Screening and Verification of Aquaporin Gene AsPIP1-3 in Garlic (Allium sativum L.) under Salt and Drought Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Drought and Salt Stress Treatment
2.2.1. Garlic Drought and Salt Stress Treatment
2.2.2. Treatment of Drought Stress in Transgenic Arabidopsis thaliana
2.3. Measurement Indicators and Methods
2.3.1. Growth Indicators
2.3.2. Relative Moisture Content
2.3.3. Photosynthetic Pigment Content
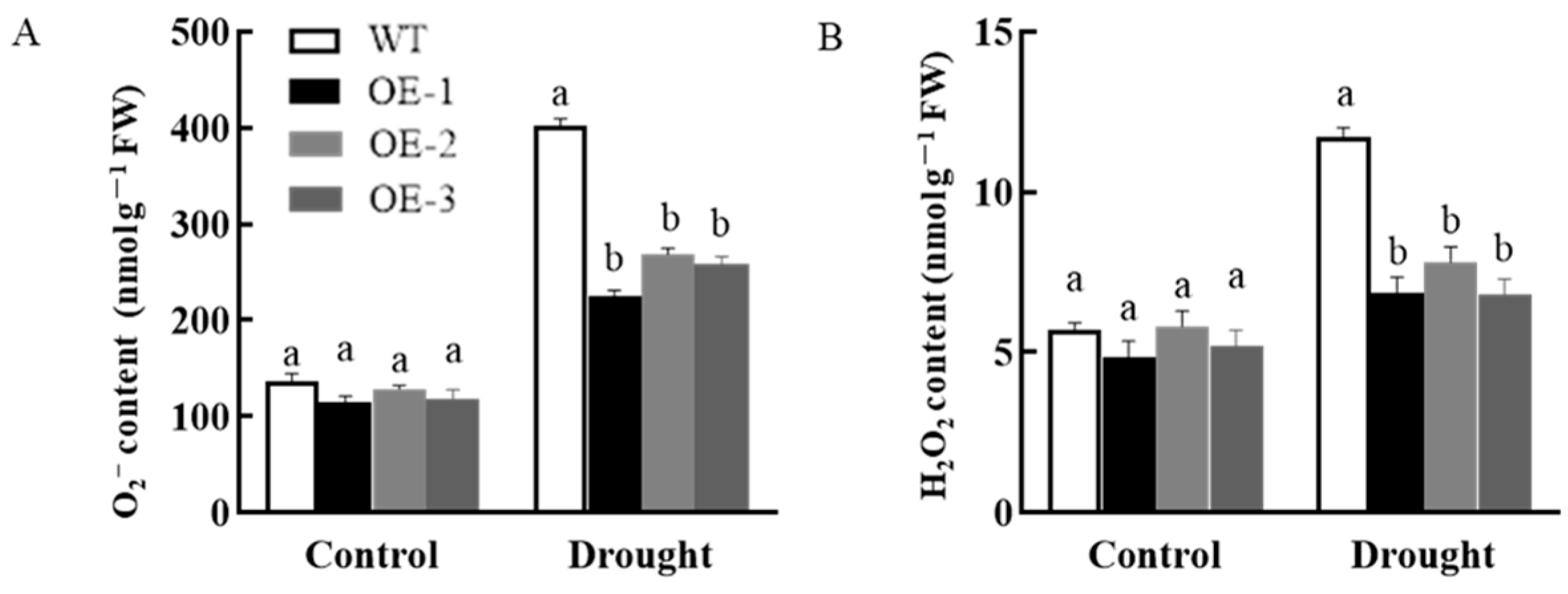
2.3.4. Superoxide Anion (O2−) Generation Rate
2.3.5. Hydrogen Peroxide (H2O2) Content
2.3.6. Antioxidant Enzyme Activity
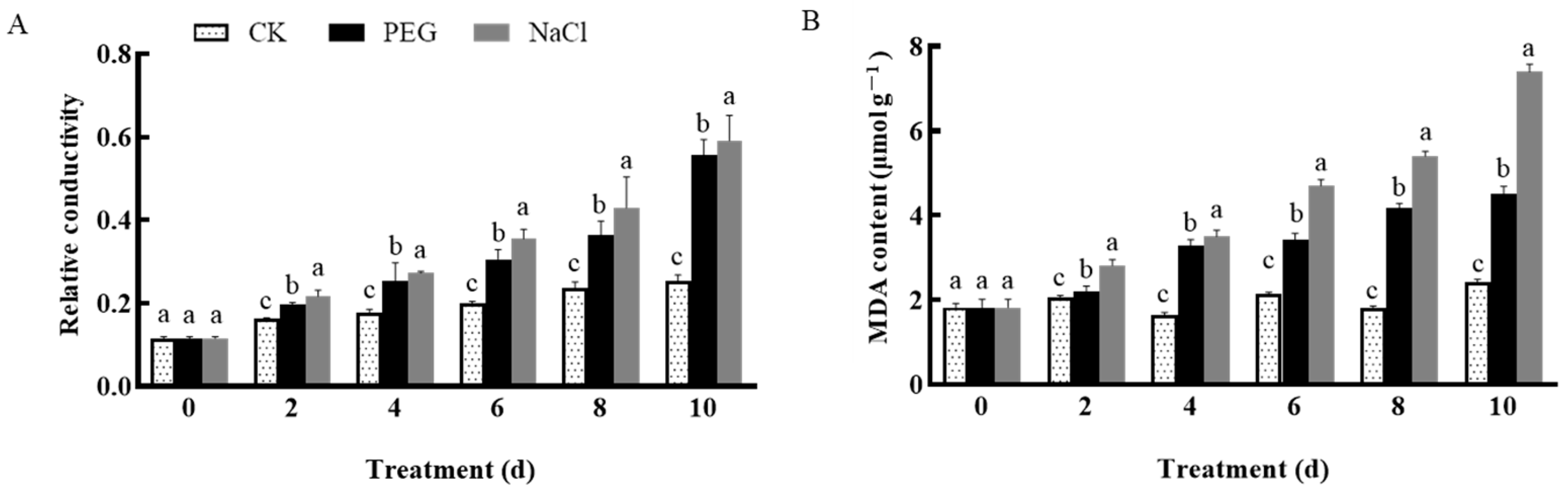
2.3.7. MDA Content
2.3.8. Relative Conductivity
2.3.9. Soluble Sugar Content
2.3.10. Proline Content
2.4. Expression Analysis of Garlic AQP Genes under Abiotic Stress
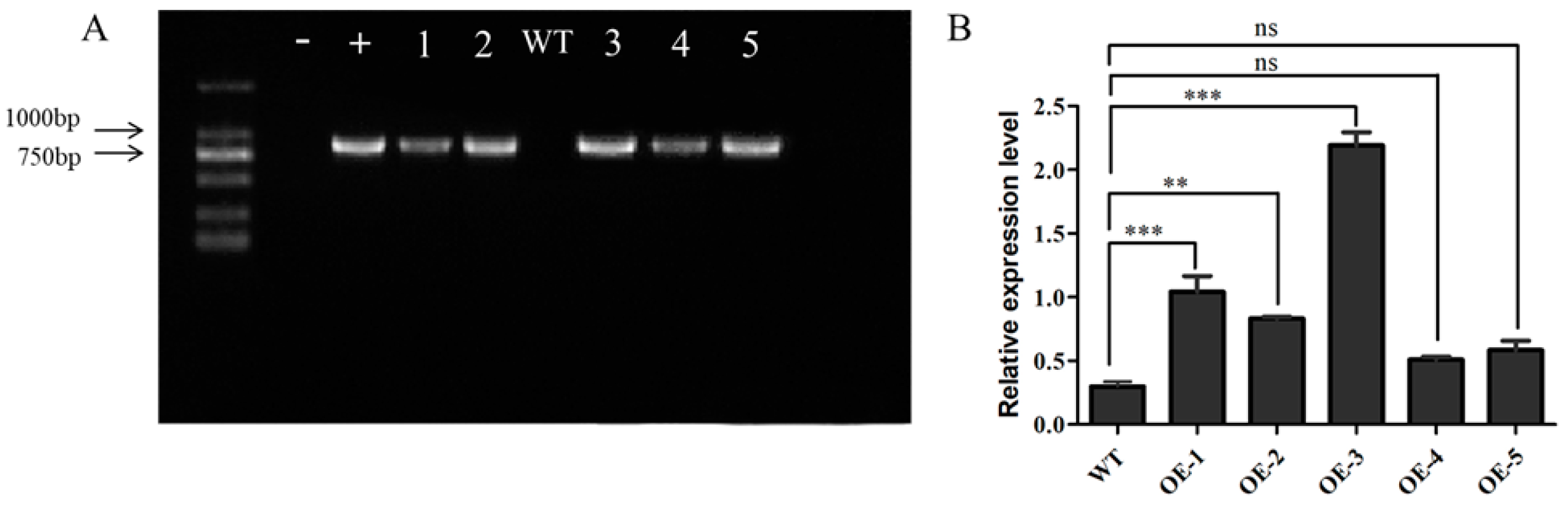
2.5. Garlic AsPIP3-1 Overexpression in Arabidopsis thaliana
2.6. Subcellular Localization of AsPIP3-1
2.7. Analysis of Stress-Related Gene Expression in Overexpressed Strains
2.8. Data Analysis
3. Results
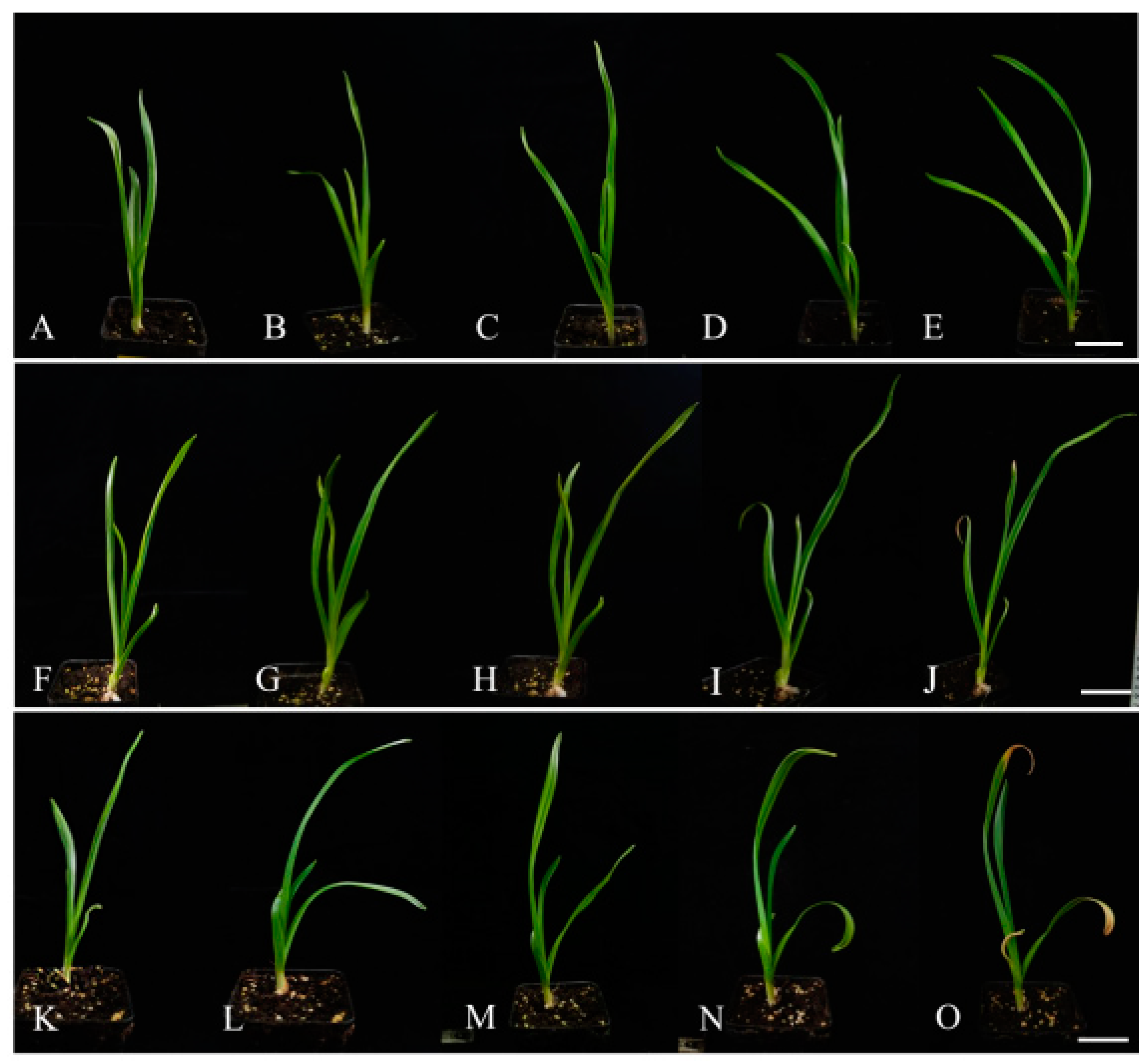
3.1. Effects of Drought and Salt Stress on Garlic Plant Growth
3.1.1. Effect on Garlic Morphology
3.1.2. Effect on Garlic Growth
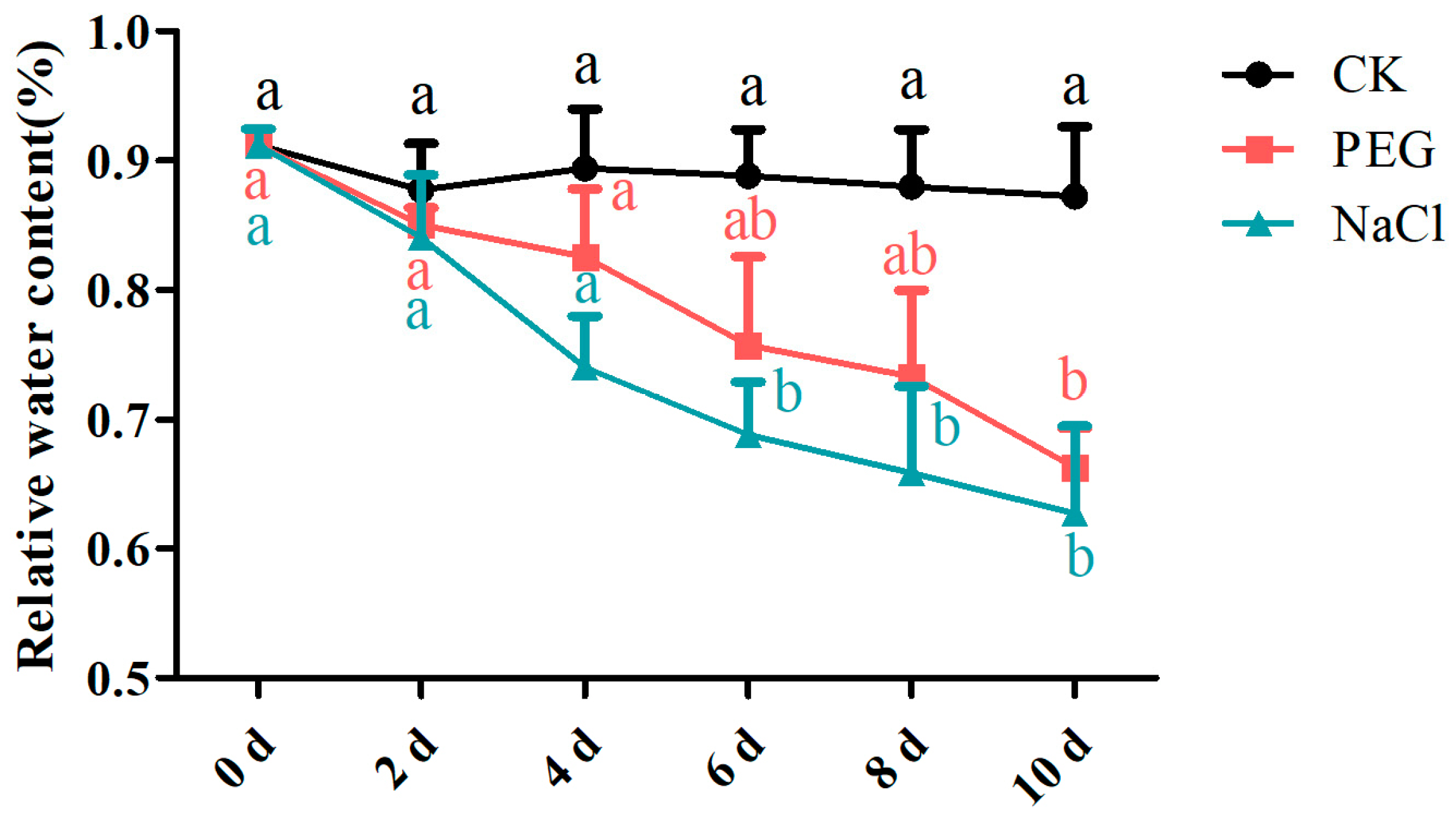
3.2. Changes in Leaf Relative Water Content and Chlorophyll Content under Drought and Salt Stress
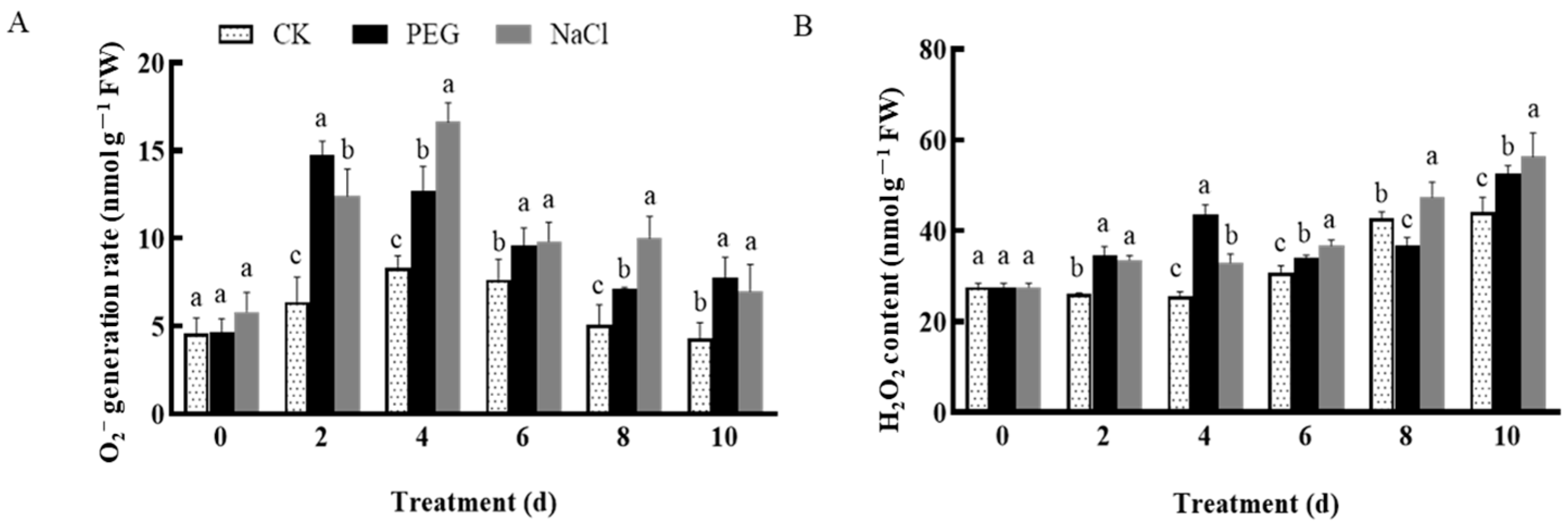
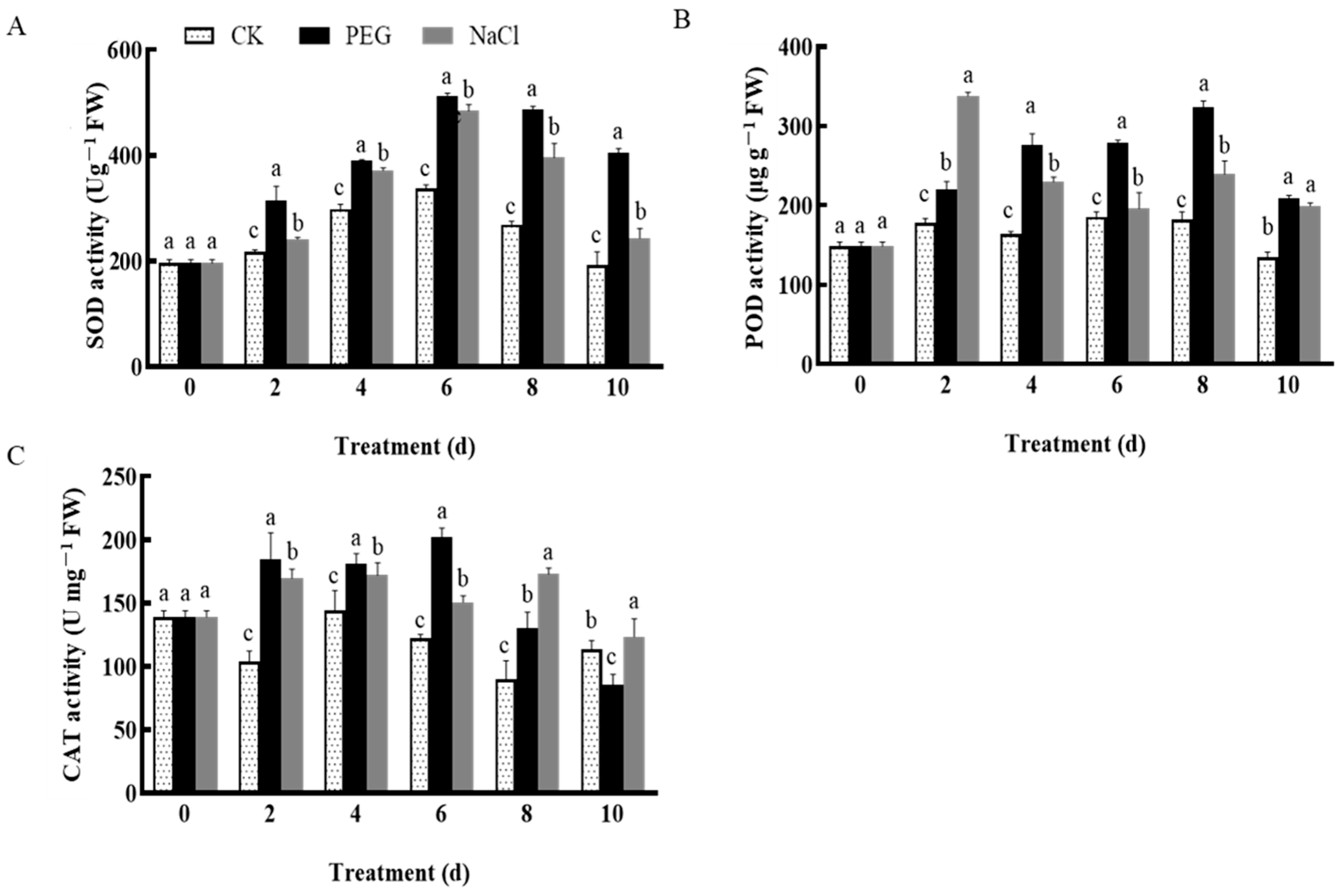
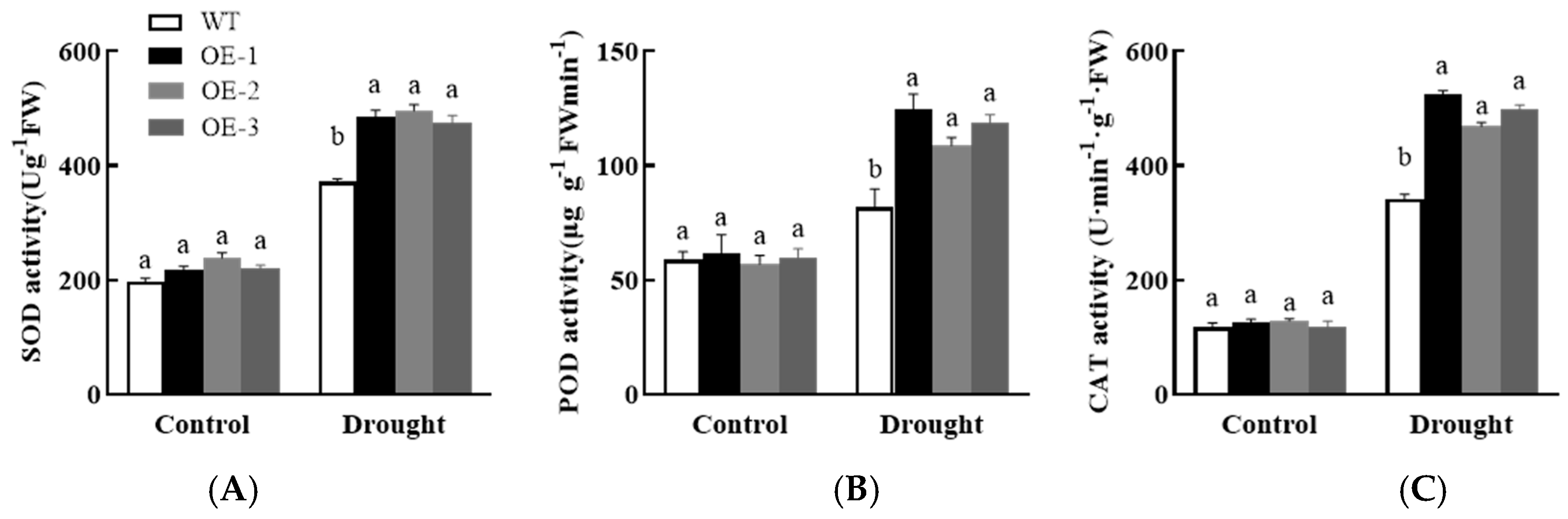
3.3. Changes in Active Oxygen Species and Antioxidant Enzymes in Garlic under Drought and Salt Stress
3.3.1. Changes in Reactive Oxygen Species
3.3.2. Changes in Antioxidant Enzyme Activity
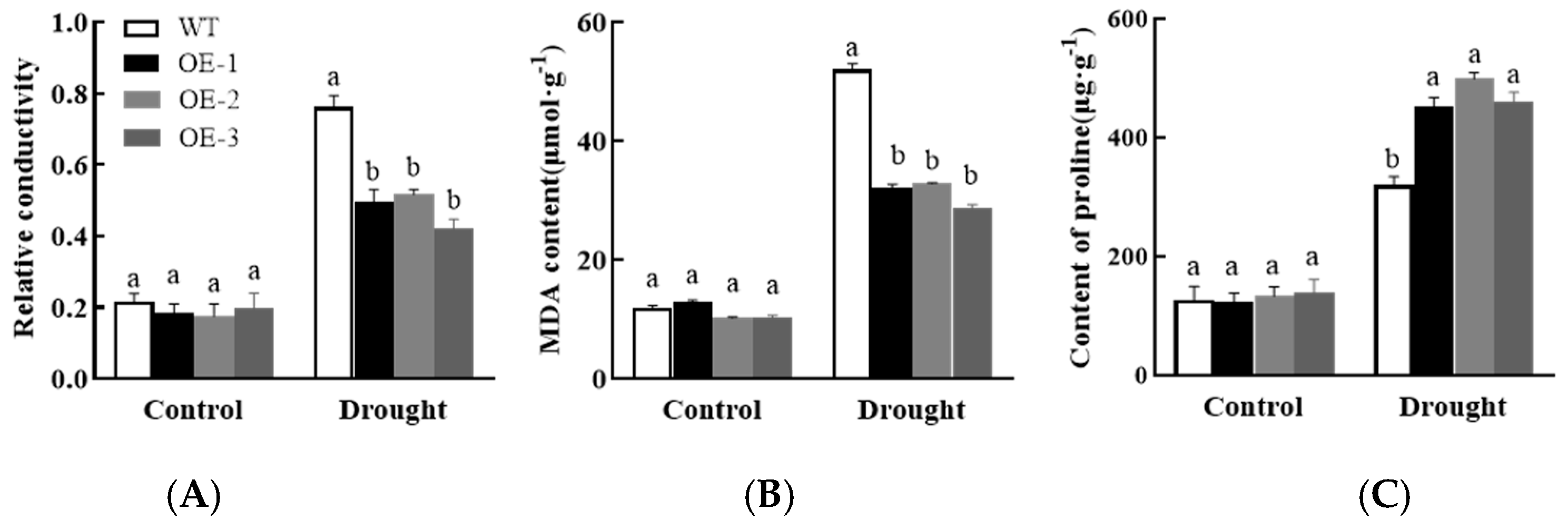
3.4. Effects of Drought and Salt Stress on Garlic Plasma Membrane Permeability and Osmotic Regulatory Substance Content
3.4.1. Changes in Garlic Plasma Membrane Permeability
3.4.2. Changes in the Content of Osmotic Regulating Substances
3.5. Expression Analysis of AsAQPs Genes under Drought and Salt Stress
3.6. AsPIP1-3 Protein Subcellular Localization Results
3.7. Functional Identification of Transgenic Arabidopsis thaliana Overexpressing AsPIP1-3
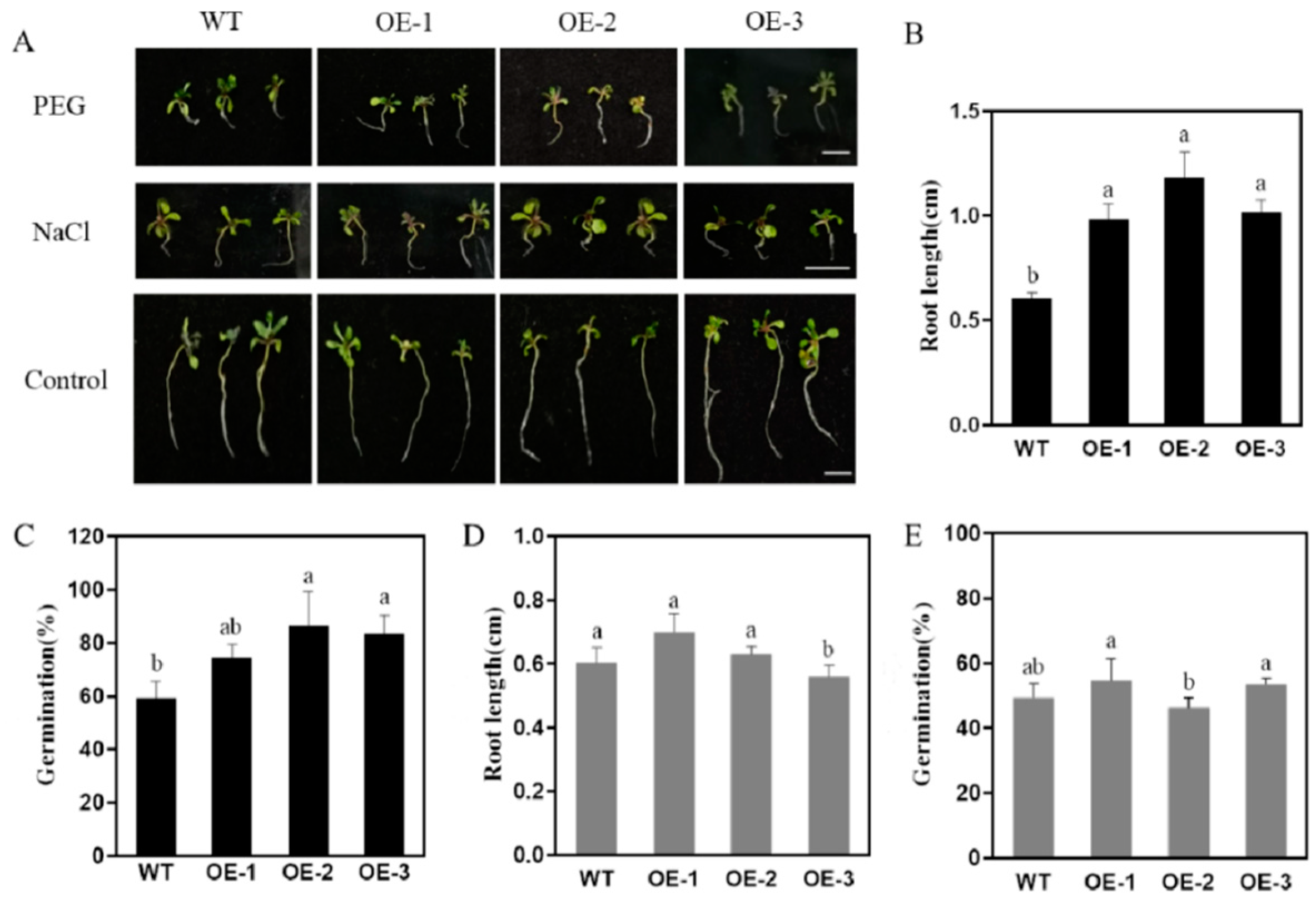
3.7.1. Phenotypic Analysis of Arabidopsis thaliana Seedlings Transgenic with AsPIP1-3 under Osmotic Stress
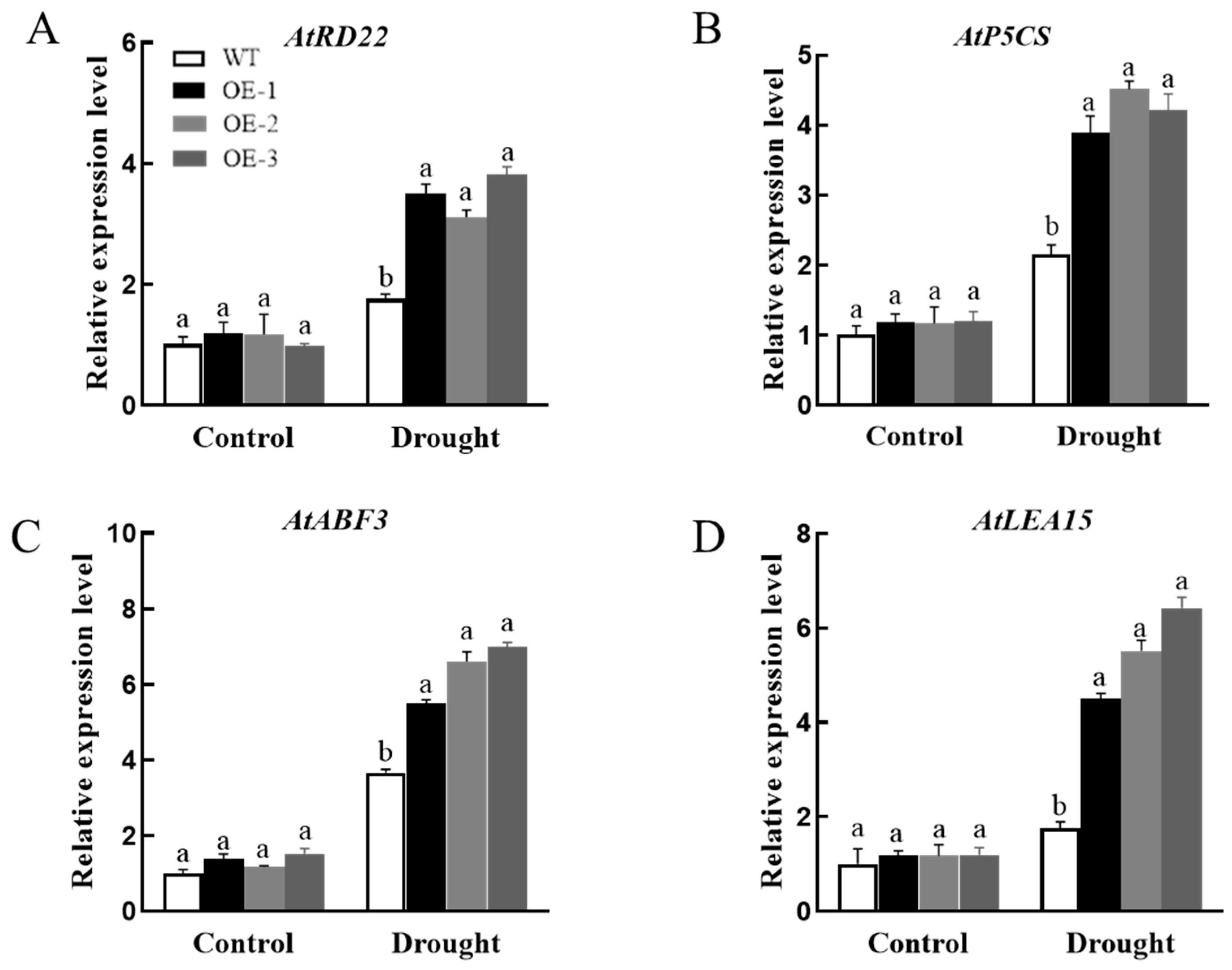
3.7.2. Analysis of Physiological, Biochemical, and Drought Stress-Related Gene Expression in Plants Overexpressing AsPIP1-3
4. Discussion
4.1. Response of Garlic Aquaporin to Drought and Salt Stress
4.2. Overexpression of AsPIP1-3 Improves Drought Tolerance of Transgenic Plants
4.3. Mechanism of Overexpression of AsPIP1-3 to Improve Drought Tolerance in Transgenic Plants
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Namazi, H. The role of garlic in the prevention of ischemia-reperfusion injury: A new mechanism. Mol. Nutr. Food Res. 2010, 52, 739. [Google Scholar] [CrossRef] [PubMed]
- Botas, J.; Fernandes, N.; Barros, L.; Alves, M.J.; Carvalho, A.M.; Ferreira, I. A comparative study of black and white Allium sativum L: Nutritional composition and bioactive properties. Molecules 2019, 24, 2194. [Google Scholar] [CrossRef] [PubMed]
- Kumari, N.; Kumar, M.; Radha Lorenzo, J.M.; Sharma, D.; Puri, S.; Pundir, A.; Dhumal, S.; Bhuyan, D.J.; Jayanthy, G.; Selim, S.; et al. Onion and garlic polysaccharides: A review on extraction, characterization, bioactivity, and modifications. Int. J. Biol. Macromol. 2022, 219, 1047–1061. [Google Scholar] [CrossRef] [PubMed]
- Murtaza, G.; Usman, M.; Iqbal, J.; Tahir, M.N.; Elshikh, M.S.; Alkahtani, J.; Toleikienė, M.; Iqbal, R.; Akram, M.I.; Gruda, N.S. The impact of biochar addition on morpho-physiological characteristics, yield and water use efficiency of tomato plants under drought and salinity stress. BMC Plant Biol. 2024, 24, 356. [Google Scholar] [CrossRef] [PubMed]
- Tester, M.; Davenport, R. Na+ tolerance and Na+ transport in higher plants. Ann. Bot. 2003, 91, 503–527. [Google Scholar] [CrossRef] [PubMed]
- Saha, D.; Choyal, P.; Mishra, U.N.; Dey, P.; Bose, B.; Md, P.; Gupta, N.K.; Mehta, B.K.; Kumar, P.; Pandey, S.; et al. Drought stress responses and inducing tolerance by seed priming approach in plants. Plant Stress 2022, 4, 100066. [Google Scholar] [CrossRef]
- Khan, M.N.; Khan, Z.; Luo, T.; Liu, J.; Rizwan, M.; Zhang, J.; Xu, Z.; Wu, H.; Hu, L. Seed priming with gibberellic acid and melatonin in rapeseed: Consequences for improving yield and seed quality under drought and non-stress conditions. Ind. Crops Prod. 2020, 156, 112850. [Google Scholar] [CrossRef]
- Zhou, L.; Zhou, J.; Xiong, Y.; Liu, C.; Wang, J.; Wang, G.; Cai, Y. Overexpression of a maize plasma membrane intrinsic protein ZmPIP1;1 confers drought and salt tolerance in Arabidopsis. PLoS ONE 2018, 13, e0198639. [Google Scholar] [CrossRef]
- Liu, C.; Fukumoto, T.; Matsumoto, T.; Gena, P.; Frascaria, D.; Kaneko, T.; Katsuhara, M.; Zhong, S.; Sun, X.; Zhu, Y.; et al. Aquaporin OsPIP1;1 promotes rice salt resistance and seed germination. Plant Physiol. Biochem. 2013, 63, 151–158. [Google Scholar] [CrossRef]
- Else, M.A.; Coupland, D.; Dutton, L.; Jackson, M.B. Decreased root hydraulic conductivity reduces leaf water potential, initiates stomatal closure and slows leaf expansion in flooded plants of castor oil (Ricinus communis) despite diminished delivery of ABA from the roots to shoots in xylem sap. Physiol. Plant. 2001, 111, 46–54. [Google Scholar] [CrossRef]
- Pedrosa, A.M.; Martins, C.P.; Gonçalves, L.P.; Costa, M.G. Late embryogenesis abundant (LEA) constitutes a large and diverse family of proteins involved in development and abiotic stress responses in sweet orange (Citrus sinensis L. Osb.). PLoS ONE 2015, 10, e0145785. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Yang, S.; Shi, K.; Yang, L.; An, M.; Wang, F.; Qi, F.; Feng, M.; Wang, M.; Gen, P.; et al. Genome-wide identification of the LRX gene family in Cucurbitaceae and expression analysis under salt and drought stress in Cucumber. Veg. Res. 2024, in press. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, L.; Zhang, Q.; Yan, X.; Zuo, C.; Song, X.; Yuan, M. Comprehensive identification of GSK3 gene family in celery and functional characterization of AgSK22 involvement in salt stress. Veg. Res. 2023, 3, 23. [Google Scholar] [CrossRef]
- Shen, L.; Yang, S.; Xia, X.; Nie, W.; Yang, X. Genome-wide identification of Kip-related protein (KRP) gene family members in eggplant and the function of SmKRP3 under salt stress. Veg. Res. 2024, 4, e013. [Google Scholar] [CrossRef]
- Guo, L.; Wang, Z.Y.; Lin, H.; Cui, W.E.; Chen, J.; Liu, M.; Chen, Z.L.; Qu, L.J.; Gu, H. Expression and functional analysis of the rice plasma-membrane intrinsic protein gene family. Cell Res. 2006, 16, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Raturi, G.; Kumawat, S.; Mandlik, R.; Duhan, D.; Thakral, V.; Sudhakaran, S.; Ram, C.; Sonah, H.; Deshmukh, R. Deciphering the Role of Aquaporins Under Different Abiotic Stress Conditions in Watermelon (Citrullus lanatus). J. Plant Growth Regul. 2023, 42, 3137–3149. [Google Scholar] [CrossRef]
- Mahdieh, M.; Mostajeran, A.; Horie, T.; Katsuhara, M. Drought stress alters water relations and expression of PIP-type aquaporin genes in Nicotiana tabacum plants. Plant Cell Physiol. 2008, 49, 801–803. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.D.; Guo, D.L.; Pei, M.S.; Liu, H.N.; Weng, T.L.; Yu, Y.H. Identification of grapevine AQP family and prediction for transcriptional regulatory network underdrought stress. J. Fruit Sci. 2021, 38, 1638–1652. [Google Scholar]
- Boursiac, Y.; Boudet, J.; Postaire, O.; Luu, D.T.; Tournaire-Roux, C.; Maurel, C. Stimulus-induced downregulation of root water transport involves reactive oxygen species-activated cell signalling and plasma membrane intrinsic protein internalization. Plant J. 2008, 56, 207–218. [Google Scholar] [CrossRef]
- Chen, Q.; Yang, S.; Kong, X.; Wang, C.; Xiang, N.; Yang, Y.; Yang, Y. Molecular cloning of a plasma membrane aquaporin in Stipa purpurea, and exploration of its role in drought stress tolerance. Gene 2018, 665, 41–48. [Google Scholar] [CrossRef]
- Al-Quraan, N.A.; Samarah, N.H.; Tanash, A.A. Effect of drought stress on wheat (Triticum durum) growth and metabolism: Insight from GABA shunt, reactive oxygen species and dehydrin genes expression. Funct. Plant Biol. 2022, 51, FP22177. [Google Scholar] [CrossRef] [PubMed]
- Blokhina, O.; Virolainen, E.; Fagerstedt, K.V. Antioxidants, oxidative damage and oxygen deprivation stress: A review. Ann. Bot. 2003, 91, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Hu, W.; Liu, J.; Zhang, J.; Jia, C.; Miao, H.; Xu, B.; Jin, Z. A banana aquaporin gene, MaPIP1;1, is involved in tolerance to drought and salt stresses. BMC Plant Biol. 2014, 14, 59. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, C.; Wang, T.; Guo, Z.; Lu, S. Overexpression of MfPIP2-7 from Medicago falcata promotes cold tolerance and growth under NO3 deficiency in transgenic tobacco plants. BMC Plant Biol. 2016, 16, 138. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Liu, Y.; Zhou, K.; Tian, C.; Aslam, M.; Zhang, B.; Liu, W.; Zou, H. Overexpression of ZmEREBP60 enhances drought tolerance in maize. J. Plant Physiol. 2022, 275, 153763. [Google Scholar] [CrossRef] [PubMed]
- Alavilli, H.; Awasthi, J.P.; Rout, G.R.; Sahoo, L.; Lee, B.H.; Panda, S.K. Overexpression of a barley aquaporin gene, Hvpip2;5 confers salt and osmotic stress tolerance in yeast and plants. Front. Plant Sci. 2016, 7, 1566–1578. [Google Scholar] [CrossRef]
- Yamada, M.; Morishita, H.; Urano, K.; Shiozaki, N.; Yamaguchi-Shinozaki, K.; Shinozaki, K.; Yoshiba, Y. Effects of free proline accumulation in petunias under drought stress. J. Exp. Bot. 2005, 56, 1975–1981. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.A.; Cho, J.I.; Han, M.; Ahn, C.H.; Jeon, J.S.; An, G.; Park, P.B. The ABRE-binding bZIP transcription factor OsABF2 is a positive regulator of abiotic stress and ABA signaling in rice. J. Plant Physiol. 2010, 167, 1512–1520. [Google Scholar] [CrossRef]
- Trovato, M.; Mattioli, R.; Costantino, P. From A. rhizogenes RolD to plant P5CS: Exploiting proline to control plant development. Plants 2018, 7, 108. [Google Scholar] [CrossRef]





| Primer Name | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| AsPIP1-3 | TTCCGCTACTGATGCCAAGA | TGCCGGTAATCGGAATCGTA |
| AsPIP2-1 | GTAGCCACGGTTATCGGGTA | AAGATCATGCCACCGAAAGC |
| AsPIP2-7 | TATCCCTAGTGCGAGCAGTG | TCACGAATCCTACACCGCAT |
| AsPIP2-8 | GGATTTGCCGTGTTCATGGT | GTCATGCCATGCTTCGTCTT |
| AsPIP2-9 | TATGGTGCACTTGGCTACGA | AAACAACAGCAGCGCCTAAA |
| AsTIP2-6 | TTACTGGGTAGGTCCGCTTG | ATCCTGAGCACAGGCTCAT |
| AsTIP2-8 | CGGAGACAGGAACACCTACA | AGTGAGTCGATTCTCGCTGT |
| AsNIP5-1 | CGGAGACAGGAACACCTACA | AGTGAGTCGATTCTCGCTGT |
| AsACTIN | TCCTAACCGAGCGAGGCTACAT | GGAAAAGCACTTCTGGGCACC |
| Primer Name | Primer Sequence (5′-3′) |
|---|---|
| pBinGFP4-F | CAAGCAATCAAGCATTCTAC |
| pBinGFP4-R | CGGACACGCTGAACTTGTGG |
| AsPIP1-3–pBinGFP4-F | atttacgaacgatagggtaccATGGCAGAGAAAGATGAAAGTGTG |
| AsPIP1-3–pBinGFP4-R | gcccttgctcaccatggatccGTCCTTGGTTTTGAACGGTATAGC |
| AsPIP1-3–pCBIMA1300-F | cacgttgccatgcagcgtacgATGGCAGAGAAAGATGAAAGTGTG |
| AsPIP1-3–pCBIMA1300-R | gttcttggccttcttcgtacgGTCCTTGGTTTTGAACGGTATAGC |
| AtP5CS-F | GGGACAAGTTGTGGATGGAGAC |
| AtP5CS-R | TGGTACAAACCTCAAGGAACAC |
| AsLEA15-F | TTCGACTTGGTACCCTGATTAC |
| AsLEA15-R | GGAAGAAGATCAGCTACATCGA |
| AtABF3-F | GATGTGGTTAACCGTTCTCAAC |
| AtABF3-R | CAGCTTGCAGTAGATTGTTGTT |
| AtRD22-F | GACTTTCGATTTTACCGACGAG |
| AtRD22-R | CGCTACCGGTTTTACCTTTATG |
| AtACTIN-F | GAATGGAAGCTGCTGGAATCCACG |
| AtACTIN-R | AACGATTCCTGGACCTGCCTCATC |
| Treatments | Days (d) | Plant Height (cm) | Pseudo Stem Diameter (mm) | Leaf Length (cm) | Leaf Width (mm) | Fresh Weight (g) | Dry Weight (g) |
|---|---|---|---|---|---|---|---|
| CK | 0 | 17.93 ± 0.98 c | 5.16 ± 0.12 cd | 13 ± 0.58 d | 10.37 ± 0.39 ab | 4.7 ± 0.32 d | 0.8 ± 0.15 c |
| 2 | 18.87 ± 0.26 bc | 5.69 ± 0.07 bc | 13.83 ± 0.23 d | 10.56 ± 0.29 ab | 5.3 ± 0.45 d | 0.99 ± 0.11 bc | |
| 4 | 21.47 ± 1.07 abc | 5.55 ± 0.28 bc | 16.17 ± 0.6 cd | 10.07 ± 0.89 ab | 5.47 ± 0.24 cd | 1.19 ± 0.11 abc | |
| 6 | 22.53 ± 1.52 ab | 5.93 ± 0.26 b | 19.07 ± 1.41 bc | 10.67 ± 0.35 ab | 6.63 ± 0.32 bc | 1.23 ± 0.32 ab | |
| 8 | 22.53 ± 1.73 ab | 6.02 ± 0.1 ab | 17.37 ± 1.26 cd | 10.57 ± 0.37 ab | 7.83 ± 0.73 ab | 1.3 ± 0.26 ab | |
| 10 | 23.43 ± 1.42 a | 6.16 ± 0.13 a | 22.4 ± 1.51 a | 11.83 ± 0.93 a | 9.13 ± 1.62 a | 1.48 ± 0.34 a | |
| PEG | 0 | 17.93 ± 0.98 c | 5.16 ± 0.12 cd | 13 ± 0.58 d | 10.37 ± 0.39 ab | 4.37 ± 0.33 d | 0.89 ± 0.17 bc |
| 2 | 19.37 ± 0.09 bc | 5.47 ± 0.22 bc | 13.6 ± 1.62 d | 10.01 ± 0.47 ab | 4.63 ± 0.19 d | 0.97 ± 0.09 bc | |
| 4 | 21.83 ± 0.84 ab | 5.73 ± 0.3 bc | 15.73 ± 0.37 cd | 9.25 ± 0.17 b | 5.17 ± 0.69 cd | 1.01 ± 0.12 bc | |
| 6 | 20.63 ± 0.55 abc | 5.81 ± 0.35 abc | 18.4 ± 1.63 bc | 10.13 ± 0.72 ab | 5.78 ± 0.18 cd | 0.96 ± 0.09 bc | |
| 8 | 22.6 ± 1.46 ab | 5.76 ± 0.45 bc | 18.77 ± 1.66 bc | 10.27 ± 0.3 ab | 5.71 ± 0.2 cd | 0.9 ± 0.15 bc | |
| 10 | 23.37 ± 1.52 a | 5.89 ± 0.44 ab | 20.07 ± 2.49 b | 11.03 ± 0.52 ab | 6.67 ± 0.67 bc | 0.98 ± 0.2 bc | |
| NaCl | 0 | 17.93 ± 0.98 c | 5.16 ± 0.12 c | 13 ± 0.58 f | 10.37 ± 0.39 ab | 3.66 ± 0.5 d | 0.79 ± 0.16 c |
| 2 | 19.37 ± 0.09 bc | 5.11 ± 0.05 d | 14.43 ± 0.94 def | 9.96 ± 0.14 ab | 5.23 ± 0.92 cd | 1.04 ± 0.09 bc | |
| 4 | 21.83 ± 0.84 ab | 5.34 ± 0.1 cd | 16.53 ± 0.29 de | 9.25 ± 0.17 b | 5.67 ± 0.96 bc | 1.04 ± 0.16 bc | |
| 6 | 20.63 ± 0.55 abc | 5.61 ± 0.2 bc | 17.67 ± 0.52 bc | 10.1 ± 0.23 ab | 6.27 ± 0.27 bc | 1.01 ± 0.15 bc | |
| 8 | 22.1 ± 1.46 ab | 5.46 ± 0.09 bc | 18.43 ± 1.24 bc | 10.25 ± 1.02 ab | 6.51 ± 0.32 bc | 0.92 ± 0.14 bc | |
| 10 | 23.17 ± 1.52 a | 5.75 ± 0.11 bc | 19.43 ± 0.98 b | 10.63 ± 1.21 ab | 6.3 ± 0.57 bc | 0.96 ± 0.14 bc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, H.; Ruan, J.; Zhou, R.; Bai, Y.; Liu, M.; Jiang, F.; Wu, Z. Screening and Verification of Aquaporin Gene AsPIP1-3 in Garlic (Allium sativum L.) under Salt and Drought Stress. Horticulturae 2024, 10, 738. https://doi.org/10.3390/horticulturae10070738
Wei H, Ruan J, Zhou R, Bai Y, Liu M, Jiang F, Wu Z. Screening and Verification of Aquaporin Gene AsPIP1-3 in Garlic (Allium sativum L.) under Salt and Drought Stress. Horticulturae. 2024; 10(7):738. https://doi.org/10.3390/horticulturae10070738
Chicago/Turabian StyleWei, Hanyu, Jiaojiao Ruan, Rong Zhou, Yunhe Bai, Min Liu, Fangling Jiang, and Zhen Wu. 2024. "Screening and Verification of Aquaporin Gene AsPIP1-3 in Garlic (Allium sativum L.) under Salt and Drought Stress" Horticulturae 10, no. 7: 738. https://doi.org/10.3390/horticulturae10070738
APA StyleWei, H., Ruan, J., Zhou, R., Bai, Y., Liu, M., Jiang, F., & Wu, Z. (2024). Screening and Verification of Aquaporin Gene AsPIP1-3 in Garlic (Allium sativum L.) under Salt and Drought Stress. Horticulturae, 10(7), 738. https://doi.org/10.3390/horticulturae10070738









