Fruit Position, Light Exposure and Fruit Surface Temperature Affect Colour Expression in a Dark-Red Apple Cultivar
Abstract
:1. Introduction
2. Materials and Methods
2.1. Effects of Fruit Position and Light Exposure on Fruit Quality
2.1.1. Fruit Light Exposure
2.1.2. Harvest Fruit Quality
2.1.3. Statistical Analysis
2.2. Effects of Shade and UV Filtration on FST and Fruit Quality
2.2.1. Experimental Design, Sampling and Treatments
2.2.2. FST
2.2.3. Fruit Quality
2.2.4. Statistical Analysis
3. Results
3.1. Effects of Fruit Position and Light Exposure on Fruit Quality
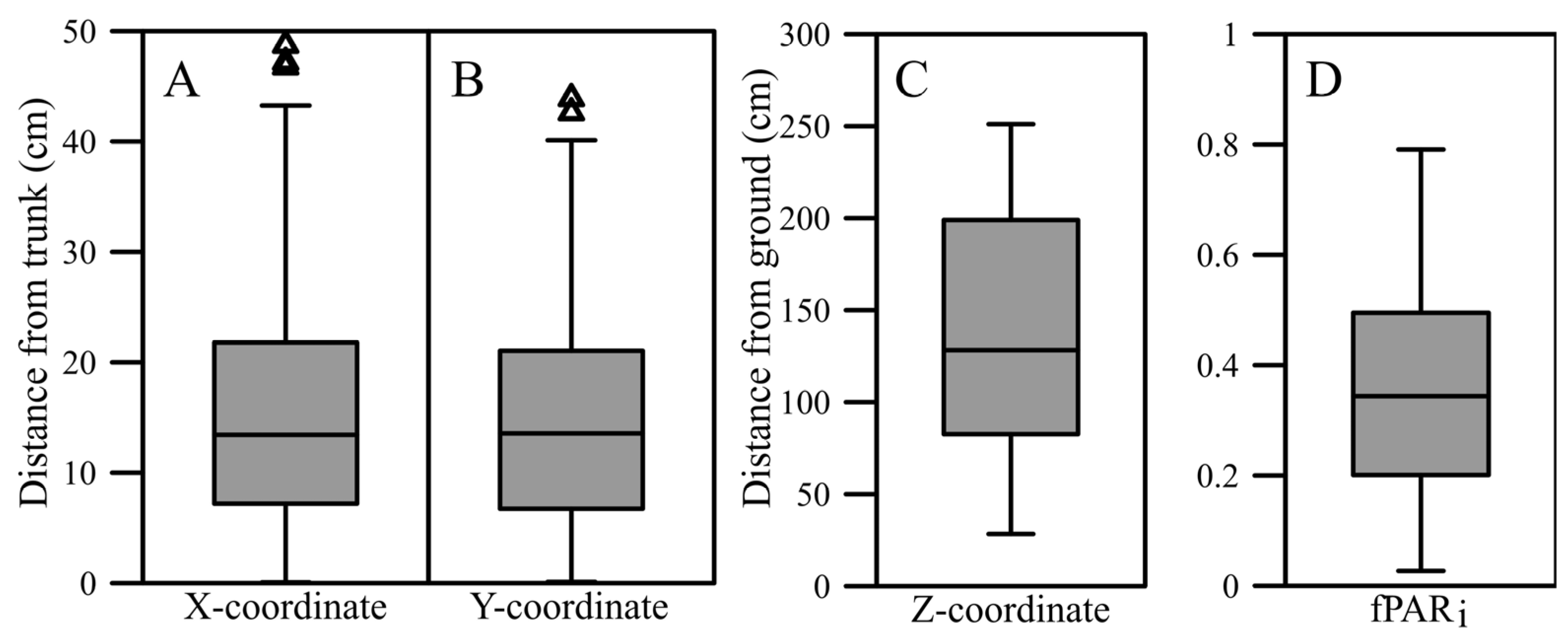
3.1.1. Sample Range
3.1.2. Correlations
Fruit Position and Light Exposure
Fruit Position and Harvest Fruit Quality
Light Exposure and Harvest Fruit Quality
3.2. Effects of Shade and UV Filtration on FST and Fruit Quality
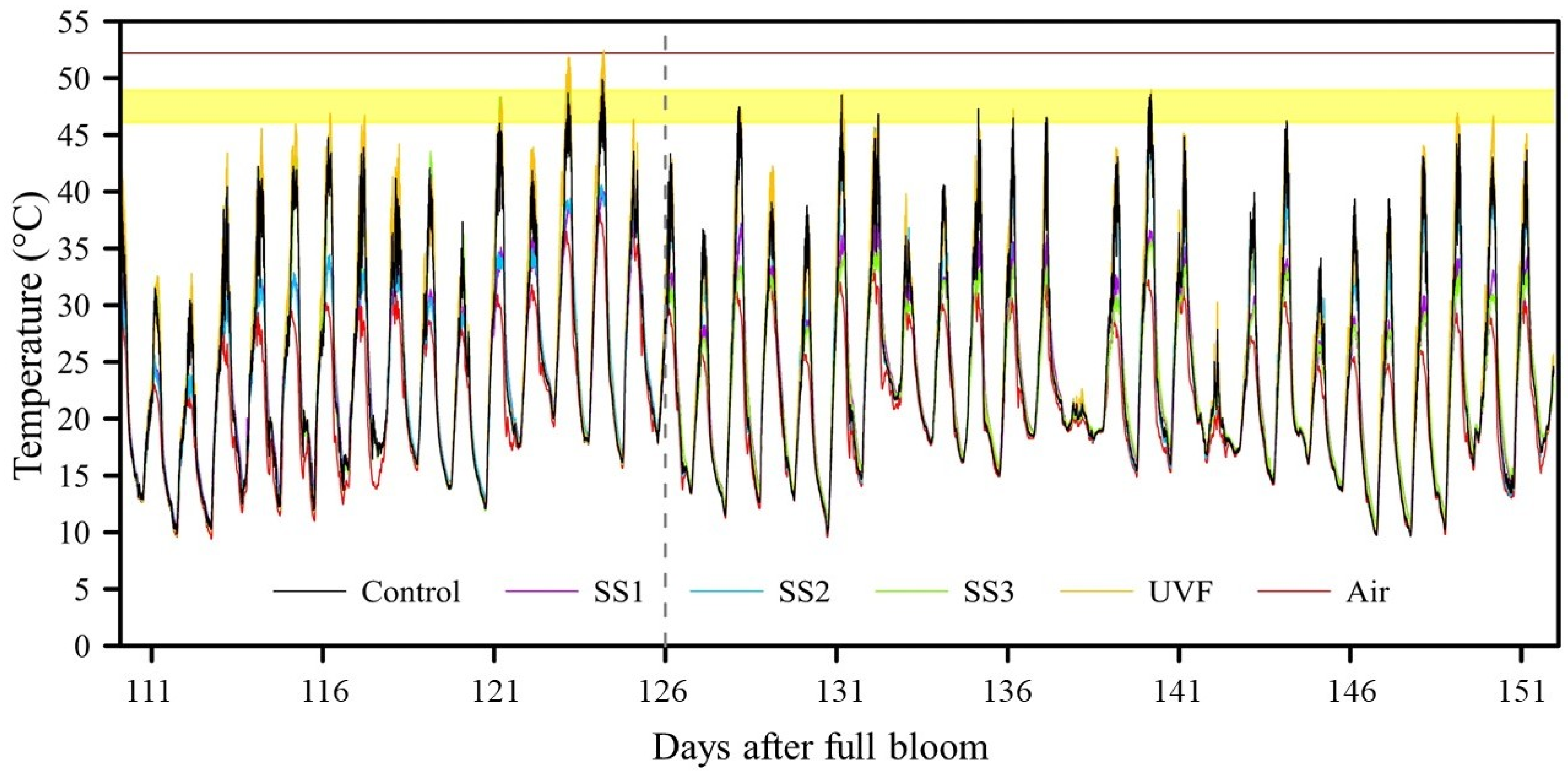
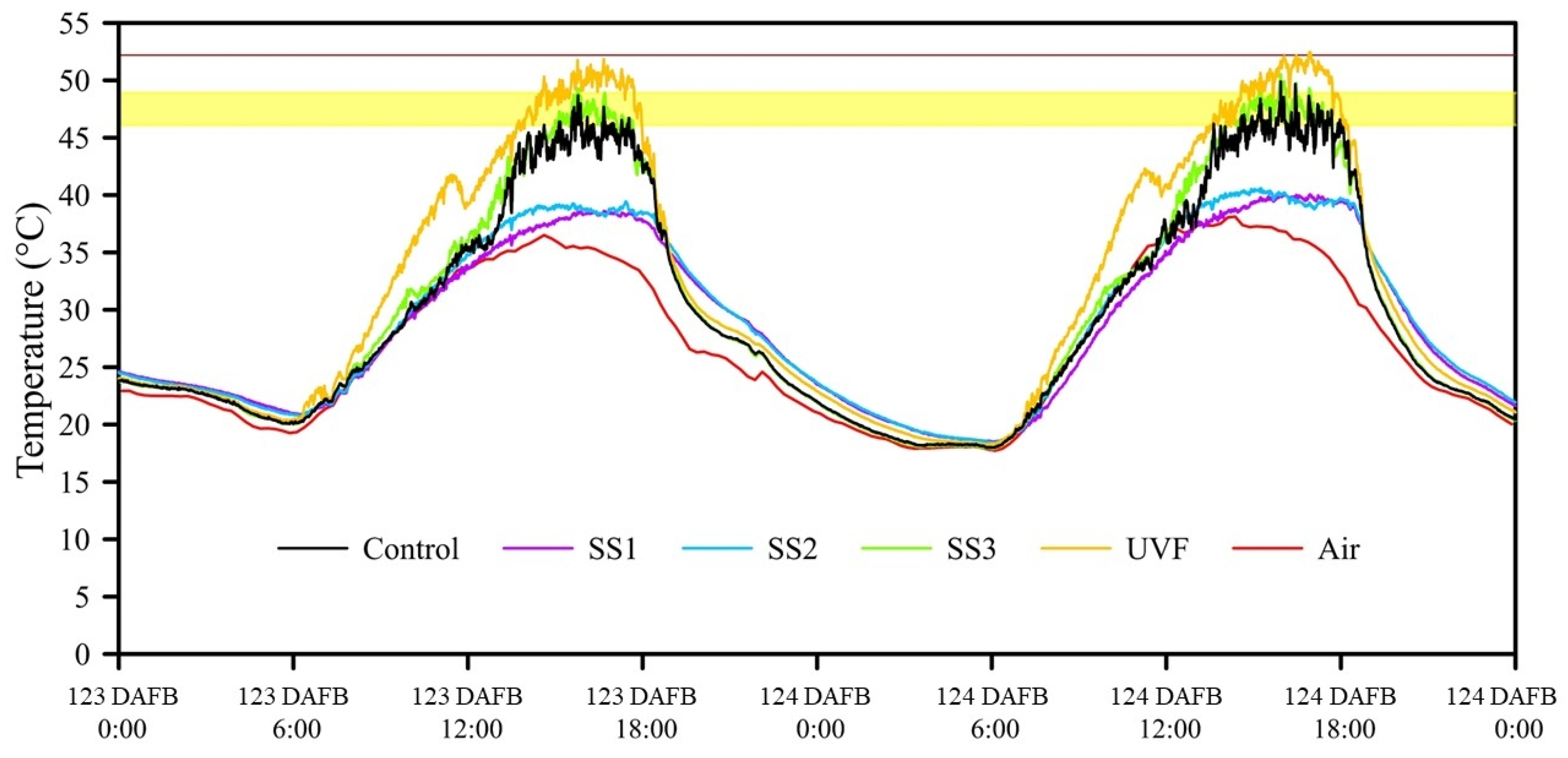
3.2.1. FST
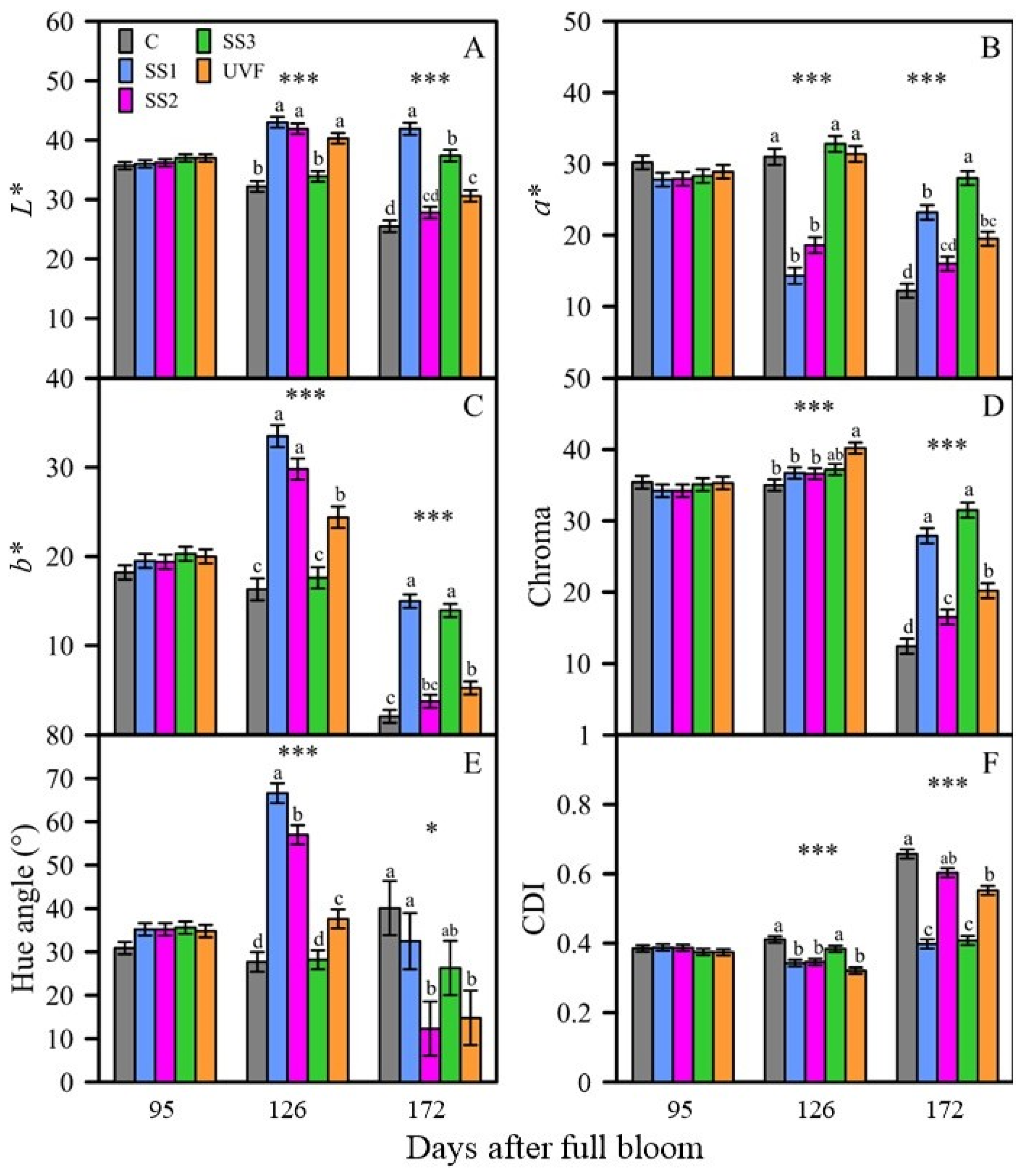
3.2.2. Fruit Quality
Peel Colour Development
Harvest Fruit Quality
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cain, J.C. Effects of Mechanical Pruning of Apple Hedgerows with a Slotting Saw on Light Penetration and Fruiting. J. Am. Soc. Hortic. Sci. 1971, 95, 664–667. [Google Scholar] [CrossRef]
- Corelli-Grappadelli, L.; Sansavini, S. Light interception and photosynthesis related to planting density and canopy management in apple. Acta Hortic. 1989, 243, 159–174. [Google Scholar] [CrossRef]
- Chen, Z.; Yu, L.; Liu, W.; Zhang, J.; Wang, N.; Chen, X. Research progress of fruit color development in apple (Malus domestica Borkh.). Plant Physiol. Biochem. 2021, 162, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Musacchi, S.; Serra, S. Apple fruit quality: Overview on pre-harvest factors. Sci. Hortic. 2018, 234, 409–430. [Google Scholar] [CrossRef]
- Steyn, N. Red Colour Improvement in Apple Fruit (Malus domestica Borkh.). Master’s Thesis, Stellenbosch University, Stellenbosch, South Africa, 2020. [Google Scholar]
- Jackson, J.E. Light Interception and Utilization by Orchard Systems. In Horticultural Reviews; Janick, J., Ed.; The AVI Publishing Company: Westport, CT, USA, 1980; pp. 208–267. [Google Scholar] [CrossRef]
- Robinson, T.; Seeley, E.J.; Barritt, B.H. Effect of Light Environment and Spur Age on ‘Delicious’ Apple Fruit Size and Quality. J. Am. Soc. Hortic. Sci. 1983, 108, 855–861. [Google Scholar] [CrossRef]
- Zhang, J.; Serra, S.; Leisso, R.; Musacchi, S. Effect of light microclimate on the quality of ‘d’Anjou’ pears in mature open-centre tree architecture. Biosyst. Eng. 2016, 141, 1–11. [Google Scholar] [CrossRef]
- Bhusal, N.; Bhusal, S.J.; Han, S.G.; Yoon, T.M. Canopy light distribution and fruit quality in excessive tall spindle apple production system. Acta Hortic. 2019, 1261, 209–217. [Google Scholar] [CrossRef]
- Kviklys, D.; Viškelis, J.; Liaudanskas, M.; Janulis, V.; Laužikė, K.; Samuolienė, G.; Uselis, N.; Lanauskas, J. Apple Fruit Growth and Quality Depend on the Position in Tree Canopy. Plants 2022, 11, 196. [Google Scholar] [CrossRef]
- Ben-Yehudah, G.; Korchinsky, R.; Redel, G.; Ovadya, R.; Oren-Shamir, M.; Cohen, Y. Colour accumulation patterns and the anthocyanin biosynthetic pathway in ‘Red Delicious’ apple variants. J. Hortic. Sci. Biotechnol. 2005, 80, 187–192. [Google Scholar] [CrossRef]
- Saure, M.C. External control of anthocyanin formation in apple. Sci. Hortic. 1990, 42, 181–218. [Google Scholar] [CrossRef]
- Arakawa, O.; Hori, Y.; Ogata, R. Relative effectiveness and interaction of ultraviolet-B, red and blue light in anthocyanin synthesis of apple fruit. Physiol. Plant. 1985, 64, 323–327. [Google Scholar] [CrossRef]
- Gouws, A.; Steyn, W.J. The effect of temperature, region and season on red colour development in apple peel under constant irradiance. Sci. Hortic. 2014, 173, 79–85. [Google Scholar] [CrossRef]
- Gurnsey, S.; Lawes, G. Improving Apple Colour; Tree Fruit Research and Extension Centre, Washington State University: Wenatchee, WA, USA, 1999. [Google Scholar]
- Curry, E.A. Temperatures for optimum anthocyanin accumulation in apple tissue. J. Hortic. Sci. 1997, 72, 723–729. [Google Scholar] [CrossRef]
- Faragher, J.D. Temperature regulation of anthocyanin accumulation in apple skin. J. Exp. Bot. 1983, 34, 1291–1298. [Google Scholar] [CrossRef]
- McClymont, L.; Goodwin, I.; Turpin, S.; Darbyshire, R. Fruit surface temperature of red-blushed pear: Threshold for sunburn damage. N. Z. J. Crop Hortic. Sci. 2016, 44, 262–273. [Google Scholar] [CrossRef]
- Schrader, L.E.; Zhang, J.; Duplaga, W.K. Two Types of Sunburn in Apple Caused by High Fruit Surface (Peel) Temperature. Plant Health Prog. 2001, 2, 3. [Google Scholar] [CrossRef]
- Department of Primary Industries and Regional Development, Government of Western Australia, Australia. Premium Grade ANABP-01 Quality Specification—2021 Season; Department of Primary Industries and Regional Development, Government of Western Australia, Australia: Perth, Australia, 2021. [Google Scholar]
- Matsuoka, K. Anthocyanins in Apple Fruit and Their Regulation for Health Benefits. In Flavonoids—A Coloring Model for Cheering up Life; Badria, F.A., Ananga, A., Eds.; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- ISO/CIE 11664-4:2019; CIE 1976 L*a*b* Colour Space. International Organisation for Standardisation: Geneva, Switzerland, 1976.
- Islam, M.S.; Scalisi, A.; O’Connell, M.G.; Morton, P.; Scheding, S.; Underwood, J.; Goodwin, I. A Ground-based Platform for Reliable Estimates of Fruit Number, Size, and Color in Stone Fruit Orchards. HortTechnology 2022, 32, 510–522. [Google Scholar] [CrossRef]
- Scalisi, A.; O’Connell, M.G.; Islam, M.S.; Goodwin, I. A Fruit Colour Development Index (CDI) to Support Harvest Time Decisions in Peach and Nectarine Orchards. Horticulturae 2022, 8, 459. [Google Scholar] [CrossRef]
- Scalisi, A.; McClymont, L.; Peavey, M.; Morton, P.; Scheding, S.; Underwood, J.; Goodwin, I. Using Green Atlas Cartographer to investigate orchard-specific relationships between tree geometry, fruit number, fruit clustering, fruit size and fruit colour in commercial apples and pears. Acta Hortic. 2023, 1360, 203–210. [Google Scholar] [CrossRef]
- Scalisi, A.; McClymont, L.; Peavey, M.; Morton, P.; Scheding, S.; Underwood, J.; Goodwin, I. Detecting, mapping and digitising canopy geometry, fruit number and peel colour in pear trees with different architecture. Sci. Hortic. 2024, 326, 112737. [Google Scholar] [CrossRef]
- Hamadziripi, E.T.; Theron, K.I.; Muller, M.; Steyn, W.J. Apple Compositional and Peel Color Differences Resulting from Canopy Microclimate Affect Consumer Preference for Eating Quality and Appearance. HortScience 2014, 49, 384–392. [Google Scholar] [CrossRef]
- Peavey, M.; Scalisi, A.; McClymont, L.; Goodwin, I. Light interception, productive performance and red colour coverage of ‘ANABP 01’ apple trees grown on different rootstocks in a multidirectional orchard. Acta Hortic. 2023, 1366, 103–110. [Google Scholar] [CrossRef]
- Lin-Wang, K.; Micheletti, D.; Palmer, J.; Volz, R.; Lozano, L.; Espley, R.; Hellens, R.P.; Chagnè, D.; Rowan, D.D.; Troggio, M.; et al. High temperature reduces apple fruit colour via modulation of the anthocyanin regulatory complex. Plant Cell Environ. 2011, 34, 1176–1190. [Google Scholar] [CrossRef]
- Steyn, W.J.; Wand, S.J.E.; Jacobs, G.; Rosecrance, R.C.; Roberts, S.C. Evidence for a photoprotective function of low-temperature-induced anthocyanin accumulation in apple and pear peel. Physiol. Plant. 2009, 136, 461–472. [Google Scholar] [CrossRef]
- Visscher, I.; Peavey, M.; McClymont, L.; Goodwin, I.; Chandra, S. The effects of light exposure on colour development in the blush pear ‘ANP-0534’. Acta Hortic. 2021, 1303, 529–536. [Google Scholar] [CrossRef]
- Brooks, C.; Fisher, D.F. Some high-temperature effects in apples: Contrast in the two sides of an apple. J. Agric. Res. 1926, 32, 1–16. [Google Scholar]
- Felicetti, D.A.; Schrader, L.E. Photooxidative sunburn of apples: Characterization of a third type of apple sunburn. Int. J. Fruit Sci. 2008, 8, 160–172. [Google Scholar] [CrossRef]
- Nilsson, T.; Gustavsson, K.E. Postharvest physiology of “Aroma” apples in relation to position on the tree. Postharvest Biol. Technol. 2007, 43, 36–46. [Google Scholar] [CrossRef]
- Solomakhin, A.; Blanke, M.M. Can coloured hailnets improve taste (sugar, sugar: Acid ratio), consumer appeal (colouration) and nutritional value (anthocyanin, vitamin C) of apple fruit? LWT—Food Sci. Technol. 2010, 43, 1277–1284. [Google Scholar] [CrossRef]
- Goodwin, I.; McClymont, L.; Turpin, S.; Darbyshire, R. Effectiveness of netting in decreasing fruit surface temperature and sunburn damage of red-blushed pear. N. Z. J. Crop Hortic. Sci. 2018, 46, 334–345. [Google Scholar] [CrossRef]
- Wünsche, J.N.; McGhie, T.; Bown, J.; Ferguson, I.; Woolf, A. Sunburn on Apples—Causes and Control Mechanisms. Acta Hortic. 2004, 636, 631–636. [Google Scholar] [CrossRef]
- Racskó, J.; Szabó, Z.; Miller, D.D.; Soltész, M.; Nyéki, J. Sunburn incidence of apples is affected by rootstocks and fruit position within the canopy but not by fruit position on the cluster. Int. J. Hortic. Sci. 2009, 15, 45–51. [Google Scholar] [CrossRef]

| Light Exposure Treatment | Time Period | |
|---|---|---|
| 95–126 DAFB | 127–172 DAFB | |
| SS1 | * | * |
| SS2 | * | – |
| SS3 | – | * |
| UVF | ** | ** |
| Control | – | – |
| X | Y | Z | fPARt | |
|---|---|---|---|---|
| fPARt | 0.054 | 0.093 | 0.607 *** | — |
| Dark-red coverage | −0.293 ** | −0.373 *** | 0.266 * | 0.286 ** |
| Light-red coverage | 0.288 ** | 0.389 *** | −0.252 * | −0.273 ** |
| Total red coverage | −0.131 | 0.015 | 0.193 | 0.207 * |
| L* | 0.286 ** | 0.350 ** | −0.274 * | −0.260 * |
| a* | 0.198 | 0.420 *** | −0.141 | −0.175 |
| b* | 0.271 * | 0.329 ** | −0.278 ** | −0.260 * |
| Hue angle (°) | 0.107 | −0.068 | −0.196 | −0.106 |
| Chroma | 0.243 * | 0.417 *** | −0.191 | −0.211 * |
| CDI | −0.266 * | −0.411 *** | 0.234 * | 0.243 * |
| Sunburn damage severity | −0.075 | −0.047 | −0.109 | 0.164 |
| Fruit diameter | −0.099 | −0.145 | 0.000 | −0.250 * |
| SSC | −0.285 ** | −0.167 | −0.040 | 0.097 |
| FF | 0.099 | 0.167 | 0.379 *** | 0.371 *** |
| SPI | −0.268 * | −0.185 | −0.211 | −0.107 |
| Light Exposure Treatment | 95–126 DAFB | 127–172 DAFB | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean Max Daily FST (°C) | SE | Mean Min Daily FST (°C) | SE | Mean Max Daily FST (°C) | SE | Mean Min Daily FST (°C) | SE | |
| SS1 | 33.3 b | 1.1 | 14.1 | 0.8 | 31.9 b | 0.9 | 15.2 | 0.5 |
| SS2 | 33.2 b | 1.1 | 14.1 | 0.8 | 38.8 a | 0.3 | 14.1 | 0.6 |
| SS3 | 42.4 a | 1.3 | 13.3 | 0.7 | 30.6 b | 0.7 | 15.1 | 0.5 |
| UVF | 43.7 a | 1.5 | 13.7 | 0.7 | 41.0 a | 1.4 | 14.5 | 0.6 |
| Control | 41.7 a | 1.3 | 13.5 | 0.8 | 40.7 a | 1.4 | 14.1 | 0.6 |
| p | <0.001 | 0.934 | <0.001 | 0.474 | ||||
| Light Exposure Treatment | FD (mm) | Sunburn Damage Severity (0–5) | FF (N) | SSC (°Brix) | DMC (%) | SPI | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | |
| SS1 | 82.2 | 1.8 | 0.0 b | 0.0 | 83.8 | 1.5 | 14.3 b | 0.3 | 17.9 | 0.4 | 2.8 b | 0.3 |
| SS2 | 82.2 | 1.0 | 1.2 a | 0.5 | 83.1 | 1.8 | 15.1 ab | 0.3 | 17.3 | 0.4 | 3.3 ab | 0.3 |
| SS3 | 82.8 | 1.3 | 0.3 ab | 0.3 | 85.3 | 1.7 | 14.2 b | 0.2 | 17.2 | 0.3 | 3.1 ab | 0.2 |
| UVF | 81.1 | 1.2 | 1.0 ab | 0.3 | 87.8 | 2.4 | 15.9 a | 0.2 | 18.5 | 0.3 | 4.0 a | 0.2 |
| Control | 79.6 | 1.3 | 0.2 ab | 0.1 | 85.5 | 2.0 | 15.4 a | 0.4 | 17.9 | 0.5 | 3.4 ab | 0.3 |
| p | 0.454 | 0.023 | 0.478 | <0.001 | 0.139 | 0.015 | ||||||
| Season | Number of Days | |||
|---|---|---|---|---|
| 30.0–31.9 °C | 32.0–34.9 °C | 35–39.9 °C | >40.0 °C | |
| 2018–2019 | 19 | 26 | 24 | 6 |
| 2019–2020 | 20 | 34 | 12 | 5 |
| 2020–2021 | 23 | 19 | 15 | 1 |
| 2021–2022 | 30 | 24 | 11 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peavey, M.; Scalisi, A.; Islam, M.S.; Goodwin, I. Fruit Position, Light Exposure and Fruit Surface Temperature Affect Colour Expression in a Dark-Red Apple Cultivar. Horticulturae 2024, 10, 725. https://doi.org/10.3390/horticulturae10070725
Peavey M, Scalisi A, Islam MS, Goodwin I. Fruit Position, Light Exposure and Fruit Surface Temperature Affect Colour Expression in a Dark-Red Apple Cultivar. Horticulturae. 2024; 10(7):725. https://doi.org/10.3390/horticulturae10070725
Chicago/Turabian StylePeavey, Madeleine, Alessio Scalisi, Muhammad S. Islam, and Ian Goodwin. 2024. "Fruit Position, Light Exposure and Fruit Surface Temperature Affect Colour Expression in a Dark-Red Apple Cultivar" Horticulturae 10, no. 7: 725. https://doi.org/10.3390/horticulturae10070725





