabi, A.; Rad, J.M.; Derera, J. Principles and utilization of combining ability in plant breeding. Biom. Biostat. Int. J. 2016, 4, 00085. [Google Scholar] [CrossRef]
- Napolitano, M.; Terzaroli, N.; Kashyap, S.; Russi, L.; Jones-Evans, E.; Albertini, E. Exploring heterosis in melon (Cucumis melo L.). Plants 2020, 9, 282. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Chen, X.; Wu, S.; Chen, X. Recent advances in understanding plant heterosis. Agric. Sci. 2015, 6, 1033–1038. [Google Scholar] [CrossRef]
- Barros, A.K.D.A.; Nunes, G.H.D.S.; Queiróz, M.A.D.; Pereira, E.W.L.; Costa Filho, J.H. Diallel analysis of yield and quality traits of melon fruits. Crop Breed. Appl. Biotechnol. 2011, 11, 313–319. [Google Scholar] [CrossRef]
- Neto, J.G.C.; Ferreira, K.T.C.; De Aragão, F.A.S.; Antônio, R.P.; De Sousa Nunes, G.H. Potential of parents and hybrids experimental of the yellow melon. Cienc. Rural 2020, 50, e20190452. [Google Scholar] [CrossRef]
- Feyzian, E.; Dehghani, H.; Rezai, A.; Jalali Javaran, M. Diallel cross analysis for maturity and yield-related traits in melon (Cucumis melo L.). Euphytica 2009, 168, 215–223. [Google Scholar] [CrossRef]
- Tomar, R.S.; Bhalala, M.K. Heterosis studies in muskmelon (Cucumis melo L.). J. Hortic. Sci. 2006, 1, 144–147. [Google Scholar] [CrossRef]
- Kaur, S.; Sharma, S.P.; Sarao, N.K.; Deol, J.K.; Gill, R.; Abd-Elsalam, K.A.; Alghuthaymi, M.A.; Hassan, M.M.; Chawla, N. Heterosis and Combining Ability for Fruit Yield, Sweetness, β-Carotene, Ascorbic Acid, Firmness and Fusarium Wilt Resistance in Muskmelon (Cucumis melo L.) Involving Genetic Male Sterile Lines. Horticulturae 2022, 8, 82. [Google Scholar] [CrossRef]
- Gvozdanovic-Varga, J.; Vasic, M.; Milic, D.; Cervenski, J. Diallel cross analysis for fruit traits in watermelon. Genetika 2011, 43, 163–174. [Google Scholar] [CrossRef]
- Hallauer, A.R. History, contribution, and future of quantitative genetics in plant breeding: Lessons from maize. Crop Sci. 2007, 47, 4–19. [Google Scholar] [CrossRef]
- Luan, F.; Sheng, Y.; Wang, Y.; Staub, J.E. Performance of melon hybrids derived from parents of diverse geographic Origins. Euphytica 2010, 173, 1–16. [Google Scholar] [CrossRef]
- Hassan, W.H.A.; Gad, A.A.; El-Salam, M.M.A.; Ismail, H.E.M. Gene action and heterosis of muskmelon. Zagazig J. Agric. Res. 2018, 45, 1953–1961. [Google Scholar] [CrossRef]
- Chikh-Rouhou, H.; Gómez-Guillamón, M.L.; Garcés-Claver, A. Melon germplasm from Tunisia with immense breeding value. Cucurbit Genet. Coop. Rep. 2021, 44, 7–12. [Google Scholar]
- Chikh-Rouhou, H.; Mezghani, N.; Mnasri, S.; Mezghani, N.; Garcés-Claver, A. Assessing the genetic diversity and population structure of a Tunisian melon (Cucumis melo L.) collection using phenotypic traits and SSR molecular markers. Agronomy 2021, 11, 1121. [Google Scholar] [CrossRef]
- Chikh-Rouhou, H.; Tlili, I.; Ilahy, R.; R’him, T.; Sta-Baba, R. Fruit quality assessment and characterization of melon genotypes. Int. J. Veg. Sci. 2021, 27, 3–19. [Google Scholar] [CrossRef]
- Chikh-Rouhou, H.; Garcés-Claver, A.; Kienbaum, L.; Ben Belgacem, A.M.; Gómez-Guillamón, M.L. Resistance of Tunisian melon landraces to Podosphaera xanthii. Horticulturae 2022, 8, 1172. [Google Scholar] [CrossRef]
- Chikh-Rouhou, H.; Gómez-Guillamón, M.L.; González, V.; Sta-Baba, R.; Garcés-Claver, A. Cucumis melo L. germplasm in Tunisia: Unexploited sources of resistance to Fusarium Wilt. Horticulturae 2021, 7, 208. [Google Scholar] [CrossRef]
- Chikh-Rouhou, H.; Ben Belgacem, A.M.; Sta-Baba, R.; Tarchoun, N.; Gómez-Guillamón, M.L. New Source of resistance to Aphis gossypii in Tunisian melon genotypes using phenotypic and molecular marker approaches. Phytoparasitica 2019, 47, 405–413. [Google Scholar] [CrossRef]
- Chikh-Rouhou, H.; Garcés-Claver, A.; Sta-Baba, R.; González, V.; Daami-Remadi, M. Screening for Resistance to Race 1 of Fusarium oxysporum f.sp melonis in Tunisian melon cultivars using molecular markers. Commun. Agric. Appl. Biol. Sci. 2018, 83, 87–92. [Google Scholar]
- Kacem, K.; Chikh-Rouhou, H. Preliminary selection and phenotypic characterization of melon landraces exhibiting resistance to powdery mildew. Int. J. Phytopathol. 2022, 11, 115–123. [Google Scholar] [CrossRef]
- Griffing, B. Concept of general and specific combining ability in relation to diallel crossing systems. Aust. J. Biol. Sci. 1956, 9, 463–493. [Google Scholar] [CrossRef]
- Covarrubias-Pazaran, G. Genome assisted prediction of quantitative traits using the R package sommer. PLoS ONE 2016, 11, e0156744. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.J. Issues in diallel analysis. Crop Sci. 1978, 18, 533–536. [Google Scholar] [CrossRef]
- Zhu, J. Methods of predicting genotype value and heterosis or offspring of hybrids. J. Biomath. 1993, 8, 32–44. [Google Scholar]
- Russell, L.; Singmann, H.; Love, J.; Buerkner, P.; Herve, M. Emmeans: Estimated Marginal Means, aka Least-Squares Means. 2018. Available online: https://rdrr.io/cran/emmeans/man/emmeans.html (accessed on 15 January 2024).
- Schloerke, B.; Cook, D.; Larmarange, J.; Briatte, F.; Marbach, M.; Thoen, E.; Elberg, A.; Crowley, J. GGally: Extension to ‘ggplot2’. R Package Version 2.2.1. 2024. Available online: https://github.com/ggobi/ggally (accessed on 15 January 2024).
- Wickham, H. ggplot2 (Elegant Graphics for Data Analysis); Springer: Cham, Switzerland, 2016; pp. 1–260. [Google Scholar] [CrossRef]
- Akanksha, A.; Jaiswal, H.K. Combining ability studies for yield and quality parameters in basmati rice (Oryza sativa L.) genotypes using diallel approach. Electron. J. Plant Breed. 2019, 10, 9–17. [Google Scholar] [CrossRef]
- Christie, B.R.; Shattuck, V.I. The diallel cross: Design, analysis, and use for plant breeders. In Plant Breeding Reviews; Wiley: New York, NY, USA, 2010; pp. 9–36. [Google Scholar]
- Melgoza, F.A.G.; Escalante, F.B.; Río, A.J.L.; Benítez, A.L.; Mendoza, A.B.; Torres, A.N.R.; Martínez, R.H.; Aranda, C.A.B. Diallel analysis for yield and quality characteristics in melon. J. Exp. Agric. Int. 2022, 44, 29–36. [Google Scholar] [CrossRef]
- Nerson, H. Heterosis in fruit and seed characters of muskmelon. Asian Aust. J. Plant Sci. Biotechnol. 2012, 6, 24–27. [Google Scholar]
- Monforte, A.J.; Iban, E.; Silvia, A.; Pere, A. Inheritance mode of fruit traits in melon: Heterosis for fruit shape and its correlation with genetic distance. Euphytica 2005, 144, 31–38. [Google Scholar]
- Paris, M.; Staub, J.E.; McCreight, J.D. Determination of fruit sampling location for quality measurements in melon (Cucumis melo L.). Cucurbit Genet. Coop. Rep. 2003, 26, 12–17. [Google Scholar]
- Rukundo, P.; Shimelis, H.; Laing, M.; Gahakwa, D. Combining Ability, Maternal Effects, and Heritability of Drought Tolerance, Yield and Yield Components in Sweet potato. Front. Plant Sci. 2017, 7, 1981. [Google Scholar] [CrossRef] [PubMed]
- Adewumi, A.S.; Asare, P.A.; Adejumobi, I.I.; Adu, M.O.; Taah, K.J.; Adewale, S.; Mondo, J.M.; Agre, P.A. Multi-Trait Selection Index for Superior Agronomic and Tuber Quality Traits in Bush Yam (Dioscorea praehensilis Benth.). Agronomy 2023, 13, 682. [Google Scholar] [CrossRef]
- Gomes, D.A.; Alves, I.M.; Maciel, G.M.; Siquieroli, A.C.S.; Peixoto, J.V.M.; Pires, P.S.; Medeiros, I.A. Genetic dissimilarity, selection index and correlation estimation in a melon germplasm. Hortic. Bras. 2021, 39, 46–51. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chikh-Rouhou, H.; Kienbaum, L.; Gharib, A.H.A.M.; Fayos, O.; Garcés-Claver, A. Combining Ability and Hybrid Breeding in Tunisian Melon (Cucumis melo L.) for Fruit Traits. Horticulturae 2024, 10, 724. https://doi.org/10.3390/horticulturae10070724
Chikh-Rouhou H, Kienbaum L, Gharib AHAM, Fayos O, Garcés-Claver A. Combining Ability and Hybrid Breeding in Tunisian Melon (Cucumis melo L.) for Fruit Traits. Horticulturae. 2024; 10(7):724. https://doi.org/10.3390/horticulturae10070724
Chicago/Turabian StyleChikh-Rouhou, Hela, Lydia Kienbaum, Amani H. A. M. Gharib, Oreto Fayos, and Ana Garcés-Claver. 2024. "Combining Ability and Hybrid Breeding in Tunisian Melon (Cucumis melo L.) for Fruit Traits" Horticulturae 10, no. 7: 724. https://doi.org/10.3390/horticulturae10070724
APA StyleChikh-Rouhou, H., Kienbaum, L., Gharib, A. H. A. M., Fayos, O., & Garcés-Claver, A. (2024). Combining Ability and Hybrid Breeding in Tunisian Melon (Cucumis melo L.) for Fruit Traits. Horticulturae, 10(7), 724. https://doi.org/10.3390/horticulturae10070724








